Abstract
Cyclooxygenases (COX-1 and COX-2) oxygenate arachidonic acid (AA) to generate prostaglandins. The enzymes associate with one leaflet of the membrane bilayer. We utilized nanodisc technology to investigate the function of human (hu) COX-2 and murine (mu) COX-2 in a lipid bilayer environment. huCOX-2 and muCOX-2 were incorporated into nanodiscs composed of POPC, POPS, DOPC, or DOPS phospholipids. Size-exclusion chromatography and negative stain electron microscopy confirm that a single COX-2 homodimer is incorporated into the nanodisc scaffold. Nanodisc-reconstituted COX-2 exhibited similar kinetic profiles for the oxygenation of AA, eicosapentaenoic acid, and 1-arachidonoyl glycerol compared to those derived using detergent solubilized enzyme. Moreover, changing the phospholipid composition of the nanodisc did not alter the ability of COX-2 to oxygenate AA or to be inhibited by various nonselective NSAIDs or celecoxib. The cyclooxygenase activity of nanodisc-reconstituted COX-2 was reduced by aspirin acetylation and potentiated by the nonsubstrate fatty acid palmitic acid to the same extent as detergent solubilized enzyme, independent of phospholipid composition. The stabilization and maintenance of activity afforded by the incorporation of the enzyme into nanodiscs generates a native-like lipid bilayer environment to pursue studies of COX utilizing solution-based techniques that are otherwise not tractable in the presence of detergents.
Keywords: cyclooxygenase, nanodisc, arachidonic acid, nonsteroidal anti-inflammatory drugs, phospholipid, aspirin
Cyclooxygenases (COX-1 and COX-2) oxygenate arachidonic acid (AA) to generate potent lipid signaling molecules collectively known as prostaglandins (1). Aspirin, nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) and COX-2 selective diaryl-heterocycle-based drugs (coxibs) inhibit COX-2 (2,3). COX-1 and COX-2 are sequence homodimers composed of tightly associated monomers. The tertiary structure of each monomer consists of an N-terminal epidermal growth factor-like domain involved in dimerization, a membrane-binding domain (MBD) and a large catalytic domain at the C-terminus (4). The catalytic domain houses physically distinct cyclooxygenase and peroxidase active sites that are functionally linked via a heme moiety. Recent biochemical studies utilizing various fatty acids and COX inhibitors indicate that COX functions as a conformational heterodimer during catalysis and inhibition, such that only one monomer of the dimer can function at a given time (5). COX also functions as an allosteric/catalytic couple, in which the cyclooxygenase activity in one monomer is modulated by the nature of the ligand bound in the cyclooxygenase active site of the opposite monomer (6–8). For example, the nonsubstrate fatty acid palmitic acid (PA) binds to one monomer and stimulates the cyclooxygenase activity of COX-2 in the partner monomer (6). However, the mechanism controlling the crosstalk between monomers has yet to be identified.
COX catalysis and inhibition takes place in the context of a membrane environment. The enzymes are localized to the lumenal membrane of the ER and the nuclear membrane, where they associate with one leaflet of the membrane bilayer (9). Bilayer association occurs via interactions with amphipathic α-helices located in the MBD of COX (10–12). Analysis of the COX-1 and COX-2 crystal structures reveals an opening between the α-helices of the MBD that leads to the entrance of the cyclooxygenase channel (13–16). It is hypothesized that substrates and inhibitors, which are hydrophobic and readily partition into membranes, travel from the lipid bilayer into the cyclooxygenase channel through the opening in the MBD (4). Indeed, detergent molecules, derived from the solubilization and purification of COX, are resolved within the opening of the MBD in some COX crystal structures, lending support to the substrate access hypothesis (14,17).
There has been limited focus on studying COX catalysis and inhibition in a more natural lipid environment. Rand Doyen and colleagues characterized the kinetics of COX-1 and COX-2 in the presence of phosphatidylcholine molecules of varying acyl chain lengths and physical state (18). These studies revealed that monomeric, micelle, and bilayer forms of phospholipids exhibit an inhibitory effect on cyclooxygenase catalysis, predominantly due to sequestration of substrate (18). MirAfzali and colleagues successfully incorporated COX-1 and COX-2 into large unilamellar vesicles produced from a mixture of DOPC:DOPS that had been doped with oleic acid (19). While incorporation of COX into the DOPS:DOPC:oleic acid vesicles did not significantly affect cyclooxygenase activity, no subsequent studies were performed to characterize the kinetics of COX catalysis and inhibition in these vesicles (11).
Nanodisc technology has proven to be a valuable tool in the study of membrane protein structure and function (20–23). Nanodiscs are comprised of a small circular patch of lipid bilayer that is rendered soluble by two amphipathic α-helical membrane scaffold proteins (MSP) that encircle the circumference of the bilayer (24). The surface area of the lipid bilayer is defined by the particular MSP used to create the nanodiscs. Various MSP constructs, derived from apo-lipoprotein A1, have been utilized to create nanodiscs with diameters typically ranging from ~9-15nm (25). Additionally, lipids with different head groups and varying acyl chain lengths can be used in nanodisc formulation. The ability to tailor the dimensions and bilayer composition of the discs makes the technology highly versatile and amenable to various membrane protein targets and experimental techniques.
We present here studies designed to further investigate COX catalysis and inhibition in a natural lipid bilayer. We reconstituted huCOX-2 and muCOX-2 into nanodiscs containing different phospholipids and isolated pure COX-2:nanodisc complexes using a dual affinity purification protocol. Incorporation of a single homodimer of COX-2 into the nanodisc was validated using size-exclusion chromatography (SEC) and negative-stain electron microscopy (EM). We subsequently used the system to investigate COX-2 catalysis and inhibition utilizing the COX-2 substrates AA, eicosapentaenoic acid (EPA), and 1-arachidonoyl glycerol (1–AG) and a variety of nonselective NSAIDs, aspirin, and COX-2 selective celecoxib. Finally, we characterized the ability of PA to potentiate the cyclooxygenase activity of COX-2. Our results indicate that nanodisc-reconstituted COX-2 exhibits the same catalytic, inhibitory, and activation properties as detergent solubilized COX-2. As such, the use of nanodisc-reconstituted COX-2 provides access to solution-based techniques to investigate COX catalysis and inhibition that have otherwise been elusive due to the presence of detergent or the large size and polydispersity of liposomes.
EXPERIMENTAL PROCEDURES
Materials
The fatty acids AA (5Z,8Z,11Z,14Z-eicosatetraenoic acid), eicosapentaenoic acid (EPA; 5Z,8Z,11Z,14Z,17Z)-eicosapentaenoic acid), and palmitic acid (PA; hexadecanoic acid), and the inhibitors ibuprofen, flurbiprofen, meclofenamic acid, naproxen, indomethacin, and aspirin were purchased from Cayman Chemical Company (Ann Arbor, MI). Celebrex® (celecoxib) was from a physician sample. Fe3+-protoporphyrin IX was purchased from Frontier Scientific (Logan, UT). Decyl maltoside (C10M) was purchased from Affymetrix (Santa Clara, CA). N-octyl β-D-glucopyranoside (βOG) was purchased from Inalco Pharmaceuticals (San Luis Obispo, CA). The QuikChange™ Mutagenesis kit II was purchased from Agilent Technologies (Santa Clara, CA). The Bac-to-Bac® baculovirus expression kit, and associated reagents, including Spodoptera frugiperda 21 (Sf21) insect cells, fetal bovine serum, fungizone, penicillin-streptomycin, and sf-900 III serum free media were purchased from Invitrogen (Carlsbad, CA). HiTrap™ HP Chelating and HiPrep™ Superdex 200 10/300-GL chromatography columns were purchased from GE Healthcare (Piscataway, NJ). Oligos used for site-directed mutagenesis were purchased from Integrated DNA Technologies (Coralville, IA). The phospholipids 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn- glycero-3-phospho-L-serine (DOPS), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS) were purchased from Avanti Polar Lipids (Alabaster, AL). Sodium cholate hydrate from ox or sheep bile ≥99%, Triton-X-100, anti-FLAG M2 affinity resin, and FLAG peptide were from Sigma-Aldrich (St. Louis, MO). BioBeads SM-2 were purchased from Bio-Rad (Hercules, MA).
Construction of human and mouse FLAG-COX-2
His6 N580A murine (mu) COX-2 in pFastbac1 from (16) was used as a template to engineer FLAG N580A muCOX-2 (FLAG muCOX-2). We utilized the QuikChange™ mutagenesis kit II to replace the His6 sequence, which was inserted between Asn-19 and Pro-20 in muCOX-2 (26), with the sequence DYKDDDDK, using the following primer (note: forward primers are listed only; inserted amino acids are boldface and underlined): 5’-GCCCTGGGGCTCAGCCAGGCAGCAAATGATTACAAGGATGACGACGATAAGCCTTGCTGTTCCAATCCATGTCAAAACC-3’. The N580A mutation prevents variable glycosylation at Asn-580 during expression (27). The resulting FLAG muCOX-2 construct was cloned into the Sma1 and Nhe1 restriction sites of pFastbac Dual so that expression was driven from the p10 promoter of the vector. The equivalent FLAG N580A human (hu) COX-2 construct (FLAG huCOX-2) was generated in the same manner using His6 N580A huCOX-2 in pFastbac1 as the template and the following primer: 5’-GCTCAGCCATACAGCAGACTACAAGGACGACGATGACAAGAATCCTTGCTGTTCCC-3’. The resulting FLAG huCOX-2 construct in pFastbac1 was used for expression, with protein expressed from the polH promoter of the vector. Each construct was verified by DNA sequence analysis at the Roswell Park Cancer Institute DNA Sequencing Laboratory.
Expression and Purification of FLAG-COX-2 Constructs
The expression of FLAG huCOX-2 and FLAG muCOX-2 was carried out in insect cells as described in (16). Cell pellet from a 2L culture was resuspended in 50mM TRIS, pH 7.4, 300mM NaCl followed by the addition of a protease inhibitor cocktail tablet (Roche, Indianapolis, IN). The cells were then lysed using a microfluidizer (Microfluidics, Newton, MA). Decyl maltoside (C10M) was added to the lysed cells to a final concentration of 0.87% (w/v) followed by stirring at 4°C for 1 hour to solubilize COX-2. The mixture was subsequently centrifuged at 100,000×g for 60 minutes to pellet insoluble debris. The supernatant was loaded onto a column (2.5cm×10cm) containing 10mL of Anti-FLAG M2 affinity resin equilibrated in 50mM TRIS, pH 7.4, 150mM NaCl, 0.87% (w/v) C10M. The resin was washed with 10 column volumes of 50mM TRIS, pH 7.4, 150mM NaCl, 0.53% (w/v) β-octyl-glucoside (βOG) before eluting bound COX-2 with 100μg/mL FLAG peptide in 50mM TRIS, pH 7.4, 150mM NaCl, 0.53% (w/v) βOG. Fractions containing COX-2 were pooled and dialyzed overnight against 50mM TRIS, pH 7.4, 150mM NaCl, 0.53% (w/v) βOG. The dialyzed protein was then concentrated to ~4mg/mL using a spin concentrator with a 50kDa molecular weight cutoff (Millipore, Billerica, MA).
Expression and Purification of Membrane Scaffold Protein 1E3D1(+)
MSP1E3D1(+) in pET-28a was obtained from AddGene (Cambridge, MA). The membrane scaffold protein construct contains an N-terminal His7 tag for purification. The plasmid was transformed into E. coli BL21(DE3) cells. For large-scale expression, 1L flasks containing TB media were inoculated with cells at 37°C and grown to an OD600 of 2.5. The cells were induced by the addition of IPTG to a final concentration of 1mM and harvested 3 hours post induction via centrifugation. Pellets were stored at −80°C until further use.
For purification, a cell pellet corresponding to a 1L growth was resuspended in 50mL of 100mM TRIS, pH 8.0, 300mM NaCl, and a protease inhibitor cocktail tablet, followed by lysis using a microfluidizer. Triton X-100 was added to the lysate to a final concentration of 1% (v/v) and the solution was stirred at 4°C for 30 minutes. The mixture was centrifuged at 30,000×g for 30 minutes and the supernatant was loaded onto two 5mL HiTrap Chelating HP columns connected in series that were equilibrated in 40mM TRIS, pH 8.0, 300mM NaCl, 1% (v/v) Triton X-100. The columns were then washed sequentially with 100mL of: a) equilibration buffer; b) 40mM TRIS, pH 8.0, 300mM NaCl, 20mM imidazole, 50mM sodium cholate; and c) 40mM TRIS, pH 8.0, 300mM NaCl, 50mM imidazole. Prior to elution, the columns were separated and MSP1E3D1(+) was eluted from each column separately using 40mM TRIS, pH 8.0, 300mM NaCl, 500mM imidazole. Fractions containing MSP1E3D1(+) were pooled and diluted to ~2mg/mL, followed by dialysis overnight at 4°C against 4L 40mM TRIS, pH 7.4, 100mM NaCl, 0.5mM EDTA. The dialyzed protein was then concentrated to a final concentration of 6–8mg/mL using a spin concentrator with a 50kDa molecular weight cutoff. The protein was dispersed into 250μL aliquots, frozen in liquid nitrogen, and stored at −80°C until further use.
Incorporation of COX-2 into Nanodiscs
COX-2 was reconstituted into nanodiscs comprised of POPC, POPS, DOPC, or DOPS phospholipids. Briefly, stock phospholipids were dissolved in chloroform, their concentrations were determined by total phosphate analysis using the manufacturer’s protocol, followed by resuspension in 50mM TRIS, pH 7.4, 150mM NaCl, and sodium cholate for use in nanodisc assembly. The sodium cholate concentration was at a concentration twice that of the phospholipid. The resulting mixtures were chilled on ice prior to the addition of MSP1E3D1(+) and detergent solubilized COX-2 to achieve a final phospholipid:MSP1E3D1(+):COX-2 molar ratio of 1950:15:1. The volume of the incorporation mixture was designed such that the final concentration of sodium cholate was at least 20mM and twice the concentration of the phospholipid. The mixtures utilizing POPC, DOPC, and DOPS were incubated at 4°C for 1 hour, followed by the addition of 0.8g of BioBeads per mL of reconstitution mixture. For POPS, the mixture was incubated at room temperature for 1 hour, followed by BioBeads addition. Incorporation mixtures were incubated with BioBeads overnight to initiate detergent removal and concomitant nanodisc assembly. The BioBeads were subsequently removed and the resulting sample was dialyzed for 4 hours at 4°C against 1L 50mM TRIS, pH 7.4, 150mM NaCl. For purification of nanodisc-reconstituted COX-2, the dialyzed sample was loaded onto a column (2.5cm × 10cm) containing 10mL of Anti-FLAG M2 affinity resin, washed with 10 column volumes of 50mM TRIS, pH 7.4, 150mM NaCl, and eluted with wash buffer containing 100μg/mL FLAG peptide. The elution fractions from the FLAG affinity column were pooled and loaded onto a 1mL HiTrap Chelating HP column. The column was washed with 10 column volumes of 50mM TRIS, pH 7.4, 150mM NaCl, followed by 10 column volumes of 50mM TRIS, pH 7.4, 150mM NaCl, 35mM imidazole. Nanodisc-reconstituted COX-2 was eluted from the column with 50mM TRIS, pH 7.4, 150mM NaCl, 350mM imidazole. Fractions were pooled and dialyzed overnight at 4°C against 25mM TRIS, pH 7.4, 150mM NaCl. For size-exclusion chromatographic analysis, dual affinity purified muCOX-2 reconstituted into POPC nanodiscs was loaded onto a Superdex 200 10/300 GL column equilibrated in 50mM TRIS, pH 7.4, 100mM NaCl.
Negative Stain Electron Microscopy
POPC nanodiscs incorporated with muCOX-2 were injected onto a Superdex 200 10/300 GL column equilibrated with 20mM TRIS, pH 7.5, 100mM NaCl. The peak fraction was collected and adsorbed onto glow discharged carbon-coated copper grids, stained with 0.7% (w/v) uranyl formate, and inspected on a JEOL 2100F electron microscope operated at 200 kV with a Tietz CCD camera. Images were acquired at a magnification of 78,473x corresponding to 2.93Å/pixel. 6,069 particles were windowed into 150 × 150 pixel individual images, aligned to each other, subjected to ten cycles of multireference alignment, and k-means classification. All image processing steps were carried out using SPIDER (28).
Activity Assays
Cyclooxygenase and peroxidase activity were measured as described in (16), with heme added to the enzyme prior to activity measurements. The stoichiometry of heme binding to nanodisc-reconstituted COX-2 was equivalent to that observed in (6). Enzyme concentrations for nanodisc-reconstituted COX-2 were determined using a combination of A280 spectroscopic measurement and densitometry analysis performed on SDS-PAGE gels with known concentrations of both bovine serum albumin and detergent solubilized COX-2 run in parallel. Assays were initiated by adding the equivalent of 5μg COX-2 in a volume of 20μL to the reaction mixtures. For inhibition studies, detergent solubilized COX-2 or nanodisc-reconstituted COX-2 at 0.25mg/mL in a volume of 150μL was incubated with 50μM of flurbiprofen, meclofenamic acid, indomethacin, naproxen, or celecoxib for 30 minutes on ice prior to injection into the oxygen electrode cuvette. For inhibition studies utilizing ibuprofen, 100μM inhibitor was utilized in the 30-minute incubation and also added to the cuvette to a final concentration of 100μM prior to the initiation of the reaction. IC50 values were also calculated for flurbiprofen and celecoxib. The values were derived from two independent measurements carried out in triplicate, and reported as ± SD. Aspirin inhibition was evaluated by incubating detergent solubilized huCOX-2 or muCOX-2 reconstituted in POPC nanodiscs at 0.25mg/mL with 500μM aspirin at room temperature, followed by measuring of the oxygenase activity at 30 minute intervals post aspirin addition. Measurements were corrected for generation of the monohydroxy product (15R)-hydroxyeicosatetraenoic acid by aspirin acetylated COX-2, which was verified using [1-14C]-AA as described in (29,30). Activation studies utilizing PA were carried out as described previously (6).
RESULTS
Incorporation of COX-2 into Nanodiscs
Analysis of the muCOX-2 x-ray crystal structure indicates that the COX-2 homodimer has a length of ~7nm when opposite extremes of the MBD are measured and ~9.5nm when opposite extremes of the catalytic domain are measured (16). As such, we chose to utilize the MSP1E3D1 scaffold, which has a diameter of 12.9nm when incorporated with phospholipids (24,31). To facilitate isolation of the COX-2:nanodisc complex, we utilized huCOX-2 and muCOX-2 constructs engineered with a FLAG tag sequence behind the native signal sequence of the enzymes (denoted as FLAG huCOX-2 and FLAG muCOX-2, respectively), along with His-tagged MSP1E3D1 scaffold protein (denoted as (MSP1E3D1(+)). FLAG COX-2 was expressed in insect cells, detergent solubilized, and purified using an anti FLAG M2 affinity column, while MSP1E3D1(+) was expressed in E. coli and purified using immobilized metal affinity chromatography (IMAC).
Our initial incorporation efforts focused on generating a muCOX-2 nanodisc complex comprised of 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC). Approximately 4 mg of affinity purified FLAG muCOX-2 and 28mg of MSP1E3D1(+) were mixed with POPC solubilized in sodium cholate. BioBeads were then added to the incorporation mixture to facilitate both detergent removal and the formation of the muCOX-2:POPC nanodisc complex (POPC:muCOX-2). After the removal of the BioBeads, a two-step affinity purification protocol consisting of anti FLAG M2 and IMAC was utilized to isolate pure POPC:muCOX-2. Figure 1A shows an SDS-PAGE gel summarizing the purity obtained after each affinity step. Approximately 1.2mg of FLAG muCOX-2 was reconstituted into POPC nanodiscs, resulting in ~30% incorporation efficiency.
Figure 1. Biophysical Characterization of POPC:muCOX-2.
(A) SDS-PAGE gel depicting the results from the dual affinity purification of nanodisc-reconstituted COX-2. Lane 1, molecular weight marker; lane 2, detergent solubilized COX-2 control; lane 3 MSP1E3D1(+) control; lane 4, crude nanodisc incorporation mixture following detergent removal with BioBeads; lane 5, FLAG column flow through; lanes 6–9, FLAG affinity column wash fractions; lane 10, purified nanodisc-reconstituted COX-2 eluted from IMAC affinity column. (B) SEC Analysis of incorporation: muCOX-2 reconstituted into POPC nanodiscs (solid line); empty POPC nanodiscs (dotted line).
We utilized SEC and negative stain EM to determine the number of COX homodimers that were incorporated into a given nanodisc. Given that COX-2 associates with one leaflet of the lipid bilayer, it is possible that a COX-2 homodimer could interact with each side of the bilayer in the nanodisc, although reconstitution ratios were designed to favor a single incorporation. The molecular weight (MW) of an empty POPC nanodisc is approximately 263kDa, assuming 260 POPC lipids per nanodisc (25). The MW for the protein portion of a COX-2 homodimer is 140kDa. A POPC nanodisc incorporated with one COX-2 homodimer would exhibit a MW of approximately 403kDa, while a POPC nanodisc incorporated with two COX-2 homodimers would exhibit a MW of 543kDa. The chromatogram resulting from SEC analysis of dual affinity purified POPC:muCOX-2 and empty POPC nanodisc is shown in Figure 1B. The empty POPC nanodisc has a retention volume of 12.3mL on the column, corresponding to an approximate MW of 270kDa when compared to the retention volumes of globular standard proteins utilized to calibrate the resolution properties of the column. The peak corresponding to POPC:muCOX-2 exhibits a retention volume of 11.1mL, corresponding to an approximate MW of 420kDa. Thus, SEC analysis is consistent with incorporation of a single COX-2 homodimer into the POPC nanodisc. There is limited separation of high MW species on a Superdex 200 10/300 GL column. To further confirm that a single homodimer of COX-2 was incorporated into the POPC nanodisc, we performed negative stain EM analysis of SEC purified POPC:muCOX-2. Analysis of the negative stain EM data indicates that POPC:muCOX-2 are predominantly incorporated with a single muCOX-2 homodimer (Figure 2).
Figure 2. Negative Stain EM Analysis of POPC:muCOX-2.
(A) Representative negative-stain EM micrograph of POPC:muCOX-2. (B) 2D k-means class averages of POPC:muCOX-2 particles. 6,069 particles were windowed into 150 × 150 pixel individual images, aligned to each other, and subjected to ten cycles of multireference alignment and k-means classification. The numbers at the bottom right designate the number of particles used in each average and the box size of each image is 44nm × 44nm. The images suggest that the COX-2 homodimer lies at the edge of the nanodisc scaffold. However, this is due to the nature of the sample preparation for negative-stain EM analysis, which flattens the sample prior to analysis. (C) Model depicting muCOX-2 (PDB id 3HS5) bound to one leaflet of the POPC nanodisc. Nanodisc scaffold proteins and POPC lipids are colored green and gray, respectively. The muCOX-2 homodimer is colored blue, with the MBD of each monomer colored red.
Cyclooxygenase Activity of Nanodisc-reconstituted COX-2
The enzyme activity of detergent solubilized FLAG muCOX-2 and POPC:muCOX-2 was evaluated by spectrophotometric measurement of the peroxidase activity and determination of the cyclooxygenase kinetics using an oxygen electrode. POPC:muCOX-2 retained complete peroxidase activity when compared to detergent solubilized FLAG muCOX-2 (data not shown). In addition, the product profiles generated from incubation of [1-14]-AA by nanodisc-reconstituted and detergent solubilized enzyme were the same (Figure 3). We utilized the fatty acid substrates AA, EPA, as well as the endocannabinoid substrate 1-AG, to characterize the cyclooxygenase kinetics of detergent solubilized FLAG muCOX-2 and POPC:muCOX-2. The kcat and KM values for the oxygenation of AA, EPA, and 1-AG by POPC:muCOX-2 were similar to the values derived for these substrates using detergent solubilized FLAG muCOX-2 and in line with those reported previously for detergent solubilized histidine-tagged huCOX-2 and muCOX-2 (16,32,33) (Table 1). Collectively, these observations confirm that incorporating muCOX-2 into a nanodisc does not adversely affect cyclooxygenase catalysis.
Figure 3. Product Formation by Nanodisc-reconstituted and Detergent Solubilized huCOX-2.
RP-HPLC product profiles derived from the incubation of [1-14C]-AA with (A) nanodisc-reconstituted and (B) detergent solubilized huCOX-2. A Waters Symmetry C18 column (4.6×250mm) was eluted at 1 mL/min flow rate with a stepwise gradient of acetonitrile:water:acetic acid 37.5:62.5:0.01 (by vol.) for 15 minutes followed by 70:30:0.01 (by vol.) for 15 minutes, and then with methanol. The peak marked with an asterisk is an artifact from switching from the first solvent to the second solvent.
Table 1. Kinetic Properties of Substrate Oxygenation by FLAG muCOX-2 and POPC:muCOX-2.
Cyclooxygenase activity was measured using an oxygen electrode with 1–200µM AA, EPA, and 1-AG utilized as COX-2 substrates. Reactions were initiated by the addition of 5µg of detergent solubilized FLAG muCOX-2 or POPC nanodisc-reconstituted COX-2 calculated to contain 5µg of muCOX-2. Values represent the mean values of three measurements ± SEM.
| Substrate | Detergent Solubilized POPC | Nanodisc-reconstituted | ||||
|---|---|---|---|---|---|---|
| Kcat (s−1) |
KM (µM) |
Kcat/KM | Kcat (s−1) |
KM (µM) |
Kcat/KM | |
| AA | 36.1 ± 0.6 | 6.8 ± 0.5 | 5.3 | 32.7 ± 0.6 | 5.4 ± 0.4 | 6.1 |
| EPA | 6.3 ± 0.9 | 1.9 ± 0.2 | 3.3 | 8.4 ± 0.2 | 1.5 ± 0.2 | 5.6 |
| 1-AG | 30.9 ± 0.7 | 6.8 ± 0.6 | 4.5 | 38.0 ± 1.5 | 10.4 ± 1.3 | 3.7 |
To evaluate the affect of various phospholipids on cyclooxygenase catalysis, we incorporated FLAG huCOX-2 into nanodiscs comprised of POPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS). COX-2 incorporation efficiencies into POPS, DOPS, and DOPC nanodiscs were estimated to be between 25%-30%, similar to that observed for the POPC:muCOX-2 incorporations. The resulting huCOX-2 incorporated POPC, POPS, DOPC, and DOPS nanodiscs were assayed for cyclooxygenase activity using 100μM AA as the substrate. The rate of oxygenation of AA by nanodisc-incorporated huCOX-2 was found to be essentially the same for each phospholipid tested when compared to each other and equivalent to that of detergent solubilized FLAG huCOX-2 (Figure 4). POPC:muCOX-2 and POPS:muCOX-2 behaved similarly as both exhibited the same rate of AA oxygenation as detergent solubilized FLAG muCOX-2 (data not shown). Taken together, the data indicate that cyclooxygenase activity is not altered when different phospholipids are utilized within the nanodisc scaffold.
Figure 4. Comparison of the Cyclooxygenase Activity of Nanodisc-reconstituted huCOX-2 Comprised of Different Phospholipids.
Bar graphs depicting the relative activity of huCOX-2 reconstituted in nanodiscs containing different phospholipids (gray bar) compared to detergent solubilized FLAG huCOX-2 (black bar). Cyclooxygenase activity was measured using an oxygen electrode and 100μM AA as the substrate. Normalized VMAX values were derived from the mean values of three measurements ± SEM and a value of 100% was assigned for the cyclooxygenase activity of detergent solubilized FLAG huCOX-2. Relative activities were calculated as: POPC:huCOX-2, 90%; DOPC:huCOX-2, 102%; DOPS:huCOX-2, 104%; POPS:huCOX-2, 100%.
Inhibition of Nanodisc-reconstituted COX-2
We assessed the ability of COX inhibitors to inhibit the cyclooxygenase activity of COX-2 that had been incorporated into nanodiscs comprised of POPC, POPS, DOPC, and DOPS and compared the results to the inhibition of detergent solubilized enzyme. Six compounds that fall into three of the four known modes of COX inhibition were utilized (34). The nonselective NSAIDs flurbiprofen, indomethacin, and meclofenamic acid are representatives of COX inhibitors that exhibit tight-binding, time-dependent properties of inhibition. Celecoxib is also a timedependent inhibitor that is selective for COX-2. Ibuprofen is classified as a nonselective, competitive, freely reversible inhibitor and naproxen is classified as “mixed” inhibitor of COX-2, given its propensity to cause initial time-dependent loss of activity that ultimately plateaus without reaching complete inhibition of the enzyme. We examined the magnitude of inhibition of detergent solubilized and nanodisc incorporated COX-2 by these compounds following a 30-minute pre-incubation. The time-dependent inhibitors were utilized at a final concentration of 50μM, while ibuprofen was utilized at a final concentration of 100μM. Ibuprofen was also included in the assay chamber at a final concentration of 100μM. Overall, detergent solubilized and nanodisc incorporated COX-2 exhibited identical inhibition profiles for all of the compounds evaluated (Figure 5). Flurbiprofen, indomethacin, meclofenamic acid, and celecoxib caused near complete inhibition of cyclooxygenase activity. Naproxen inhibition resulted in a 60-70% loss of activity, while inhibition with ibuprofen caused a 50% loss of activity. We also calculated IC50 values for inhibition of POPC:huCOX-2 and detergent solubilized FLAG huCOX-2 with flurbiprofen and celecoxib. Flurbiprofen exhibited an IC50 of 2.08 ± 0.10μM against detergent solubilized huCOX-2 and 2.10 ± 0.16μM against nanodisc-reconstituted huCOX-2, while celecoxib exhibited an IC50 of 1.09 ± 0.03μM against detergent solubilized huCOX-2 and 1.00 ± 0.05μM against nanodiscreconstituted huCOX-2. The inhibition profiles observed here for nanodisc-reconstituted COX-2 also agree with previously published results that utilized detergent solubilized COX-2 (6,17,34). Thus, the cyclooxygenase activity of nanodisc-reconstituted COX-2 can be inhibited by different classes of nonselective NSAIDs and celecoxib to the same extent that detergent solubilized enzyme is inhibited. Changing the phospholipid composition of the nanodisc also has no affect on the magnitude of inhibition by the compounds utilized in this study.
Figure 5. NSAID Mediated Inhibition of COX-2 in Detergent and Nanodiscs.
Bar graphs depicting the ability of NSAIDs to inhibit COX-2 that has been reconstituted into nanodiscs with different phospholipids compared to inhibition of detergent solubilized COX-2 is shown for (A) muCOX-2 and (B) huCOX-2. A value of 100% is assigned for the cyclooxygenase activity of uninhibited FLAG COX-2, with the remaining cyclooxygenase activity after inhibition normalized to the uninhibited enzyme. Error bars represent the standard deviation between triplicate measurements. Abbreviations are as follows: IBP, ibuprofen; Napx, naproxen; FBP, flurbiprofen; Indo, indomethacin; Meclo, meclofenamic acid; CBX, celecoxib.
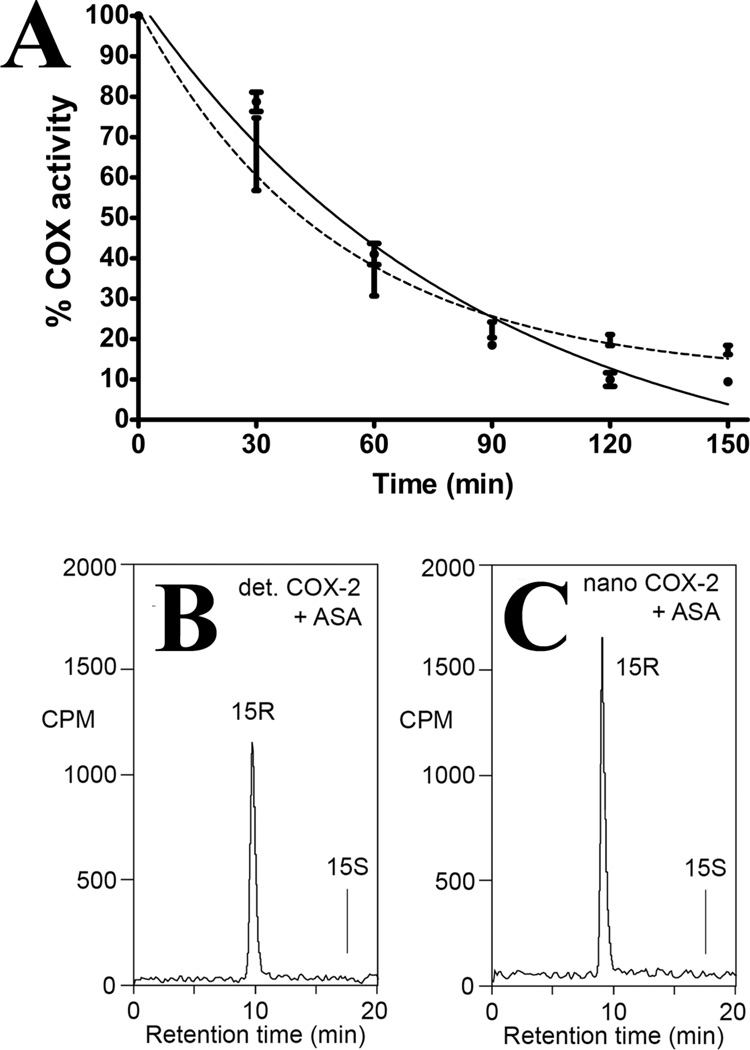
We next assessed the ability of aspirin to acetylate POPC:muCOX-2 and compared the results to the acetylation of detergent solubilized FLAG muCOX-2. The covalent acetylation of COX by aspirin represents the fourth known mode of COX inhibition (34). Aspirin acetylation of COX-1 results in complete inhibition of cyclooxygenase activity, whereas aspirin acetylation of COX-2 results in a shift in reaction specificity, converting activity from a cyclooxygenase to a lipoxygenase, generating the byproduct (15R)-hydroxyeicosatetraenoic acid (15R-HETE) (35,36). POPC:muCOX-2 and FLAG muCOX-2 were incubated with 500μM aspirin at room temperature and aliquots were removed in 30-minute intervals over the course of 150 minutes, followed by measurement of the remaining oxygenase activity using an oxygen electrode and characterization of the product profiles using [1-14C]-AA and chiral HPLC. The aspirin inhibition time-course profiles for POPC:muCOX-2 and FLAG muCOX-2 were virtually identical to one another (Figure 6A) and consistent with the inhibition studies carried out with the nonselective NSAIDs and celecoxib. The magnitude of aspirin inhibition observed for POPC:muCOX-2 was also in agreement with previously published findings (36,37). As expected, aspirin-acetylated POPC:huCOX-2 and FLAG huCOX-2 generated 15R-HETE as the major product derived from AA (Figure 6B and 6C).
Figure 6. Aspirin Inhibition of muCOX-2 in Detergent and Nanodiscs.
(A) Detergent solubilized muCOX-2 (solid line) and POPC:muCOX-2 (dashed line) were incubated with 500μM aspirin for the indicated times and then assayed for oxygenase activity using an oxygen electrode. Values represent the average of triplicate measurements ± SEM and were corrected for the production of monohydroxy product. Chiral analysis of 15-HETE formed by aspirin-acetylated (B) detergent solubilized and (C) nanodisc-reconstituted huCOX-2. A Chiralpak AD column (4.6×250mm) was eluted at 1 mL/min flow rate with a solvent of hexane/ethanol 100:2 (by vol). 15R-HETE represented greater than 98% of the total products formed for each analysis.
Activation of Nanodisc-reconstituted COX-2 by Palmitic Acid
Nonsubstrate fatty acids have been shown to stimulate cyclooxygenase catalysis in a concentration-dependent manner through an allosteric/catalytic couple (6,8). PA was found to be the most effective nonsubstrate fatty acid in modulating the cyclooxygenase activity. We assessed the ability of PA to potentiate the oxygenation of AA by COX-2 reconstituted into nanodiscs comprised of POPC, POPS, DOPC, and DOPS and compared the magnitude of the increase in cyclooxygenase activity to that observed by detergent solubilized enzyme. The rate of oxygenation of 5μM AA in the presence and absence of 25μM PA by nanodisc-reconstituted and detergent solubilized COX-2 was measured using an oxygen electrode. PA potentiated the oxygenation of AA by nanodisc-reconstituted COX-2 regardless of the phospholipid composition of the nanodisc (Figure 7). The magnitude of the increase in activity was between 130-160% for nanodisc-reconstituted huCOX-2 and 115-130% for nanodisc-reconstituted muCOX-2, which is comparable to that seen for detergent solubilized FLAG COX-2 used in this study and also in line with previously published results for huCOX-2 and muCOX-2 (6,38).
Figure 7. PA Potentiates AA Oxygenation in Nanodiscs.
Bar graphs depicting the potentiating affect of AA oxygenation in the presence of PA for detergent solubilized and nanodisc-reconstituted (A) huCOX-2 and (B) muCOX-2. The oxygenation of 5μM AA in the absence of PA is set to 100% (black bar), while the percent increase in the oxygenation of 5μM AA in the presence of 25μM PA is depicted by the gray bars. Values represent the average of triplicate measurements ± SEM.
DISCUSSION
COX enzymes have been the topic of intense research due to their wide implications in human physiology and high value as drug targets. Most of the structural and kinetic characterizations of COX-1 and COX-2 have been conducted using detergent solubilized enzyme to maintain stability of the enzyme outside of its natural membrane environment. However, molecular dynamics simulations suggest that the environment of the lipid bilayer may play an influential role in the function of COX (39). Hence, developing a system to characterize catalysis and inhibition in the membrane becomes necessary. The limited studies that have investigated a membrane bound form of COX-1 or COX-2 have largely been performed with either crude microsomal preparations or via incorporation of COX into large liposome vesicles (19). The isolation of microsomal preparations allows for the investigation of the catalytic properties of COX in a lipid environment, albeit in the presence of other protein components that can potentially limit or interfere with functional characterization. Construction of liposome vesicles provides for a homogeneous protein population for studies in a native lipid environment and even allows for the manipulation of the lipid composition within the vesicles. However, the resulting liposome vesicles are generally much larger than the target protein and typically contain many molecules of the purified membrane protein (19). In order to overcome these limitations and set the stage for the investigation of conformational dynamics involved in COX catalysis and allosteric regulation, we incorporated FLAG huCOX-2 and FLAG muCOX-2 into lipid bilayer nanodiscs of controlled size and lipid composition. The use of a dual affinity purification approach results in the isolation of highly pure nanodiscs incorporated with a single COX-2 homodimer as confirmed by SEC and negative stain EM analyses.
Given their chemical properties, it is not surprising that NSAIDs and fatty acid substrates of COX readily partition into lipid bilayers (40–42). However, in artificial lipid membrane systems, such as liposome vesicles, fatty acids and NSAIDs become sequestered in the large vesicles, subsequently limiting their movement into the enzyme active site and subsequent availability for catalysis and inhibition. As a consequence, the measured kinetic parameters become altered, making data interpretation challenging (18). In contrast to liposome vesicles, nanodisc incorporation confines COX-2 to a much smaller patch of lipid membrane such that substrates and inhibitors that enter the lipid bilayer are immediately localized in close proximity to the entrance of the cyclooxygenase channel. Indeed, we observed that huCOX-2 reconstituted into nanodiscs comprised of POPC, POPS, DOPC, and DOPS exhibited equivalent cyclooxygenase activity as that of detergent solubilized FLAG huCOX-2 when AA was utilized as the substrate. Moreover, the kinetic parameters derived from the oxygenation of AA, EPA, and 1-AG by nanodisc-reconstituted muCOX-2 were comparable to those obtained using detergent solubilized enzyme. Nonselective NSAIDs, celecoxib, and aspirin, which cover the four known modes of COX inhibition, reduced the cyclooxygenase activity of nanodisc-incorporated huCOX-2 and muCOX-2 to the same levels as that observed for detergent solubilized FLAG huCOX-2 and muCOX-2. Taken together, our results indicate that the nanodisc scaffold is a suitable membrane mimetic for the functional characterization of COX-2 in a lipid bilayer absent of detergent.
While the use of detergent greatly facilitates isolation and stabilization of COX, the role that detergent plays in influencing protein conformational dynamics related to catalysis and inhibition is not well understood. For example, x-ray crystallographic characterization of PA binding to COX-2 confirmed biochemical results indicating that PA binds to only one monomer of the COX homodimer, but did not provide any insight into the mechanism of crosstalk as both monomers were virtually identical when superimposed upon one another (6). Similarly, there were no differences observed between structures when x-ray crystallography was used to characterize the nuances associated with the inhibition of COX-1 by time-dependent versus time-independent inhibitors (43). Coupled with the observation that detergent molecules bind within the opening of the MBD of some COX crystal structures, it is interesting to speculate that the use of detergent to mimic lipid interactions combined with the restricted conformational freedom of the protein within a crystal lattice masks the conformational dynamics associated with allosteric modulation and time-dependent inhibition of COX. The allosteric regulation of COX-2 by nonsubstrate fatty acids has until now been only observed utilizing detergent solubilized enzyme. Our demonstration of PA activation of AA oxygenation by nanodisc-reconstituted COX-2 rules out that this observed modulation of catalysis is related to the presence of detergent in the system.
In summary, these studies validate the use of nanodisc technology to investigate the monotopic membrane protein COX-2 in a natural lipid environment devoid of detergent. The COX-2:nanodisc complexes generated here contain a single COX-2 homodimer and exhibit characteristic kinetic profiles for substrates that are comparable to those derived using detergent solubilized enzyme. Changing the phospholipid composition of the nanodisc also had no bearing on the ability of COX-2 to oxygenate AA or to be inhibited by nonselective NSAIDs or celecoxib. In addition, the activity of nanodisc-reconstituted COX-2 was reduced by aspirin acetylation and potentiated by PA to equivalent levels observed for detergent solubilized enzyme. The use of nanodisc-reconstituted COX-2 opens up a wide range of solution-based experiments that were otherwise intractable given the presence of detergent or large liposome vesicles and provides for the potential to elucidate the structural dynamics that drive the mechanisms of allosteric activation and time-dependent inhibition.
Highlights.
A single COX-2 homodimer is incorporated into the nanodisc scaffold.
Nanodisc reconstituted COX-2 retains its catalytic and inhibitory behaviors.
Nanodiscs provide sufficient maintenance of the integrity of COX-2.
Nanodiscs provide access to solution-based techniques for investigation of COX.
ACKNOWLEDGEMENTS
This research was supported by NIH grant R01 GM077176 to MGM, R01 GM076592 to CS, and UIUC start-up funds and a Research Board grant to AD. BJO is supported by graduate fellowships from KeyBank. DRM is supported by a Block grant. Cryo EM data collection at the NYSBC was made possible by a grant from NYSTAR and NIH RR017528.
The abbreviations used are
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PG
prostaglandin
- AA
arachidonic acid
- PA
palmitic acid
- EPA
eicosapentaenoic acid
- 1-AG
1-arachidonoyl glycerol
- mu
murine
- hu
human
- C10M
decyl maltoside
- βOG
N-octyl β-D-glucopyranoside
- POPC
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- DOPS
1,2-dioleoyl-sn-glycero-3-phospho-L-serine
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- POPS
1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine
- SEC
size-exclusion chromatography
- EM
electron microscopy
- IMAC
immobilized metal affinity chromatography
- FLAG muCOX-2
detergent solubilized FLAG-tagged mouse COX-2
- FLAG huCOX-2
detergent solubilized FLAG-tagged human COX-2
- POPC:muCOX-2
muCOX-2 incorporated into POPC nanodiscs
- POPC:huCOX-2
huCOX-2 incorporated into POPC nanodiscs
- MW
molecular weight
- MSP
membrane scaffold protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev. 2011;111:5821–5865. doi: 10.1021/cr2002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blobaum AL, Marnett LJ. Structural and functional basis of cyclooxygenase inhibition. J Med Chem. 2007;50:1425–1441. doi: 10.1021/jm0613166. [DOI] [PubMed] [Google Scholar]
- 3.DeWitt DL. Cox-2-selective inhibitors: the new super aspirins. Mol Pharmacol. 1999;55:625–631. [PubMed] [Google Scholar]
- 4.Garavito RM, Malkowski MG, DeWitt DL. The structures of prostaglandin endoperoxide H synthases-1 and -2. Prostaglandins Other Lipid Mediat. 2002;68–69:129–152. doi: 10.1016/s0090-6980(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 5.Yuan C, Rieke CJ, Rimon G, Wingerd BA, Smith WL. Partnering between monomers of cyclooxygenase-2 homodimers. Proc Natl Acad Sci U S A. 2006;103:6142–6147. doi: 10.1073/pnas.0601805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong L, Vecchio AJ, Sharma NP, Jurban BJ, Malkowski MG, Smith WL. Human cyclooxygenase-2 is a sequence homodimer that functions as a conformational heterodimer. J Biol Chem. 2011;286:19035–19046. doi: 10.1074/jbc.M111.231969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prusakiewicz JJ, Duggan KC, Rouzer CA, Marnett LJ. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry. 2009;48:7353–7355. doi: 10.1021/bi900999z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan C, Sidhu RS, Kuklev DV, Kado Y, Wada M, Song I, Smith WL. Cyclooxygenase Allosterism, Fatty Acid-mediated Cross-talk between Monomers of Cyclooxygenase Homodimers. J Biol Chem. 2009;284:10046–10055. doi: 10.1074/jbc.M808634200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer AG, Woods JW, Arakawa T, Singer II, Smith WL. Subcellular localization of prostaglandin endoperoxide H synthases-1 and -2 by immunoelectron microscopy. J Biol Chem. 1998;273:9886–9893. doi: 10.1074/jbc.273.16.9886. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Smith T, Grabski S, DeWitt DL. The membrane association sequences of the prostaglandin endoperoxide synthases-1 and -2 isozymes. J Biol Chem. 1998;273:29830–29837. doi: 10.1074/jbc.273.45.29830. [DOI] [PubMed] [Google Scholar]
- 11.MirAfzali Z, Leipprandt JR, McCracken JL, DeWitt DL. Topography of the prostaglandin endoperoxide H2 synthase-2 in membranes. J Biol Chem. 2006;281:28354–28364. doi: 10.1074/jbc.M605206200. [DOI] [PubMed] [Google Scholar]
- 12.Otto JC, Smith WL. Photolabeling of prostaglandin endoperoxide H synthase-1 with 3-trifluoro-3-(m-[125I]iodophenyl)diazirine as a probe of membrane association and the cyclooxygenase active site. J Biol Chem. 1996;271:9906–9910. doi: 10.1074/jbc.271.17.9906. [DOI] [PubMed] [Google Scholar]
- 13.Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 14.Malkowski MG, Ginell SL, Smith WL, Garavito RM. The productive conformation of arachidonic acid bound to prostaglandin synthase. Science. 2000;289:1933–1937. doi: 10.1126/science.289.5486.1933. [DOI] [PubMed] [Google Scholar]
- 15.Picot D, Loll PJ, Garavito RM. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994;367:243–249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- 16.Vecchio AJ, Simmons DM, Malkowski MG. Structural basis of fatty acid substrate binding to cyclooxygenase-2. J Biol Chem. 2010;285:22152–22163. doi: 10.1074/jbc.M110.119867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duggan KC, Walters MJ, Musee J, Harp JM, Kiefer JR, Oates JA, Marnett LJ. Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen. J Biol Chem. 2010;285:34950–34959. doi: 10.1074/jbc.M110.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rand Doyen J, Yucer N, Lichtenberger LM, Kulmacz RJ. Phospholipid actions on PGHS-1 and -2 cyclooxygenase kinetics. Prostaglandins Other Lipid Mediat. 2008;85:134–143. doi: 10.1016/j.prostaglandins.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MirAfzali Z, Leipprandt JR, McCracken JL, DeWitt DL. Fast, efficient reconstitution of the cyclooxygenases into proteoliposomes. Arch Biochem Biophys. 2005;443:60–65. doi: 10.1016/j.abb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Baas BJ, Denisov IG, Sligar SG. Homotropic cooperativity of monomeric cytochrome P450 3A4 in a nanoscale native bilayer environment. Arch Biochem Biophys. 2004;430:218–228. doi: 10.1016/j.abb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Boldog T, Grimme S, Li M, Sligar SG, Hazelbauer GL. Nanodiscs separate chemoreceptor oligomeric states and reveal their signaling properties. Proc Natl Acad Sci U S A. 2006;103:11509–11514. doi: 10.1073/pnas.0604988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frauenfeld J, Gumbart J, Sluis EO, Funes S, Gartmann M, Beatrix B, Mielke T, Berninghausen O, Becker T, Schulten K, Beckmann R. Cryo-EM structure of the ribosome-SecYE complex in the membrane environment. Nat Struct Mol Biol. 2011;18:614–621. doi: 10.1038/nsmb.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi L, Shen QT, Kiel A, Wang J, Wang HW, Melia TJ, Rothman JE, Pincet F. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science. 2012;335:1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J Am Chem Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, Sligar SG. Chapter 11 - Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009;464:211–231. doi: 10.1016/S0076-6879(09)64011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith T, Leipprandt J, DeWitt D. Purification and characterization of the human recombinant histidine-tagged prostaglandin endoperoxide H synthases-1 and -2. Arch Biochem Biophys. 2000;375:195–200. doi: 10.1006/abbi.1999.1659. [DOI] [PubMed] [Google Scholar]
- 27.Otto JC, DeWitt DL, Smith WL. N-glycosylation of prostaglandin endoperoxide synthases-1 and -2 and their orientations in the endoplasmic reticulum. J Biol Chem. 1993;268:18234–18242. [PubMed] [Google Scholar]
- 28.Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 29.Schneider C, Boeglin WE, Brash AR. Enantiomeric separation of hydroxy eicosanoids by chiral column chromatography: effect of the alcohol modifier. Anal Biochem. 2000;287:186–189. doi: 10.1006/abio.2000.4847. [DOI] [PubMed] [Google Scholar]
- 30.Schneider C, Brash AR. Stereospecificity of hydrogen abstraction in the conversion of arachidonic acid to 15R-HETE by aspirin-treated cyclooxygenase-2. Implications for the alignment of substrate in the active site. J Biol Chem. 2000;275:4743–4746. doi: 10.1074/jbc.275.7.4743. [DOI] [PubMed] [Google Scholar]
- 31.McDougle DR, Palaria A, Magnetta E, Meling DD, Das A. Functional studies of N-terminally modified CYP2J2 epoxygenase in model lipid bilayers. Protein Sci. 2013;22:964–979. doi: 10.1002/pro.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, Smyth EM, Wilson SJ, FitzGerald GA, Garavito RM, Sui de X, Regan JW, Smith WL. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282:22254–22266. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- 33.Vecchio AJ, Malkowski MG. The structure of NS-398 bound to cyclooxygenase-2. J Struct Biol. 2011;176:254–258. doi: 10.1016/j.jsb.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gierse JK, Koboldt CM, Walker MC, Seibert K, Isakson PC. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem J. 1999;339 ( Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 35.Rimon G, Sidhu RS, Lauver DA, Lee JY, Sharma NP, Yuan C, Frieler RA, Trievel RC, Lucchesi BR, Smith WL. Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase-1. Proc Natl Acad Sci U S A. 2010;107:28–33. doi: 10.1073/pnas.0909765106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma NP, Dong L, Yuan C, Noon KR, Smith WL. Asymmetric acetylation of the cyclooxygenase-2 homodimer by aspirin and its effects on the oxygenation of arachidonic, eicosapentaenoic, and docosahexaenoic acids. Mol Pharmacol. 2010;77:979–986. doi: 10.1124/mol.109.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecomte M, Laneuville O, Ji C, DeWitt DL, Smith WL. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J Biol Chem. 1994;269:13207–13215. [PubMed] [Google Scholar]
- 38.Vecchio AJ, Orlando BJ, Nandagiri R, Malkowski MG. Investigating substrate promiscuity in cyclooxygenase-2: the role of Arg-120 and residues lining the hydrophobic groove. J Biol Chem. 2012;287:24619–24630. doi: 10.1074/jbc.M112.372243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan S, Coveney PV. A comparative study of the COX-1 and COX-2 isozymes bound to lipid membranes. J Comput Chem. 2009;30:1038–1050. doi: 10.1002/jcc.21130. [DOI] [PubMed] [Google Scholar]
- 40.Boggara MB, Krishnamoorti R. Partitioning of nonsteroidal antiinflammatory drugs in lipid membranes: a molecular dynamics simulation study. Biophys J. 2010;98:586–595. doi: 10.1016/j.bpj.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunes C, Brezesinski G, Lima JL, Reis S, Lucio M. Synchrotron SAXS and WAXS study of the interactions of NSAIDs with lipid membranes. J Phys Chem B. 2011;115:8024–8032. doi: 10.1021/jp2025158. [DOI] [PubMed] [Google Scholar]
- 42.Nunes C, Brezesinski G, Pereira-Leite C, Lima JL, Reis S, Lucio M. NSAIDs interactions with membranes: a biophysical approach. Langmuir. 2011;27:10847–10858. doi: 10.1021/la201600y. [DOI] [PubMed] [Google Scholar]
- 43.Selinsky BS, Gupta K, Sharkey CT, Loll PJ. Structural analysis of NSAID binding by prostaglandin H2 synthase: time-dependent and time-independent inhibitors elicit identical enzyme conformations. Biochemistry. 2001;40:5172–5180. doi: 10.1021/bi010045s. [DOI] [PubMed] [Google Scholar]