Supplementation with the probiotic Lactobacillus rhamnosus GG (LGG) is safe, decreases repeated episodes of diarrhea, improves intestinal permeability, and increases IgG antibody response in rotavirus diarrhea in Indian children.
Keywords: probiotics, LGG, gastroenteritis, immune response, intestinal function
Abstract
Background. Probiotics have a possible role in the treatment of pediatric acute gastroenteritis. We report the effect of the probiotic Lactobacillus rhamnosus GG (LGG) on intestinal function, immune response, and clinical outcomes in Indian children with cryptosporidial or rotavirus diarrhea.
Methods. Children with gastroenteritis aged 6 months to 5 years, testing positive for either rotavirus or Cryptosporidium species in stool (coinfections were excluded), were randomized to LGG (ATCC 53103) or placebo, once daily for 4 weeks. Baseline demographic and clinical details were obtained. Sera were tested for immunoglobulin G (IgG) and immunoglobulin A (IgA) antibodies to Cryptosporidium and rotavirus, and the lactulose to mannitol ratio for intestinal permeability was determined at baseline and at the end of follow-up.
Results. Of the 124 children enrolled, 82 and 42 had rotavirus and cryptosporidial diarrhea, respectively. Median diarrheal duration was 4 days; one-third of the children had severe diarrhea. Baseline and clinical parameters were comparable between children receiving LGG and placebo. At the end of follow-up, fewer children with rotavirus diarrhea on LGG had repeated diarrheal episodes (25% vs 46%; P = .048) and impaired intestinal function (48% vs 72%; P = .027). Significant increase in IgG levels postintervention (456 vs 2215 EU; P = .003) was observed in children with rotavirus diarrhea receiving LGG. Among children with cryptosporidial diarrhea, those receiving LGG showed significant improvement in intestinal permeability.
Conclusions. LGG has a positive immunomodulatory effect and may be useful in decreasing repeated episodes of rotavirus diarrhea. Improvement in intestinal function in children with rotavirus and cryptosporidial gastroenteritis emphasizes the role of probiotics in treating intestinal impairment after infection.
Clinical Trials Registration. CTRI/2010/091/000339.
Rotavirus and Cryptosporidium species are the etiological agents most commonly associated with diarrhea in India [1, 2]. Oral rehydration therapy prevents the related dehydration [3], but does not affect frequency of bowel movements, diarrheal duration, or intestinal barrier function [4, 5]. To limit and to heal intestinal damage, appropriate treatment modalities are needed.
Probiotics, predominantly Lactobacillus rhamnosus GG (LGG), have been used in the treatment of diarrheal illness, with minimal side effects [6–9]. Reviews showed benefit in acute rotavirus diarrhea, with significant reduction in duration [10]. In animal models, probiotics reduce Cryptosporidium shedding [11, 12], but no studies have been performed in children. Studies suggest that probiotics modulate the innate and adaptive immune response, particularly in the gastrointestinal tract [13–15], and intestinal barrier function [16, 17]. This study assessed the effect of LGG (ATCC 53103) on intestinal function of children with rotavirus or cryptosporidial diarrhea.
METHODS
Study Design
A randomized, double-blind, placebo-controlled clinical trial was conducted at the Christian Medical College, Vellore, India, between May 2010 and July 2011. Children between the ages of 6 months and 5 years with diarrhea, positive for either rotavirus or Cryptosporidium species and resident within 20 km, were eligible. Children with coinfections (presence of both rotavirus and Cryptosporidium), severe malnutrition, probiotic consumption in the preceding month (other than yogurt), allergy to probiotics, or acute abdomen or colitis were excluded. Written informed consent was obtained, and children were randomized to LGG (ATCC 53103) or placebo. The study was approved by the institutional review boards of Christian Medical College, Vellore and Tufts University School of Medicine, Boston and registered with the Clinical Trials Registry of India (CTRI/2010/091/000339).
Study Intervention
The randomization code was provided by a nonstudy statistician to a research pharmacist who dispensed the LGG and the placebo, labeled by subject identification numbers. Randomization assignments were made in permuted blocks of sizes 2 and 4 for a 1 : 1 randomization.
The probiotic LGG was a gelatin capsule with 1 × 1010 organisms and 170 mg of microcrystalline cellulose; the placebo contained 170 mg cellulose. The intervention started at recruitment. However, children prescribed bactericidal antibiotics started the intervention after the antibiotics, usually 3 days later. The intervention was provided weekly, with room temperature storage, with the contents of 1 capsule given once daily in boiled and cooled milk. The LGG (Culturelle) and the placebo were supplied by i-Health Inc (Cromwell, Connecticut).
Baseline Assessment and Data Collection
Data on sociodemographic, anthropometric, and clinical parameters, including the duration of diarrhea, associated vomiting, fever, dehydration, severity of diarrhea, and treatment, were collected at recruitment. Diarrhea was defined as ≥3 loose watery stools within a 24-hour period [18]. On resolution of the diarrheal episode, a stool sample and 1 serum (preintervention) sample were collected and a lactulose to mannitol (L:M) test was conducted to assess intestinal function. A high L:M ratio is suggestive of an increased intestinal permeability due to disruption of the intestinal barrier system [19].
On alternate days, field workers visited study homes to obtain morbidity information and assess compliance. Illnesses were treated by the study physicians or referred to hospital. After 4 weeks, postintervention stool and serum, and anthropometric measurements were obtained and an L:M test was performed.
Sample Size
In a study involving Gambian children, the mean L:M ratio in children affected by intestinal infections was 0.3 (SD, 0.2) [20]. With an expected reduction with probiotics of 40% for rotavirus and 50% for Cryptosporidium, an α error of 5%, a power of 80%, and an allocation ratio of 1:1, the sample sizes were calculated to be 44 children with rotavirus diarrhea and 28 children with Cryptosporidium diarrhea in each arm.
Screening for Rotavirus and Cryptosporidium Species
Rotavirus detection was by VP6 antigen enzyme-linked immunosorbent assay (ELISA) (ProSpecT Rotavirus, Oxoid, Basingstoke, UK), and genotyping as previously described [21]. Fecal samples were screened for Cryptosporidium species by microscopic examination and 18S ribosomal RNA polymerase chain reaction [22], followed by restriction fragment-length polymorphism analysis for species determination [23].
Tests of Intestinal Permeability
After 4 hours of fasting, children were given a test solution containing 5 g lactulose (Duphar Laboratories, Southampton, UK) and 1 g mannitol (Dr Reddy's Labs, Hyderabad, India) in 20 mL water. Urine was collected for 5 hours and the total volume recorded. An aliquot was preserved with chlorhexidine (0.236 mg/mL of urine; Sigma Chemical, St Louis, Missouri). The lactulose and mannitol concentrations were measured by high-pressure liquid chromatography with light scatter detection [24]. Based on the mean + 2 SD L:M ratio in a group of healthy southern Indian children, a value of >0.0832 was considered indicative of impaired intestinal function [25].
ELISA for Assessment of Serological Response to Rotavirus and Cryptosporidial Diarrhea
For every child with rotavirus, the pre- and postintervention serum samples were analyzed for antirotavirus immunoglobulin A (IgA) and immunoglobulin G (IgG) antibodies by an antibody-sandwich enzyme immunoassay [26]. Serum IgG and IgA levels to Cryptosporidium glycopeptide (gp)15 were quantified by ELISA using a recombinant gp15 antigen [27].
Assessment of Malnutrition, Hemoglobin and Serum Ferritin Levels
Height-for age (HAZ), weight-for-height (WHZ) and weight-for-age (WAZ) z-scores were calculated using the 2006 World Health Organization child growth standards [28]. Children were classified as stunted (HAZ less than −2 SD), wasted (WHZ less than −2 SD), underweight (WAZ less than −2 SD) or normal based on their z-scores.
Hemoglobin was measured on venous blood (Becton Dickinson Automated Hematology Analyzer, Franklin Lakes, NJ, USA). Serum ferritin was measured by chemiluminescence (Siemens ADVIA Centaur® XP Immunoassay System, Erlangen, Germany).
Statistical Analysis
All variables were examined using descriptive statistics, dispersion for continuous variables, frequency counts, and marginal percentages with 95% confidence intervals for categorical variables. Comparisons between the 2 groups were done using t tests for normally distributed variables (or nonparametric tests for nonnormally distributed variables) and χ2 tests for categorical variables. All differences were considered statistically significant if the 2-tailed P value was ≤.05. Data analysis was performed using SAS software, version 9.2 for Windows (SAS Institute, Cary, North Carolina). A sensitivity analysis excluding children on antibiotics at recruitment was also performed.
RESULTS
Study Enrollment and Follow-up
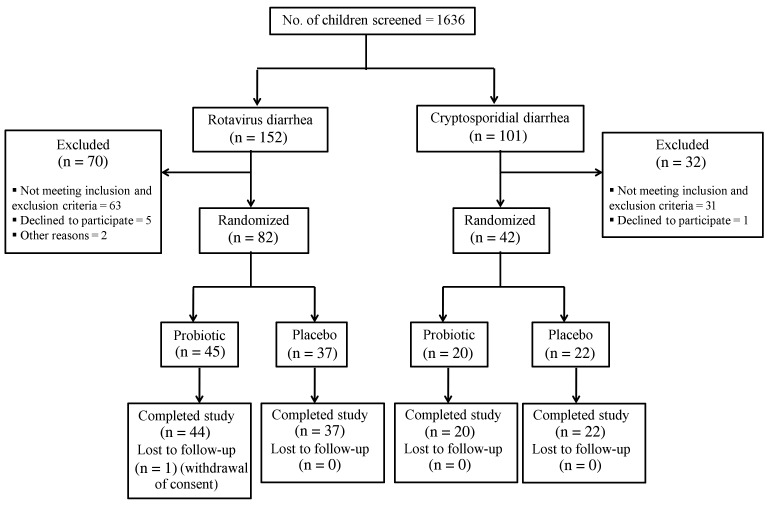
Of the 1636 children screened, 152 and 101 children had rotavirus and cryptosporidial diarrhea, respectively, and 124 children (82 with rotavirus and 42 with cryptosporidial diarrhea) who were available for follow-up were enrolled (Figure 1). All children except 1 completed the study. A majority of children (89 [72%]) were 100% compliant, and all 123 children who completed the study had >80% compliance. None of the enrolled children received a rotavirus vaccine.
Figure 1.
Flow diagram of study recruitment and follow-up.
Baseline Characteristics
The median age at recruitment was 13 months (interquartile range [IQR], 10–19 months). Children with rotavirus diarrhea were younger at 11 months (IQR, 9–16 months) than those with cryptosporidial diarrhea at 18 months (IQR, 14–21 months) (P < .001). Most (76/124 [61%]) children were male. Of the 122 families for whom socioeconomic status was available, 93 (76%) were of low socioeconomic status using the Kuppuswamy scale [29].
Children were weaned at a median age of 6 months (IQR, 5–6 months), and most children (69/124 [56%]) were being exclusively or partially breastfed at recruitment. Twenty-five (20%) children had a history of yogurt intake. Eighteen children (15%) were prescribed antibiotics by the treating physician for the current diarrheal episode; most (14/18 [78%]) were bactericidal antibiotics. The baseline characteristics in the LGG and placebo groups were comparable, including (Table 1) and excluding (Supplementary Table 1) children who were prescribed antibiotics.
Table 1.
Comparison of Baseline Characteristics of Children With Rotavirus and Cryptosporidial Gastroenteritis in the Probiotic and Placebo Groups
| Characteristic | Overall (N = 124) |
Rotavirus Diarrhea (n = 82) |
Cryptosporidial Diarrhea (n = 42) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Probiotics (n = 65), No. (%) | Placebo (n = 59), No. (%) | P Value | Probiotics (n = 45), No. (%) | Placebo (n = 37), No. (%) | P Value | Probiotics (n = 20), No. (%) | Placebo (n = 22), No. (%) | P Value | |
| Age at recruitment, mo, median (IQR) | 13 (10–20) | 12 (10–17) | .579 | 11 (9–15) | 11 (10–16) | .874 | 19 (16–22) | 16 (11–21) | .124 |
| Sex | |||||||||
| Male | 42 (65) | 34 (58) | .425 | 29 (64) | 22 (60) | .643 | 13 (65) | 12 (55) | .491 |
| Female | 23 (35) | 25 (42) | 16 (36) | 15 (41) | 7 (35) | 10 (46) | |||
| Socioeconomic statusa | |||||||||
| Low | 48 (76) | 45 (76) | .617 | 32 (74) | 30 (81) | .563 | 16 (80) | 15 (68) | .384 |
| Middle | 14 (22) | 14 (24) | 10 (23) | 7 (19) | 4 (20) | 7 (32) | |||
| High | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | |||
| Age at weaning, mo, median (IQR)b | 6 (5–6) | 6 (5–6.5) | .446 | 6 (5–6) | 6 (4–6) | .230 | 6 (5.5–6) | 6 (5–7) | .787 |
| History of yogurt intake | 11 (17) | 14 (24) | .345 | 4 (9) | 7 (19) | .210 | 7 (35) | 7 (32) | .827 |
| Antibiotic usage | 8 (13) | 10 (17) | .486 | 5 (11) | 8 (22) | .210 | 3 (15) | 2 (10) | .555 |
Abbreviation: IQR, interquartile range.
a Data not available for 2 children.
b Data not available for 1 child.
Clinical Characteristics of Diarrhea at Enrollment
The median duration of diarrhea was 4 days (IQR, 3–6 days), with 2 children (1 each with rotavirus and Cryptosporidium) having diarrhea for >14 days. Eighty-eight (72%) children had associated vomiting, for a median of 2 days (IQR, 1–2 days). Similarly, 104 of 121 (86%) children had associated fever. Using the Vesikari scoring system for diarrhea severity [30], the median score was 9 (IQR, 7–11), with 34 of 116 (29%) children with complete data having severe diarrhea (Vesikari score ≥11). A significant proportion of children (P = .044) with rotavirus infection (23%) had more severe disease than those with cryptosporidial infection (6%).
The clinical parameters of the diarrheal episodes (duration and severity of diarrhea, associated vomiting, fever, and dehydration) were comparable (Table 2 and Supplementary Table 2).
Table 2.
Comparison of Clinical Characteristics of Diarrhea in Children With Rotavirus and Cryptosporidial Gastroenteritis, by the Probiotic and Placebo Groups
| Characteristic | Overall (N = 124) |
Rotavirus Diarrhea (n = 82) |
Cryptosporidial Diarrhea (n = 42) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Probiotics (n = 65), No. (%) | Placebo (n = 59), No. (%) | P Value | Probiotics (n = 45), No. (%) | Placebo (n = 37), No. (%) | P Value | Probiotics (n = 20), No. (%) | Placebo (n = 22), No. (%) | P Value | |
| Duration of diarrheaa, d, median (IQR) | 4 (3–6) | 4 (3–6) | .447 | 4 (3–5) | 4 (3–6) | .268 | 4.5 (3–6) | 4 (2–6) | .809 |
| Associated vomitingb | 49 (77) | 39 (66) | .199 | 39 (89) | 29 (76) | .124 | 10 (50) | 11 (50) | 1.000 |
| Associated feverc | 52 (84) | 52 (88) | .500 | 36 (86) | 32 (87) | .921 | 16 (80) | 20 (91) | .313 |
| Associated dehydrationb | 7 (11) | 1 (2) | .063 | 6 (13) | 1 (3) | .121 | 1 (5) | 0 (0) | .463 |
| Severe diarrhea (Vesikari score >10)d | 19 (33) | 15 (25) | .382 | 16 (40) | 12 (32) | .490 | 3 (17) | 3 (14) | 1.000 |
| Diarrhea requiring hospitalization | 15 (23) | 13 (22) | .890 | 13 (29) | 9 (24) | .642 | 2 (10) | 4 (18) | .665 |
| Diarrhea during follow-up | 18 (26) | 25 (42) | .098 | 11 (24) | 17(46) | .048 | 7 (35) | 8 (37) | .926 |
Abbreviation: IQR, interquartile range.
a Data not available for 2 children.
b Data not available for 1 child.
c Data not available for 3 children.
d Data not available for 8 children.
Intestinal Permeability
At recruitment, 80 of 115 (70%) children had impaired intestinal function by the L:M test. At the end of follow-up, 67 of 121 (55%) children had impaired intestinal permeability. A significantly lower proportion of children in the LGG group (30/64 [47%]) had impaired function postintervention than those in the placebo group (37/57 [65%]; P = .046). When this comparison was repeated separately for either rotavirus or cryptosporidial diarrhea, the difference in the proportion of children with impaired intestinal function between the LGG and placebo groups was statistically significant for those with rotavirus (21/44 [48%] vs 26/36 [72%]; P = .027), but not for those with cryptosporidial diarrhea (9/20 [45%] vs 11/21 [52%]; P = .636). When children prescribed antibiotics prior to recruitment were excluded from analysis, the numbers decreased to 69 rotavirus-infected and 37 Cryptosporidium-infected children; the proportion of children with impaired intestinal function was not significantly different between the probiotic and placebo groups both pre- and postintervention (Supplementary Table 3), although a trend in improvement of intestinal function was seen.
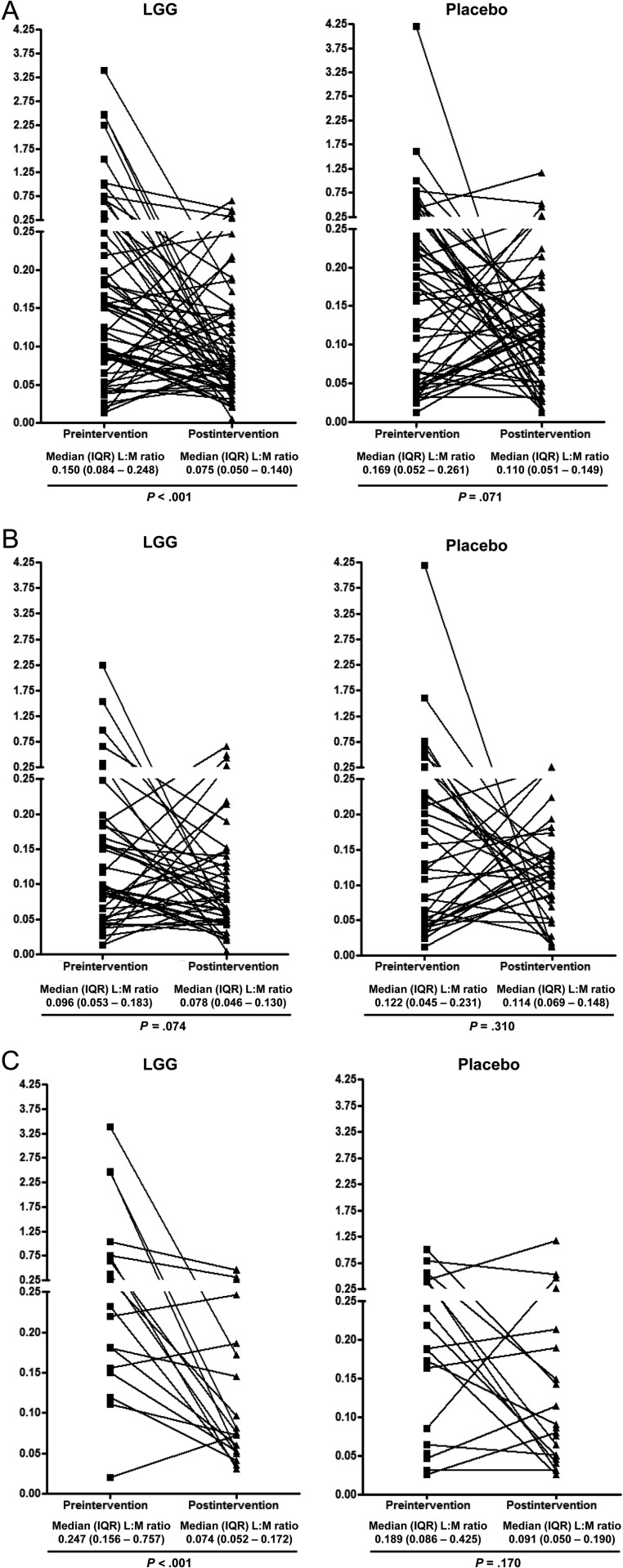
When the preintervention L:M ratios were compared, children with cryptosporidial diarrhea (median, 0.219 [IQR, 0.150–0.639]) had a significantly higher L:M ratio than those with rotavirus diarrhea (median, 0.104 [IQR, 0.050–0.201]; P < .001). Among children with cryptosporidial diarrhea, a reduction in the postintervention L:M ratio was observed both in the LGG (from a median L:M ratio of 0.247 preintervention to 0.074 postintervention; P < .001) and the placebo groups (from 0.189 to 0.091; P = .170), but was statistically significant only in children receiving LGG (Figure 2C). In contrast, among children with rotavirus diarrhea, those receiving LGG showed some evidence of improved intestinal function (reduction in the median L:M ratio from 0.096 preintervention to 0.078 postintervention), although it was not statistically significant (P = .074; Figure 2B).
Figure 2.
Pre- and postintervention lactulose to mannitol (L:M) ratio in all children (A), children with rotavirus diarrhea (B), and children with cryptosporidial diarrhea (C). The squares and triangles represent pre- and postintervention L:M ratio, respectively, for each child. The lines represent intrachild changes in the L:M ratio. Abbreviations: IQR, interquartile range; LGG, Lactobacillus rhamnosus GG; L:M, lactulose to mannitol ratio.
Of the 79 children with impaired intestinal function at recruitment, for whom postintervention L:M results were available, 38 (48%) had improved intestinal function. Children given probiotics (27/46 [59%]) were more likely to show improvement than those given placebo (11/33 [33%]; P = .026). Repeating the analysis separately for children with rotavirus (59% for probiotic group vs 30% for placebo group; P = .048) and cryptosporidial (59% for probiotic group vs 38% for placebo group; P = .269) diarrhea yielded similar results, although the difference was not statistically significant for children with cryptosporidial diarrhea. The proportion of children with diarrhea during follow-up was comparable between those with and without improved intestinal permeability (34% vs 32%; P = .813), irrespective of whether they had rotavirus (44% vs 27%; P = .224) or cryptosporidial (20% vs 40%; P = .232) diarrhea, or received probiotics (33% vs 32%; P = .901) or placebo (36% vs 32%; P = .794).
Serological Response to Rotavirus Diarrhea
Rotavirus IgA and IgG Response
The median IgA (130 vs 196 ELISA units [EU]) and IgG (819 vs 1597 EU) antibody levels in all seropositive children increased from baseline to postintervention, but only IgG was statistically significant in all children (P = .004) and those receiving probiotics (P = .003) (Table 3). Results were similar when children on antibiotics at the time of recruitment were excluded (Supplementary Table 4).
Table 3.
Comparison of Antibodies Against Rotavirus and Cryptosporidium gp15 at Baseline and at the End of 4 Weeks of Follow-up
| IgA, EU, Median (IQR) |
IgG, EU, Median (IQR) |
|||||
|---|---|---|---|---|---|---|
| Preintervention | Postintervention | P Value | Preintervention | Postintervention | P Value | |
| Rotavirus | ||||||
| All | 130 (31–312) | 196 (128–316) | .062 | 819 (155–2152) | 1597 (804–4135) | .004 |
| LGG | 156 (30–378) | 211 (144–319) | .258 | 456 (69–2110) | 2215 (851–6026) | .003 |
| Placebo | 112 (35–287) | 148 (68–306) | .212 | 1268 (759–3582) | 1276 (774–3932) | .510 |
| Cryptosporidium | ||||||
| All | 5027 (2527–22 984) | 4490 (2703–7913) | .051 | 73 554 (38 154–132 001) | 72 448 (52 128–178 049) | .020 |
| LGG | 6198 (2527–24 671) | 5590 (3699–7913) | .584 | 109 734 (22 758–155 021) | 65 306 (54 922–163 522) | .967 |
| Placebo | 4756 (2303–22 984) | 3923 (2501–8746) | .212 | 56 106 (39 323–77 403) | 61 884 (32 082–146 229) | .634 |
Abbreviations: EU, enzyme-linked immunosorbent assay units; IgA, immunoglobulin A; IgG, immunoglobulin G; IQR, interquartile range; LGG, Lactobacillus rhamnosus GG.
Serological Response to Cryptosporidial Diarrhea
Cryptosporidium gp15 IgA and IgG Response
The median IgA and IgG antibody levels declined significantly from pre- to postintervention when all children were considered (IgA: from 5027 to 4490 EU, P = .051; IgG: from 73 554 to 72 448 EU, P = .020), but not when analyzed by the probiotic and the placebo groups (Table 3). Excluding children on antibiotics at the time of recruitment, however, resulted in a statistically significant decrease in the postintervention IgG among children receiving LGG (109 734 EU vs 64 652 EU; P = .048; Supplementary Table 4).
Diarrhea During Follow-up
During the follow-up period, 36 (29%) children had 1 episode and 7 (6%) had 2 further episodes of diarrhea. Children with rotavirus diarrhea receiving probiotics had significantly fewer subsequent diarrheal episodes than children receiving placebo (11/44 [25%] vs 17/37 [46%]; P = .048). This difference was not seen in children with cryptosporidial diarrhea (7/20 [35%] vs 8/22 [36%]; P = .927) or when both groups were combined (25/59 [42%] vs 18/64 [28%]; P = .098). When children on antibiotics at the time of recruitment were excluded from analysis, the trend was similar but not statistically significant (Supplementary Table 2).
Other Illnesses During Follow-up
The majority of children (104/123 [85%]) had upper respiratory infections, and 12 had other illnesses during follow-up. These were distributed equally among children in the probiotic (56/64 [88%]) and placebo (49/59 [83%]; P = .486) groups.
Serious Adverse Events
Five children experienced serious adverse events requiring hospitalization; these were for lower respiratory infections, vulval abscess, and measles. Four were in the probiotic group, but no events were considered related to the intervention. All the children recovered.
Anthropometry, Hemoglobin, and Serum Ferritin Levels
The proportion of stunted children was greater in the LGG group than in the placebo group both pre- and postintervention, although the proportions of wasted and underweight children were comparable (Supplementary Tables 5 and 6). The proportions of children stunted, wasted, and underweight were comparable between children with rotavirus and cryptosporidial infection, pre- and postintervention.
Anemia (hemoglobin <11 g/dL) was diagnosed in 75 of 123 (61%) children and low serum ferritin (<12 ng/mL) in 53 of 120 (44.2%) children (Supplementary Table 5); proportions were comparable between children given probiotics and placebo, both pre- and postintervention (Supplementary Tables 5 and 6).
DISCUSSION
Supplementation with LGG for 4 weeks after acute infection improved intestinal permeability in children with rotavirus and cryptosporidial diarrhea, reduced the number of subsequent diarrheal episodes, and increased IgG response in children with rotavirus diarrhea. Although small, this is one of the few studies to examine mechanisms by which probiotics may prevent further damage or promote intestinal integrity in children, following infections of defined etiology.
In 3 earlier randomized controlled studies with 105 children given LGG and 96 placebo controls, who started supplementation during acute diarrhea, the duration of diarrhea was significantly reduced (weighted mean difference, −2 days, 95% confidence interval, −2.3 to −1.7 days) [10]. One trial reported no effect on rotavirus diarrhea [10]. In our review, there are no studies on probiotics in cryptosporidial diarrhea, although a case study reported that LGG in treatment of cryptosporidiosis in a patient with celiac disease and chronic diarrhea resulted in resolution within 10 days of probiotic initiation [31]. In our study, administration of LGG did not decrease the duration of diarrhea in rotavirus and cryptosporidial diarrhea. This could be because the intervention was started later for children on antibiotics, but no differences in diarrheal duration were detected between antibiotic-treated and untreated children.
In a systematic review on the use of probiotics in infectious diarrhea [6], 23 studies involving 1917 participants who received various probiotics, including LGG, were evaluated. Twelve of 23 studies reported no adverse events, 8 had no information on adverse events, and 3 reported adverse events, mostly vomiting, considered unlikely to be related to probiotics. In our study, 34% of the participants had 1 or more episodes of diarrhea during the follow-up period, with probiotics decreasing further diarrheal episodes in children with rotavirus at recruitment.
To our knowledge, this is the first study to investigate the effects of LGG on both immune response and intestinal permeability in diarrhea due to specific pathogens. Although a majority of the children had preexisting IgA and IgG antibodies to rotavirus (79/82) and IgA antibodies to Cryptosporidium species (33/42), nearly a third of all children (29/82 with rotavirus infection and 10/42 with cryptosporidiosis) showed a 4-fold decrease in IgA antibody levels. These findings and our previous studies have shown high levels of exposure in early childhood to both rotavirus and Cryptosporidium [32, 33], and it is likely that the infection causing diarrhea in these children was not a primary infection. IgA levels would be expected to peak very early in nonprimary infection; hence, the large decrease in IgA levels during the convalescent phase possibly reflects an early peak in the antibody response at the time the baseline sample was obtained, followed by the rapid decline of IgA, which has a short half-life, to a 4-fold lower level 4 weeks following baseline sampling.
The mechanisms by which probiotics exert an immunomodulatory effect are not completely understood. Previous studies have shown that they modulate innate and adaptive immune responses [13–15], particularly to gastrointestinal pathogens, increasing serum IgG and secretory IgA to enteric pathogens, including rotavirus, and to oral vaccines for poliovirus [34], Salmonella typhi [35, 36], and rotavirus [37].
A few studies have shown that probiotics have a positive effect on intestinal permeability [38] whereas others have shown no association [39, 40]. In our study, almost 70% of children had impaired intestinal function at recruitment, decreasing to 55% at the end of follow-up. Children given LGG showed improvement in their intestinal function compared with those given placebo. A significantly elevated preintervention L:M ratio was observed among children with cryptosporidial diarrhea, indicative of more extensive acute intestinal damage caused by Cryptosporidium species. Although a significant intrachild reduction in median L:M ratio was observed in children with cryptosporidial diarrhea receiving LGG, the proportion of children with impaired intestinal function was comparable between the probiotic group and the placebo group at the end of follow-up, possibly due to the smaller sample size. In comparison, among children with rotavirus diarrhea, a significantly lower proportion of children receiving LGG had impaired intestinal function postintervention, even though the pre-/postintervention reduction in median L:M ratio was not statistically significant. Taken together, these findings suggest a beneficial role of LGG in restoring intestinal integrity in children with diarrhea due to either Cryptosporidium or rotavirus.
A major limitation of this study was the size, which may have resulted in insufficient statistical power to detect differences between the LGG and placebo groups for some of the outcome variables, especially among children with cryptosporidial diarrhea. Larger numbers of children and a long-term follow-up will, therefore, help us better understand the effect of probiotics on childhood enteric infections. Moreover, even though mothers were trained by the study nurse to reconstitute, boil, and sufficiently cool the milk (tested on the back of the hand) before addition of the contents of the capsule, the temperature of the milk was not recorded in the homes. Hence, it is possible that the probiotic was inactivated in incompletely cooled milk, although such instances would have been rare.
In conclusion, improvement in intestinal integrity in children with gastroenteritis treated with LGG emphasizes the role of probiotics in treating intestinal impairment postinfection. The positive immunomodulatory effect of LGG may also be useful in decreasing reinfections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are indebted to the parents and children who participated in the study for their cooperation and support. We also thank the field workers and all the support staff of the Department of Gastrointestinal Sciences, Christian Medical College, Vellore, for making this study possible.
Financial support. This work was supported by the National Institute of Child Health and Human Development (grant number R03 HD057736 to H. D. W.) and the Indian Council of Medical Research (grant number 5/7/180-Indo-US/RHN to G.K.) under the Indo-US Program on Maternal and Child Health and Human Development Research. A. P., S. B., and R. S. were supported by the Fogarty International Center (training grant number D43 TW007392 to G. K.). The LGG and the placebo capsules were provided by i-Health Inc (Cromwell, Connecticut).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ajjampur SS, Sankaran P, Kang G. Cryptosporidium species in HIV-infected individuals in India: an overview. Natl Med J India. 2008;21:178–84. [PubMed] [Google Scholar]
- 2.Ramani S, Kang G. Burden of disease and molecular epidemiology of group A rotavirus infections in India. Indian J Med Res. 2007;125:619–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Bellemare S, Hartling L, Wiebe N, et al. Oral rehydration versus intravenous therapy for treating dehydration due to gastroenteritis in children: a meta-analysis of randomised controlled trials. BMC Med. 2004;2:11. doi: 10.1186/1741-7015-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum LS, Oria PA, Olson CK, Breiman RF, Ram PK. Examining the use of oral rehydration salts and other oral rehydration therapy for childhood diarrhea in Kenya. Am J Trop Med Hyg. 2011;85:1126–33. doi: 10.4269/ajtmh.2011.11-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desjeux JF, Briend A, Butzner JD. Oral rehydration solution in the year 2000: pathophysiology, efficacy and effectiveness. Baillieres Clin Gastroenterol. 1997;11:509–27. doi: 10.1016/s0950-3528(97)90029-4. [DOI] [PubMed] [Google Scholar]
- 6.Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;11:CD003048. doi: 10.1002/14651858.CD003048.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pant AR, Graham SM, Allen SJ, et al. Lactobacillus GG and acute diarrhoea in young children in the tropics. J Trop Pediatr. 1996;42:162–5. doi: 10.1093/tropej/42.3.162. [DOI] [PubMed] [Google Scholar]
- 8.Raza S, Graham SM, Allen SJ, Sultana S, Cuevas L, Hart CA. Lactobacillus GG promotes recovery from acute nonbloody diarrhea in Pakistan. Pediatr Infect Dis J. 1995;14:107–11. doi: 10.1097/00006454-199502000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bousvaros A, Guandalini S, Baldassano RN, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn's disease. Inflamm Bowel Dis. 2005;11:833–9. doi: 10.1097/01.mib.0000175905.00212.2c. [DOI] [PubMed] [Google Scholar]
- 10.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33(suppl 2):S17–25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 11.Alak JI, Wolf BW, Mdurvwa EG, et al. Supplementation with Lactobacillus reuteri or L. acidophilus reduced intestinal shedding of Cryptosporidium parvum oocysts in immunodeficient C57BL/6 mice. Cell Mol Biol (Noisy-le-grand) 1999;45:855–63. [PubMed] [Google Scholar]
- 12.Alak JI, Wolf BW, Mdurvwa EG, Pimentel-Smith GE, Adeyemo O. Effect of Lactobacillus reuteri on intestinal resistance to Cryptosporidium parvum infection in a murine model of acquired immunodeficiency syndrome. J Infect Dis. 1997;175:218–21. doi: 10.1093/infdis/175.1.218. [DOI] [PubMed] [Google Scholar]
- 13.Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93(suppl 1):S41–8. doi: 10.1079/bjn20041356. [DOI] [PubMed] [Google Scholar]
- 14.Galdeano CM, Perdigon G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol. 2006;13:219–26. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen K. Probiotics and the immune response. J Clin Gastroenterol. 2006;40:232–4. doi: 10.1097/00004836-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Petrof EO, Kojima K, Ropeleski MJ, et al. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–87. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Marco ML, Pavan S, Kleerebezem M. Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol. 2006;17:204–10. doi: 10.1016/j.copbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Geneva: WHO; 1995. The treatment of diarrhoea: a manual for physicians and other senior health workers. [Google Scholar]
- 19.Mishra A, Makharia GK. Techniques of functional and motility test: how to perform and interpret intestinal permeability. J Neurogastroenterol Motil. 2012;18:443–7. doi: 10.5056/jnm.2012.18.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell DI, McPhail G, Lunn PG, Elia M, Jeffries DJ. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr. 2004;39:153–7. doi: 10.1097/00005176-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Sowmyanarayanan TV, Ramani S, Sarkar R, et al. Severity of rotavirus gastroenteritis in Indian children requiring hospitalization. Vaccine. 2012;30(suppl 1):A167–72. doi: 10.1016/j.vaccine.2011.07.145. [DOI] [PubMed] [Google Scholar]
- 22.Xiao L, Escalante L, Yang C, et al. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999;65:1578–83. doi: 10.1128/aem.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen S, Dalle F, Gallay A, Di Palma M, Bonnin A, Ward HD. Identification of Cpgp40/15 type Ib as the predominant allele in isolates of Cryptosporidium spp. from a waterborne outbreak of gastroenteritis in South Burgundy, France. J Clin Microbiol. 2006;44:589–91. doi: 10.1128/JCM.44.2.589-591.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barboza Junior MS, Silva TM, Guerrant RL, Lima AA. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz J Med Biol Res. 1999;32:1499–504. doi: 10.1590/s0100-879x1999001200008. [DOI] [PubMed] [Google Scholar]
- 25.Vieira MM, Paik J, Blaner WS, et al. Carotenoids, retinol, and intestinal barrier function in children from northeastern Brazil. J Pediatr Gastroenterol Nutr. 2008;47:652–9. doi: 10.1097/MPG.0b013e31816bf4bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward RL, Bernstein DI, Smith VE, et al. Rotavirus immunoglobulin a responses stimulated by each of 3 doses of a quadrivalent human/bovine reassortant rotavirus vaccine. J Infect Dis. 2004;189:2290–3. doi: 10.1086/421248. [DOI] [PubMed] [Google Scholar]
- 27.Ajjampur SS, Sarkar R, Allison G, et al. Serum IgG response to Cryptosporidium immunodominant antigen gp15 and polymorphic antigen gp40 in children with cryptosporidiosis in South India. Clin Vaccine Immunol. 2011;18:633–9. doi: 10.1128/CVI.00464-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Geneva, Switzerland: WHO; 2006. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. [Google Scholar]
- 29.Kuppuswamy B. Manual of socioeconomic status scale (urban) Delhi, India: Manasayan; 1981. [Google Scholar]
- 30.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–67. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 31.Pickerd N, Tuthill D. Resolution of cryptosporidiosis with probiotic treatment. Postgrad Med J. 2004;80:112–3. doi: 10.1136/pmj.2003.014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gladstone BP, Ramani S, Mukhopadhya I, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med. 2011;365:337–46. doi: 10.1056/NEJMoa1006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar R, Ajjampur SS, Prabakaran AD, et al. Cryptosporidiosis among children in an endemic semiurban community in southern India: does a protected drinking water source decrease infection? Clin Infect Dis. 2013;57:398–406. doi: 10.1093/cid/cit288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukushima Y, Kawata Y, Hara H, Terada A, Mitsuoka T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol. 1998;42:39–44. doi: 10.1016/s0168-1605(98)00056-7. [DOI] [PubMed] [Google Scholar]
- 35.Fang H, Elina T, Heikki A, Seppo S. Modulation of humoral immune response through probiotic intake. FEMS Immunol Med Microbiol. 2000;29:47–52. doi: 10.1111/j.1574-695X.2000.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 36.Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol. 1994;10:55–63. doi: 10.1111/j.1574-695X.1994.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 37.Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310–2. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- 38.Noone C, Menzies IS, Banatvala JE, Scopes JW. Intestinal permeability and lactose hydrolysis in human rotaviral gastroenteritis assessed simultaneously by non-invasive differential sugar permeation. Eur J Clin Invest. 1986;16:217–25. doi: 10.1111/j.1365-2362.1986.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 39.Johansen K, Stintzing G, Magnusson KE, et al. Intestinal permeability assessed with polyethylene glycols in children with diarrhea due to rotavirus and common bacterial pathogens in a developing community. J Pediatr Gastroenterol Nutr. 1989;9:307–13. doi: 10.1097/00005176-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Stintzing G, Johansen K, Magnusson KE, Svensson L, Sundqvist T. Intestinal permeability in small children during and after rotavirus diarrhoea assessed with different-size polyethyleneglycols (PEG 400 and PEG 1000) Acta Paediatr Scand. 1986;75:1005–9. doi: 10.1111/j.1651-2227.1986.tb10331.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.