Abstract
For a subpopulation of acute myeloid leukemia (AML) patients, the constitutively activated tyrosine kinase, mutant FLT3, has emerged as a promising target for therapy. The development of drug resistance, however, is a growing concern for mutant FLT3 inhibitors, such as PKC412. Potential therapeutic benefit can arise from the combination of two structurally diverse inhibitors that target- but bind differently to- the same protein or from two inhibitors with completely different mechanisms of action. Thus, there is a need for identification and development of novel FLT3 inhibitors that have the ability to positively combine with PKC412 or standard chemotherapeutic agents used to treat AML as a way to suppress the development of drug resistance and consequently prolong disease remission. Here, we report the effects of the novel type II ATP competitive inhibitors, HG-7-85-01 and HG-7-86-01, which potently and selectively target mutant FLT3 protein kinase activity, and inhibit the proliferation of cells harboring FLT3-ITD or FLT3 kinase domain point mutants via induction of apoptosis and cell cycle inhibition. Anti-leukemic activity of HG-7-85-01 was demonstrated in vivo to be comparable to that observed with PKC412 in a bioluminescence assay utilizing NCr nude mice harboring Ba/F3-FLT3-ITD-luc+ cells. HG-7-85-01 was also observed to override PKC412 resistance. Finally, HG-7-85-01 and HG-7-86-01 synergized with PKC412 and standard chemotherapeutic agents against mutant PKC412-sensitive and some PKC412-resistant, FLT3-positive cells. Thus, we present a structurally novel class of FLT3 inhibitors that warrants consideration for clinical testing against drug-resistant disease in AML patients.
Introduction
Acute myelocytic leukemia (AML), which occurs in approximately 10,000 Americans per year, is characterized by aberrant proliferation of myeloid progenitor cells and a partial block in cellular differentiation (1). Approximately 30% of AML patients, and a portion of ALL patients, harbor a mutant form of the class III receptor tyrosine kinase, FLT3 (Fms-Like Tyrosine kinase-3; STK-1, human Stem Cell Tyrosine Kinase-1; or FLK-2, Fetal Liver Kinase-2) (2). Constitutively activated FLT3 occurs most often as internal tandem duplications (ITD) within the juxtamembrane domain (3), and is observed in approximately 20–25% of AML patients, but in less than 5% of patients with myelodysplastic syndrome (MDS) (3–8). The transplantation in mice of murine bone marrow cells infected with a retrovirus expressing a FLT3-ITD mutant leads to the development of a rapidly lethal myeloproliferative disease (8).
Approximately 7% of AML patients harbor point mutations within the "activation loop" of FLT3 which are believed to force the kinase into an "activated" configuration (9), The majority harbor a missense mutation at position 835. Other less prevalent point mutations in the kinase domain have been identified, including N841I (10) and Y842C (11).
PKC412 is a broad spectrum small molecule kinase inhibitor that is effective against mutant FLT3-expressing cells (12). A Phase Ib clinical trial was conducted in which newly diagnosed AML patients were treated with PKC412 (at 50 mg po bid) in sequential and simultaneous combinations with daunorubicin and cytarabine induction and high-dose cytarabine consolidation. Patients in this trial experienced transient and/or reversible side effects, and clinical complete responses (CR) were observed in 100% of patients harboring mutant FLT3 (13). PKC412 is currently in late-stage, Phase III clinical trials. In addition there are two FDA approved ‘multi-targeted’ kinase inhibitor drugs, sorafenib, sunitinib, which are potent FLT3 inhibitors. Ambit’s AC220 (14) exhibited low nanomolar potency in biochemical and cellular assays, excellent kinase selectivity and good activity in tumor xenograft model and a mouse bone marrow engraftment model. Millenium Pharmaceuticals’ MLN 518 is an orally active inhibitor of FLT3, PDGFRa/b, c-KIT and has demonstrated limited activity as a single agent in Phase I/II clinical trials in patients with AML and myelodysplastic syndrome (15). There are several other reported FLT3 inhibitors including CGP52421 (metabolite of PKC 412), CEP 701, ABT 869 (16).
While small molecule inhibitors like PKC412 are showing promise in the clinic, up to now none has achieved sustained clinical responses as a single agent in AML patients. In addition, a growing problem for the treatment of acute leukemia is the development of resistance due to acquired point mutations in the molecular targets (17, 18). Indeed, the detection of drug-resistant leukemic blast cells in AML patients undergoing PKC412 therapy has sparked the screening and characterization of novel, structurally diverse inhibitors of FLT3 that, if used in combination with anti-leukemic agents, are predicted to prevent the development of drug resistance.
We report here initial characterization of HG-7-85-01 and HG-7-86-01 as potent and selective inhibitors of mutant FLT3 protein kinase activity. These compounds selectively inhibit FLT3 kinase activity and the proliferation, viability, and cell cycle progression of leukemic cells harboring mutant FLT3, with no apparent effect on cells harboring wild-type FLT3. HG-7-85-01 significantly inhibits mutant FLT3-positive leukemia growth in vivo to a degree comparable to that observed with PKC412. We also show that HG-7-85-01 and HG-7-86-01 effectively combine with PKC412, as well as the standard chemotherapeutic agents, Ara-c and doxorubicin, to kill mutant FLT3-expressing cells to a significantly higher degree than any one agent alone. These results support the notion that FLT3 is an attractive therapeutic target for AML, and point to the emergence of a novel class of inhibitors that could potentially be used in combination with other anti-leukemic agents to treat mutant FLT3-positive AML.
Materials and Methods
Cell lines and cell culture
The IL-3-dependent murine hematopoietic cell line Ba/F3 was transduced with either FLT3-ITD- or FLT3-D835Y--containing MSCV retroviruses harboring a neomycin selectable marker, and selected for resistance to neomycin (8). FLT3-ITD- and D835Y-expressing Ba/F3 cells were a gift from Dr. Gary Gilliland. Cell lines were obtained proximal to the time of publication of two manuscripts from Dr. James Griffin’s and Dr. Gary Gilliland’s laboratories, respectively, in which these constructs were used (8, 12). FLT3 mutations expressed in these lines were confirmed by sequencing and the entire cDNA was subcloned from pGEM to MSCV-EB neo or MSCV2.2 GFP (kindly provided by W. Pear, University of Pennsylvania) into the HpaI site in each case. PKC412-responsiveness of the FLT3-ITD-expressing cells and D835Y-expressing cells was observed to be pharmacologically similar in the current study to results obtained previously with these cell lines (12).
Y572C is a point mutation in the juxtamembrane domain of FLT3 (19). We developed Y572C-expressing Ba/F3 cells in 2004 via transfection using a pCIneo expressing vector, followed by selection for neomycin resistance. The Y572 mutation was confirmed by sequencing using both plasmid and genomic DNA, and the mutation has been characterized as an activating mutation (unpublished data).
PKC412-resistant Ba/F3 cell lines expressing FLT3 harboring mutations in the ATP-binding pocket were previously developed (20). These cell lines were developed as a result of identification of FLT3-inhibitor resistant mutants through vector and library construction and verification of mutant FLT3 sequences (20). The differential PKC412 resistance of these drug-resistant cell lines was investigated and confirmed several times by our lab (21, 22). Cell lines were additionally confirmed pharmacologically to be PKC412 resistant in the current study, with results similar to those observed previously (G697R was observed to be the most highly PKC412-resistant; N676D was observed to be moderately PKC412-resistant in comparison).
We developed the Ba/F3-N841I cell line by transfection using a pCIneo expressing vector, followed by selection for resistance to neomycin (10). We confirmed the sequence of N841I via DNA sequencing. At the time of preparation of reference (22), the PKC412-responsiveness of Ba/F3 cells expressing the N841I mutant was also confirmed and observed to be similar to what was reported in (10).
The human AML-derived, FLT3-ITD-expressing cell line, MOLM-13 (DSMZ (German Resource Centre for Biological Material), was engineered to express luciferase fused to neomycin phosphotransferase (pMMP-LucNeo) by transduction with a VSVG-pseudotyped retrovirus as previously described (23, 24). The MOLM13 cell line was confirmed pharmacologically to be PKC412 sensitive in the current study, with drug responsiveness comparable to that previously published (21). The KG-1a cell line, a less well-differentiated variant of the KG-1 cell line, was a gift from Dr. Anthony Letai’s laboratory. Both the KG-1 and KG-1a cell lines, originally purchased from the American Type Culture Collection (ATCC) (Manassas, VA), showed a similar pharmacological responsiveness to HG-7-85-01.
All cell lines were cultured with 5% CO2 at 37°C in RPMI (Mediatech, Inc., Herndon, VA) with 10% fetal calf serum (FCS) and supplemented with 1% L-glutamine. Parental Ba/F3 cells were similarly cultured with 15% WEHI-conditioned medium as a source of IL-3. Parental Ba/F3 cells are routinely checked for growth factor-dependence by culturing in the absence of WEHI and monitoring viability status. Transfected cell lines were cultured in media supplemented with 1mg/ml G418.
Early passages of all lines obtained from ATCC or provided as a gift were expanded and frozen in multiple aliquots at the time of receipt to limit the passage number of the different lines. The cell lines used in this study have not been recently authenticated, however Dana Farber Cancer Institute is in the process of doing this now through DNA sequencing and profiling experiments using DNA fingerprinting with small tandem repeat (STR) profiling.
Chemical compounds and biologic reagents
HG-7-85-01 and HG-7-86-01 were synthesized in the laboratory of Dr. Nathanael Gray, Boston, MA. PKC412 was synthesized by Novartis Pharma AG, Basel, Switzerland. Compounds were initially dissolved in DMSO to make 10 mM stock solutions, and then were serially diluted to obtain final concentrations for in vitro experiments. Ara-c and doxorubicin were purchased from Sigma Chemical Co (St Louis, MO).
Antibodies and immunoblotting
Anti-p-Tyr (clone 4G10, Upstate Biotechnology, NY) was used at 1:1000 for immunoblotting. Anti-FLT3/Flk-2 (C-20, Santa Cruz Biotechnology, Inc., CA) was used at 1:200 for immunoblotting. The monoclonal anti-β-actin antibody (clone AC-15) (Sigma-Aldrich, St. Louis, MO) and α-tubulin antibody (clone DM1A) (Sigma Aldrich, St. Louis, MO) were each used at a 1:2000 dilution. The phospho-S6 ribosomal protein (Ser240/244) antibody (#2215) (Cell Signaling Technology, Danvers, MA) was used at a dilution of 1:2000. Anti-p-STAT5 (#9359, Cell Signaling Technology, MA) was used at a 1:1000 dilution and STAT5 (sc-835, Santa Cruz Biotechnology, Inc. CA) was used at 1:10,000 for immunoblotting. Anti-PARP (# 9542, Cell Signaling) was used at 1:10,000. The following antibodies were used at a 1:2500 dilution and were purchased from Cell Signaling (Danvers, MA): p-MAPK 9101; MAPK 9102.
Protein lysate preparation and immunoblotting were carried out as previously described (12).
Proliferation studies, cell cycle analysis, and apoptosis assay
Cell counts for proliferation studies were obtained using the trypan blue exclusion assay, as previously described (12). Error bars represent the standard error of the mean for each data point. Programmed cell death of inhibitor-treated cells was determined using the Annexin-V-Fluos Staining Kit (Boehringer Mannheim, Indianapolis, IN), as previously described (12). Cell cycle analysis was performed as previously described (12).
Drug combination studies
For drug combination studies, compounds were added simultaneously at fixed ratios to cells, and cell viability was determined by trypan blue exclusion and expressed as the function of growth affected (FA) drug-treated versus control cells. Synergy was assessed by Calcusyn software (Biosoft, Ferguson, MO and Cambridge, UK), using the Chou-Talalay method (25). The combination index=[D]1 [Dx]1 + [D]2/[Dx]2, where [D]1 and [D]2 are the concentrations required by each drug in combination to achieve the same effect as concentrations [Dx]1 and [Dx]2 of each drug alone. Generally, values less than one indicate synergy, whereas values greater than one indicate antagonism.
Mouse studies and in vivo imaging
Ba/F3-FLT3-ITD cells were transduced with a VSVG-pseudotyped retrovirus comprised of the firefly luciferase coding region (from pGL3-basic; Promega, Madison, WI) cloned into PMSCV puro (Clonetech, Mountain View, CA). Cells were neomycin selected to produce the Ba/F3-FLT3-ITD (luc+) cell line.
For administration to male NCR-nude mice (5–6 weeks of age; Taconic, NY), virus- and Mycoplasma-free cells were washed and resuspended in Hank’s Balanced Salt Solution (HBSS; Mediatech, Inc.,VA) and administered via IV tail vein injection (800,000 cells/mouse). Anesthesized mice were imaged 1–3 days post IV-injection to generate a baseline used to establish treatment cohorts with matched tumor burden, and total body luminescence was measured as previously described (24).
AML patient cells
Frozen vials of bone marrow from AML patients identified as harboring mutant FLT3 were thawed prior to processing using Ficoll-Plaque-Plus (Amersham Pharmacia Biotech AB, Uppsala, Sweden) for the isolation of mononuclear cells. Mononuclear cells were isolated from normal bone marrow by density gradient centrifugation through Ficoll-Plaque Plus at 2000 rpm for 30 minutes, followed by two washes in 1× PBS. Mononuclear cells were then tested in liquid culture (Iscove’s MDM, supplemented with 20% FCS) in the presence of different concentrations of HG-7-85-01. All bone marrow samples from AML patients were obtained under approval of the Dana Farber Cancer Institute Institutional Review Board.
AML patient information is presented as supplementary data.
Results
Discovery of HG-7-85-01 and HG-7-86-01 as inhibitors of mutant FLT3
HG-7-85-01 and HG-7-86-01 were originally designed as a hybrid between the type I inhibitor, dasatinib, and the type II inhibitor, nilotinib (Figure 1A). Details about the structure-based design strategy, synthesis of HG-7-85-01 and co-crystalization with Src are described elsewhere (24). Details regarding the synthesis of HG-7-86-01 are shown in Supplementary Figure S1.
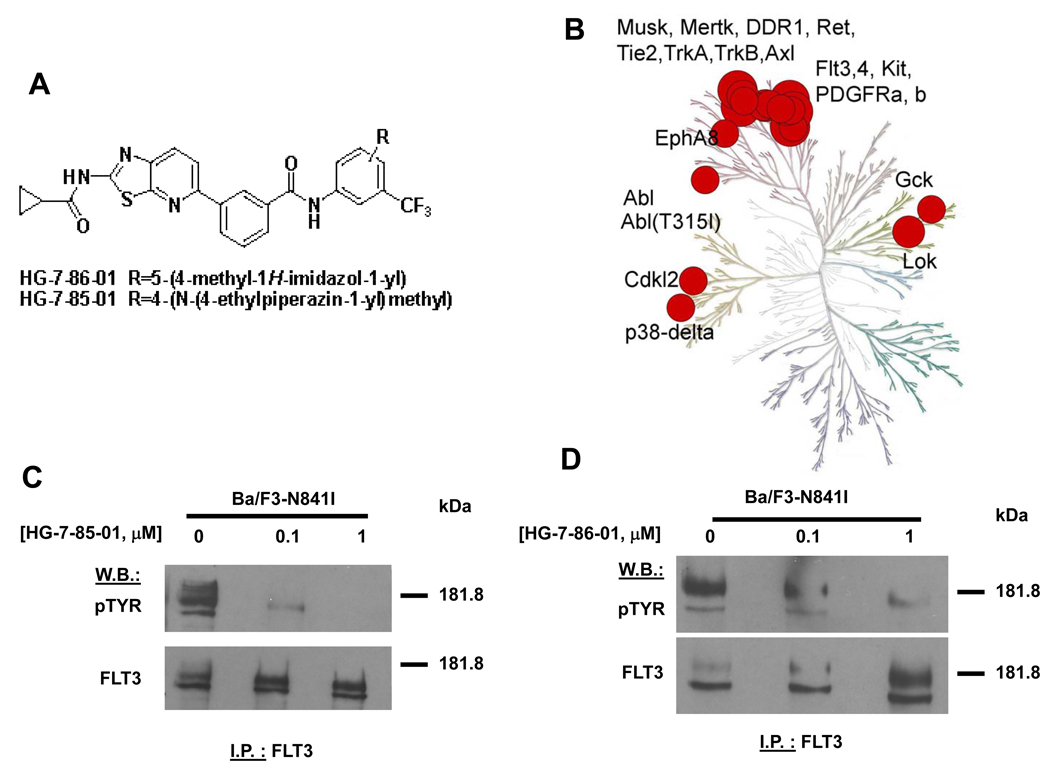
Figure 1. HG-7-85-01 and HG-7-86-01 as inhibitors of mutant FLT3.
(A) Molecular structures of HG-7-85-01 and HG-7-86-01. (B) Kinase selectivity of HG-7-86-01 based on screening 400 kinases. Kinases where significant binding affinity was detected at 10 uM were retested in dose-response format to determine a dissociation constant (Kd). The size of the red circle is proportion to Kd. (C) FLT3 I.P./western: Approximately 2-hour treatment of Ba/F3-N841I with HG-7-85-01 at the indicated concentrations. (D) FLT3 I.P./western: Approximately two-hour treatment of Ba/F3-N841I with HG-7-86-01 at the indicated concentrations. The p-TYR signal corresponds to autophosphorylation of mutant FLT3.
Kinase selectivity was assessed for HG-7-85-01 and HG-7-86-01 using KINOMEscan™ (Ambit Biosciences, San Diego, CA), a high-throughput method for screening kinase inhibitors against a panel of human kinases (26). This technology enables compounds of interest to be assayed for their ability to compete for binding to the ATP-site of a panel of 353 kinases, each fused to a proprietary tag. The quantity of each kinase bound to an immobilized, ATP-site directed ligand was measured in the presence and absence of a 10 µM concentration of HG-7-85-01 and HG-7-86-01.
Kinases that displayed greater than 90% displacement relative to the DMSO control were subject to determination of a dissociation constant (Kd). These studies revealed that, for both compounds, a number of wild-type and mutant forms of kinases such as BCR-ABL, PDGFRα/β, FLT3, Ret, Tie-2, Kit, and DDR1 exhibited potent binding (27) (Figure 1B and Supplementary Table S1). As the results of KINOMEscan™ suggested that mutant FLT3 may be one of several potential and clinically relevant targets of HG-7-85-01 and HG-7-86-01, this finding required confirmation in cell-based assays.
Effect of HG-7-85-01 and HG-7-86-01 FLT3 kinase activity and FLT3 signaling in mutant FLT3-expressing cells
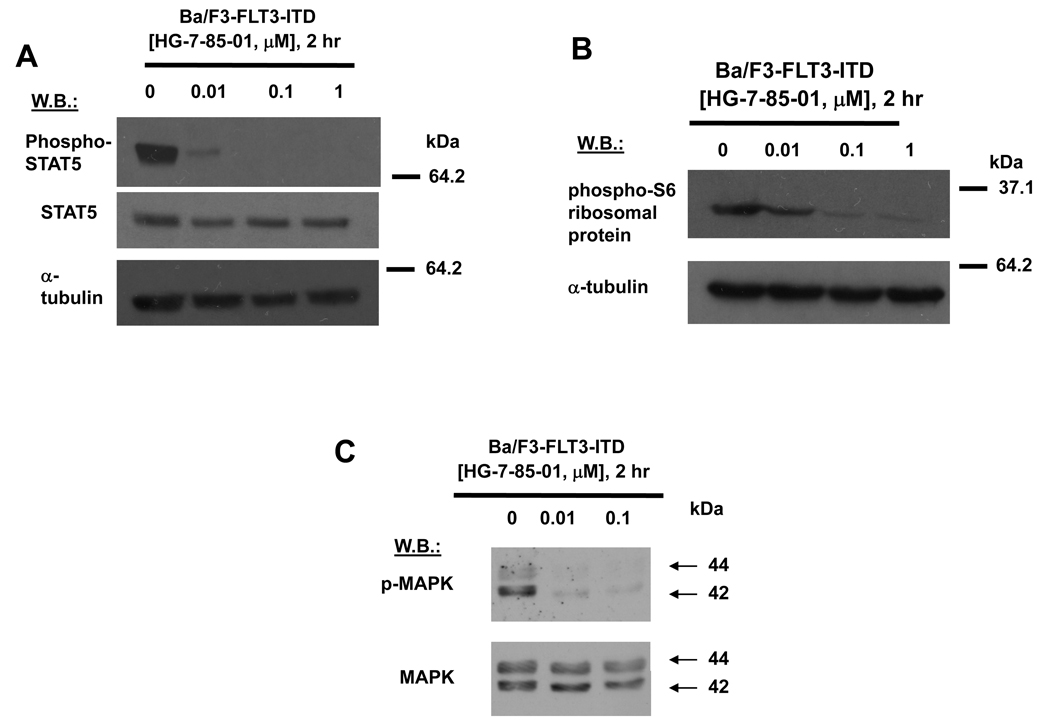
Both HG-7-85-01 and HG-7-86-01 inhibited mutant FLT3 autophosphorylation in a concentration-dependent manner, with no apparent decrease in mutant FLT3 protein levels (Figure 1C, D). These results suggest that mutant FLT3 is a direct target of HG-7-85-01 and HG-7-86-01, with HG-7-85-01 showing higher potency than HG-7-86-01. HG-7-85-01 also inhibited phosphorylation of STAT5, an integral component of the JAK/STAT signaling pathway downstream of FLT3, as well as of phospho-S6 ribosomal protein phosphorylation, a component of the mTOR pathway (Figure 2A–B and Supplementary Figure S2 (C)). Similar results were observed with HG-7-86-01, albeit at a lower potency (Supplementary Figure S2 (A, B, D)). HG-7-85-01 also inhibited p44/42 MAPK (Erk1/2) of the RAS/Raf/MEK/ERK signaling pathway, another pathway downstream of FLT3 (Figure 2C). The inhibition by HG-7-85-01 and HG-7-86-01 of multiple components of signaling pathways downstream of mutant FLT3 supports the notion that mutant FLT3 is, indeed, a protein kinase target of these agents and is inhibited as their primary mechanism of action.
Figure 2. Effect of HG-7-85-01 on FLT3 signaling in cells harboring mutations in the juxtamembrane domain of FLT3.
(A–C) Approximately 2-hour treatment of Ba/F3-FLT3-ITD cells with HG-7-85-01 prior to protein lysis and immunoblot assays.
Effect of HG-7-85-01 and HG-7-86-01 on proliferation and total cellular tyrosine phosphorylation of cells harboring mutations in the juxtamembrane domain of FLT3
HG-7-85-01 selectively inhibited the growth and viability of FLT3-ITD-expressing MOLM13-luc+ cells, and had comparatively little effect toward wt FLT3-expressing KG-1 cells, or KG1a cells that were serum-starved prior to FLT3 ligand stimulation (Supplementary Figure S3 (A,B) and Supplementary Figure S4(A)). HG-7-85-01 potently inhibited the growth of cells expressing mutations in the juxtamembrane domain of FLT3 (IC50 for FLT3-ITD=2–4 nM; IC50 for Y572C=1.25–2.5nM, Table I), with complete cyto-protection observed when cells were co-cultured with drug and WEHI (IL-3), and no effect on the growth and viability of parental Ba/F3 cells at similar concentrations (Supplementary Figure S3 (C–D) and Supplementary Figure S4(B)). The cyto-protective effect of IL-3 suggests that inhibition of mutant FLT3-expressing cells is due to selective inhibition of the mutant FLT3 kinase, and that there is an absence of nonselective inhibitory effects on growth factor (IL-3)-mediated signaling. HG-7-85-01 also shows activity against AML patient bone marrow samples harboring FLT3 mutations at concentrations ≤1 µM (Supplementary Figure S5A–E), which are concentrations that are both IL-3 rescue-able and nontoxic toward parental Ba/F3 cells in the murine cell line system. Indeed, one peripheral blood sample tested (PB, AML-35) showed less sensitivity to HG-7-85-01 in the presence of cytokines as compared to their absence (Supplementary Figure S5(E), which is similar to the observed IL-3 rescue of mutant FLT3-expressing Ba/F3 cells treated with HG-7-85-01, and supportive of the notion of HG-7-85-01 being selective toward mutant FLT3 as a target. In addition, WT-FLT3-expressing AML patient samples were generally less sensitive to HG-7-85-01 at concentrations up to 1 uM than AML patient samples expressing mutant FLT3 (Supplementary Figure S5(F)).
Table I. IC50 values for HG-7-85-01 and HG-7-86-01 against mutant FLT3.
IC50 values were calculated based on five concentration linear dose-response curves generated for HG-7-85-01 and HG-7-86-01. Trypan blue exclusion was performed to obtain viable cell counts, and data were plotted as percent of untreated controls.
| Cell Line | IC50 (nM) HG-7-85-01 |
IC50 (nM) HG-7-86-01 |
|---|---|---|
| Ba/F3 | >1000 | >1000 |
| Ba/F3-FLT3-ITD | 2–4 | 125–250 |
| Ba/F3-Y572C | 1.25–2.5 | 12.5–25 |
| Ba/F3-N841I | 12.5–25 | 200–400 |
| Ba/F3-D835Y | 50–100 | 500–1000 |
| Ba/F3-N676D | 50 | 50–100 |
| Ba/F3-G697R | 500 | 1000–2000 |
Similar results were achieved with HG-7-86-01 when tested on cell lines expressing FLT3-juxtamembrane mutations, however this agent is considerably less potent than HG-7-85-01 (IC50 for FLT3-ITD=125–250 nM; IC50 for Y572C=12.5–25nM, Supplementary Figure S6, Table I). Cells were killed by 1 µM HG-7-86-01, with complete protection in the presence of drug when media was supplemented with growth factor (IL-3), and parental Ba/F3 cells were mostly unaffected when in the presence of up to 1 µM HG-7-86-01 (Supplementary Figure S6).
The ability of HG-7-85-01 and HG-7-86-01 to affect overall cellular tyrosine phosphorylation levels as a measure of selectivity for mutant FLT3 as a target was investigated. HG-7-85-01 and HG-7-86-01 inhibited total cellular tyrosine phosphorylation in FLT3-ITD- and Y572C-expressing cells in a concentration-dependent manner with no effect on basal levels of tyrosine phosphorylation observed in parental Ba/F3 cells (as compared to the higher tyrosine phosphorylation signal in mutant FLT3-expressing cells) and no reduction of levels of FLT3 protein (Supplementary Figure S7 and data not shown). In contrast, levels of FLT3 protein appear to increase in a drug concentration-dependent manner for HG-7-85-01, which is a finding consistent with previous studies carried out with ABL inhibitors like imatinib and nilotinib (28). These results suggest a possible drug-protein complex formation and stabilization, concurrent with inhibition of kinase activity.
Effect of HG-7-85-01 and HG-7-86-01 on viability and cell cycle progression of cells harboring mutations in the juxtamembrane domain of FLT3 in vitro
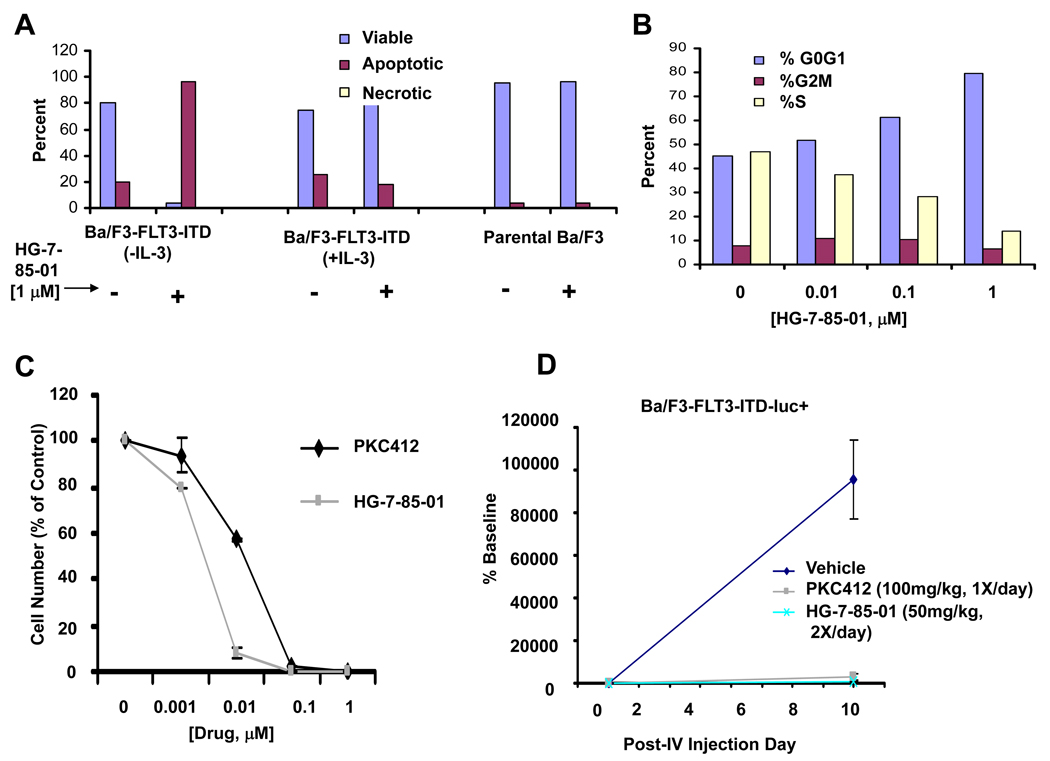
We were interested in investigating the underlying mechanism(s) whereby HG-7-85-01 and HG-7-86-01 inhibit the proliferation of mutant FLT3-expressing cells. HG-7-85-01 caused G0/G1 arrest and induced apoptosis of mutant FLT3-expressing cells as evidenced by an increased apoptotic fraction in drug-treated cells, with no effect on viability of parental Ba/F3 cells and no effect on mutant FLT3-positive cell viability in the presence of WEHI (IL-3) (Figure 3A–B, Supplementary Figure S4(B)). Similarly, HG-7-86-01 caused G0/G1 arrest and induced apoptosis of FLT3-ITD-expressing cells in the absence of IL-3, with accompanying PARP cleavage, and no effect on viability of parental Ba/F3 cells (Supplementary Figure S8 and Supplementary Figure S4(C)). Effects of HG-7-86-01 are selective for mutant FLT3 as evidenced by a lack of toxicity toward normal bone marrow cells in a normal bone marrow colony assay (Supplementary Figure S8). These results suggest that both induction of apoptosis and inhibition of cell cycle progression contribute to the observed inhibition of cellular proliferation of mutant FLT3-expressing cells by HG-7-85-01 and HG-7-86-01.
Figure 3. Effect of HG-7-85-01 on viability and cell cycle progression of cells harboring mutations in the juxtamembrane domain of FLT3 in vitro and cell proliferation in vivo.
(A) Induction of apoptosis by HG-7-85-01 of mutant FLT3-expressing cells (following 3 days of treatment). (B) Effect of HG-7-85-01 on cell cycle progression of mutant FLT3-expressing cells (following a 24 hour treatment). (C) In vitro effects of HG-7-85-01 on proliferation of cells harboring the FLT3-ITD mutation, shown alongside PKC412, for comparison. (D) In vivo effects of HG-7-85-01 on proliferation of cells harboring the FLT3-ITD mutation, shown alongside PKC412, for comparison. This study is representative of two independent studies, in which similar results were observed. Approximately 800,000 Ba/F3-FLT3-ITD-luc+ cells injected into the tail veins of NCr athymic nude mice and treated with either vehicle, PKC412 (100mg/kg), or HG-7-85-01 (50mg/kg, 2× daily). Graphed bioluminescence values are shown as percent baseline.
Student t-test comparison
Veh vs HG85 day 9 post IV injection, p<0.0278
Veh vs PKC412 [100mg/kg], day 9 post-IV injection, p<0.0294
Antiproliferative effect of HG-7-85-01 on mutant FLT3-expressing cells in vivo
HG-7-85-01 is approximately 10-fold more potent than PKC412 against mutant FLT3-positive Ba/F3 cells in vitro (Figure 3C), although efficacy between the two agents is comparable in vivo (Figure 3D). In comparison with vehicle-treated mice injected via tail vein with Ba/F3-FLT3-ITD cells, leukemia burden, as assessed by bioluminescence, in drug-treated mice was observed to be significantly suppressed and to a similar degree following 8 days of treatment with HG-7-85-01 (100mg/kg/day) or PKC412 (100mg/kg/day), respectively (Figure 3D). Details about pk characteristics of HG-7-85-01 and HG-7-86-01 are shown in the supplementary data section.
Effect of HG-7-85-01 and HG-7-86-01 on mutant FLT3-expressing cells harboring a point mutation in the activation loop
HG-7-85-01 inhibited the proliferation of cells expressing N841I- and D835Y, with the majority of cells killed at 1 µM, however the potency observed was significantly less than that demonstrated against cells expressing FLT3 juxtamembrane domain mutations (IC50 for N841I=12.5–25 nM; IC50 for D835Y=50–100 nM, Table I and Supplementary Figure S3(E–F). HG-7-86-01 showed only weak activity against the N841I and D835Y mutants (IC50 for N841I=200–400 nM; IC50 for D835Y=500–1000nM, Table I).
HG-7-85-01 potently inhibited total cellular tyrosine phosphorylation in Ba/F3-N841I and Ba/F3-D835Y cells in a concentration-dependent manner with no reduction of levels of FLT3 protein (Supplementary Figure S7). HG-7-86-01 inhibited total cellular tyrosine phosphorylation in Ba/F3-N841I cells at 1 µM, with very little effect on Ba/F3-D835Y cellular tyrosine phosphorylation (data not shown).
Overcoming PKC412 resistance with HG-7-85-01
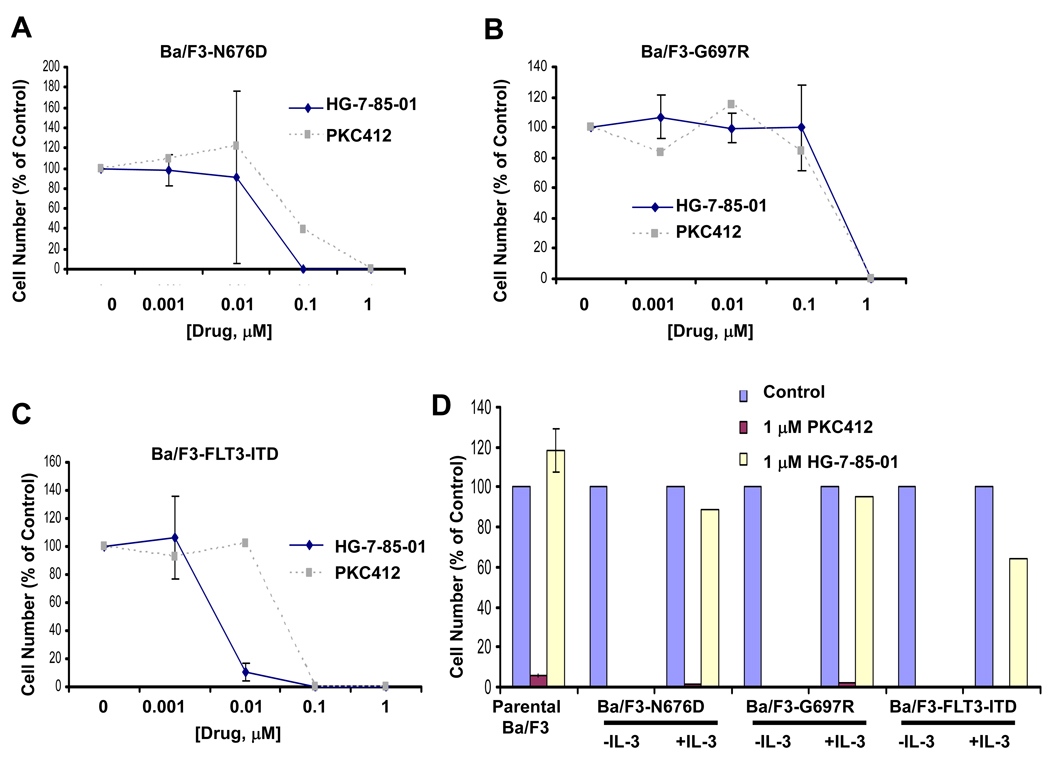
In addition to killing PKC412-sensitive mutant FLT3-expressing cells, HG-7-85-01 is able to override some forms of PKC412 resistance (Figure 4). The PKC412-resistant line, Ba/F3-N676D, displayed slightly higher sensitivity to HG-7-85-01 than PKC412 (Figure 4A), as evidenced by a modest shift of the HG-7-85-01 dose-response curve to the left of the PKC412 dose-response curve. More impressively, however, the highly PKC412-resistant line, Ba/F3-G697R, was sensitive to 1 µM HG-7-85-01, a concentration that does not kill parental Ba/F3 cells and is IL-3 rescue-able (Figure 4B, D). In contrast, 1 µM PKC412 kills parental Ba/F3 cells and is not IL-3 rescue-able, making this concentration of PKC412 nonselectively toxic (Figure 4D). These results suggest that HG-7-85-01 is able to kill some PKC412-resistant cells with higher potency than PKC412 and/or at concentrations that are physiologically relevant.
Figure 4. Overcoming PKC412 resistance with HG-7-85-01.
Approximately 3-day treatment of Ba/F3-N676D (A), Ba/F3-G697R (B), and Ba/F3-FLT3-ITD (C) with HG-7-85-01 or PKC412. (D) Approximately 3-day treatment of parental Ba/F3 cells, N676D-Ba/F3 cells (−/+ IL-3), G697R-Ba/F3 cells (−/+ IL-3), and FLT3-ITD-Ba/F3 cells (−/+ IL-3) with 1 uM PKC412 vs 1 uM HG-7-85-01. Both 1 uM PKC412 and 1 uM HG-7-85-01 are effective at killing mutant FLT3-expressing cells. However, 1 uM PKC412 kills parental Ba/F3 cells and is not IL-3 rescue-able. 1 uM HG-7-85-01 does not kill parental Ba/F3 cells and is IL-3 rescue-able.
Combination studies: HG-7-85-01 and HG-7-86-01, alone and combined with PKC412 and standard chemotherapeutic agents, Ara-c and doxorubicin, against cells harboring FLT3 mutations
We first investigated the ability of HG-7-85-01 to positively combine with PKC412, Ara-c, and doxorubicin against mutant FLT3-positive leukemia. HG-7-85-01 in combination with PKC412 against FLT3-ITD-positive cells was observed to cause a leftward shift in the dose response curve as compared to any one agent alone (Calcusyn combination indices: 1.03167 (ED50), 0.90664 (ED75), 0.80147 (ED90), or additive to synergistic effects, Table II). Combination effects between HG-7-85-01 and doxorubicin similarly suggested synergy, while the combination of HG-7-85-01 and the standard chemotherapeutic agent, Ara-c, resulted in values ranging from synergy (ED90) to antagonism (ED25) (Table II).
Table II. Combination indices calculated for HG-7-85-01 and HG-7-86-01, respectively, combined with other agents against mutant FLT3.
Values 0.3–0.7 indicate synergy. Values 0.7–0.85 indicate moderate synergy. Values 0.85–0.90 indicate slight synergy. Values 0.9–1.1 indicate nearly additive effects. Values 1.10–1.20 indicate slight antagonism. Values 1.20–1.45 indicate moderate antagonism. Values 1.45–3.3 indicate antagonism. Those values that indicate moderate synergy to synergy are shaded gray.
| Combination Indices: | |||||
|---|---|---|---|---|---|
| Drug Combination |
Cell Lines | ED25 | ED50 | ED75 | ED90 |
| HG-7-85-01+PKC412 | MOLM13 | 1.18086 | 1.03167 | 0.90664 | 0.80147 |
| HG-7-85-01+Ara-c | Ba/F3-FLT3-ITD | 1.68776 | 1.07047 | 0.71292 | 0.50237 |
| HG-7-85-01+Doxorubicin | Ba/F3-FLT3-ITD | 0.68494 | 0.67599 | 0.67836 | 0.69213 |
| HG-7-86-01+PKC412 | Ba/F3-FLT3-ITD | 0.59352 | 0.56710 | 0.58033 | 0.63352 |
| HG-7-86-01+PKC412 | MOLM13 | 1.05116 | 0.81017 | 0.67430 | 0.59844 |
| HG-7-86-01+PKC412 | Ba/F3-D835Y | 1.02964 | 0.79888 | 0.63317 | 0.51291 |
| HG-7-86-01+PKC412 | Ba/F3-Y572C | 0.68173 | 0.54498 | 0.44014 | 0.35923 |
| HG-7-86-01+PKC412 | Ba/F3-N676D | 2.44639 | 2.13308 | 1.85997 | 1.62191 |
| HG-7-86-01+PKC412 | Ba/F3-G697R | 1.17645 | 0.97361 | 0.84182 | 0.76069 |
| HG-7-86-01+Ara-c | Ba/F3-FLT3-ITD | 1.08827 | 0.73803 | 0.50054 | 0.33950 |
| HG-7-86-01+Doxorubicin | Ba/F3-FLT3-ITD | 0.73602 | 0.67835 | 0.63407 | 0.60052 |
HG-7-86-01 in combination with PKC412 against Ba/F3-FLT3-ITD and MOLM13-luc+ cells was observed to cause a leftward shift in the dose-response curve as compared to HG-7-86-01 alone or PKC412 alone (Calcusyn: synergy) (Table II). Similarly, HG-7-86-01 in combination with PKC412 led to more killing of Ba/F3-D835Y cells and Ba/F3-Y572C cells than either drug alone (Calcusyn: synergy) (Table II). The combination between HG-7-86-01 and PKC412 led to more killing of the PKC412-resistant cell line, Ba/F3-G697R, than either drug alone (Calcusyn: nearly additive effects to moderate synergism for ED50 to ED90) (Table II). However, antagonism was observed for the combination of HG-7-86-01 and PKC412 against the PKC412-resistant line, Ba/F3-N676D. These results suggest that HG-7-86-01 may be able to be effectively combined with FLT3 inhibitors in late-stage clinical development, like PKC412, in a non-antagonistic fashion, against mutant FLT3-positive disease and some forms of PKC412-resistant disease.
Lastly, we investigated the combination effects of HG-7-86-01 and the standard chemotherapeutic agents, Ara-c and doxorubicin, against mutant FLT3-expressing cells. The combination of HG-7-86-01 and Ara-c led to leftward shifts in the dose-response curves as compared to each single agent alone when tested against Ba/F3-FLT3-ITD cells (Calcusyn: moderate synergism to synergy for ED25-ED90) (Table II). Similar results were observed for the combination of HG-7-86-01 and doxorubicin (Calcusyn: nearly additive to synergistic results for ED25-ED90). These results suggest that HG-7-86-01 could potentially be combined with standard chemotherapy agents, like Ara-c and doxorubicin, in way that is more efficacious than single agent therapy.
Discussion
Drug resistance in the form of development of point mutations in the target protein is a growing concern in patients treated with tyrosine kinase inhibitors. Combination therapy, using two structurally diverse agents that either bind to different sites on the same protein, or interact and inhibit distinct signaling components of the same or different signaling pathways, offers a promising therapeutic strategy designed to suppress the emergence of these point mutations. In the case of CML, combinations of distinct inhibitors of Bcr-Abl, including dasatinib and imatinib, effectively reduced the occurrence of drug-resistant clones (29, 30). Similarly, the combination of imatinib with the dual Scr/Abl inhibitors AP23848 and dasatinib, respectively, has shown promise in preclinical in vitro studies involving treatment of non-resistant and imatinib-resistant Bcr-Abl point mutant-expressing cells (31). In the case of PKC412-resistant leukemia, because of the nature of resistance mechanisms, such as emergence of drug-resistant clones (32) it will likely be of significant clinical benefit to simultaneously administer more than one mutant FLT3 inhibitor to patients as a way to suppress the development of these drug-resistant mutants.
FLT3 naturally possesses a phenylalanine residue at the ‘gatekeeper’ position which has been demonstrated to be responsible for the inability of imatinib to inhibit this kinase (33). The ability of HG-7-85-01 and HG-7-86-01 to potently inhibit FLT3 demonstrates their ability to tolerate large hydrophobic amino acids at the gatekeeper position consistent with the ability of both compounds to target the T315I mutation of Bcr-Abl. HG-7-85-01 and HG-7-86-01 are distinct amino-thiazolopyridine based type II ATP competitive inhibitor. HG-7-85-01 is a low nanomolar FLT3 inhibitor in biochemical and cellular assays. It is more potent than MLN518, PKC 412, CGP 5241, Sunitinib and has comparable potency with AC220, CEP-701, Sorafenib as a FLT3 inhibitor. HG-7-85-01 and HG-7-86-01 exhibited considerably greater kinase selectivity than PKC412, CEP 701, CGP52421, Sorafenib and Sunitinib. In contrast to HG compounds, the majority of FLT3 inhibitors reported in the literature are type I ATP-competitive inhibitors. Type II ATP-competitive kinase inhibitors recognize the unique DFG-out conformation of kinases and may be advantageous in terms of potency and selectivity as demonstrated in HG compounds and other reported kinase inhibitors (34).
In addition to its anti-proliferative effects against gatekeeper mutants (27), HG-7-85-01 selectively induces apoptosis of leukemic cells harboring mutant FLT3 with a significantly higher potency than that of PKC412, with no apparent effect on cells harboring wild-type FLT3. HG-7-85-01 inhibits the proliferation of patient blasts harboring mutant FLT3, is active against PKC412-sensitive and –resistant isoforms of mutant FLT3, and significantly suppresses FLT3-ITD-induced leukemia burden in mice. HG-7-85-01 also positively combines with PKC412 and standard chemotherapeutic agents, like Ara-c and doxorubicin, to more effectively inhibit the proliferation of mutant FLT3-expressing cells than any one agent alone. The ability of HG-7-85-01 to inhibit mutant FLT3 is a distinctive feature that, in combination with its activity as a Type II ATP competitive inhibitor against a diverse panel of tyrosine kinase gatekeeper mutants (27), underscores its structural uniqueness and potential clinical versatility.
Both compounds were demonstrated to act synergistically with PKC412 to inhibit mutant FLT3-dependent cell proliferation. Thus, either agent could be combined with PKC412 to potentially achieve higher patient responsiveness as well as reduce the likelihood of emergence of some FLT3 point mutations conferring resistance to PKC412. Combinations of mutant FLT3 inhibitors could theoretically be administered on either a sequential, rotating schedule, or administered simultaneously, provided the drugs did not interfere with each other.
HG-7-85-01 and HG-7-86-01 are reasonably selective kinase inhibitors. The selectivity of HG-7-85-01 is intermediate between compounds such as imatinib and nilotinib, which are more selective than HG-7-85-01, and dasatinib, which is less selective (27). Thus, despite the ability of both HG-7-85-01 and HG-7-86-01 to inhibit more than one kinase target (Figure 1), they are not considered promiscuous kinase inhibitors based on their calculated selectivity score metric (26, 27).
Taken together, these results support the idea that FLT3 is an attractive therapeutic target for AML. Our findings with HG-7-85-01 and HG-7-86-01 also highlight the emergence of a novel class of FLT3 inhibitors that exhibit high selectivity and potency toward mutant FLT3 as an oncogenic target.
Supplementary Material
Acknowledgments
Grant Support: J.D.G is supported by NIH grant CA66996, and a Specialized Center of Research Award from the Leukemia and Lymphoma Society. J.D.G. is also supported by NIH grants CA36167 and DK50654. J.D.G., R.S., N.G. and A.L.K. have a financial interest with Novartis Pharma AG.
References
- 1.McKenzie SB. Advances in understanding the biology and genetics of acute myelocytic leukemia. Clin Lab Sci. 2005;18:28–37. [PubMed] [Google Scholar]
- 2.Stirewalt DL, Radich JP. The role of FLT3 in hematopoeitic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 3.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the FLT3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 4.Horiike S, Yokota S, Nakao M, et al. Tandem duplications of the FLT3 receptor gene are associated with leukemic transformation of myelodysplasia. Leukemia. 1997;11:1442–1446. doi: 10.1038/sj.leu.2400770. [DOI] [PubMed] [Google Scholar]
- 5.Kiyoi H, Towatari M, Yokota S, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- 6.Kondo M, Horibe K, Takahashi Y, et al. Prognostic value of internal tandem duplication of the FLT3 gene in childhood acute myelogenous leukemia. Med Pediatr Oncol. 1999;33:525–529. doi: 10.1002/(sici)1096-911x(199912)33:6<525::aid-mpo1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Rombouts WJ, Blokland I, Lowenberg B, Ploemacher RE. Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the FLT3 gene. Leukemia. 2000;14:675–683. doi: 10.1038/sj.leu.2401731. [DOI] [PubMed] [Google Scholar]
- 8.Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99:310–318. doi: 10.1182/blood.v99.1.310. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 10.Jiang J, Paez JG, Lee JC, et al. Identifying and characterizing a novel activating mutation of the FLT3 tyrosine kinase in AML. Blood. 2004;104:1855–1858. doi: 10.1182/blood-2004-02-0712. [DOI] [PubMed] [Google Scholar]
- 11.Kindler T, Breitenbuecher F, Kasper S, et al. Identification of a novel activating mutation (Y842C) within the activation loop of FLT3 in patients with acute myeloid leukemia (AML) Blood. 2005;105:335–340. doi: 10.1182/blood-2004-02-0660. [DOI] [PubMed] [Google Scholar]
- 12.Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 13.Stone RM, Fischer T, Paquette R, et al. Phase 1B study of PKC412, an oral FLT3 kinase inhibitor, in sequential and simultaneous combinations with daunorubicin and cytarabine (DA) induction and high-dose cytarabine consolidation in newly diagnosed patients with AML. Blood. 2005;106:404a. [Google Scholar]
- 14.Zarrinkar P, Gunawardane R, Cramer M, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of AML. Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y, Paz K. Tantutinib, an oral, small molecule inhibitor of FLT3 for the treatment of AML and other cancer conditions I. Drugs. 2008;11:46–56. [PubMed] [Google Scholar]
- 16.Weisberg E, Barrett R, Liu Q, et al. FLT3 inhibition and mechanism of drug resistance in mutant FLT3-positive AML Drug Resist. Updates. 2009;12:81–89. doi: 10.1016/j.drup.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 18.Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 19.Frohling S, Scholl C, Levine RL, et al. Identification of driver and passenger mutations of FLT3 by high-throughput DNA sequence analysis and functional assessment of candidate alleles. Cancer Cell. 2007;12:501–513. doi: 10.1016/j.ccr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Cools J, Mentens N, Furet P, et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res. 2004;64:6385–6389. doi: 10.1158/0008-5472.CAN-04-2148. [DOI] [PubMed] [Google Scholar]
- 21.Weisberg E, Kung AL, Wright RD, et al. Potentiation of antileukemic therapies by Smac mimetic, LBW242: effects on mutant FLT3-expressing cells. Mol Cancer Ther. 2007;6:1951–1961. doi: 10.1158/1535-7163.MCT-06-0810. [DOI] [PubMed] [Google Scholar]
- 22.Weisberg E, Roesel J, Bold G, et al. Antileukemic effects of the novel, mutant FLT3 inhibitor NVP-AST487: effects on PKC412-sensitive and -resistant FLT3-expressing cells. Blood. 2008;112:5161–5170. doi: 10.1182/blood-2008-02-138065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo Y, MacLeod RA, Uphoff CC, et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23) Leukemia. 1997;11:1469–1477. doi: 10.1038/sj.leu.2400768. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong SA, Kung AL, Mabon ME, et al. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3:173–183. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 25.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enz. Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 27.Weisberg E, Choi HG, Ray A, et al. Discovery of a small molecule type II inhibitor of wild-type and gatekeeper mutants of BCR-ABL, PDGFRα, Kit, and Src kinases. Blood. doi: 10.1182/blood-2009-11-251751. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Burgess MR, Skaggs BJ, Shah NP, et al. Comparative analysis of two clinically active Bcr-Abl kinase inhibitors reveals the role of conformation-specific binding in resistance. Proc Natl Acad Sci USA. 2005;102(9):3395–3400. doi: 10.1073/pnas.0409770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradeen HA, Eide CA, O’Hare T, et al. Comparison of imatnib, dasatinib (BMS-354825), and nilotinib (AMN107) in an n-ethyl-n-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood. 2006;108(7):2332–2338. doi: 10.1182/blood-2006-02-004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Hare T, Walters DK, Stoffregen EP, et al. Combined Abl inhibitor therapy for minimizing drug resistance in chronic myeloid leukemia: Src/Abl inhibitors are compatible with imatinib. Clin. Cancer Res. 2005;11(10 Pt 1):6987–6993. doi: 10.1158/1078-0432.CCR-05-0622. [DOI] [PubMed] [Google Scholar]
- 32.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107:293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 33.Bohmer FD, Karagyozov L, Uecker A, et al. A single amino acid exchange inverts susceptibility of related receptor tyrosine kinases for the ATP site inhibitor STI-571. J Biol Chem. 2003;278:5148–5155. doi: 10.1074/jbc.M209861200. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nat. Chem. Biol. 2006;2(7):358–364. doi: 10.1038/nchembio799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.