Abstract
Commensal microorganisms live in association with the mucosal surfaces of all vertebrates. The skin of teleost fish is known to harbor commensals. In this study we report for the first time the presence of an intracellular Gram positive bacteria, Staphylococcus warneri that resides in the skin epidermis of rainbow trout (Oncorhynchus mykiss). S. warneri was isolated from healthy hatchery trout skin epithelial cells. In situ hybridization confirmed the intracellular nature of the bacterium. Skin explants exposed in vitro to S. warneri or the extracellular pathogen Vibrio anguillarum show that S. warneri is able to induce an anti-inflammatory cytokine status via TGF-β 1b compared to the pro-inflammatory responses (IL-1β , IL-6 and TNF-α) elicited by V. anguillarum. In vivo experiments showed that S. warneri is not pathogenic to rainbow trout when injected intraperitoneally at high concentrations. However, S. warneri is able to stimulate V. anguillarum growth and biofilm formation on rainbow trout scales. Our results demonstrate that rainbow trout skin commensals such as S. warneri have the potential to become indirect pathobionts by enhancing growth and biofilm formation of pathogens such as V. anguillarum. These results show that fish farming practices (i.e. handling and other manipulations) can alter the skin microbiota and compromise the skin health of rainbow trout.
Keywords: rainbow trout, skin, Staphylococcus warneri, Vibrio anguillarum, pathobiont
1. Introduction
Commensal microorganisms provide metabolic, developmental and immunological functions to the host (Sommer and Bäckhed, 2013). but they are, however, not always innocuous. When homeostasis is breached, invasive symbionts, or pathobionts, prompt abnormal inflammatory responses potentially causing disease (Chow et al., 2011; Belkaid et al., 2013; Kamada et al., 2013). Pathobionts are overrepresented during dysbiosis situations, which arise from genetic predispositions, exposure to environmental or metabolic stressors, or alteration of the normal microbial communities (Round and Mazmanian, 2009; Packey and Sartor, 2009).
The delineation between pathogens and commensals is not always easy to make and it is mostly defined by the immune responses they trigger. One of the current dogmas establishes that successful colonization by a commensal microorganism relies on the induction of anti-inflammatory responses, often mediated by the cytokine TGF-β (Detournay et al., 2012) that leads to tolerance of the commensal by the host. Other theories point to the opposite, postulating that commensals must generate a stereotypical inflammatory cascade when establishing a symbiosis with their host (Nussbaum and Locksley, 2012).
In teleost fish, the gills, gut and skin are the main mucosal surfaces harboring diverse microbial communities. The skin is thought to be the largest immunologically active organ and commensals may often assist in the homeostasis of this barrier and contribute to the hosts’ repertoire of immune defense mechanisms against pathogens. In fish, the skin is composed of a layer of living epithelial cells, with no keratinization and abundant mucus-secreting cells. Teleost SALT (skin-associated lymphoid tissue) is continuously exposed to diverse microbial stimuli (including commensals and pathogens) as well as environmental and mechanical stressors (Salinas et al., 2011; Esteban, 2012) and it is able to mount gut-like immune responses (Xu et al., 2013).
The Gram positive Staphylococcus spp. can be pathogenic to their fish hosts, causing exophthalmia and septicaemia-like symptoms in fish that have been infected (Shah and Tyagy, 1986), although they have also been reported from fish in the absence of disease (Spangaard et al., 2000; Cantas et al., 2012). S. warneri includes several strains reported as pathogenic to humans (Campoccia et al., 2010). Moreover, S. warneri has been isolated and grown from discolored kidneys and livers of diseased rainbow trout (Oncorhynchus mykiss) that displayed ulcerations on the fins and exophthalmia, along with ascetic fluid in the abdomens (Gil et al., 2000). In this study, we identify for the first time S. warneri as a resident commensal of rainbow trout skin. The aim of the study was to examine the role S. warneri as a possible pathobiont for rainbow trout as well as the interactions between S. warneri and the common Gram negative pathogen, Vibrio anguillarum.
2. Materials and methods
2.1. Isolation and identification of S. warneri
The bacterium was isolated from the skin of healthy adult triploid rainbow trout (mean weight 250g) obtained from Lisboa Springs Hatchery, Pecos, New Mexico where fish were maintained in concrete raceways with continuous water flow. Fish were sampled during the months of November and December when the water temperature is between 8 – 13° C. Health status was only evaluated based on external signs: no ulcers, no bleeding, lack of external parasites, active swimming and feeding behavior. No further tests were conducted to assess presence of internal infections. Both skin mucus samples and skin samples without mucus were used for bacterial isolation. Bacteria present in the mucus were isolated as explained elsewhere (Xu et al., 2013). Ten µ l aliquots were plated in Luria broth (LB) agar plates or Tryptic Soy Agar (TSA) plates. Additionally, three fish were used to isolate possible bacteria living in the skin. To that end, after the mucus had been scraped, the skin was sprayed with 70% ethanol and a 2 cm2 section of skin was immediately dissected and placed in HBSS containing penicillin and streptomycin (100 U/ml and 100 µg/ml, respectively). After shaking at room temperature for 2 h, the skin sample was transferred to a Petri dish containing HBSS without antibiotics. At this point the skin was finely minced (over 100 times) and cells were further lysed using a 1 ml syringe with a needle. The suspension was vortexed and then centrifuged at 3000 g, 10 min. The pellet was resuspended in sterile PBS and plated in either LB or TSA plates.
After 72 h, three different types of colonies could be observed. One of these was sub- cultured onto TSA plates. Colonies were small, pale yellow with round, smooth edges. The cultures matched the definition of S. warneri according to Bergey’s Manual of Systematic Bacteriology (2011). The same type of colony was found in the mucus- derived cultures. The pure isolate culture was identified at the Tricore laboratories (Albuquerque, New Mexico) by means of Gram stain and MALDI-TOF. These tests revealed that the isolate was a Gram positive cocci, S. warneri. The identity was further confirmed by PCR using S. warneri specific 16s rDNA primers (Table 1) (Iwase et al., 2007), cloning and sequencing. PCR used the following cycles: 94°C for 5 min, then 30 cycles of 94°C for 30 s, 50°C for 30 s, 72°C for 1.5 min and a final extension of 72 °C for 10 min. All PCR products were cloned and sequenced to confirm their identity as explained previously (Tacchi et al., 2013). The obtained sequence had a homology of 100% with S. warneri.
Table 1.
Primers used in the present study
| Gene | Primer | Sequence (5'–3') | Application |
|---|---|---|---|
| IL1β | IL1bF | ACATTGCCAACCTCATCATCG | qPCR |
| IL1bR | TTGAGCAGGTCCTTGTCCTTG | qPCR | |
| TNFα | TNFα F | GGGGACAAACTGTGGACTGA | qPCR |
| TNFα R | GAAGTTCTTGCCCTGCTCTG | qPCR | |
| IL6 | IL6F | ACTCCCCTCTGTCACACACC | qPCR |
| IL6R | GGCAGACAGGTCCTCCACTA | qPCR | |
| TGFβ 1a | TGFb1aF | CTCACATTTTACTGATGTCACTTCCTGT | qPCR |
| TGFb1aR | GGACAACTGCTCCACCTTGTG | qPCR | |
| TGFβ 1b | TGFb1bF | CATGTCCATCCCCCAGAACT | qPCR |
| TGFb1bR | GGACAACTGTTCCACCTTGTGTT | qPCR | |
| EF-1α | EF-1aF | CAACGATATCCGTCGTGGCA | qPCR |
| EF-1aR | ACAGCGAAACGACCAAGAGG | qPCR | |
| Swar 16S | SwarF | TGTAGCTAACTTAGATAGTGTTCCTTCT | RT-PCR |
| SwarR | CCGCCACCGTTATTTCTT | RT-PCR |
2.2. Fish and stress experiments
Rainbow trout (n=6) were either sampled directly from the hatchery rafts or after a 5 h transport from the Lisboa Spring hatchery to Tingley beach, Albuquerque. The transport was conducted in a truck, using raceway water from the hatchery without any sedation.
2.3. Localization of S. warneri by Fluorescent in situ hybridization (FISH)
In order to investigate the localization of S. warneri in the skin of rainbow trout, a FISH probe was designed. The probe (Eurofins MWG Operon) was labeled with Cy5 in the 5’ end and targeted the superoxide dismutase A (sodA) gene of S. warneri. Control trout skin cryosections (n=6) (5 µ m-thick) were fixed in formalin for 10 min, permeabilized overnight in 70% ethanol, hybridized at 37° C with the S. warneri probe, stained with the nuclear stain DAPI and then observed under a Nikon Ti fluorescent microscope. Images were analyzed using Nis Elements Advanced Research software.
2.4. Quantification of S. warneri in the skin of control and stressed rainbow trout by PCR
The presence of S. warneri in the skin of rainbow trout was quantified by qPCR using a standard curve made of serial dilutions of a pure S. warneri culture. The standard curve ranged from 109 to 10 S. warneri colony forming units (cfu). Total DNA was obtained from skin tissue samples of control or stressed rainbow trout. The sodA gene of S. warneri was amplified according to Iwase (2007) using specific primers (Table 1). 100 ng of S. warneri or trout skin DNA were amplified by qPCR. The qPCR was performed as previously described (Tacchi et al., 2013).
2.5. In vitro exposure of skin explants to S. warneri
Control rainbow trout skin explants (n=5) (0.5 cm2) were surface sterilized and placed in 24-well plates. Explants were exposed to 0, 102, 104 or 106 S. warneri cells or 106 V. anguillarum cells for 6, 24 or 48 h. At each time point, explants were collected and placed in Trizol for RNA extraction. cDNA synthesis was performed using 1 µ g of total RNA, which was denatured (65°C, 5 min) in the presence of 1 µl of oligo-dT17, 1 µl dNTP (deoxynucleoside triphosphate mix 10 mM each (Promega) and RNA/DNA free water (Sigma) in a volume of 13 µ l. Synthesis was carried out using 1 µ l Superscript III enzyme reverse transcriptase (Invitrogen) in the presence of 5 µ l of 5x first strand buffer, 1 µ l 0.1 M DTT (final volume of 25 µ l) and incubated at 55°C for 1 h.
2.6. qPCR studies: expression of pro-inflammatory and anti-inflammatory cytokines
The expression of the pro-inflammatory cytokines IL-1β , Il-6 and TNF-α as well as the anti-inflammatory cytokines TGF-β 1a and TGF-β 1b was studied by RT-qPCR using specific primers (Table 1). The qPCR was performed as described above using 3 ìl of a diluted cDNA template. Rainbow trout elongation factor EF-1α was used as control gene for normalization of expression. The relative expression level of the genes was determined using the Pfaffl method (Pfaffl, 2001) as previously described (Tacchi et al., 2013). The sequences of all the PCR products amplified by qPCR were further confirmed by cloning.
2.7. Effect of S. warneri on the growth of the pathogen Vibrio anguillarum
GFP-V. anguillarum was grown in the presence or absence of S. warneri. V. anguillarum was grown for 24 h in LB at 24°C and cultures were adjusted to an absorbance of 1.4 at 600 nm for a concentration of 8.4x108 cfu/ml. A total of 104 cfu were added to each well of 96-well plates in triplicate. S. warneri cultures grown for 24 h (also in LB) were added to the wells at 10, 102, 104 or 106 cfu/well. Positive control consisted of wells containing V. anguillarum only, while negative controls consisted of wells containing S. warneri only and LB only. GFP fluorescence was measured at 3, 6, 21 and 29 h in a Synergy H1 plate reader at an excitation wavelength of 485 nm and emission wavelength of 538 nm.
2.8. Effect of S. warneri on Vibrio anguillarum biofilm formation
Biofilm formation on rainbow trout scales was assayed as described previously by Croxatto et al., 2007. Briefly, scales were collected from the lateral line and kept at 12°C for 48 h before infection. GFP-expressing V. anguillarum was added (104 cells/well) as well as S. warneri (in dilutions of 102, 104, 106 cells/well). After 48 h the media was removed and the scales were fixed with 100% methanol, air-dried and mounted. The images acquired were analyzed using NIS Elements Advanced Research software. The mean green fluorescence intensity of each captured image was divided by the surface area value. This ratio was used was used to quantify the mean relative biofilm formation value for each treatment.
2.9. In vivo injection of S. warneri
S. warneri was grown overnight in LB, washed in PBS and injected intraperitoneally (i.p) into healthy rainbow trout (mean weight=3 g). Rainbow trout (n=40) were obtained from Clear Springs Foods Inc. (Buhl, Idaho) and received a 50 µ l intraperitoneal (i.p) injection containing 1×107, 3×108 or 1×109 S. warneri cfu/fish. Control group received an i.p injection of PBS. Fish were monitored for any signs of disease or mortality for four weeks after the injection.
2.10. Statistical analysis
Data is presented as mean ± standard error (SE). qPCR measurements were analyzed by t-test by comparing values with the control group. One-way ANOVA analysis followed by Tukey’s posthoc test was used to identify differences between treatments in the biofilm formation experiments. p-values < 0.05 were considered significant.
3. Results
3.1. Localization of S. warneri in the skin of rainbow trout
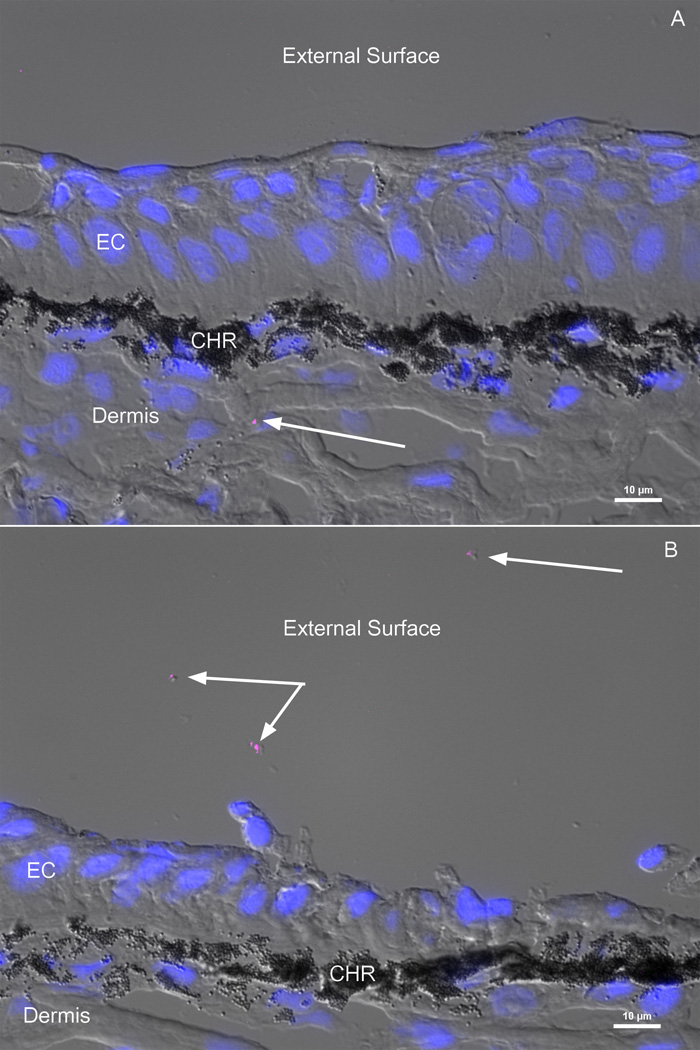
FISH staining on rainbow trout skin cryosections revealed the presence of S. warneri both in the mucus (Fig. 1A) and in the skin tissue, mostly in the dermis compartment (Fig. 1B).
Figure 1.
Localization of S. warneri in hatchery rainbow trout skin by FISH. Skin cryosections were hybridized with a S. warneri specific probe labeled with cynanine 5 (magenta). DAPI-stained cell nuclei are shown in blue. The fluorescent images were overlaid with a differential interference contrast (DIC) image of the same field. A) S. warneri is present in the dermis layer of the skin B) S. warneri is present in the mucus layer of rainbow trout. Arrows point S. warneri cells. CHR: chromatophores, EC: epithelial cells.
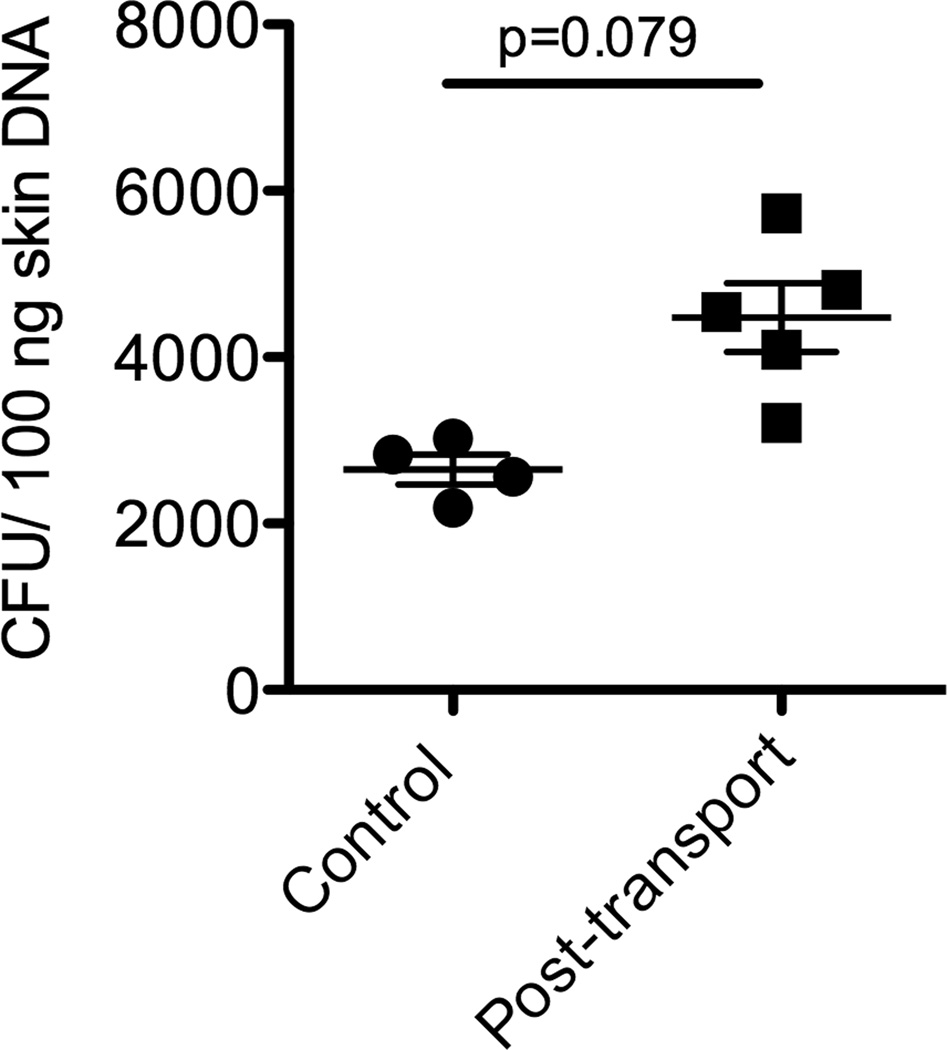
3.2. The numbers of S. warneri cfu in the skin increase during a transport event
Using a standard curve and a qPCR assay, we were able to quantify the number of cfu present in the skin of rainbow trout before and after a transport event. As Fig. 2 shows, the numbers of S. warneri cfu present in the skin of rainbow trout were significantly higher in the “handled” group (post transport) compared to the control group.
Figure 2.
Quantification of the number of S. warneri colony forming units in the skin (both mucus and tissue) of rainbow trout before (control) or after a transport event by qPCR (n=6).
3.3. Expression of pro-inflammatory and anti-inflammatory cytokines in rainbow trout skin explants exposed to S. warneri or V. anguillarum
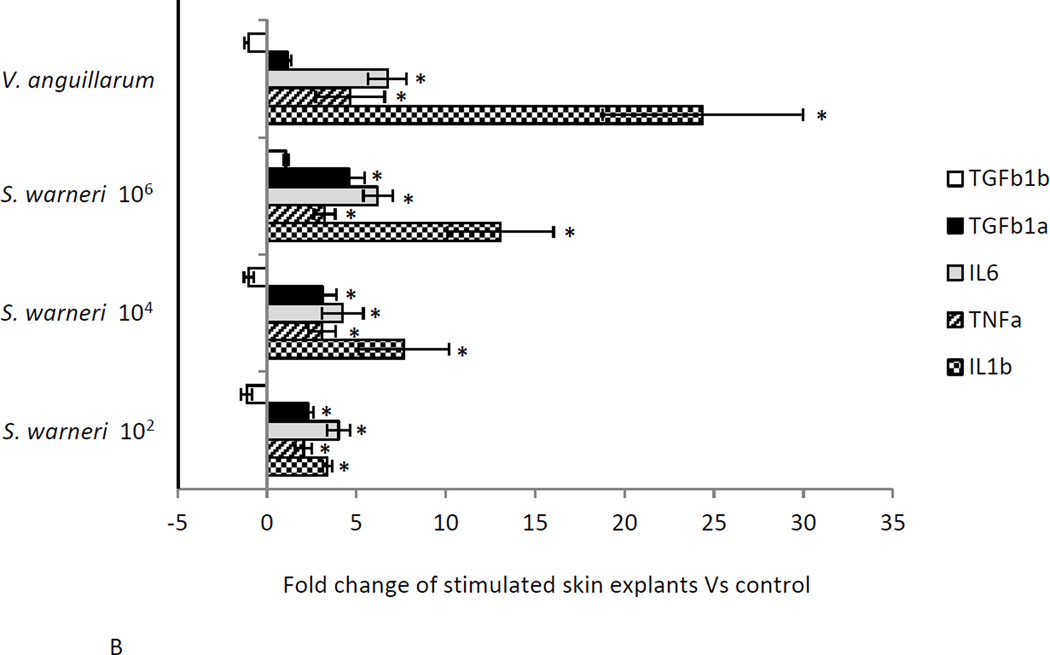
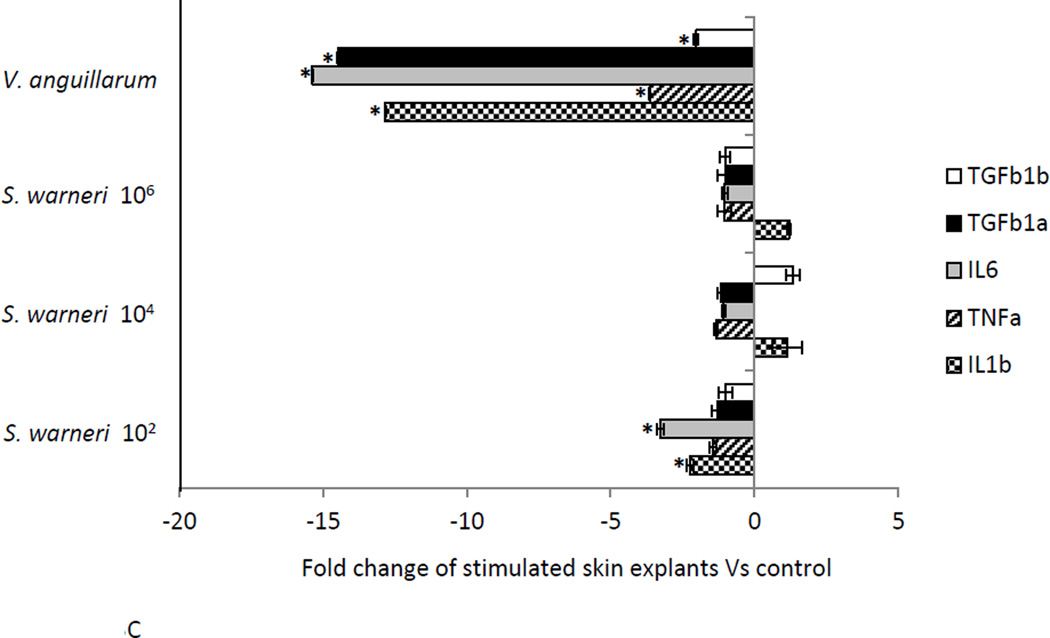
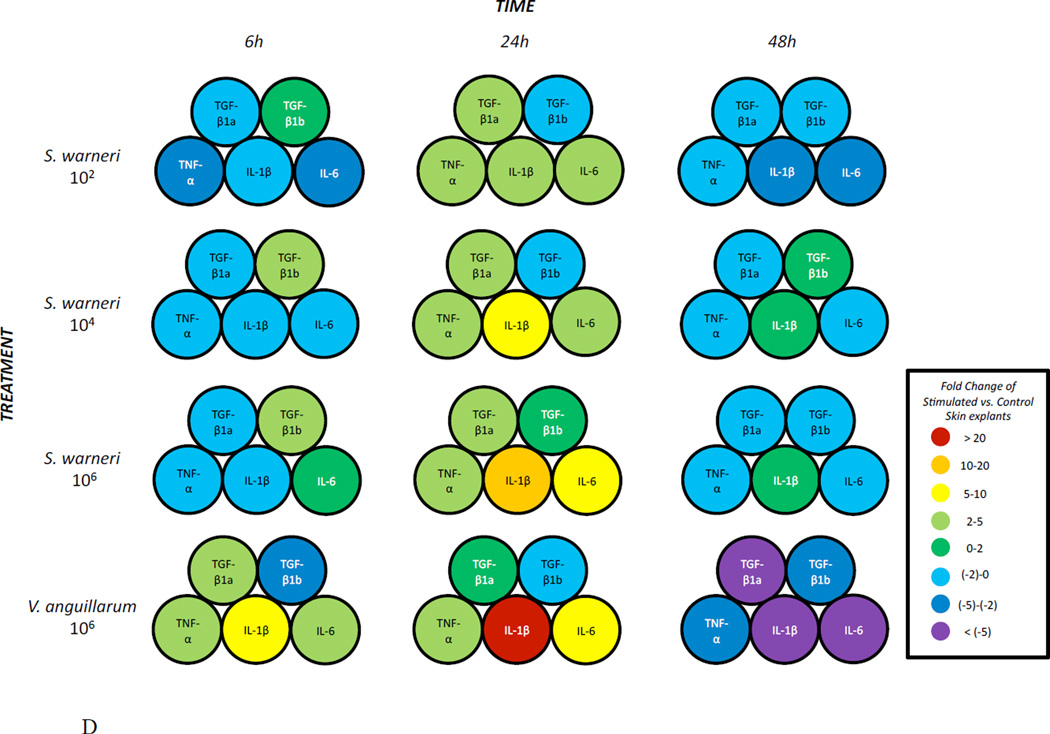
The expression of three pro-inflammatory (IL1β , TNFα and IL6) and two anti-inflammatory cytokines (TGFβ 1a and TGFβ 1b) was measured in the skin of rainbow trout at three different time points following in vitro exposure to three different concentrations of S. warneri. As shown in Fig. 3A-D, the commensal S. warneri and the pathogen V. anguillarum induced different cytokine expression profiles in the skin of rainbow trout. At 6 h, S. warneri stimulated cells showed a suppression of the inflammatory response with all the pro-inflammatory molecules (and TGFβ 1a) down-regulated and the down-regulation decreased with increasing concentrations of the commensal. However, the anti-inflammatory cytokine TGF1β b was found to be up-regulated significantly at 6 h (P values < 0.05) when cells were stimulated with S. warneri. V. anguillarum, on the other hand, induced the expression of IL1β , TNFα, IL6 and TGFβ 1a and a suppression of TGF1β b. 24 h following stimulation, both bacteria induced a dramatic up-regulation of IL1β, TNFα, IL6 and TGFβ 1a (in a dose dependent manner in S. warneri stimulated cells), whilst TGF1β b was not modified in expression. At 48 h, a significant down-regulation of the genes studied was observed in the treatment with the lowest concentration of S. warneri (P value < 0.05) and in the V. anguillarum treatment (P value < 0.01). The suppression was more evident in V. anguillarum-stimulated skin explants compared to S. warneri stimulated skin explants.
Figure 3.
Expression of pro- and anti-inflammatory cytokines in rainbow trout skin explants following incubation with S. warneri or V. anguillarum at different concentrations by qPCR. A) Cytokine expression levels after 6 h B) Cytokine expression levels after 24 h C) Cytokine expression levels after 48 h D) Visual summary of all results from A, B and C. Expression levels were put into eight different categories according to the figure legend. Asterisks indicate significant differences compared with the control treatment.
3.4. Direct effects of S. warneri on the growth of GFP-V. anguillarum
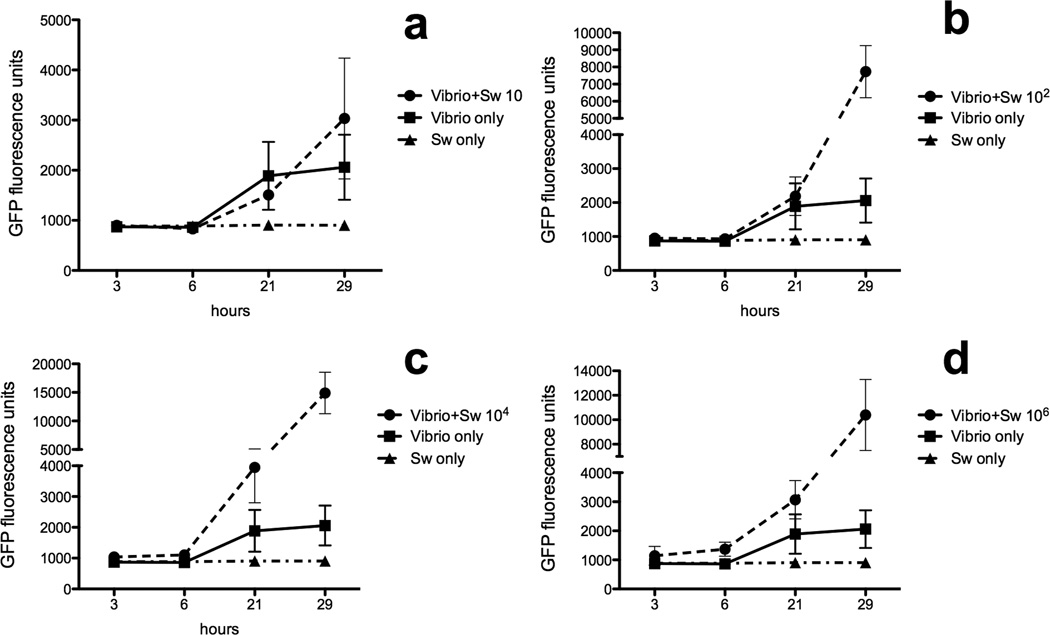
The growth of V. anguillarum in the presence of different S. warneri concentrations was measured over a period of 29 h. Four concentrations of S. warneri were tested. The lowest dose (10 cfu/ml) had no effect on V. anguillarum growth but the rest of the concentrations tested, all enhanced V. anguillarum growth (Fig 4A-D). The greatest enhancement occurred after 29 h of culture although the two highest concentrations tested already had an effect after 21 h. At 29 h, V. anguillarum growth was > 4 times, 7 times and 6 times higher in the presence of 102, 104 and 106 S. warneri cfu/ml, respectively, compared to V. anguillarum alone.
Figure 4.
V. anguillarum growth curves in the presence of absence of S. warneri at A) 10 cfu/ml, B) 102 cfu/ml, C) 104 cfu/ml and D) 106 cfu/ml. Growth was measured by quantifying the GFP fluorescence units at each time point in a plate reader. Results are representative of three independent experiments.
3.5. Effect of S. warneri on V. anguillarum biofilm formation on trout scales
We measured the ability of GFP-V. anguillarum to form biofilms on the scales of control rainbow trout in the presence or absence of S. warneri. Biofilm formation was enhanced after 48 h by the presence of S. warneri at all doses tested (Fig. 5).
Figure 5.
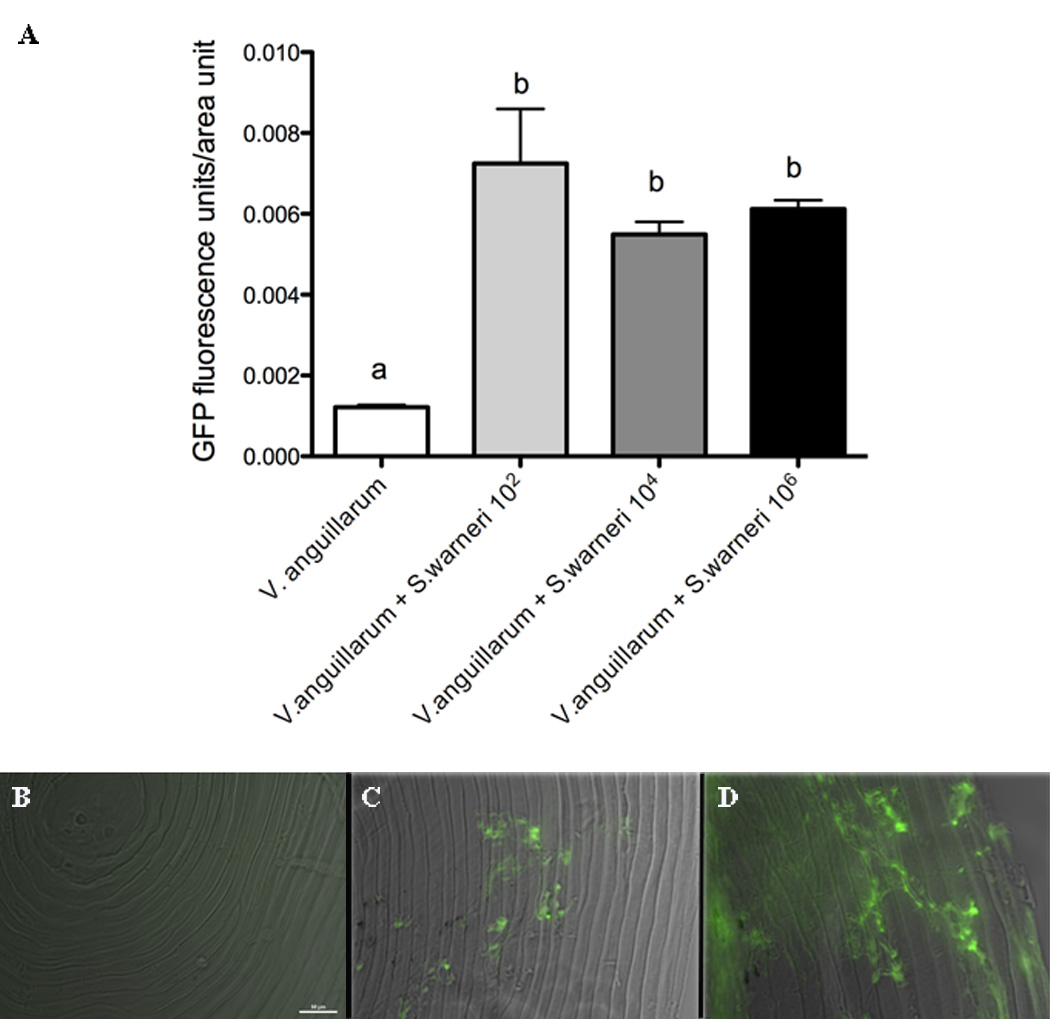
V. anguillarum biofilm formation on rainbow trout skin is enhanced by the presence of S. warneri. A) Results are shown as the mean +/− SE of the GFP fluorescence units per unit of area after analyzing ten different scales per experimental group by fluorescence microscopy. B) Fluorescent microscopy image and DIC overlay of a control scale incubated with S. warneri for 48 h. C) Fluorescent microscopy image and DIC overlay of a scale incubated with GFP-V. anguillarum for 48 h. D) Fluorescent microscopy image and DIC overlay of a control scale incubated with GFP-V. anguillarum and S. warneri (102 cfu/ml) for 48 h. Different letters indicate significantly different groups.
3.6. S. warneri is not pathogenic to rainbow trout
Challenge experiments using three different doses of S. warneri by i.p injection caused no mortality or morbidity in rainbow trout over a period of four weeks. Only one fish died at the highest assayed dose (109 cfu/fish) 6 days post-injection. Bacterial colonies with similar morphological characteristics to S. warneri were recovered on TSA plates from the kidney of the only fish that died. The ID of the bacterial colonies was confirmed by 16s rDNA sequencing (sequence homology 100%).
Discussion
Commensal microorganisms bring many benefits to the vertebrate host colonizing different compartments within the mucosal epithelia but they are not always innocuous to the host if homeostasis is breached at the mucosal barriers.
In the present study, we report for the first time the presence of a Gram positive bacterium, S. warneri that resides in the skin of hatchery-reared rainbow trout in New Mexico. S. warneri is a common resident of the human skin that can cause disease or even be fatal in immunocompromised patients and infants (Campoccia et al., 2010). Interestingly, in the present study, S. warneri was not only found in association with the mucus but also inside the skin. This was shown both by FISH staining with an S. warneri specific probe and also by culturing lysates of skin cells on agar plates. Other Staphylococcus spp, such as S. aureus? has been shown to invade host cells, replicating in the cytoplasm or in the phagolysosome of phagocytes (Fraunholz and Sinha, 2012), and can also internalize within keratinocytes (Kintarak et al., 2004). From our current study, it appears that S. warneri has similar abilities of internalizing within host; specifically in rainbow trout skin epithelial cells. We found that S. warneri is both present in the skin mucus and the skin tissue of rainbow trout.
We quantified the number of S. warneri cfu in the skin of control and “handled” rainbow trout following a 5h transport event. Our results clearly indicate that transport causes important changes in some skin commensal bacteria. In the case of S. warneri, numbers were considerably higher following transport than before indicating that this commensal has the ability to grow better when the host is under stress. Our results are in agreement with a previous report on rainbow trout hindgut microbiota, which showed that acute stress alters the concentration of Staphyloccocus spp. (Olsen et al., 2005). In contrast to our results, Staphylococcus spp. concentrations decreased compared to the increased concentrations reported here. Our observations in the skin have important implications for the management of hatcheries and any other fish farming facilities, where transport events are common and therefore the composition of fish skin-associated bacteria is likely to change.
Pathogens and commensals are known to induce different signatures of cytokine production by the host (Maehr et al., 2013). Whilst pathogens induce pro-inflammatory responses, commensals, in turn, elicit anti-inflammatory cytokines. In rainbow trout, there are two isoforms of the cytokine TGF-β1 currently known, TGF-β1a and TGF-β1b. Whether TGF-β plays a role in the skin of rainbow trout in response to commensals or pathogens had not been investigated to date. In order to test our hypothesis that anti-inflammatory and pro-inflammatory cytokines may be differentially modulated in response to commensal or pathogen colonization, we studied the expression of five cytokines in skin explants exposed to S. warneri or V. anguillarum in vitro. Our results show that the skin of rainbow trout responds to commensals and pathogens in a different way, by inducing the expression of different cytokine subsets. Generally speaking, the pathogen V. anguillarum induces a pro-inflammatory cytokine signature whereas the commensal S. warneri induces anti-inflammatory cytokines (TGF-β1b) when present at low concentrations. High S. warneri concentrations, in turn, led to induction of IL1-β expression mirroring an inflammatory response against the commensal similar to that of the pathogen. Interestingly, the two TGF-β1 forms studied behaved in different fashions, with TGF-β1a displaying a more pro-inflammatory like response, and TGF-β1b being the stereotypical anti-inflammatory cytokine. These results highlight the delicate line that divides commensals and pathogens and how, if present at high concentrations, commensals evoke pathogen-like responses.
Extensive studies have revealed that disruption of the host commensal communities increases host susceptibility to pathogenic infections (Belkaid et al., 2013; Kamada et al., 2013). Our results indicate that the presence of S. warneri has a direct effect on V. anguillarum growth. This may be due to production of certain metabolites by S. warneri that are beneficial for V. anguillarum, although the exact mechanism for this interaction remains to be elucidated. Next, we examined whether V. anguillarum biofilm formation on rainbow trout scales may be affected by S. warneri in vitro. Biofims contain complex polysaccharide polymers within its matrix and can be produced by various bacteria. Biofilm formation can be tolerated and sometimes beneficial to the host (Visick, 2009), but overproduction, especially in the case of pathogens, may lead to increase bacterial colonization and disease (Wahl et al., 2012). Our results show that in the presence of S. warneri the ability of V. anguillarum to form biofilms was significantly enhanced. Together, these experiments demonstrate that the skin residing commensal, S. warneri, can act as a catalyst for the growth and biofilm formation of the pathogen V. anguillarum, likely increasing infection success.
Staphyloccocus spp. such as S. aureus and S. epidermidis have been associated with the aquatic environment and diseased aquatic organisms (Kusuda and Sugiyama, 1981). Furthermore, S. warneri was once isolated from diseased rainbow trout in Spain (Gil et al., 2000). The isolate in that study, Y-13-L, was tested for pathogenicity in brown trout exposed to a high temperature stress (20°C). However, pathogenicity was not tested in rainbow trout. According to the present study, S. warneri is not pathogenic (or has a very low pathogenicity) to rainbow trout (at least to the strain here used) even when injected at very high concentrations (109/fish). The differences between both studies may be due to differences between the bacterial isolates, the hosts (brown versus rainbow trout) and/or the challenge conditions (temperature stress versus thermal comfort).
In conclusion, S. warneri is part of the resident microbiota of the trout skin. At low concentrations, S. warneri appears to be innocuous to the host inducing anti- inflammatory cytokines. However, dysbiosis or any other break down of skin homeostasis may lead to overgrowth of this commensal. In this situation, an inflammatory response in the skin takes place. Despite the facts that S. warneri does not appear to be a direct pathobiont in rainbow trout (causing disease if reaching the systemic circulation) it acts as an indirect pathobiont aiding pathogens (in this case V. anguillarum) to grow and colonize the host. The present study underscores the delicate balance between the fish mucosal immune system and its associated commensal microbial communities and highlights the potential role of commensals in the onset of aquatic skin diseases.
Acknowledgements
We thank Dr D. Milton for kindly providing the GFP-V. anguillarum strain and the Lisboa Spring Hatchery for the rainbow trout. This work was funded by NIH COBRE grant P20GM103452.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- De Vos Paul, Boone David R, Garrity George M, Castenholz Richard W, Brenner Don J, Krieg Noel R, Staley Springer James T, De Vos Paul. Bergey’s Manual of Systematic Bacteriology: volume 3: The Firmicutes. 2011 [Google Scholar]
- Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 2013;14:646–653. doi: 10.1038/ni.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoccia D, Montanaro L, Visai L, Corazzari T, Poggio C, Maso A, Pirini V, Ravaioli S, Cangini I, Speziale P, Arciola CR. Characterization of 26 Staphylococcus warneri isolates from orthopedic infections. Int. J. Artif. Organs. 2010;33:575–581. doi: 10.1177/039139881003300903. [DOI] [PubMed] [Google Scholar]
- Cantas L, Sørby JR, Aleström P, Sørum H. Culturable gut microbiota diversity in zebrafish. Zebrafish. 2012;9:e0712. doi: 10.1089/zeb.2011.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br. J. dermatol. 2008;158:442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto A, Lauritz J, Chen C, Milton D. Vibrio anguillarum colonization of rainbow trout integument requires a DNA locus involved in exopolysaccharide transport and biosynthesis. Environ. Microbiol. 2007;9:370–382. doi: 10.1111/j.1462-2920.2006.01147.x. [DOI] [PubMed] [Google Scholar]
- Detournay O, Schnitzler CE, Poole A, Weis VM. Regulation of cnidarians-dinoflagellate mutualisms: Evidence that activation of a host TGFβ innate immune innate immune pathway promotes tolerance of the symbiont. Dev. Comp. Immunol. 2012;38:525–537. doi: 10.1016/j.dci.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Esteban M. An Overview of the Immunological Defenses in Fish Skin. ISRN Immunology. 2012;2012:0–29. [Google Scholar]
- Fraunholz M, Sinha B. Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect. Microbiol. 2012;2:43. doi: 10.3389/fcimb.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Vivas J, Gallardo CS, Rodriguez LA. First isolation of Staphylococcus warneri, from diseased rainbow trout, Oncorhynchus mykiss (Walbaum), in Northwest Spain. J. Fish Dis. 2000;23:295–298. [Google Scholar]
- Iwase T, Seki K, Shinji H, Mizunoe Y, Masuda S. Development of a real-time PCR assay for the detection and identification of Staphylococcus capitis, Staphylococcus haemolyticus and Staphylococcus warneri. J. Med. Microbiol. 2007;56:1346–1349. doi: 10.1099/jmm.0.47235-0. [DOI] [PubMed] [Google Scholar]
- Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintarak S, Nair SP, Speight PM, Whawell SA. A recombinant fragment of the fibronectin-binding protein of Staphylococcus aureus inhibits keratinocyte migration. Arch. Dermatol. Res. 2004;296:250–257. doi: 10.1007/s00403-004-0515-y. [DOI] [PubMed] [Google Scholar]
- Kusuda R, Sugiyama A. Studies on the characters of Staphylococcus epidermidis isolated from diseased fish.1.On the morphological, biological, and biochemical properties. Fish Pathol. 1981;16:15–23. [Google Scholar]
- Littman D, Pamer E. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr T, Costa MM, Vecino JL, Wadsworth S, Martin SA, Wang T, Secombes CJ. Transforming growth factor-β1b: A second TGF-β1 paralogue in the rainbow trout (Oncorhynchus mykiss) that has a lower constitutive expression but is more responsive to immune stimulation. Fish Shellfish Immunol. 2013;34:420–432. doi: 10.1016/j.fsi.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Nussbaum JC, Locksley RM. Infectious (Non)tolerance—frustrated commensalism gone awry? Cold Spring Harb. Perspect. Biol. 2012 doi: 10.1101/cshperspect.a007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RE, Sundell K, Mayhew TE, Myklebust R, Ringø E. Acute stress alters intestinal function of rainbow trout, Oncorhynchus mykiss (Walbaum) Aquaculture. 2005;250:480–495. [Google Scholar]
- Packey C, Sartor R. Commensal Bacteria, Traditional and Opportunistic Pathogens, Dysbiosis and Bacteria Killing in Inflammatory Bowel Diseases. Curr. Opin. Infect. Dis. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29 doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round J, Mazmanian S. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas I, Zhang YA, Sunyer JO. Mucosal immunoglobulins and B cells of Teleost fish. Dev. Comp. Immunol. 2011;35:1346–1365. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah KL, Tyagy BC. An eye disease in silver carp, Hypophthalmichthys molitrix, held in tropical ponds, associated with the bacterium Staphylococcus aureus. Aquaculture. 1986;55:1–4. [Google Scholar]
- Sommer S, Bäckhed F. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- Spanggaard B, Huber I, Nielsen J, Nielsen T, Appel KF, Gram L. The microflora of rainbow trout intestine: a comparison of traditional and molecular identification. Aquaculture. 2000;182:1–15. [Google Scholar]
- Tacchi L, Larragoite E, Salinas I. Discovery of J Chain in African Lungfish (Protopterus dolloi, Sarcopterygii) Using High Throughput Transcriptome Sequencing: Implications in Mucosal Immunity. PLoS One. 2013;8(8):e70650. doi: 10.1371/journal.pone.0070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL. An intricate network of regulators controls biofilm formation and colonization by Vibrio fischeri. Mol. Microbiol. 2009;74:782–789. doi: 10.1111/j.1365-2958.2009.06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Goecke F, Labes A, Dobretsov S, Weinberger F. The second skin: ecological role of epibiotic biofilms on marine organisms. Front. Microbiol. 2012 doi: 10.3389/fmicb.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelaar Bonga S. The stress response in fish. Physiol. Rev. 1997;77:591–625. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]