Where HIV and food insecurity coexist, ready-to-use supplementary foods (RUSFs) are increasingly prescribed to improve patient outcomes. We found no statistically significant differences in outcome with RUSFs vs less expensive corn-soy blend plus rations for HIV patients in rural Haiti.
Keywords: HIV, food security, food insecurity, nutrition, macronutrient supplement
Abstract
Background. The epidemics of food insecurity, malnutrition, and human immunodeficiency virus (HIV) frequently overlap. HIV treatment programs increasingly provide nutrient-dense ready-to-use supplementary foods (RUSFs) to patients living with HIV and food insecurity, but in the absence of wasting, it is not known if RUSF confers benefit above less costly food commodities.
Methods. We performed a randomized trial in rural Haiti comparing an RUSF with less costly corn-soy blend plus (CSB+) as a monthly supplement to patients with HIV infection who were on antiretroviral therapy (ART) <24 months prior to study start. We compared 6- and 12-month outcomes by ration type in terms of immunologic response, body mass index (BMI), adherence to ART, general health quality of life, household food insecurity, and household wealth.
Results. A cohort of 524 patients with HIV receiving ART was randomized and followed over time. Median CD4 cell count at baseline was 339 cells/µL (interquartile range [IQR], 197–475 cells/µL) for the CSB+ group, and 341 cells/µL (IQR, 213–464/µL) for the RUSF group. Measured outcomes improved from baseline over time, but there were no statistically significant differences in change for BMI, household wealth index, hunger, general health perception score, or adherence to ART by ration type at 6 or 12 months. The RUSF group had higher CD4 count at 12 months, but this was also not statistically significant.
Conclusions. In 12 months of follow-up, there was no statistically significant difference in outcomes between those receiving RUSF-based compared with CSB+-based rations in a cohort of HIV-infected adults on ART in rural Haiti.
Food insecurity, undernutrition, and human immunodeficiency virus (HIV) infection are structurally linked and often geographically overlapping problems that have negative impacts on individuals as well as on their households [1, 2]. As HIV infection progresses, it causes a catabolic state and increased susceptibility to other infections, which are both compounded by lack of caloric and other nutrient intake, leading to progressive worsening of malnutrition [3]. In persons with HIV infection, low body mass index (BMI) is associated with increased early mortality [4], and food insecurity is associated with reduced adherence to antiretroviral therapy (ART) and incomplete viral RNA suppression [5, 6].
To address the problems of undernutrition and food insecurity, HIV programs increasingly provide food assistance to patients in the form of food rations [7]. In addition to the most obvious benefit of reducing hunger, food assistance has been associated with improved adherence to ART, improved attendance at routine clinical appointments, and increases in BMI compared with no food assistance [8, 9]. However, the commodities used for supplementary food assistance vary greatly, and although the role of micronutrients in improving HIV-related outcomes has been studied extensively [10, 11], few studies examine the relationship between macronutrient supplements and morbidity or mortality. As a result, there is little evidence to guide providers on the most appropriate type, quantity, or duration of food assistance or on the most appropriate eligibility criteria for those receiving food supplements to achieve maximum benefit.
Corn-soy blends (CSBs) are fortified blended foods that have been used extensively as food aid for decades [12]. Concerns about low nutrient content and ration sharing have resulted in the creation of newer CSB varieties with added micronutrients such as corn-soy blend plus (CSB+) [13]. Increasing attention is also being focused on the use of ready-to-use spreads known as ready-to-use therapeutic foods (RUTFs) or ready-to use supplementary foods (RUSFs). RUTFs/RUSFs have been used as treatment and supplements for malnourished children with great success and are increasingly being studied for use in adults with HIV wasting [14, 15]. They are energy-dense, lipid-based spreads that resist bacterial contamination and require no cooking [16]. These characteristics may contribute to increased consumption by adults with HIV infection, who often have poor appetite and/or mouth lesions. They are often individually packaged, which could dissuade sharing by the targeted individual. Despite potential advantages, the cost of RUSF may be up to 3 times greater than CSB [14]. Although local production is increasing, RUSF must often be imported, thus adding to logistics costs and potentially diverting resources for food assistance away from local economies [17, 18]. RUSFs are regularly distributed to and marketed for adults living with HIV [19].
In Haiti, food insecurity is very common [20], and the prevalence of HIV infection among 15- to 49-year-olds is 2.2% [21]. This paper describes a prospective randomized trial in rural Haiti in which adults with HIV infection on ART were randomized to receive either an RUSF- or CSB+-based food ration. We hypothesized that RUSF might lead to better nutritional and nonnutritional outcomes for adults with HIV infection, compared to CSB+.
METHODS
The study took place in the context of an ongoing HIV program at 3 health centers in the Artibonite Department of Haiti (St Marc, Petite Rivière de l'Artibonite, and Verrettes) between June 2010 and July 2011. Partners In Health (PIH) is a nongovernmental organization that provides healthcare in Haiti in support of the Haitian Ministry of Health. In addition to providing comprehensive medical care that is free of charge, PIH has collaborated with the World Food Programme (WFP) since 2004 to distribute food assistance to eligible recipients with HIV infection.
Eligibility
Individuals were eligible for the study if they were documented to have HIV infection by standard laboratory procedures, lived in the geographic catchment area of PIH services, were ≥18 years of age, and had started ART in the 24 months prior to study enrollment. Individuals were excluded if another household member was also eligible for food assistance or if they were pregnant at the time of enrollment.
Study Procedures
During enrollment, patients with HIV infection were screened for study eligibility during routine monthly clinic visits and using ART registers. When enrollment was complete, participants were randomized within health centers to receive either a standard CSB+-based food ration or an RUSF ration for the entire duration of follow-up, without crossover. Randomization occurred by having each participant draw a lot that corresponded to either the CSB+ or RUSF group from an envelope. The study was not blinded. The target sample size of 600 participants assumed an 8% annual dropout rate and would have yielded >80% power to detect a difference in CD4 cell count of 25 cells at 12 months for a 2-sided test of superiority.
Food Assistance
Food assistance in the study had 2 components to ensure that the study rations were in line with the standard of care at the time. The first component, or “individual ration,” was specifically targeted to the individual with HIV infection (ie, index patient). The second component, or “family ration,” was intended to offset the monthly needs of the index patient's family. The individual ration was comprised of either CSB+ (distributed with sugar and vitamin A- and D-fortified oil) or sachets of peanut-based RUSF (Supplementary Plumpy, Edesia). The rations were of similar caloric composition (Table 1). Food rations were distributed to study subjects by prescription after monthly HIV clinic attendance. For those patients too severely ill to travel to clinic, community health workers were allowed to deliver rations to the patient's home if needed. RUSF was individually packaged, and recipients were instructed to eat 2 sachets per day of this pre-prepared food. CSB+ requires cooking and was presented in bulk packaging along with oil and sugar. Both groups received instructions encouraging consumption of the individual ration only by the index patient and the use of the rest of the ration to support the family's needs. CSB+ was provided in-kind by WFP. We estimated cost, excluding shipping, at US$0.19 per day [22]. The RUSF ration purchased for the study cost US$0.34 per day excluding shipping.
Table 1.
Nutritional Composition of Supplementary Food Rations
| Corn-Soy Blend Plus With Fortified Oil and Sugar (235 g/day) | Ready-to-Use Supplementary Food (184 g/day) | |
|---|---|---|
| Energy, kcal | 1000 | 1000 |
| Protein, g | 26.6 | 26 |
| Lipids, g | 25 | 62 |
| Calcium, mg | 1024 | 552 |
| Phosphorus, mg | 1192 | 552 |
| Potassium, mg | 1448 | 2014 |
| Magnesium, mg | … | 166 |
| Zinc, mg | 15.4 | 26 |
| Copper, mg | … | 3.2 |
| Iron, mg | 23.5 | 22 |
| Iodine, µg | 80 | 184 |
| Selenium, µg | … | 56 |
| Sodium, mg | … | <534 |
| Vitamin A, µg | 2539–2629 | 1680 |
| Vitamin D, µg | 30.6–32.6 | 30 |
| Vitamin E, mg | 19.3 | 36.8 |
| Vitamin C, mg | 182.4 | 98 |
| Vitamin B1, mg | 1.36 | 1.2 |
| Vitamin B2, mg | 3.5 | 3.4 |
| Vitamin B6, mg | 3.1 | 1.2 |
| Vitamin B12, µg | 4 | 3.4 |
| Vitamin K, µg | 116 | 38.6 |
| Biotin, µg | 16.4 | 120 |
| Folic acid, µg | 400 | 386 |
| Pantothenic acid, mg | 4.4 | 5.8 |
| Niacin, mg | 22.4 | 9.8 |
Data Collection
Index patients and their households were assessed by surveys at the household at baseline as well as at 6-month and 12-month follow-up time points. Information was collected on the index patients’ age, sex, marital status, education level, income sources, assets, and housing. Data were also collected on clinical status, quality of life, food security status, and nutrition outcomes. CD4 cell count data, weights, and heights were abstracted from the electronic medical record and from paper registries. We assessed household food security using the Household Hunger Scale [23], which classifies households as having little to no hunger (score of 0–1), moderate hunger (score of 2–3), or severe hunger (score of 4–6). BMI was calculated by the following formula:  . A quality-of-life measure was adapted for local use based on the general health perceptions subscale of the AIDS Clinical Trials Group Short Form–21 [24], with responses measured on a 5-point Likert scale. Suboptimal adherence was defined as self-report of missing any doses of ART in the past 30 days. This definition was selected because self-reported adherence tends to be an upper-bound estimate of true adherence and because a large majority of participants reported no missed doses. A household wealth index was generated based on principal components analysis of the following economic indicators at baseline: radio ownership, household floor material, land ownership, roof material, access to a latrine, electricity, water access, and livestock ownership.
. A quality-of-life measure was adapted for local use based on the general health perceptions subscale of the AIDS Clinical Trials Group Short Form–21 [24], with responses measured on a 5-point Likert scale. Suboptimal adherence was defined as self-report of missing any doses of ART in the past 30 days. This definition was selected because self-reported adherence tends to be an upper-bound estimate of true adherence and because a large majority of participants reported no missed doses. A household wealth index was generated based on principal components analysis of the following economic indicators at baseline: radio ownership, household floor material, land ownership, roof material, access to a latrine, electricity, water access, and livestock ownership.
Statistical Analysis
We conducted intention-to-treat repeated measure profile analyses of all randomized patients. We conducted analyses using generalized estimating equations (GEEs) with an unstructured correlation structure and robust standard errors. Continuous variables (CD4 cell count, BMI, general health perceptions score, and household wealth index) were modeled using the identity link and normal distribution, with CD4 cell count and BMI modeled on the square root and inverse square root scales, respectively. For dichotomous outcomes (adherence and hunger), we used the logit link and a binary distribution. Baseline outcome measurements were modeled as part of the outcome vector, assuming equal baseline means [25]. For all outcomes, difference in differences between baseline and 6-month timepoints and baseline and 12-month timepoints were assessed by examining the interaction between the food ration assignment and time.
Following unadjusted analyses, we assessed for possible confounding by examining changes in the effect estimates for ration type when adjusting for age, sex, and variables that appeared imbalanced between the 2 groups (ie, access to improved hygienic services, electricity in the home). We also examined whether unadjusted results differed from the model-based estimates from GEEs adjusting for baseline variables that predicted missingness in an outcome value (ie, site, baseline CD4 cell count) and used missing indicator variables as required. Finally, we assessed for selection bias due to missing 12-month CD4 cell count data. To do this, we conducted a sensitivity analysis in which we compared the within-person baseline to 12-month change in CD4 cell count, by ration type, and used inverse probability weighting with stabilized weights and a linear regression model to account for predictors of missing 12-month CD4 cell count, including 6-month CD4 cell count [26]. We conducted post hoc secondary analyses of CD4 cell count among individuals with advanced immune suppression (CD4 count <200 cells/µL) and those who initiated ART within 12 months of enrollment, and of BMI among those with a BMI in the lowest quartile (<19.68) and those who initiated ART within 12 months of enrollment. For subgroup analyses, we did not assume equal baseline means across ration groups. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, North Carolina).
Ethical Considerations
This study received ethics approval from the Brigham and Women's/Partners Institutional Review Board (Boston, Massachusetts) and the Zanmi Lasante Institutional Review Board (Cange, Haiti).
RESULTS
Baseline Characteristics
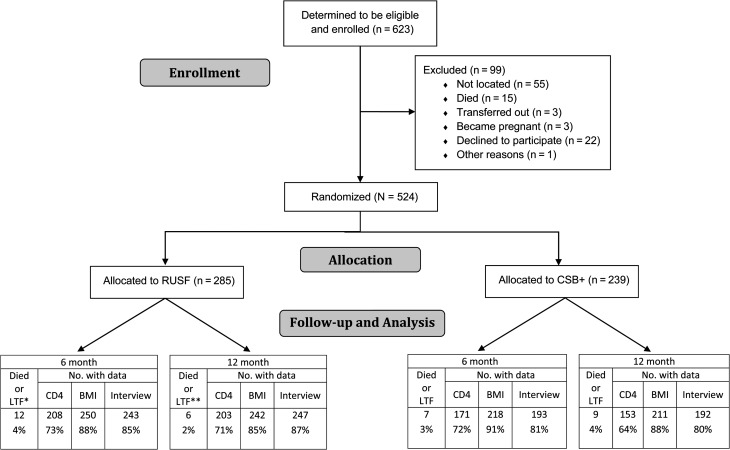
From May 2010 to June 2011, 623 eligible individuals were identified and agreed to enroll in the study (Figure 1). Of these, 524 were ultimately randomized. The percentage of participants with CD4 cell count, BMI, and interview data at baseline, 6-month, and 12-month time points is shown in Figure 1. Participants had been on ART for a median of 11.8 months (CSB+ group) and 10.2 months (RUSF group). Median CD4 cell count was 339 cells/µL (interquartile range [IQR], 197–475 cells/µL) for the CSB+ group, and 341 cells/µL (IQR, 213–464 cells/µL) for the RUSF group; Table 2 describes the study cohort. Missing baseline data was <8% for most covariates. Groups were similar on key measured baseline characteristics and baseline measures of the outcome variables of interest. Almost two-thirds of participants were women and most were very poor, with little access to electricity, low literacy, and high rates of food insecurity.
Figure 1.
Overview of randomization and data collection in a study of 2 supplementary food rations for patients living with human immunodeficiency virus in rural Haiti. *Only 1 loss from care, a recipient of ready-to-use supplementary food, was identified in this study, **The number of deaths/losses to follow-up at 12 months is not inclusive of 6-month numbers. Abbreviations: BMI, body mass index; CSB+, corn-soy blend plus; LTF, loss to follow-up; RUSF, ready-to-use supplementary food.
Table 2.
Baseline Characteristics of the Study Cohort (N = 524)a
| Variable | CSB+ Group (n = 239) | RUSF Group (n = 285) |
|---|---|---|
| Female sex | 144 (60.3) | 178 (62.5) |
| Age, y, mean (SD) | 39.1 (10.3) | 38.7 (11.0) |
| Health center | ||
| Petite Riviere de l'Artibonite | 91 (38.1) | 106 (37.2) |
| St Marc | 93 (38.9) | 127 (44.6) |
| Verrettes | 55 (23.0) | 52 (18.3) |
| Time on antiretroviral therapy, mo, median (IQR) | 11.8 (7.2–17.6) | 10.2 (5.6–17.1) |
| Marital status (n = 490) | ||
| Single | 59 (26.6) | 79 (29.5) |
| Cohabitating | 91 (41.0) | 96 (35.8) |
| Married | 27 (12.2) | 21 (7.8) |
| Separated or divorced | 25 (11.3) | 49 (18.3) |
| Widowed | 20 (9.0) | 23 (8.6) |
| Able to read and write (n = 490) | 119 (53.6) | 155 (57.8) |
| If literate, highest level of education (n = 274) | ||
| None | 2 (1.7) | 0 (0) |
| Some primary | 53 (44.5) | 83 (53.6) |
| Completed primary | 17 (14.3) | 17 (11.0) |
| Some secondary | 45 (37.8) | 51 (32.9) |
| Completed secondary | 2 (1.7) | 2 (1.3) |
| Higher than secondary | 0 (0) | 2 (1.3) |
| No. of people per household, median (IQR) (n = 488) | 4.5 (3–6) | 5 (3–6) |
| Has income-generating activity (n = 490) | 74 (33.3) | 74 (27.6) |
| Poverty scoreb, mean (SD) (n = 484) | 25.3 (10.2) | 26.2 (11.0) |
| Access to improved hygienic services (latrine or flush toilet) (n = 490) | 152 (68.5) | 161 (60.1) |
| Main water source is improved (n = 489) | 191 (86.0) | 219 (82.0) |
| Home has electricity (n = 490) | 71 (32.0) | 114 (42.5) |
| Has a community health worker (n = 489) | 194 (87.4) | 235 (88.0) |
| Very or severely food insecurec (n = 446) | 196 (94.7) | 228 (95.4) |
| Received food ration from PIH in the month prior to survey (n = 489) | 77 (34.7) | 93 (34.8) |
| If received a PIH ration, shared it outside of household (n = 169) | 58 (75.3) | 66 (71.7) |
| Received food ration from other organization in the month prior to survey (n = 488) | 3 (1.4) | 4 (1.5) |
| Obtains food from garden/field (n = 488) | 35 (15.8) | 53 (19.9) |
| Measurements of outcome variables | ||
| CD4 cell count, median (IQR) (n = 509) | 339 (197–475) | 341 (213–464) |
| Body mass index, median (IQR) (n = 490) | 22.0 (20.0–24.5) | 21.6 (19.5–24.0) |
| General health perception scored, mean (SD) (n = 489) | 43.7 (20.5) | 46.5 (21.1) |
| Suboptimal adherence (n = 488) | 53 (24.0) | 60 (22.5) |
| Little or no hungere (n = 482) | 25 (11.3) | 28 (10.7) |
| Wealth index scoref, mean (SD) (n = 486) | 1.31 (0.60) | 1.30 (0.71) |
Data are presented as No. (%) unless otherwise specified. For continuous variables, median (IQR) are shown for nonnormally distributed variables and mean (SD) are shown for normally distributed variables.
Abbreviations: CSB+, corn-soy blend plus; IQR, interquartile range; PIH, Partners in Health; RUSF, ready-to-use supplementary food; SD, standard deviation.
a Unless noted otherwise.
b Range of possible scores: 0 (worst) to 85 (best).
c Latin American and Caribbean Food Security Scale.
d Range of possible scores: 0 (worst) to 100 (best).
e Household Hunger Scale.
f Range of possible scores: 0 (worst) to 2.38 (best).
Changes in Study Outcomes Over Time
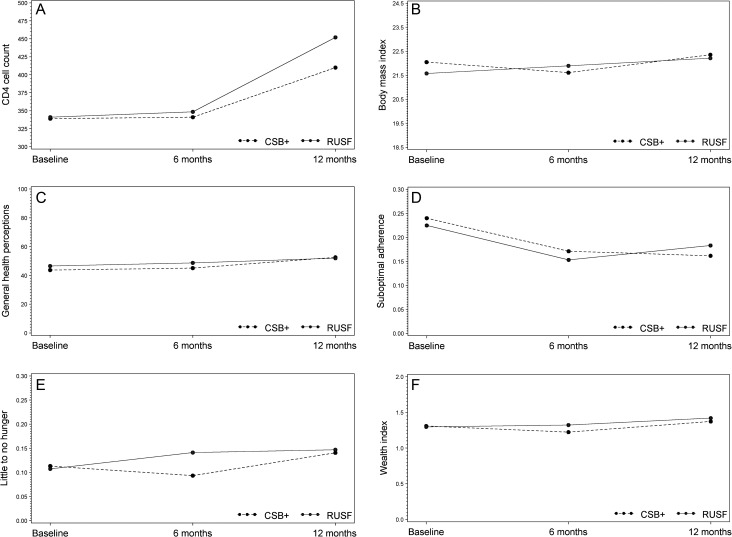
Results were consistent between unadjusted and adjusted analyses, and between those that did and did not adjust for predictors of missing outcomes; therefore, unadjusted analyses are presented here. In general, outcomes improved from baseline to 12 months for most of the 6 study outcomes (Figure 2A–F). The 33 deaths that occurred during the follow-up period were comparably distributed by ration type (6.7% of patients died in the CSB group vs 6.0% in the RUSF group), although participants receiving CSB were more often missing an interview or CD4 cell count at 12 months than those receiving RUSF (20% vs 13% for interviews, P = .05; 36% vs 29% for CD4 cell count, P = .08).
Figure 2.
Study outcomes by group in a study of 2 supplementary food rations for patients living with human immunodeficiency virus in rural Haiti. A, Median CD4 cell count, by food ration type. B, Median body mass index, by food ration type. C, Mean general health perception score, by food ration type. D, Prevalence of suboptimal adherence, by food ration type. E, Prevalence of little to no hunger, by food ration type. F, Mean wealth index, by food ration type. Results from regression analyses showed statistically significant increases in CD4 cell count, body mass index, household wealth index, and general health perception score (all P ≤ .0001 for baseline to 12-month change), and a statistically significant decrease in the proportion of individuals who met the definition of suboptimal adherence (P = .03). An increase in the percentage of participants reporting little to no hunger at 12 months was borderline statistically significant (P = .06). Abbreviations: CSB+, corn-soy blend plus; RUSF, ready-to-use supplementary food.
Table 3 and Figure 2A–F display the differences in changes over time for each of the 6 study outcomes by food ration type. With the exception of a greater baseline to 6-month change in wealth index among those receiving RUSF of borderline statistical significance, no statistically significant differences in change were observed from baseline to 6 months or baseline to 12 months, suggesting that changes in these outcomes during the 12-month study period occurred similarly between the 2 groups. These results were similar for all post hoc subset analyses (Supplementary Table 1) and for the sensitivity analysis using inverse probability weighting.
Table 3.
Differences in Change in Study Outcomes at 6 and 12 Months, by Food Ration Type
| Outcome | 6-mo Estimate (95% CI) | P Valuea | 12-mo Estimate (95% CI) | P Valueb |
|---|---|---|---|---|
| CD4 cell count (square root) | n = 379 | n = 356 | ||
| RUSF | 18.1 (17.4–18.8) | .82 | 19.9 (19.1–20.8) | .10 |
| CSB+ | 18.0 (17.2–18.8) | 19.1 (18.0–20.1) | ||
| Body mass index (inverse square root) | n = 468 | n = 453 | ||
| RUSF | 0.215 (.213–.217) | .16 | 0.212 (.210–.214) | .52 |
| CSB+ | 0.215 (.212–.217) | 0.210 (.208–.212) | ||
| General health perceptions score | n = 435 | n = 438 | ||
| RUSF | 49 (46–51) | .28 | 52 (49–54) | .55 |
| CSB+ | 46 (43–48) | 52 (49–55) | ||
| Suboptimal adherence | n = 435 | n = 438 | ||
| RUSF | 16% (12–21) | .61 | 19% (14–24) | .52 |
| CSB+ | 18% (13–24) | 17% (12–22) | ||
| Little to no hunger | n = 434 | n = 437 | ||
| RUSF | 14% (10–19) | .10 | 15% (11–20) | .70 |
| CSB+ | 9% (6–14) | 14% (10–20) | ||
| Wealth index | n = 431 | n = 437 | ||
| RUSF | 1.30 (1.22–1.38) | .05 | 1.41 (1.33–1.50) | .26 |
| CSB+ | 1.22 (1.14–1.31) | 1.37 (1.29–1.45) |
Abbreviations: CI, confidence interval; CSB+, corn-soy blend plus; RUSF, ready-to-use supplementary food.
a For difference in 0–6 mo change by group.
b For difference in 0–12 mo change by group.
DISCUSSION
Food insecurity is a complex problem that may cause ill health through multiple interconnected pathways [27]. In the context of public health programs, how to best mitigate the negative impact of food insecurity on HIV outcomes is not yet known. We conducted a randomized study comparing the effectiveness of 2 commonly used but different types of food supplementation.
Previous research in our programs in Haiti demonstrated that macronutrient supplements, in the form of a monthly dry food ration provided to HIV-infected patients, was associated with improved adherence to clinic visits, BMI, and food security at 6 and 12 months compared to receiving no supplement [8]. Other evidence supports the finding that food assistance provided to patients with HIV results in weight gain and can improve adherence to ART, in addition to alleviating hunger [9, 28, 29]. In this trial, most study outcomes, including BMI and CD4 count, improved in subjects over time. This may be expected over time for patients on ART and food supplementation, but the results were not different between the RUSF and CSB+ groups. Additional outcomes measured in our study, including food insecurity, general health perception, quality of life, and household wealth index, did not differ significantly between the study groups over time.
CD4 cell count correlates highly with progression of HIV disease and is the main surrogate marker for immunologic function [30, 31]. It is used clinically in monitoring ART, determining the risk for opportunistic infections, and assessing prognosis [30–32]. Although an increase in CD4 cell count over time is expected on ART, there is biologic plausibility to the hypothesis that nutrient-dense supplements could result in an incremental improvement in immune function over CSB+, even in the absence of weight gain, particularly if the effect was related to the micronutrient benefits of RUSF. Selenium, for example, was associated in one study with small improvements in CD4 cell count compared to placebo in patients with HIV infection [33]. In our study, although CD4 cell counts were nearly identical across groups at baseline and after 6 months, the RUSF group had notably, but not statistically significant, higher CD4 cell counts at 12 months. It is possible that our study was underpowered to detect a statistically significant difference of this size, as only 356 (68%) subjects had a CD4 cell count available for analysis at 12 months. Given the potential important implications of an effect on CD4 cell count, we believe that this finding merits further investigation.
BMI is a screening measure that uses weight and height to estimate body fatness, with measures <18.5 considered underweight, 18.5–24.9 normal weight, and >25.0 overweight. In a randomized study in underweight adults with HIV infection in Malawi, RUTF was associated with better BMI and lean body mass outcomes compared with CSB [15]. Other studies have demonstrated the effectiveness of RUTF as a treatment for wasting in patients with HIV [14, 34]. In our study, food insecurity was very high, but median BMI in both study groups was within the normal range. To our knowledge, this is the first study that compares RUSF to CSB+ in a cohort of patients with HIV infection in which food insecurity rather than wasting is the primary vulnerability. We found no particular benefit to RUSF compared to CSB+ in these patients.
This study has several limitations. Only 68% of participants had CD4 counts available at the 12-month follow-up time point, reducing power to detect a difference in this measure. This was largely due to programmatic challenges at the study sites related to the availability and functionality of CD4 testing machines. The study began enrollment 6 months after a major earthquake in Haiti in 2010 and 4 months before a massive cholera epidemic began in the region. Although the study proceeded per protocol, these disasters had an impact on the health system in which the study took place [35]. Because consumption of the food ration was not observed, we cannot be sure of what happened to the food once it was given to participants. Foods are generally shared within households and may be allocated differently within the household based on a number of factors [7, 36]. CSB+ is not as distinguishable from the family food stock as RUSF is, and this difference in packaging may confer a special status to RUSF such that it may be less likely to be shared by the index patient. Qualitative studies currently under way with the subjects of this study may help to further elucidate the personal as well as intra- and extra-household use of food rations. Finally, our study targeted individuals for enrollment who had been on ART for <2 years, but despite this, the median CD4 count of the cohort was not as low as in other study settings [9, 14]. This may be because the study occurred in a well-established HIV program in which cases of HIV infection are increasingly diagnosed before advanced disease occurs [37].
CONCLUSIONS
In this randomized study of food-insecure individuals living with HIV infection on ART in rural Haiti, improvements were observed in CD4 cell count, BMI, general health perceptions, adherence to ART, and household wealth index over time, regardless of food ration type. We found no significant benefit to using a peanut-based RUSF ration compared to the less expensive CSB+ ration in terms of study outcomes. Although CD4 cell counts were higher in the RUSF group than the CSB+ group at 12 months, this difference was not statistically significant. Missing CD4 cell counts at 12 months resulted in lower than anticipated power for this outcome. Further comparison of RUSF and CSB+ in HIV programs is warranted, particularly to determine the impact of such supplements on CD4 cell count and to determine what subgroups, if any, might benefit preferentially from the use of one ration type over the other.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank the study participants. We are grateful to Zanmi Lasante and PIH staff in Haiti. Special thanks go to the World Food Programme in Haiti, in particular to Michelle Doura, Paola de Santos, and Myrta Kaulard, for providing food rations and for their support to the project. We also acknowledge programming support from Zibiao Zhang and statistical analysis advice from Miguel Hernán.
Financial support. This study was funded by the National Institute of Child Health and Human Development at the National Institutes of Health (grant number R01HD057627 to L. C. I.). M. F. F. was supported by the Global Health Research Core at the Department of Global Health and Social Medicine, Harvard Medical School. Support was also provided by the Harvard University Center for AIDS Research, a National Institutes of Health–funded program (grant number P30AI060354).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chopra M, Darnton-Hill I. Responding to the crisis in sub-Saharan Africa: the role of nutrition. Public Health Nutr. 2006;9:544–50. doi: 10.1079/phn2006948. [DOI] [PubMed] [Google Scholar]
- 2.World Food Programme/ World Health Organization/UNAIDS. Policy brief: HIV, food security and nutrition. Geneva, Switzerland: WHO: 2008. [Google Scholar]
- 3.Ivers LC, Cullen KA, Freedberg KA, Block S, Coates J, Webb P. HIV/AIDS, undernutrition, and food insecurity. Clin Infect Dis. 2009;49:1096–102. doi: 10.1086/605573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zachariah R, Fitzgerald M, Massaquoi M, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–60. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 5.Weiser SD, Frongillo EA, Ragland K, Hogg RS, Riley ED, Bangsberg DR. Food insecurity is associated with incomplete HIV RNA suppression among homeless and marginally housed HIV-infected individuals in San Francisco. J Gen Intern Med. 2009;24:14–20. doi: 10.1007/s11606-008-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Au J, Kayitenkore K, Shutes E, et al. Access to adequate nutrition is a major potential obstacle to antiretroviral adherence among HIV-infected individuals in Rwanda. AIDS. 2006;20:2116–8. doi: 10.1097/01.aids.0000247580.16073.1b. [DOI] [PubMed] [Google Scholar]
- 7.President's Emergency Plan for AIDS Relief. Washington, DC: PEPFAR; 2006. Report on food and nutrition for people living with HIV/AIDS. [Google Scholar]
- 8.Ivers LC, Chang Y, Gregory Jerome J, Freedberg KA. Food assistance is associated with improved body mass index, food security and attendance at clinic in an HIV program in central Haiti: a prospective observational cohort study. AIDS Res Ther. 2010;7:33. doi: 10.1186/1742-6405-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantrell RA, Sinkala M, Megazinni K, et al. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food-insecure adults in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;49:190–5. doi: 10.1097/QAI.0b013e31818455d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fawzi W, Msamanga G, Spiegelman D, Hunter D. Studies of vitamins and minerals and HIV transmission and disease progression. J Nutr. 2005;135:938–44. doi: 10.1093/jn/135.4.938. [DOI] [PubMed] [Google Scholar]
- 11.Fawzi W, Msamanga G. Micronutrients and adverse pregnancy outcomes in the context of HIV infection. Nutr Rev. 2004;62:269–75. doi: 10.1301/nr.2004.jul.269-275. [DOI] [PubMed] [Google Scholar]
- 12.US Agency for International Development. Corn soy blend/plus commodity fact sheet. Available at: http://www.usaid.gov/what-we-do/agriculture-and-food-security/food-assistance/resources/implementation-tools/corn-soy. Accessed 20 August 2013.
- 13.Wood J, Sibanda-Mulder F. Improvement of fortified blended foods: CSB products and Unimix. Available at: http://www.unicef.org/supply/files/8._Improvement_of_Fortified_Blended_Foods_CSB_Products_and_Unimix.pdf. Accessed 28 October 2013.
- 14.Manary M, Ndekhat M, van Oosterhout JJ. Supplementary feeding in the care of the wasted HIV infected patient. Malawi Med J. 2010;22:46–8. doi: 10.4314/mmj.v22i2.58792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ndekha MJ, van Oosterhout JJ, Zijlstra EE, Manary M, Saloojee H, Manary MJ. Supplementary feeding with either ready-to-use fortified spread or corn-soy blend in wasted adults starting antiretroviral therapy in Malawi: randomised, investigator blinded, controlled trial. BMJ. 2009;338:b1867. doi: 10.1136/bmj.b1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Agency for International Development. Ready-to-use therapeutic food commodity fact sheet. Available at: http://www.usaid.gov/what-we-do/agriculture-and-food-security/food-assistance/resources/ready-use-therapeutic-food. Accessed 20 August 2013.
- 17.Rice A. The peanut solution. New York Times, 2 September 2013; [Google Scholar]
- 18.Komrska J. Increasing access to ready-to-use therapeutic foods (RUTF) Field Exchange. 2012;42:46–7. [Google Scholar]
- 19.Nutriset. Plumpy'Soy: food for adults at risk of malnutrition. Available at: http://www.nutriset.fr/en/product-range/produit-par-produit/plumpysoy-food-for-adults-at-risk-of-malnutrition.html. Accessed 20 August 2013.
- 20.Coordination Nationale de la Sécurité Alimentaire (CNSA) National survey of food security ENSA 2011 (French) Available at: http://www.cnsa509.org/Web/Etudes/Rapport%20final%20enquete%20nationale(ENSA).pdf. Accessed 20 August 2013.
- 21.Measure DHS. La prévalence du VIH: résultats de EMMUS-V 2012 (the prevalence of HIV) Available at: www.measuredhs.com/pubs/pdf/HF45/HF45.pdf . Accessed 20 August 2013.
- 22.World Food Programme (WFP) WFP nutrition policy, nutrition programs and food supplements. Available at: http://home.wfp.org/stellent/groups/public/documents/resources/wfp247204.pdf. Accessed 29 August 2013.
- 23.Deitchler M, Ballard T, Swindale A, Coates J. Introducing a simple measure of household hunger for cross-cultural use. Washington, DC: Food and Nutrition Technical Assistance II project, AED; 2011. [Google Scholar]
- 24.Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV) Qual Life Res. 1997;6:481–93. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- 25.Fitzmaurice G, Laird N, Ware J. Applied longitudinal analysis. Hoboken: John Wiley & Sons; 2008. [Google Scholar]
- 26.Toh S, Hernan MA. Causal inference from longitudinal studies with baseline randomization. Int J Biostat. 2008;4 doi: 10.2202/1557-4679.1117. article 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiser SD, Young SL, Cohen CR, et al. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr. 2011;94:1729S–39S. doi: 10.3945/ajcn.111.012070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwenk A, Steuck H, Kremer G. Oral supplements as adjunctive treatment to nutritional counseling in malnourished HIV-infected patients: randomized controlled trial. Clin Nutr. 1999;18:371–4. doi: 10.1016/s0261-5614(99)80018-1. [DOI] [PubMed] [Google Scholar]
- 29.Clark RH, Feleke G, Din M, et al. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. JPEN J Parenter Enteral Nutr. 2000;24:133–9. doi: 10.1177/0148607100024003133. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 31.Hogg RS, Yip B, Chan K, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 32.Ledergerber B, Egger M, Erard V, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282:2220–6. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 33.Hurwitz BE, Klaus JR, Llabre MM, et al. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med. 2007;167:148–54. doi: 10.1001/archinte.167.2.148. [DOI] [PubMed] [Google Scholar]
- 34.Oosterhout JJV, Ndekha M, Moore E, Kumwenda JJ, Zijlstra EE, Manary M. The benefit of supplementary feeding for wasted Malawian adults initiating ART. AIDS Care. 2010;22:737–42. doi: 10.1080/09540120903373581. [DOI] [PubMed] [Google Scholar]
- 35.Vertefeuille JF, Dowell SF, Domercant JW, Tappero JW. Cautious optimism on public health in post-earthquake Haiti. Lancet. 2013;381:517–9. doi: 10.1016/S0140-6736(13)60051-3. [DOI] [PubMed] [Google Scholar]
- 36.Haddad L, Peña C, Nishida C, Quisumbing A, Slack A. Food security and nutrition implications of intrahousehold bias: a review of literature. Washington, DC: International Food Policy Research Institute; 1996. [Google Scholar]
- 37.Koenig S, Ivers L, Pace S, et al. Successes and challenges of HIV treatment programs in Haiti: aftermath of the earthquake. HIV Ther. 2010;4:145–60. doi: 10.2217/hiv.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.