Abstract
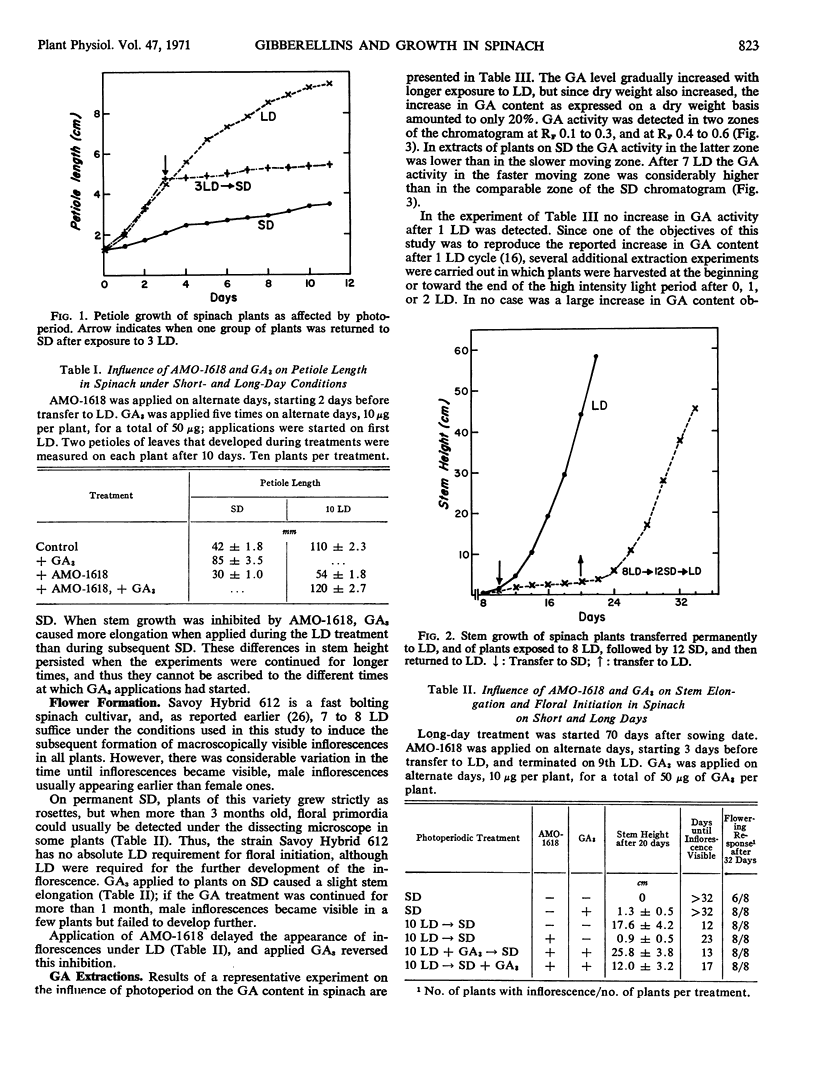
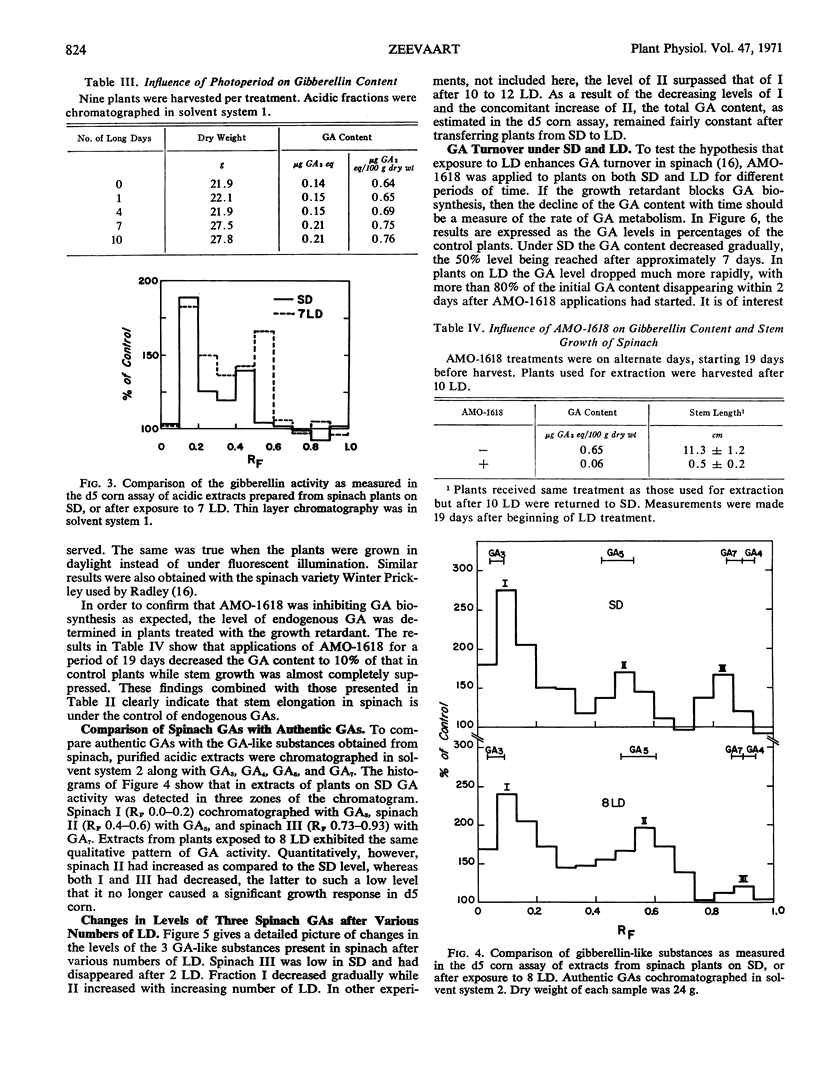
The earliest visible responses of spinach plants (Spinacia oleracea L., cv. Savoy Hybrid 612) transferred from short to long days (8 hours of high intensity light supplemented with 16 hours of low intensity illumination from incandescent lamps) were upright leaf orientation and increased elongation of the petioles. The effect of long days on growth rate was direct; i.e., there was no after-effect if the plants were transferred to short days. Gibberellin A3 applied to plants under short days had an effect similar to that of long days, whereas application of the growth retardant AMO-1618 [2′-isopropyl-4′-(trimethylammonium chloride)-5′-methylphenyl piperidinel-carboxylate] under long days caused a growth habit typical of short-day conditions. Gibberellin A3 caused more stem growth in plants under long days in which the endogenous gibberellin content had been reduced by AMO-1618 than in plants under short days not treated with the growth retardant.
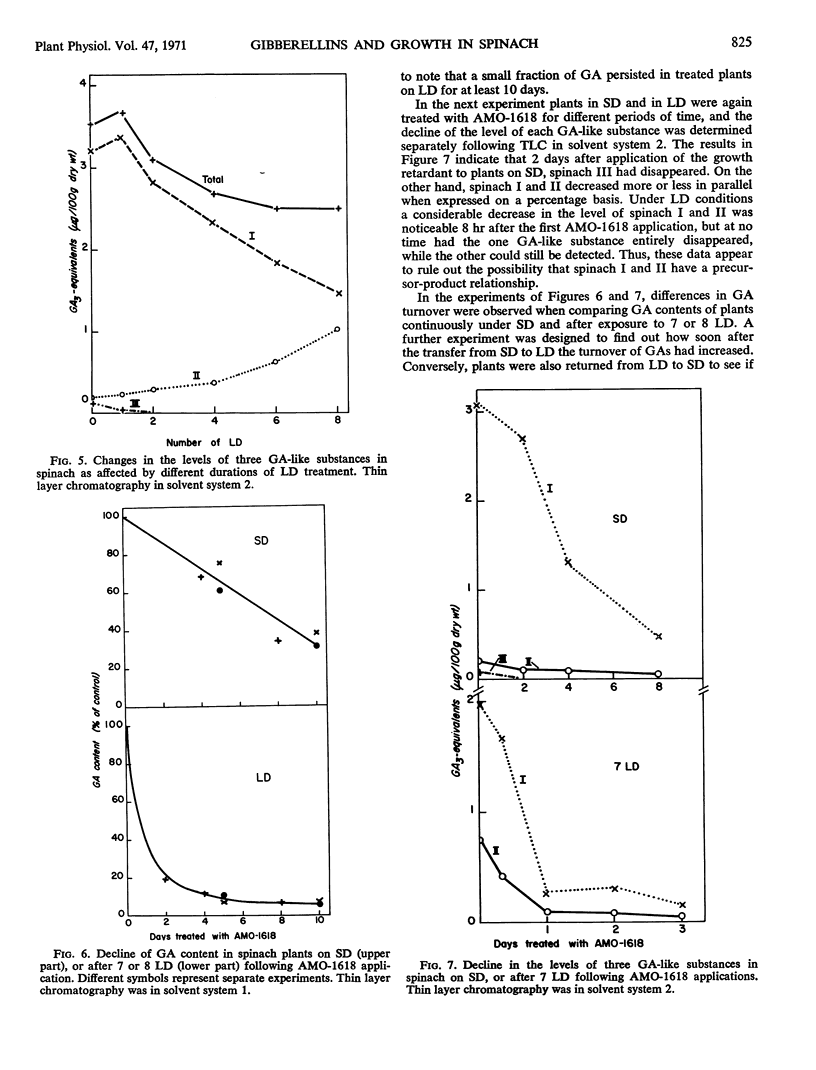
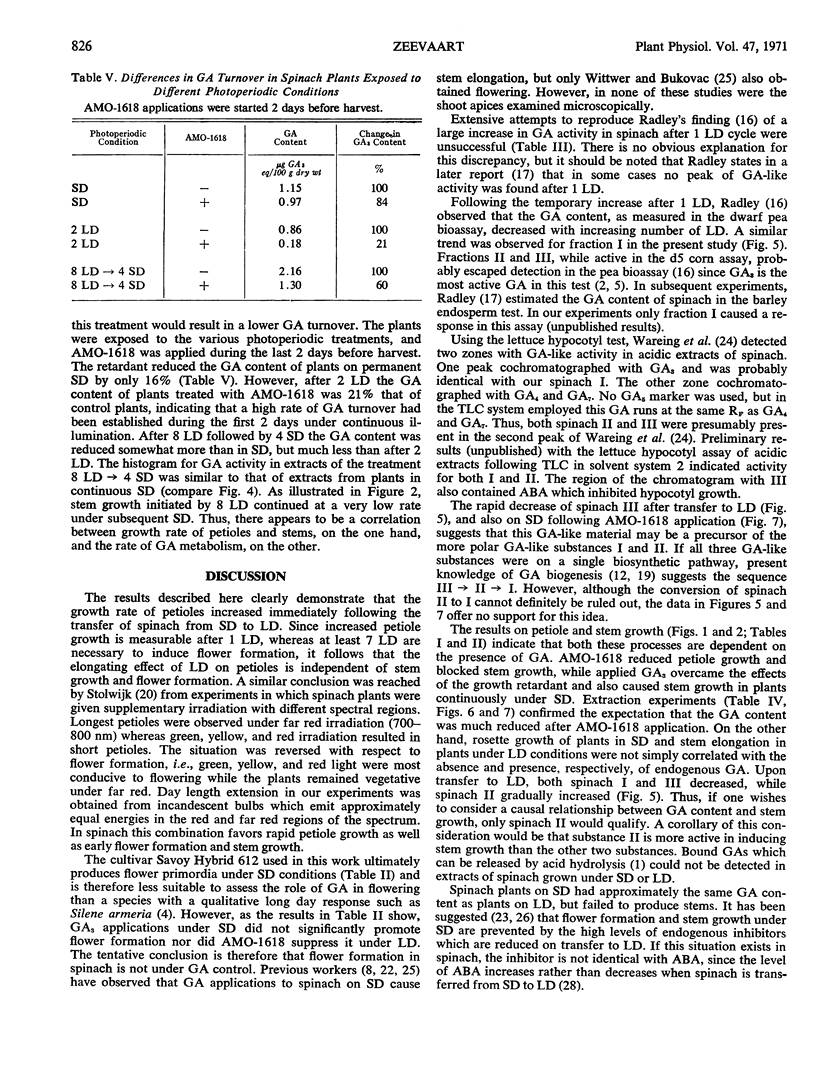
Three gibberellin-like substances, called I, II, and III in order of increasing RF value, were present in acidic extracts of spinach under short days. After transfer to long days, II increased, whereas I and III decreased, the latter below the level of detection in the d5 corn assay. Following application of AMO-1618 the gibberellin content of plants under long days fell off more rapidly than in those under short days, indicating that gibberellin turnover was markedly higher under long days. This increased rate of gibberellin metabolism was established after 2 long days. When plants were returned to short days, the turnover of gibberellins declined. It is suggested that a higher rate of gibberellin biosynthesis combined with increased sensitivity to gibberellin is responsible for the observed growth responses in spinach under long days.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barendse G. W., Kende H., Lang A. Fate of radioactive gibberellin a(1) in maturing and germinating seeds of peas and Japanese morning glory. Plant Physiol. 1968 May;43(5):815–822. doi: 10.1104/pp.43.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland C. F., Zeevaart J. A. Gibberellins in Relation to Flowering and Stem Elongation in the Long Day Plant Silene armeria. Plant Physiol. 1970 Sep;46(3):392–400. doi: 10.1104/pp.46.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. Preparation of radioactive gibberellin a(1) and its metabolism in dwarf peas. Plant Physiol. 1967 Nov;42(11):1612–1618. doi: 10.1104/pp.42.11.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave A., Kende H. Radioactive gibberellin a(5) and its metabolism in dwarf peas. Plant Physiol. 1970 Jan;45(1):56–61. doi: 10.1104/pp.45.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEMBDNER G., GROSS R., SCHREIBER K. [Thin layer chromatography of gibberellins]. Experientia. 1962 Dec 15;18:584–585. doi: 10.1007/BF02172196. [DOI] [PubMed] [Google Scholar]
- Wittwer S. H., Bukovac M. J. Gibberellin Effects on Temperature and Photoperiodic Requirements for Flowering of Some Plants. Science. 1957 Jul 5;126(3262):30–31. doi: 10.1126/science.126.3262.30. [DOI] [PubMed] [Google Scholar]


