Abstract
Background
Iron deficiency contributes to anaemia in patients with chronic kidney disease. I.v. iron is therefore widely used for anaemia treatment, although it may induce oxidative stress and activate monocytes. Different i.v. iron preparations are available, but interestingly their substance-specific immunologic effects are poorly studied.
Methods
We analysed the effect of iron sucrose, ferric carboxymaltose, iron isomaltoside 1000, low-molecular-weight iron dextran and ferumoxytol on classical, intermediate and nonclassical monocyte biology. We therefore stimulated in vitro mature monocytes and haematopoietic CD34+ stem cells during their differentiation into monocytes with different concentrations (0.133, 0.266, 0.533 mg/mL) of i.v. iron preparations. Alterations of monocyte subset distribution, expression of surface markers (CD86, CCR5, CX3CR1), as well as production of pro-inflammatory cytokines (TNF-α, IL-1β) and reactive oxygen species were measured using flow cytometry. Additionally, we analysed phagocytosis and antigen presentation capacity.
Results
We found specific immunologic effects after stimulation with iron sucrose which were not induced by the other iron preparations. Iron sucrose activated monocyte subsets leading to significantly increased CD86 expression. Simultaneously CD16 and CX3CR1 expression and monocytic phagocytosis capacity were decreased. Additionally, differentiation of monocytes from haematopoietic CD34+ stem cells was almost completely abolished after stimulation with iron sucrose.
Conclusions
Our findings demonstrate that specific immunologic effects of distinct i.v. iron preparations exist. The clinical relevance of these findings requires further investigation.
Keywords: CD14, CD16, immune deficiency, iron therapy, monocyte subsets
INTRODUCTION
Iron deficiency is highly prevalent in chronic kidney disease (CKD) patients [1]. After erythropoiesis-stimulating agents failed to improve cardiovascular disease burden among CKD patients in several recent large-scale randomized clinical trials [2–4], the idea of iron supplementation for anaemia treatment has re-gained interest in nephrology in recent years [5].
Iron supplementation may be provided either orally or intravenously. However, oral absorption of iron is poor in many CKD patients due to their chronic micro-inflammatory status with commensurately high hepcidin levels [6]. Hepcidin itself impairs intestinal iron absorption and transport from the enterocytes to the plasma [7].
Therefore the use of intravenous (i.v.) iron preparations has been recommended [8, 9]. Several i.v. iron preparations have been developed, most of which (like iron sucrose, ferric carboxymaltose, low-molecular-weight iron dextran and ferumoxytol) consist of a ferritin-like polynuclear, non-ionic ferric-oxyhydroxide core and a carbohydrate shell. Another i.v. iron preparation, iron isomaltoside 1000, differs in structure from these molecules, since it has been described as a matrix-like structure with interchanging layers of linear isomaltoside 1000 oligomers and iron atoms placed in cavities between, and within, the oligosaccharide molecules [10]. I.v. iron preparations vary in their carbohydrate ligands as well as in the chemistry of the coupling process and thus in physiochemical and pharmacokinetic parameters such as molecular weight, reactivity as well as thermodynamic stability [11]. These differences result in a substance-specific kinetic of non-transferrin-bound iron (NTBI; ‘free iron’) release from iron complexes after their i.v. administration [12]. Within the cell, NTBI can induce the formation of reactive oxygen species (ROS) [13, 14]. Oxidative stress, in turn, results in endothelial damage and dysfunction and triggers inflammation [15, 16]. Moreover, recent reports suggest that i.v. iron administration may impair the function of circulating leukocytes with subsequently increased susceptibility to bacterial infections [17]. Of note, most scientific work has focused on implications of ‘free iron’ on neutrophils [18], and only few reports have been published on interaction of ‘free iron’ with monocytes [19], despite the central role of monocytes in iron metabolism and host defence [20].
Moreover, previous research on iron toxicity has completely neglected the existence of monocyte heterogeneity, constituting a knowledge gap regarding the effects of i.v. iron preparations on different monocyte subsets. Three distinct monocyte subsets, namely, classical CD14++CD16− monocytes, intermediate CD14++CD16+ monocytes and nonclassical CD14+CD16++ monocytes have been acknowledged by the recent consensus statement [21]. Intermediate monocytes are characterized as inflammatory and potentially proatherogenic cells; high cell counts of intermediate monocytes predict adverse cardiovascular outcome in CKD patients [22, 23].
Against this background, the purpose of this study was to investigate the immunoactivation of different monocyte subsets by five i.v. iron preparations which are commonly used in clinical nephrology, namely iron sucrose, ferric carboxymaltose, iron isomaltoside 1000, low-molecular-weight iron dextran and ferumoxytol [24].
SUBJECTS AND METHODS
Subjects
For our in vitro analysis, we recruited two groups of study participants:
control subjects without overt CKD (depending on the analyses, three to seven subjects per experiment, as indicated below; all subjects had serum creatinine <1.5 mg/dL), and
patients with severe CKD [four patients with CKD stage G 4 - 5 (GFR< 30 mL/min/1.73 m2) not yet on renal replacement therapy, and four haemodialysis patients].
All participants gave informed consent. The study protocol was approved by the local Ethics Committee and was conducted in accordance with the Declaration of Helsinki.
Flow cytometric analysis
Monocyte subsets were flow cytometrically identified in cell culture or in whole-blood assays according to our standardized and validated gating strategy using FACS Canto II with FACSDiva Software (BD Biosciences, Heidelberg, Germany) [22]. In brief, using a side scatter/CD86 dot plot, monocytes were detected as CD86-positive cells with monocytic scatter properties. Subsequently the three monocyte subsets classical, intermediate and nonclassical monocytes were gated based on their surface expression pattern of CD14 (LPS receptor) and CD16 (FcγIII receptor).
In the whole-blood assays we stimulated 150 µL blood with different i.v. iron preparations: iron sucrose (Venofer®), ferric carboxymaltose (ferinject®) (both from Vifor Pharma AG, Glattbrugg, Switzerland), iron isomaltoside 1000 (MonoFer®), low-molecular-weight iron dextran (CosmoFer®) (both from Pharmacosmos, Holbæk, Denmark) and ferumoxytol (Feraheme®, from AMAG Pharmaceuticals, Lexington, MA, USA).
All stimulations were done at 37°C and 5% CO2, and three iron concentrations were used: 0.133, 0.266 and 0.533 mg/mL.
Protein surface expression and intracellular cytokine production were quantified as mean fluorescence intensity (MFI). The following antibodies were used: anti-CD14 PerCP (Mφ9), anti-CD16 PeCy7 (3G8) and anti-CD195 APC (2D7/CCR5) and anti-TNF FITC (MAb11) (BD Biosciences, Heidelberg, Germany), anti-CD86 PE (HA5.2B7) (Beckman-Coulter, Krefeld, Germany), anti-CX3CR1 FITC (2A9-1) (Biozol, Eching, Germany), and anti-IL-1β Alexa Fluor® 647 (JK1B-1) (Biolegend, Fell, Germany).
Surface expression of CD14, CD16, CD86, CCR5 and CX3CR1 was determined after incubating 150 µL EDTA anticoagulated blood with iron preparations for 5 h.
For intracellular measurement of TNF-α and IL-1β, 150 µL Li-hep anticoagulated blood was incubated with iron preparations for 2 h before and for 1 h after addition of 350 µM of brefeldin A (Sigma-Aldrich, Taufkirchen, Germany). After staining for CD14, CD16 and CD86 and lysis, cells were fixed with paraformaldehyde (4%), washed with a saponin-containing buffer, stained with antibodies against TNF-α and IL-1β, washed and fixed with paraformaldehyde (1%).
In vitro generation of monocytes from haematopoietic CD34+ stem cells
For in vitro generation of monocytes, haematopoietic CD34+ stem cells were isolated and monocytes generated according to our standardized protocol (manuscript in preparation). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from EDTA anticoagulated blood by Ficoll-Paque (Lymphocyte Separation Medium; PAA, Cölbe, Germany) gradient density centrifugation. CD34+ cells were isolated using the CD34 MicroBead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). For determining quality of isolation, CD34+ cells were stained with anti-CD34 APC (581; BD Biosciences) and anti-CD45 PE (HI30; BD Biosciences) antibodies. Mean purity assessed by flow cytometry was 85.3 ± 4.8%.
Isolated CD34+ haematopoietic stem cells were expanded in 6-well plates (1 × 104 cells/mL) for 13 days in the Haematopoietic Progenitor Cell Expansion Medium DXF, enriched with Cytokine Mix E (PromoCell GmbH, Heidelberg, Germany). After expansion, cells were seeded in 12-well plates for differentiation to monocytes in the Haematopoietic Progenitor Medium (PromoCell GmbH) supplemented with iron preparations. Daily progress was flow cytometrically monitored after anti-CD14, anti-CD16 and anti-CD86 staining, subdividing cells into monocyte subsets. At Day 7 of differentiation, surface expression of CCR5 and CX3CR1 was additionally measured.
Measurement of ROS
For measurement of ROS, PBMCs were isolated from EDTA anticoagulated blood and stimulated with iron preparations for 3 h. Afterwards cells were incubated with 10 µM of the cell-permanent carboxy-H2DFFDA (Life technologies, Darmstadt, Germany) for 15 min at 37°C and 5% CO2. Samples were stained with CD14, CD16 and CD86 and the amount of ROS (MFI of H2DFFDA) was flow cytometrically determined as described above.
In analogy, for ROS determination of in vitro generated monocytes at Day 7 of differentiation, 200 µL of cell suspension was incubated with carboxy-H2DFFDA. Afterwards samples were stained and MFI-values were flow cytometrically calculated.
Phagocytosis assay
For measurement of monocytic phagocytosis capacity Fluoresbrite Yellow Green (YG) Carboxylate Microspheres (0.75 µm, Polysciences, Eppelheim, Germany) were opsonized with heterologous serum (mixed with Krebs Ringers PBS), adjusted to 108 particles/mL and shaken gently for 30 min at 37°C. After stimulation of 150 µL of citrate anticoagulated blood with iron preparations for 4.5 h, 50 µL of opsonized particles were added and incubated for additional 30 min at 37°C with mild shaking. Samples were stained as described above, and counts of FITC-positive cells were determined flow cytometrically in each monocyte subset.
Within in vitro generated monocytes, phagocytosis capacity was measured at Day 7 of differentiation. Therefore, 90 µL of cell suspension was incubated with opsonized particles as described before.
Proliferation assay
Monocyte subset-specific ability to induce T-cell proliferation was measured using the cytoplasmic dilution of CFDA-SE (Vybrant CFDA-SE Cell Tracer Kit; Life technologies). In detail, PBMCs were isolated from EDTA anticoagulated blood and labelled with 5 μM CFDA-SE for 10 min at 37°C. Cells were cultivated in 96-well plates at a density of 6 × 105 cells and stimulated with iron preparations in the presence of 2.5 µg/mL staphylococcal enterotoxin B (SEB; Sigma-Aldrich). After 3 days, counts of proliferating T-cells were analysed flow cytometrically by measuring CFDA-SE dilution, identifying T-cells by anti-CD3 APC staining (SK7; Biolegend).
Measurement of iron uptake
For measurement of monocytic iron uptake, 150 µL of Li-hep anticoagulated blood was stimulated with iron preparations for 1 h. Samples were stained with antibodies against CD14, CD16 and CD86 as well as with calcein acetoxymethyl ester (Biomol, Hamburg, Germany) in a final concentration of 0.2 µM. Iron uptake was determined based on the ability of iron to bind calcein and to quench its fluorescence.
Determination of viability
For measurement of cellular viability, PBMCs were isolated from EDTA anticoagulated blood and stimulated with iron preparations for 3 h. Subsequently, samples were stained with Annexin V and 7-AAD in Annexin V Binding Buffer (FITC Annexin V Apoptosis Detection Kit with 7-AAD, Biolegend). Flow cytometrically, monocyte subsets were identified as described above, and viable cells were defined as 7-AAD-negative monocytes.
Statistics
Statistical analysis was performed for each iron preparation using one-way analysis of variance (ANOVA), followed by Dunnett's multiple comparison test as post hoc test. Data were presented as percentages or MFI ± SEM (standard error of the mean).
RESULTS
Impact of iron preparations on human monocyte subsets
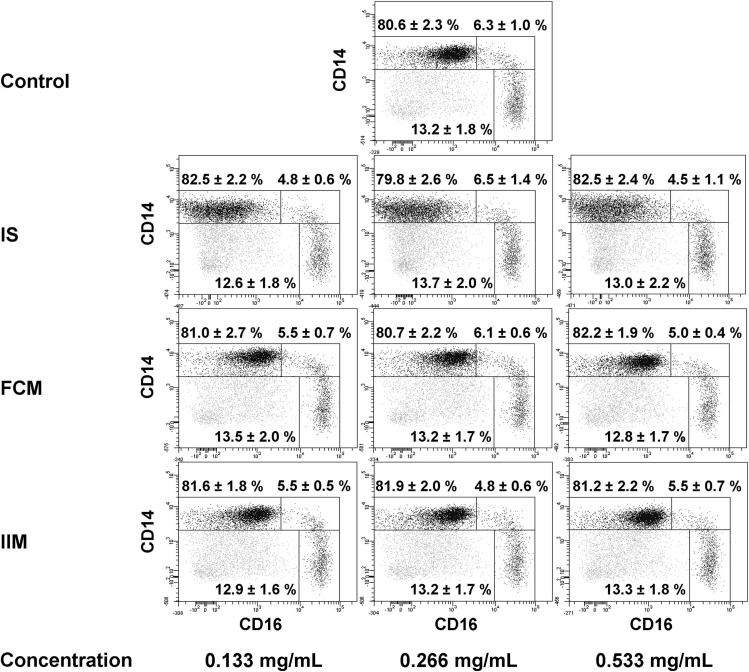
We first aimed to analyse the impact of the three iron preparations iron sucrose, ferric carboxymaltose and iron isomaltoside 1000 on human monocyte subsets collected from control subjects without overt CKD. Therefore, we stimulated whole blood with the different iron preparations and analysed their effect on the expression of CD14, CD16 and CD86 on classical, intermediate and nonclassical monocytes. We found iron sucrose dose-dependently to increase CD86 surface expression on all monocyte subsets. In addition, we observed a significant reduction of CD16 expression on classical monocytes, and on nonclassical monocytes (Table 1). Despite this down-regulation of CD16 expression, no significant changes in monocyte subset distribution occurred (Figure 1), and CD14 expression did not change (Table 1). Ferric carboxymaltose and iron isomaltoside 1000 did not affect the expression of CD14, CD16 or CD86 (Table 1).
Table 1.
Expression of CD14, CD16 and CD86 on circulating mature classical, intermediate and nonclassical monocytes from control subjects
| Monocyte subset | Control | Iron sucrose |
Ferric carboxymaltose |
Iron isomaltoside 1000 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | |||
| CD14 | Classical monocytes | 5453 ± 302 | 4766 ± 258 | 5202 ± 265 | 5166 ± 360 | 5324 ± 419 | 5469 ± 328 | 5147 ± 345 | 5194 ± 318 | 5116 ± 310 | 5094 ± 363 |
| Intermediate monocytes | 4842 ± 349 | 4258 ± 380 | 4385 ± 338 | 3960 ± 276 | 4503 ± 275 | 4620 ± 235 | 4554 ± 415 | 4620 ± 423 | 4512 ± 367 | 4530 ± 384 | |
| Nonclassical monocytes | 292 ± 25 | 320 ± 37 | 356 ± 23 | 335 ± 23 | 307 ± 31 | 317 ± 39 | 296 ± 27 | 280 ± 24 | 318 ± 35 | 313 ± 31 | |
| CD16 | Classical monocytes | 892 ± 119 | 233 ± 43** | 201 ± 43** | 116 ± 24** | 847 ± 70 | 863 ± 94 | 808 ± 90 | 839 ± 81 | 820 ± 74 | 836 ± 84 |
| Intermediate monocytes | 6608 ± 1256 | 9159 ± 1598 | 7841 ± 1596 | 7348 ± 1530 | 8364 ± 2273 | 7556 ± 1902 | 7353 ± 1593 | 8186 ± 2300 | 8157 ± 1994 | 7425 ± 1697 | |
| Nonclassical monocytes | 34 563 ± 1021 | 29 593 ± 1772 | 29 186 ± 1904 | 22 271 ± 2376** | 31 979 ± 1680 | 34 913 ± 2671 | 31 873 ± 1637 | 33 687 ± 1982 | 31 530 ± 2027 | 33 258 ± 1785 | |
| CD86 | Classical monocytes | 553 ± 51 | 582 ± 36 | 809 ± 49** | 1291 ± 63** | 551 ± 56 | 552 ± 52 | 540 ± 53 | 551 ± 53 | 535 ± 53 | 541 ± 60 |
| Intermediate monocytes | 1100 ± 128 | 1564 ± 117 | 1881 ± 192** | 2207 ± 154** | 1202 ± 186 | 1202 ± 156 | 1200 ± 143 | 1222 ± 146 | 1262 ± 154 | 1202 ± 154 | |
| Nonclassical monocytes | 1419 ± 148 | 1551 ± 122 | 2047 ± 241 | 2204 ± 249* | 1376 ± 138 | 1421 ± 132 | 1397 ± 131 | 1388 ± 146 | 1355 ± 122 | 1386 ± 131 | |
Indicated are mean fluorescence intensity [MFI] ± SEM; n = 7
*P < 0.05, **P < 0.01
FIGURE 1:
Representative example of monocyte subset distribution after stimulation with iron sucrose (IS), ferric carboxymaltose (FCM) or iron isomaltoside 1000 (ISM) (0.133, 0.266 and 0.533 mg/mL). Blood was collected from control subjects. Classical monocytes are shown in the upper left gate, intermediate monocytes in the upper right gate and nonclassical monocytes in the lower right gate of each dot plot. Statistical analysis was performed for each iron preparation using ANOVA, followed by Dunnett's multiple comparison test as post hoc test; data of seven independent experiments are presented as mean ± SEM.
Effect of iron preparations on monocytic chemokine receptor expression, inflammation response and functional characteristics
We analysed the effect of the three different iron preparations iron sucrose, ferric carboxymaltose and iron isomaltoside 1000 on subset-specific expression of chemokine receptors CCR5 and CX3CR1, on production of pro-inflammatory cytokines (TNF-α and IL-1β) and on ROS production. CX3CR1 expression was specifically and dose-dependently reduced by iron sucrose, while CCR5 expression was not. Moreover, iron sucrose tended to increase IL-1β and ROS production, but not TNF-α production. Ferric carboxymaltose and iron isomaltoside 1000 affected neither cytokine production, chemokine receptor expression nor ROS production significantly (Table 2).
Table 2.
Expression of CCR5 and CX3CR1 as well as production of TNF-α, IL-1β and ROS in circulating mature classical, intermediate and nonclassical monocytes from control subjects
| Monocyte subset | Control | Iron sucrose |
Ferric carboxymaltose |
Iron isomaltoside 1000 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | |||
| CCR5 | Classical monocytes | 76 ± 10 | 115 ± 16 | 102 ± 18 | 82 ± 25 | 73 ± 10 | 77 ± 9 | 76 ± 9 | 77 ± 10 | 76 ± 9 | 75 ± 8 |
| Intermediate monocytes | 297 ± 86 | 369 ± 121 | 340 ± 118 | 277 ± 126 | 303 ± 100 | 284 ± 82 | 285 ± 96 | 300 ± 91 | 311 ± 93 | 290 ± 91 | |
| Nonclassical monocytes | 99 ± 19 | 115 ± 24 | 99 ± 23 | 63 ± 28 | 94 ± 20 | 96 ± 12 | 88 ± 13 | 95 ± 19 | 80 ± 11 | 95 ± 16 | |
| CX3CR1 | Classical monocytes | 1138 ± 73 | 845 ± 57** | 660 ± 50** | 496 ± 35** | 1156 ± 87 | 1158 ± 75 | 1164 ± 80 | 1190 ± 75 | 1242 ± 73 | 1202 ± 86 |
| Intermediate monocytes | 2180 ± 209 | 1755 ± 198 | 1505 ± 189* | 1130 ± 120** | 2221 ± 206 | 2160 ± 229 | 2180 ± 196 | 2310 ± 254 | 2341 ± 275 | 2708 ± 467 | |
| Nonclassical monocytes | 4860 ± 231 | 4141 ± 293 | 3368 ± 303** | 2697 ± 223** | 4922 ± 266 | 4863 ± 252 | 4815 ± 204 | 4904 ± 189 | 4946 ± 182 | 4827 ± 348 | |
| TNF-α | Classical monocytes | 168 ± 30 | 140 ± 20 | 123 ± 14 | 119 ± 14 | 185 ± 32 | 191 ± 36 | 191 ± 30 | 183 ± 40 | 182 ± 36 | 182 ± 32 |
| Intermediate monocytes | 183 ± 29 | 216 ± 44 | 181 ± 26 | 177 ± 27 | 207 ± 34 | 228 ± 47 | 205 ± 35 | 220 ± 36 | 189 ± 39 | 187 ± 20 | |
| Nonclassical monocytes | 125 ± 18 | 162 ± 19 | 141 ± 16 | 131 ± 15 | 148 ± 21 | 148 ± 23 | 144 ± 20 | 155 ± 23 | 142 ± 23 | 145 ± 17 | |
| IL-1β | Classical monocytes | 73 ± 19 | 133 ± 38 | 128 ± 35 | 148 ± 40 | 85 ± 12 | 84 ± 12 | 82 ± 15 | 95 ± 19 | 93 ± 23 | 92 ± 31 |
| Intermediate monocytes | 122 ± 26 | 173 ± 46 | 168 ± 28 | 169 ± 28 | 137 ± 28 | 160 ± 32 | 137 ± 32 | 162 ± 39 | 136 ± 30 | 123 ± 24 | |
| Nonclassical monocytes | 90 ± 15 | 112 ± 23 | 89 ± 12 | 89 ± 14 | 108 ± 14 | 104 ± 11 | 105 ± 14 | 110 ± 15 | 104 ± 15 | 103 ± 17 | |
| ROS | Classical monocytes | 2899 ± 684 | 3403 ± 767 | 3503 ± 781 | 3449 ± 769 | 3043 ± 691 | 3260 ± 706 | 3252 ± 718 | 3201 ± 822 | 3403 ± 873 | 3613 ± 937 |
| Intermediate monocytes | 3760 ± 898 | 4758 ± 1266 | 4400 ± 893 | 4687 ± 989 | 3973 ± 987 | 4173 ± 948 | 4264 ± 1024 | 4184 ± 1158 | 4351 ± 1141 | 4768 ± 1321 | |
| Nonclassical monocytes | 3179 ± 806 | 3795 ± 1026 | 4011 ± 1105 | 3784 ± 1027 | 3248 ± 878 | 3503 ± 922 | 3554 ± 970 | 3531 ± 1103 | 3686 ± 1152 | 3972 ± 1233 | |
Indicated are mean fluorescence intensity [MFI] ± SEM; n = 7 for analysis of CCR5 and CX3CR1 expression, n = 4 for analysis of TNF-α and IL-1β production, n = 6 for analysis of ROS production
*P < 0.05, **P < 0.01
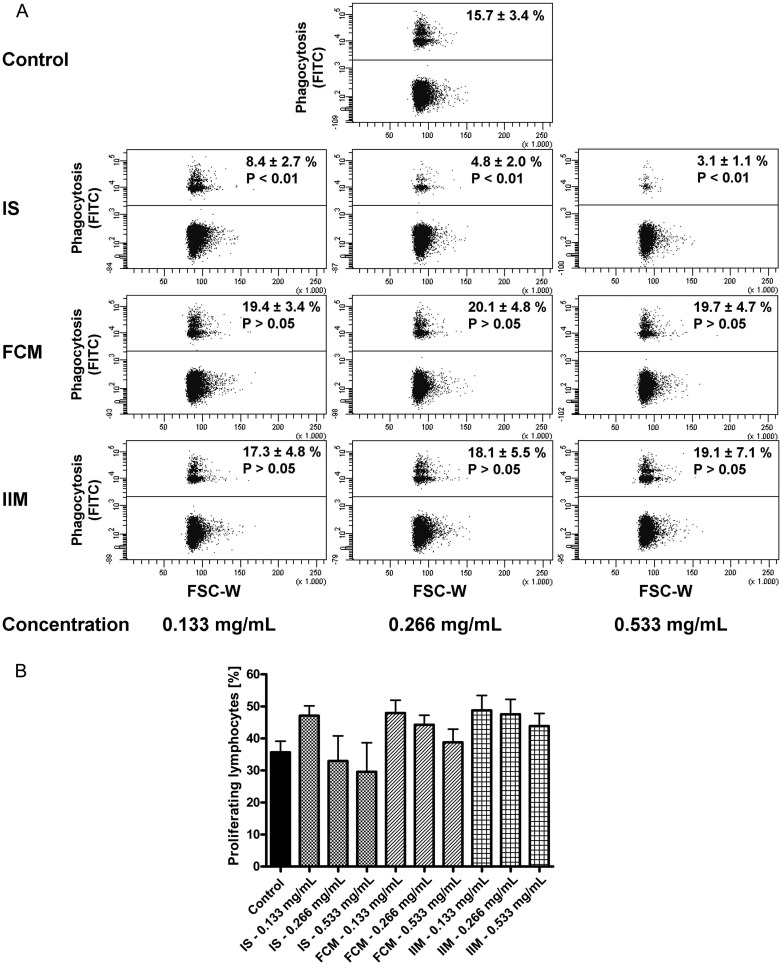
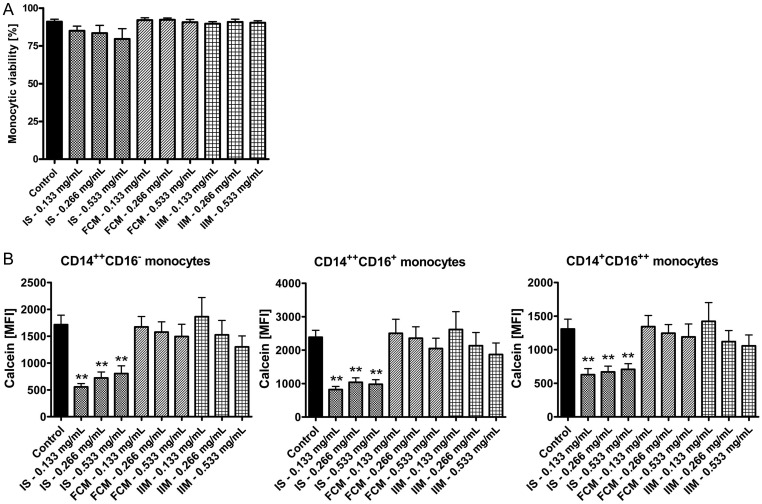
Next, we tested the effect of iron preparations on the main functional characteristics of monocytes, namely phagocytosis capacity and antigen presentation (via analysis of T-cell proliferation). In contrast to ferric carboxymaltose and iron isomaltoside 1000, iron sucrose significantly reduced the capacity of classical monocytes to phagocyte microspheres (Figure 2A). T-cell proliferation tended to be decreased after stimulation with the highest dosage of iron sucrose, but tended to be increased after stimulation with lower dosages of iron sucrose, ferric carboxymaltose and iron isomaltoside 1000 (Figure 2B). To prove biological plausibility of these results, we first excluded that the effects of iron sucrose are caused by increased apoptosis of monocytes (Figure 3A), and next showed that iron sucrose is more avidly taken up by monocytes than ferric carboxymaltose or iron isomaltoside 1000 (Figure 3B), which may account for the preparation-specific effects of iron sucrose on monocyte function.
FIGURE 2:
(A). Representative example of classical monocytes to phagocyte opsonized carboxylate microspheres (0.75 µm, Yellow Green) within 30 min after stimulation with iron sucrose (IS), ferric carboxymaltose (FCM) or iron isomaltoside 1000 (IIM) (0.133, 0.266 and 0.533 mg/mL). Blood was collected from control subjects. Counts of FITC-positive cells (upper population) were determined flow cytometrically and statistical analysis was performed for each iron preparation by using ANOVA followed by Dunnett's multiple comparison test as post hoc test; data of six independent experiments are presented as mean ± SEM. (B). Flow cytometric analysis of T-cell proliferation after stimulation with staphylococcal enterotoxin B (SEB) (2.5 µg/mL) and the different iron preparations iron sucrose (IS), ferric carboxymaltose (FCM) and iron isomaltoside 1000 (IIM) (0.133, 0.266 and 0.533 mg/mL) for 3 days. Blood was collected from control subjects. Statistical analysis was performed for each iron preparation by using ANOVA followed by Dunnett's multiple comparison test as post hoc test; data of seven independent experiments are presented as mean ± SEM.
FIGURE 3:
(A) Flow cytometric analysis of monocytic viability after stimulation with iron sucrose (IS), ferric carboxymaltose (FCM) or iron isomaltoside 1000 (IIM) (0.133, 0.266 and 0.533 mg/mL) by 7-AAD staining. Blood was collected from control subjects. Statistical analysis was performed for each iron preparation by using ANOVA and the Dunnett's multiple comparison test as post hoc test; data of five independent experiments are presented as mean ± SEM. (B). Flow cytometric calcein assay for the analysis of the iron content in classical, intermediate and nonclassical monocytes after stimulation of whole blood for 1 h with iron sucrose (IS), ferric carboxymaltose (FCM) or iron isomaltoside 1000 (IIM) (0.133, 0.266 and 0.533 mg/mL). As iron intracellularly binds calcein and quenches its fluorescence, lower fluorescence intensity represents higher intracellular iron content. Blood was collected from control subjects. Data were measured as mean fluorescence intensity (MFI). Statistical analysis was performed using ANOVA and the Dunnett's multiple comparison test as post hoc test; data of six independent experiments are presented as mean ± SEM; **P < 0.01.
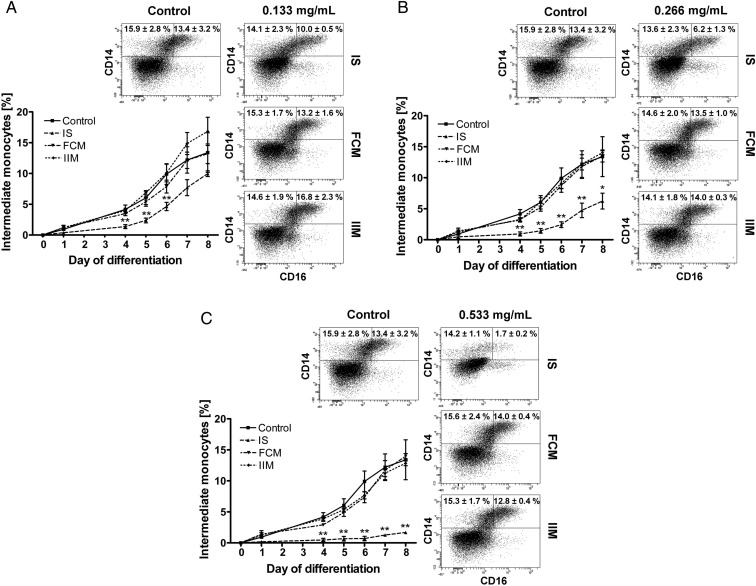
Impact of iron preparations on monocyte differentiation
In order to test whether specific iron preparations (iron sucrose, ferric carboxymaltose and iron isomaltoside 1000) differently affect development of monocytes, we analysed in vitro differentiation of isolated CD34+ haematopoietic stem cells into classical and intermediate monocytes. Iron sucrose significantly reduced in vitro differentiation into intermediate monocytes even at the lowest dose (Figure 4), which is mirrored by a strongly decreased CD14 and CD16 expression on these cells (Table 3). Simultaneously it significantly increased CD86 expression on in vitro differentiated monocytes, which is in line with its effect on circulating mature monocytes. Although a significant reduction of CD14 on classical monocytes was observed after stimulation with ferric carboxymaltose and iron isomaltoside 1000, these iron preparations affected neither in vitro differentiation of monocyte subsets nor their expression of CD16 and CD86.
FIGURE 4:
In vitro differentiation of haematopoietic CD34+ stem cells into classical and intermediate monocytes after stimulation with iron sucrose (IS), ferric carboxymaltose (FCM) or iron isomaltoside 1000 (IIM) [0.133 mg/mL (A), 0.266 mg/mL (B) and 0.533 mg/mL (C)]. Iron preparations were added at Day 0. Blood was collected from control subjects. Statistical analysis was performed using ANOVA and the Dunnett's multiple comparison test as post hoc test; data of three independent experiments are presented as mean ± SEM; *P < 0.05, **P < 0.01.
Table 3.
Expression of CD14, CD16 and CD86 on classical and intermediate monocytes after differentiation from haematopoietic stem cells from control subjects
| Monocyte subset | Control | Iron sucrose |
Ferric carboxymaltose |
Iron isomaltoside 1000 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | |||
| CD14 | Classical monocytes | 10 546 ± 394 | 6835 ± 702* | 7027 ± 536 | 6088 ± 1474* | 7329 ± 218** | 7050 ± 264** | 7842 ± 726** | 8352 ± 469 | 7376 ± 430** | 8528 ± 830 |
| Intermediate monocytes | 25 929 ± 2713 | 19 929 ± 820 | 18 009 ± 348* | 17 709 ± 1948* | 19 417 ± 1022 | 19 272 ± 801 | 20 952 ± 1991 | 21 481 ± 1579 | 19 247 ± 1429 | 20 862 ± 2394 | |
| CD16 | Classical monocytes | 1147 ± 33 | 867 ± 32** | 769 ± 60** | 630 ± 35** | 1335 ± 88 | 1342 ± 121 | 1316 ± 81 | 1232 ± 140 | 1272 ± 82 | 1177 ± 104 |
| Intermediate monocytes | 5549 ± 638 | 6462 ± 103 | 5654 ± 445 | 4806 ± 475 | 5125 ± 355 | 5173 ± 225 | 5227 ± 86 | 7327 ± 1545 | 5801 ± 83 | 5364 ± 164 | |
| CD86 | Classical monocytes | 838 ± 106 | 945 ± 15 | 1259 ± 110* | 1659 ± 91** | 940 ± 118 | 964 ± 102 | 894 ± 60 | 707 ± 57 | 779 ± 54 | 762 ± 19 |
| Intermediate monocytes | 3178 ± 550 | 3074 ± 462 | 3370 ± 380 | 3654 ± 816 | 3819 ± 563 | 3814 ± 555 | 3233 ± 285 | 2751 ± 42 | 3011 ± 199 | 2834 ± 170 | |
Indicated are mean fluorescence intensity [MFI] ± SEM; n = 3
*P < 0.05, **P < 0.01
Implication of iron preparations on characteristics of in vitro-differentiated monocytes
We then determined the expression of CCR5 and CX3CR1, as well as ROS production, by in vitro differentiated monocyte subsets (Table 4). CX3CR1 expression was increased after treatment with iron sucrose and ferric carboxymaltose, whereas CCR5 expression and ROS production were not significantly altered.
Table 4.
Expression of CCR5 and CX3CR1 and ROS production by in vitro differentiated classical and intermediate monocytes from control subjects
| Monocyte subset | Control | Iron sucrose |
Ferric carboxymaltose |
Iron isomaltoside 1000 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | 0.133 mg/mL | 0.266 mg/mL | 0.533 mg/mL | |||
| CCR5 | Classical monocytes | 643 ± 154 | 433 ± 62 | 418 ± 44 | 337 ± 5 | 599 ± 112 | 547 ± 107 | 551 ± 110 | 508 ± 115 | 519 ± 97 | 524 ± 116 |
| Intermediate monocytes | 1365 ± 195 | 1274 ± 103 | 1135 ± 133 | 862 ± 15 | 1326 ± 142 | 1291 ± 139 | 1285 ± 190 | 1246 ± 120 | 1247 ± 142 | 1278 ± 219 | |
| CX3CR1 | Classical monocytes | 3982 ± 370 | 5936 ± 386 | 6057 ± 678 | 3308 ± 510 | 4980 ± 117 | 5481 ± 164* | 5415 ± 326* | 5304 ± 1085 | 5029 ± 588 | 4731 ± 463 |
| Intermediate monocytes | 5602 ± 420 | 10 118 ± 491* | 10 906 ± 957** | 7885 ± 1148 | 6961 ± 183* | 7612 ± 182** | 7506 ± 395** | 7869 ± 1943 | 7064 ± 718 | 6570 ± 483 | |
| ROS | Classical monocytes | 2714 ± 919 | 7471 ± 2622 | 8710 ± 3032 | 10 715 ± 4186 | 5104 ± 1888 | 5550 ± 2158 | 5831 ± 2147 | 3697 ± 1160 | 4103 ± 1384 | 4959 ± 1724 |
| Intermediate monocytes | 3628 ± 1141 | 10 642 ± 3877 | 12 392 ± 4802 | 11 998 ± 5096 | 7231 ± 2547 | 7635 ± 2820 | 8783 ± 3102 | 5347 ± 1580 | 5910 ± 1886 | 7044 ± 2453 | |
Indicated are mean fluorescence intensity [MFI] ± SEM; n = 3
*P < 0.05, **P < 0.01
Finally, in vitro differentiated classical and intermediate monocytes displayed a dose-dependent reduction in their phagocytosis capacity after iron sucrose treatment, even though the level of significance was not reached (data not shown).
Impact of iron preparations on human monocyte subsets from CKD patients
We next analysed (i) whether these observed effects of iron sucrose can be transferred from control subjects without overt CKD to patients with severe CKD and (ii) whether two other iron preparations, namely low-molecular-weight iron dextran and ferumoxytol, induce monocyte activation similar to iron sucrose.
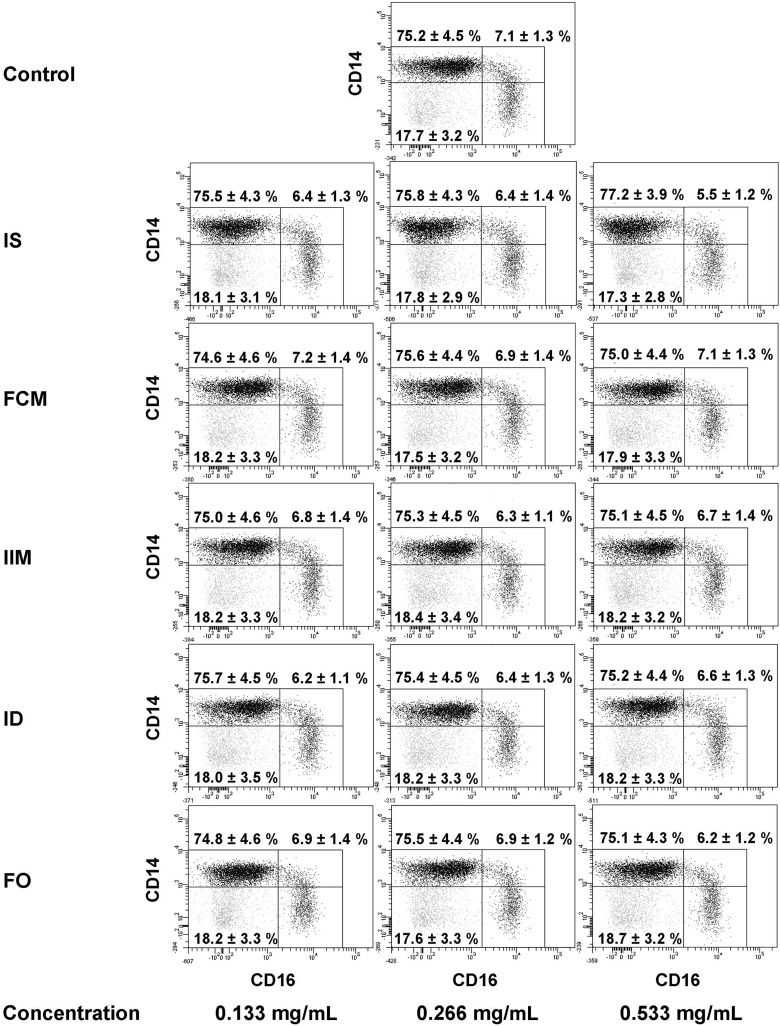
We first found iron sucrose to dose-dependently increase CD86 surface expression on classical and intermediate monocytes and to significantly reduce CD16 expression on classical monocytes, whereas monocyte subset distribution and CD14 expression were not changed (Figure 5, Supplementary Table S1). Ferric carboxymaltose, iron isomaltoside 1000, low-molecular-weight iron dextran and ferumoxytol affected neither monocyte subset distribution nor expression of CD14, CD16 or CD86 on monocyte subsets (Supplementary Table S1).
FIGURE 5:
Representative example of monocyte subset distribution after stimulation with iron sucrose (IS), ferric carboxymaltose (FCM), iron isomaltoside 1000 (ISM), low-molecular-weight iron dextran (ID) and ferumoxytol (FO) (0.133, 0.266 and 0.533 mg/mL). Blood was collected from patients with severe CKD. Classical monocytes are shown in the upper left gate, intermediate monocytes in the upper right gate and nonclassical monocytes in the lower right gate of each dot plot. Statistical analysis was performed for each iron preparation using ANOVA, followed by Dunnett's multiple comparison test as post hoc test; data of eight independent experiments are presented as mean ± SEM.
Effect of iron preparations on monocytic chemokine receptor expression and functional characteristics on monocytes from CKD patients
CX3CR1 expression was dose-dependently reduced by iron sucrose, while CCR5 expression and ROS production were not significantly altered. Ferric carboxymaltose, iron isomaltoside 1000, low-molecular-weight iron dextran and ferumoxytol affected neither chemokine receptor expression nor ROS production (Supplementary Table S1).
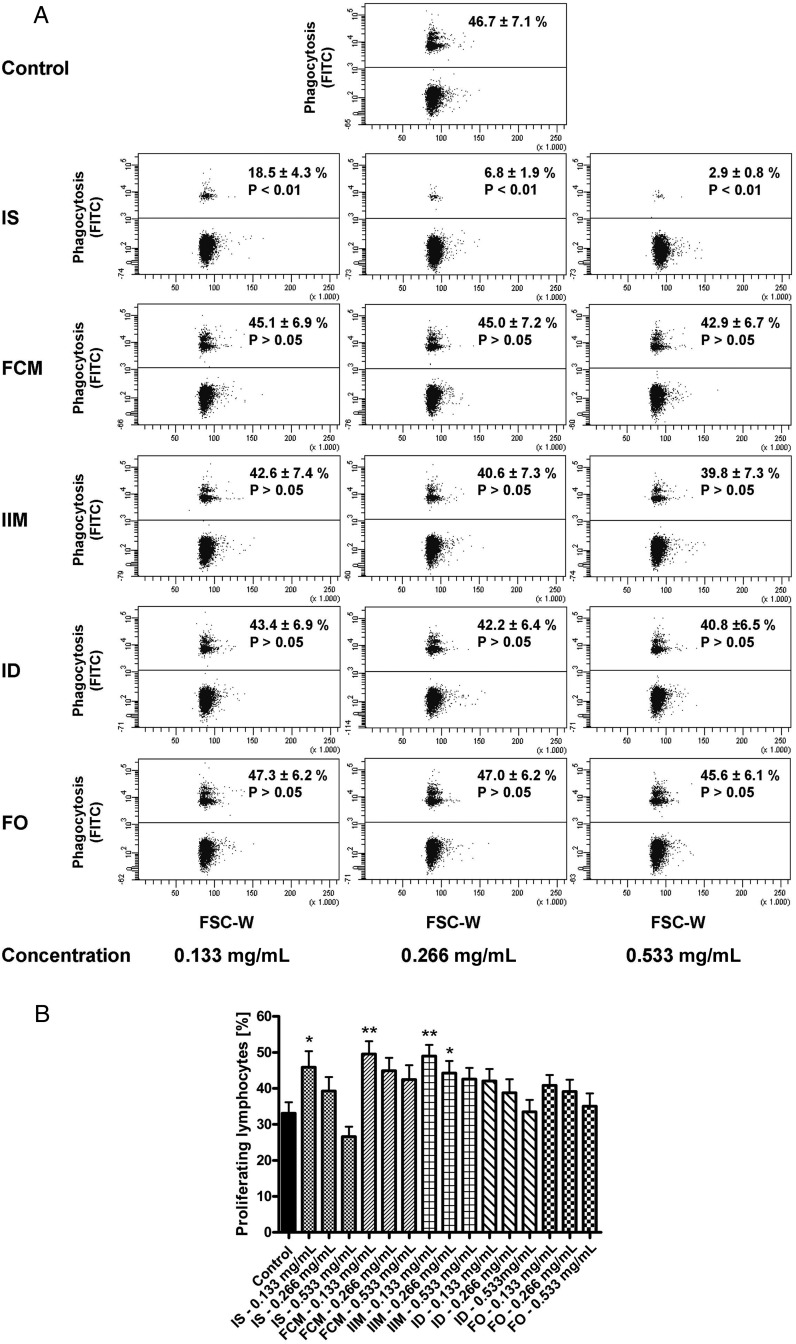
Next, we found a significant reduction of the phagocytosis capacity of classical monocytes after stimulation with iron sucrose (Figure 6A). Moreover, T-cell proliferation was heterogeneously affected by different iron preparations in different dosages (Figure 6B). Finally, compared with the other four iron preparations, iron sucrose is more avidly taken up by monocytes as found in the calcein assay (Supplementary Figure S1).
FIGURE 6:
(A) Representative example of classical monocytes to phagocyte opsonized carboxylate microspheres (0.75 µm, Yellow Green) within 30 min after stimulation with iron sucrose (IS), ferric carboxymaltose (FCM), iron isomaltoside 1000 (IIM), low-molecular-weight iron dextran (ID) and ferumoxytol (FO) (0.133, 0.266 and 0.533 mg/mL). Blood was collected from patients with severe CKD. Counts of FITC-positive cells (upper population) were determined flow cytometrically and statistical analysis was performed for each iron preparation by using ANOVA followed by Dunnett's multiple comparison test as post hoc test; data of eight independent experiments are presented as mean ± SEM. (B). Flow cytometric analysis of T-cell proliferation after stimulation with staphylococcal enterotoxin B (SEB) (2.5 µg/mL) and the different iron preparations iron sucrose (IS), ferric carboxymaltose (FCM) and iron isomaltoside 1000 (IIM), low-molecular-weight iron dextran (ID) and ferumoxytol (FO) (0.133, 0.266 and 0.533 mg/mL) for 3 days. Blood was collected from patients with severe CKD. Statistical analysis was performed for each iron preparation by using ANOVA followed by Dunnett's multiple comparison test as post hoc test; data of eight independent experiments are presented as mean ± SEM; *P < 0.05, **P < 0.01.
In summary, effects of iron sucrose on monocytes from patients with severe CKD were in line with effects observed in monocytes from subjects without overt CKD. For technical reasons we did not analyse in vitro differentiation of monocytes from haematopoietic CD34+ stem cells and monocytic viability.
DISCUSSION
Use of i.v. iron preparations for anaemia treatment has attracted substantial interest in recent years. Despite an inverse association between haemoglobin levels and cardiovascular event rate [25, 26] and mortality [27] in observatory studies, large intervention trials that used erythropoietin or other erythropoietin-stimulating agents (ESA) for normalizing haemoglobin levels in CKD patients failed to reduce cardiovascular events and death. Therefore, recent KDIGO consensus guidelines advocate the use of iron supplements with the aim both to increase haemoglobin and to reduce ESA doses [1]. Moreover KDIGO guidelines acknowledge the need for i.v. iron supplements in dialysis patients, in whom gastrointestinal absorption of oral iron supplements is generally poor [1].
The increasing use of i.v. iron preparations necessitates a critical analysis of their side effects [28, 29], which are mainly attributed to the abruptly increasing amount of NTBI (‘free iron’) after i.v. iron infusion.
It has long been acknowledged that i.v. iron preparations impair leukocyte immune function and trigger bacterial infections [17]. Interestingly, previous experimental work focussed on the effect of i.v. iron preparations on neutrophil biology, whereas their impact on monocytes received little interest in the past.
Against this background, we now analysed the effect of the three different i.v. iron preparations, iron sucrose, ferric carboxymaltose and iron isomaltoside 1000, in both therapeutically recommended and supratherapeutic dosages on monocytes from control subjects in vitro. To verify the relevance of these effects in clinical nephrology, we further tested the immunological in vitro effects of these three preparations on monocytes collected from patients with severe CKD; this additionally allowed us to test two further preparations, namely low-molecular-weight iron dextran and ferumoxytol. Iron sucrose induced significant changes in monocytic immune function, which occurred even at lower, therapeutically recommended dosages, whereas ferric carboxymaltose, iron isomaltoside 1000, low-molecular-weight iron dextran and ferumoxytol had no relevant effects at any dosage. We first found that iron sucrose changed expression of CD16, without affecting subset distribution of mature circulating monocytes. In differentiation experiments, the reduced CD16 expression after i.v. iron sucrose stimulation occurred in conjunction with a dose-dependent inhibition of stem cell differentiation into classical and intermediate monocytes.
We next assessed the effect of iron stimulation on specific characteristics of intermediate monocytes, namely cytokine and ROS production and chemokine receptor expression. We hereby firstly confirmed that intermediate monocytes have the highest capacity to produce pro-inflammatory cytokines [30] and ROS [22]. Next, we found that iron sucrose tended to increase IL-1β and ROS production. Interestingly, Martin-Malo et al. reported that iron sucrose and ferric carboxymaltose increased percentages of ROS-producing cells and apoptotic cells both in vitro—in PBMCs from healthy donors and patients CKD stage 5—and in vivo during haemodialysis sessions [31].
While intermediate monocytes are prone to production of pro-inflammatory cytokines and ROS, and to antigen presentation, classical monocytes have the highest phagocytosis capacity [32], which is verified in the present analysis. Confirming our hypothesis of an impaired monocytic function after iron sucrose stimulation, we found a dose-dependent reduction of phagocytosis even at the lowest dose. This effect is in line with previous studies testing the effect of iron sucrose on phagocytosis capacity of polymorphonuclear leukocytes [17, 33].
Taken together, only iron sucrose impaired monocytic functions in vitro. This may result from pharmacokinetic differences between i.v. iron preparations, which differ in their carbohydrate ligands, consequently their structural build-up, distinct molecular weights (iron sucrose: 140 100 Da, ferric carboxymaltose: 233 100 Da, iron isomaltoside 1000: 150 000 Da, low-molecular-weight iron dextran: 165 000 Da, ferumoxytol: 275 700 Da) [10] and half-lives (iron sucrose: 5.3 h; ferric carboxymaltose: 7.4–9.4 h, iron isomaltoside 1000: 23.2 h, low-molecular-weight iron dextran: 27–30 h, ferumoxytol: 14.7 h) [34, 35]. Among those iron preparations, iron sucrose has the lowest molecular weight, the shortest half-life and the lowest stability, which in conjunction determine the amount and the kinetic of free iron release. Moreover, in relation to the size of the iron particles, iron sucrose has the highest content of labile iron when compared with the other iron preparations [10, 36]. It is proposed that stable i.v. iron preparations, like ferric carboxymaltose, iron isomaltoside 1000, low-molecular-weight iron dextran and ferumoxytol, release few NTBI and are mainly taken up as complex by phagocytosis [37]. In contrast, less stable iron preparations, such as iron sucrose, release higher levels of NTBI. Even though most of this free iron is taken up by transferrin and other proteins, some amount of NTBI may directly be taken up in an unregulated way by different cell types or freely translocate across cell membranes [34, 38, 39]. Intracellularly, ‘free iron’ constitutes the labile iron pool, consisting of iron that is not linked to its storage protein ferritin [13, 38]. This labile iron pool may induce distinct toxic effects such as ROS production and subsequently DNA damage and lipid peroxidation [40, 41].
In our work, we were able to demonstrate a more rapid uptake of iron sucrose compared with ferric carboxymaltose, iron isomaltoside 1000, low-molecular-weight iron dextran and ferumoxytol in all three monocyte subsets, using the calcein assay. These results are in line with the findings of Sonnweber et al. [19], who had shown that i.v. injected iron sucrose is taken up by monocytes and increases circulating ferritin levels resulting in an impaired monocytic immune function, cytokine expression and activation of the NF-κB pathway.
Our study has several limitations. In most experiments, we incubated whole blood or blood cells only for 5 h for technical reasons: previous experiments have shown that monocyte subsets change their surface marker expression after a longer incubation period in vitro. This particularly affects surface expression of CD16, which renders flow cytometric distinction of monocyte subsets impossible. This short incubation time might have biased our study results, as monocyte effects of ferric carboxymaltose, iron isomaltoside 1000, low-molecular-weight iron dextran and ferumoxytol may become evident only after longer incubation. Nonetheless, even those experiments that lasted several days, e.g. in vitro differentiation of haematopoietic stem cells into monocytes, pointed towards a particular effect of iron sucrose on monocyte biology.
As a further limitation, we cannot formally prove the biological relevance of our study results. We deliberately chose to focus our study on in vitro effects of iron on mature circulating monocytes and haematopoietic stem cells. In vivo monocytes might be exposed to lower iron concentrations than in our in vitro analysis, after iron complexes may rapidly be taken up in vivo by cells of the reticuloendothelial system via endocytosis [37].
Nonetheless, given that monocytes are the central regulators of the innate immune systems, and that CKD by itself negatively affects monocytic functions, we are confident that repetitive infusion of iron sucrose for treatment of anaemia in CKD may be considered as potentially immunoactivating. To further unravel the biological impact of different iron preparations, a randomized controlled trial with clinical end-points comparing iron sucrose and other i.v. iron preparations would be informative. Nonetheless, until such a trial will be initiated, in vitro data will be necessary in order to better appreciate the risk of distinct iron preparations.
Taken together, when comparing the i.v. iron preparations iron sucrose, ferric carboxymaltose, iron isomaltoside 1000, low-molecular-weight iron dextran and ferumoxytol, we found strong and selective immunologic effects of iron sucrose on monocyte subsets even at pharmacological dosages. These findings underscore the notion that each specific i.v. iron preparations may exert particular biological functions and may confer specific side effects.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
Study design, laboratory work and data interpretation were performed only by the authors. In addition, the manuscript was solely written by the authors. They declare that the results presented in this paper have not been published previously in whole or part, except in abstract format.
Supplementary Material
ACKNOWLEDGEMENTS
The organizational skills of Marie-Theres Blinn and Martina Wagner are deeply appreciated. The study was supported by a grant from Pharmacosmos (Holbaek, Denmark).
REFERENCES
- 1.Group KDIGOKAW. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 2.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. doi:10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 3.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. doi:10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. doi:10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 5.Joshi AD, Holdford DA, Brophy DF, et al. Utilization patterns of IV iron and erythropoiesis stimulating agents in anemic chronic kidney disease patients: a multihospital study. Anemia. 2012;2012:248430. doi: 10.1155/2012/248430. doi:10.1155/2012/248430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Regidor DL, McAllister CJ, et al. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3070–3080. doi: 10.1681/ASN.2005040423. doi:10.1681/ASN.2005040423. [DOI] [PubMed] [Google Scholar]
- 7.Theurl I, Theurl M, Seifert M, et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111:2392–2399. doi: 10.1182/blood-2007-05-090019. doi:10.1182/blood-2007-05-090019. [DOI] [PubMed] [Google Scholar]
- 8.Alleyne M, Horne MK, Miller JL. Individualized treatment for iron-deficiency anemia in adults. Am J Med. 2008;121:943–948. doi: 10.1016/j.amjmed.2008.07.012. doi:10.1016/j.amjmed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne DW, Kapoian T, Suki W, et al. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients’ Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol. 2007;18:975–984. doi: 10.1681/ASN.2006091034. doi:10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- 10.Jahn MR, Andreasen HB, Futterer S, et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm. 2011;78:480–491. doi: 10.1016/j.ejpb.2011.03.016. doi:10.1016/j.ejpb.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Wang SX. Intravenous iron sucrose in peritoneal dialysis patients with renal anemia. Perit Dial Int. 2008;28:149–154. [PubMed] [Google Scholar]
- 12.Kooistra MP, Kersting S, Gosriwatana I, et al. Nontransferrin-bound iron in the plasma of haemodialysis patients after intravenous iron saccharate infusion. Eur J Clin Invest. 2002;32(Suppl 1):36–41. doi: 10.1046/j.1365-2362.2002.0320s1036.x. doi:10.1046/j.1365-2362.2002.0320s1036.x. [DOI] [PubMed] [Google Scholar]
- 13.Brissot P, Ropert M, Le Lan C, et al. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta. 2012;1820:403–410. doi: 10.1016/j.bbagen.2011.07.014. doi:10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Pai AB, Conner T, McQuade CR, et al. Non-transferrin bound iron, cytokine activation and intracellular reactive oxygen species generation in hemodialysis patients receiving intravenous iron dextran or iron sucrose. Biometals. 2011;24:603–613. doi: 10.1007/s10534-011-9409-6. doi:10.1007/s10534-011-9409-6. [DOI] [PubMed] [Google Scholar]
- 15.Collard K, White D, Copplestone A. The effect of maximum storage on iron status, oxidative stress and antioxidant protection in paediatric packed cell units. Blood Transfus. 2013;11:419–425. doi: 10.2450/2012.0046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens. 2004;13:93–99. doi: 10.1097/00041552-200401000-00013. doi:10.1097/00041552-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Ichii H, Masuda Y, Hassanzadeh T, et al. Iron sucrose impairs phagocytic function and promotes apoptosis in polymorphonuclear leukocytes. Am J Nephrol. 2012;36:50–57. doi: 10.1159/000339285. doi:10.1159/000339285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patruta SI, Edlinger R, Sunder-Plassmann G, et al. Neutrophil impairment associated with iron therapy in hemodialysis patients with functional iron deficiency. J Am Soc Nephrol. 1998;9:655–663. doi: 10.1681/ASN.V94655. [DOI] [PubMed] [Google Scholar]
- 19.Sonnweber T, Theurl I, Seifert M, et al. Impact of iron treatment on immune effector function and cellular iron status of circulating monocytes in dialysis patients. Nephrol Dial Transplant. 2011;26:977–987. doi: 10.1093/ndt/gfq483. doi:10.1093/ndt/gfq483. [DOI] [PubMed] [Google Scholar]
- 20.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790:682–693. doi: 10.1016/j.bbagen.2008.08.006. doi:10.1016/j.bbagen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. doi:10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 22.Zawada AM, Rogacev KS, Rotter B, et al. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–e61. doi: 10.1182/blood-2011-01-326827. doi:10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 23.Rogacev KS, Seiler S, Zawada AM, et al. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. doi:10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 24.Bailie GR, Larkina M, Goodkin DA, et al. Variation in intravenous iron use internationally and over time: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2013;28:2570–2579. doi: 10.1093/ndt/gft062. doi:10.1093/ndt/gft062. [DOI] [PubMed] [Google Scholar]
- 25.Foley RN, Parfrey PS, Harnett JD, et al. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28:53–61. doi: 10.1016/s0272-6386(96)90130-4. doi:10.1016/S0272-6386(96)90130-4. [DOI] [PubMed] [Google Scholar]
- 26.Harnett JD, Foley RN, Kent GM, et al. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47:884–890. doi: 10.1038/ki.1995.132. doi:10.1038/ki.1995.132. [DOI] [PubMed] [Google Scholar]
- 27.Regidor DL, Kopple JD, Kovesdy CP, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191. doi: 10.1681/ASN.2005090997. doi:10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 28.Zager RA, Johnson AC, Hanson SY, et al. Parenteral iron formulations: a comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis. 2002;40:90–103. doi: 10.1053/ajkd.2002.33917. doi:10.1053/ajkd.2002.33917. [DOI] [PubMed] [Google Scholar]
- 29.Macdougall IC, Geisser P. Use of intravenous iron supplementation in chronic kidney disease: an update. Iran J Kidney Dis. 2013;7:9–22. [PubMed] [Google Scholar]
- 30.Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Malo A, Merino A, Carracedo J, et al. Effects of intravenous iron on mononuclear cells during the haemodialysis session. Nephrol Dial Transplant. 2012;27:2465–2471. doi: 10.1093/ndt/gfr711. doi:10.1093/ndt/gfr711. [DOI] [PubMed] [Google Scholar]
- 32.Zawada AM, Rogacev KS, Schirmer SH, et al. Monocyte heterogeneity in human cardiovascular disease. Immunobiology. 2012;217:1273–1284. doi: 10.1016/j.imbio.2012.07.001. doi:10.1016/j.imbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Deicher R, Ziai F, Cohen G, et al. High-dose parenteral iron sucrose depresses neutrophil intracellular killing capacity. Kidney Int. 2003;64:728–736. doi: 10.1046/j.1523-1755.2003.00125.x. doi:10.1046/j.1523-1755.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 34.Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3:12–33. doi: 10.3390/pharmaceutics3010012. doi:10.3390/pharmaceutics3010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordfjeld K, Andreasen H, Thomsen LL. Pharmacokinetics of iron isomaltoside 1000 in patients with inflammatory bowel disease. Drug Des Devel Ther. 2012;6:43–51. doi: 10.2147/DDDT.S30015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Futterer S, Andrusenko I, Kolb U, et al. Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD) J Pharm Biomed Anal. 2013;86:151–160. doi: 10.1016/j.jpba.2013.08.005. doi:10.1016/j.jpba.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Danielson BG. Structure, chemistry, and pharmacokinetics of intravenous iron agents. J Am Soc Nephrol. 2004;15(Suppl 2):S93–S98. doi: 10.1097/01.ASN.0000143814.49713.C5. [DOI] [PubMed] [Google Scholar]
- 38.Cabantchik ZI, Breuer W, Zanninelli G, et al. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–287. doi: 10.1016/j.beha.2004.10.003. doi:10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Pootrakul P, Breuer W, Sametband M, et al. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded beta-thalassemia/HbE patients treated with an oral chelator. Blood. 2004;104:1504–1510. doi: 10.1182/blood-2004-02-0630. doi:10.1182/blood-2004-02-0630. [DOI] [PubMed] [Google Scholar]
- 40.Evans RW, Rafique R, Zarea A, et al. Nature of non-transferrin-bound iron: studies on iron citrate complexes and thalassemic sera. J Biol Inorg Chem. 2008;13:57–74. doi: 10.1007/s00775-007-0297-8. doi:10.1007/s00775-007-0297-8. [DOI] [PubMed] [Google Scholar]
- 41.Chrichton RR, Danielson BG, Geisser P. Iron Therapy with Special Emphasis on Intravenous Administration. Bremen, Germany: UNI-MED Science; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.