Abstract
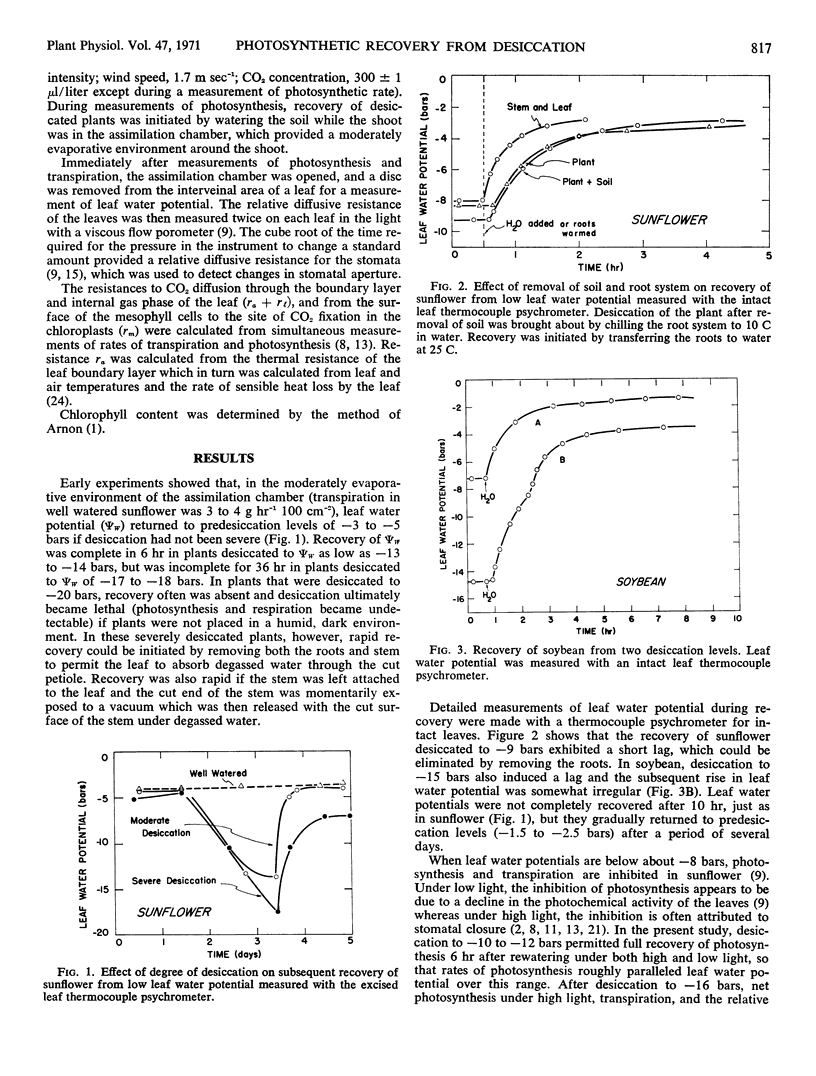
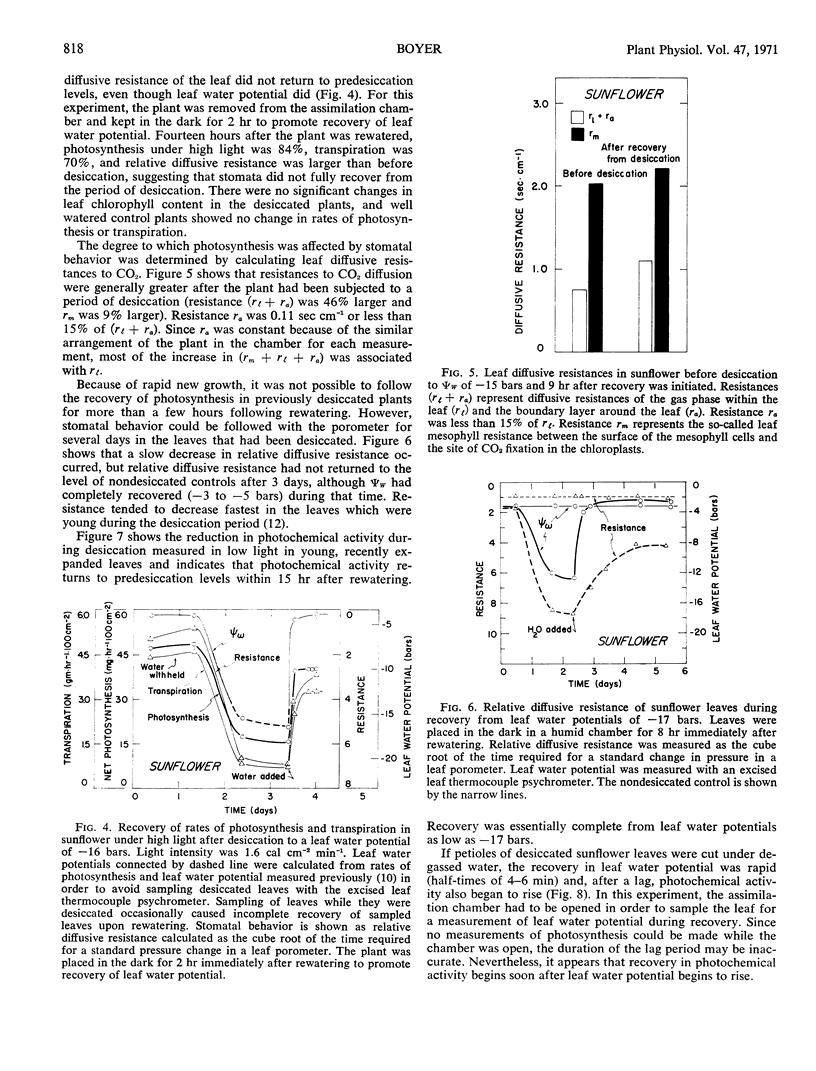
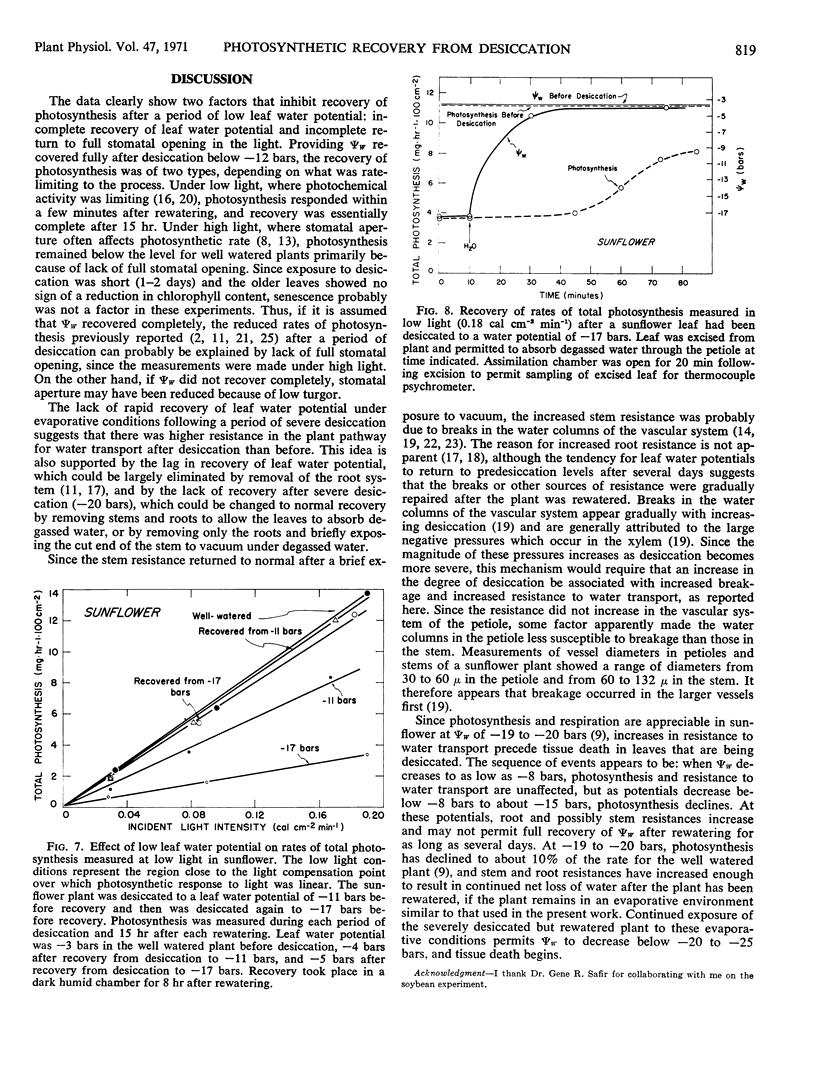
Photosynthesis was studied in sunflower plants subjected to 1 to 2 days of desiccation and then permitted to recover. The leaf water potential to which leaves returned after rewatering was dependent on the severity of desiccation and the evaporative conditions. Under moderately evaporative conditions, leaf water potential returned to predesiccation levels after 3 to 5 hours when desiccation was slight. Leaf water potentials remained below predesiccation levels for several days after rewatering when leaf water potentials decreased to −13 to −19 bars during desiccation. Leaf water potential showed no sign of recovery when leaf water potentials decreased to −20 bars or below during desiccation. The lack of full recovery of leaf water potential was attributable to increased resistance to water transport in the roots and stem. The resistance ultimately became large enough to result in death of the leaves because net water loss continued even after the soil had been rewatered.
Measurements of photosynthesis were made at high light intensities, where stomatal aperture often affects photosynthesis, and at low light intensities, where the photochemical activity of the leaves limits photosynthesis. Providing leaf water potentials remained above −12 bars during the desiccation period and returned to predesiccation levels during recovery, photosynthesis under both low and high light paralleled the recovery in leaf water potential after rewatering. After desiccation to leaf water potentials below −12 bars, recovery was incomplete under high light and could be attributed to lack of full stomatal opening. Lack of full opening persisted for 3 days and showed no sign of eventual recovery even though leaf water potentials recovered fully. Under low light, however, recovery in photochemical activity was complete within 15 hours after desiccation to leaf water potentials as low as −17 bars.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton F. M. Effects of a Series of Cycles of Alternating Low and High Soil Water Contents on the Rate of Apparent Photosynthesis in Sugar Cane. Plant Physiol. 1956 Jul;31(4):266–274. doi: 10.1104/pp.31.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Bowen B. L. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 1970 May;45(5):612–615. doi: 10.1104/pp.45.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Differing sensitivity of photosynthesis to low leaf water potentials in corn and soybean. Plant Physiol. 1970 Aug;46(2):236–239. doi: 10.1104/pp.46.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Free-energy transfer in plants. Science. 1969 Mar 14;163(3872):1219–1220. doi: 10.1126/science.163.3872.1219. [DOI] [PubMed] [Google Scholar]
- Boyer J. S. Isopiestic technique: measurement of accurate leaf water potentials. Science. 1966 Dec 16;154(3755):1459–1460. doi: 10.1126/science.154.3755.1459. [DOI] [PubMed] [Google Scholar]
- Boyer J. S. Leaf water potentials measured with a pressure chamber. Plant Physiol. 1967 Jan;42(1):133–137. doi: 10.1104/pp.42.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Relationship of water potential to growth of leaves. Plant Physiol. 1968 Jul;43(7):1056–1062. doi: 10.1104/pp.43.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel H. T. Freezing of xylem sap without cavitation. Plant Physiol. 1967 Jan;42(1):55–66. doi: 10.1104/pp.42.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G. W., Childers N. F. INFLUENCE OF SOIL MOISTURE ON PHOTOSYNTHESIS, RESPIRATION, AND TRANSPIRATION OF APPLE LEAVES. Plant Physiol. 1941 Jul;16(3):565–583. doi: 10.1104/pp.16.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander P. F., Love W. E., Kanwisher J. W. The Rise of Sap in Tall Grapevines. Plant Physiol. 1955 Mar;30(2):93–104. doi: 10.1104/pp.30.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander P. F., Ruud B., Leivestad H. The Rise of Sap in a Tropical Liana. Plant Physiol. 1957 Jan;32(1):1–6. doi: 10.1104/pp.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Upchurch R. P., Peterson M. L., Hagan R. M. Effect of Soil-Moisture Content on the Rate of Photosynthesis and Respiration in Ladino Clover (Trifolium Repens L.). Plant Physiol. 1955 Jul;30(4):297–303. doi: 10.1104/pp.30.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]


