Abstract
Background
Trans-fatty acid (TFA) consumption is associated with risk of coronary heart disease, and trans-18:2, but not trans-18:1, in red blood cells membranes has been associated with sudden cardiac arrest. Abnormal heart rate variability (HRV) reflects autonomic dysfunction and predicts cardiac death. Relationships between TFA consumption and HRV remain under-studied. We determined whether total TFA consumption, as well as trans-18:1 and trans-18:2 TFA consumption, were independently associated with HRV in two independent cohorts in the US and Portugal.
Methods and Results
In two independent cohorts of older US adults (Cardiovascular Health Study ([CHS], age=72±5yrs, 1989/1995) and young Portuguese adults (Porto, age=19±2yrs, 2008/2010), we assessed habitual TFA intake by food frequency questionnaires in CHS (separately estimating trans-18:1 and trans-18:2) and multiple 24-hour recalls in Porto (estimating total TFA only, which in a subset correlated with circulating trans-18:2, but not trans-18:1, suggesting that we captured the former). HRV was assessed using 24-hour Holters in CHS (N=1,076) and repeated short-term (5-min) ECGs in Porto (N=160). We used multivariate-adjusted linear regression to relate TFA consumption to HRV cross-sectionally (CHS, Porto) and longitudinally (CHS). In CHS, higher trans-18:2 consumption was associated with lower 24-hour standard-deviation-of-all-normal-to-normal-intervals (SDNN) both cross-sectionally (−12%, 95%CI=6–19%, p=0.001) and longitudinally (−15%, 95%CI=4–25 %, p= 0.009), and lower 24-hour SDANN and SDNN-index (p<0.05 each). Higher trans-18:1 consumption in CHS was associated with more favorable 24-hour HRV, in particular time-domain indices (SDNN, SDANN, SDNN-index; p<0.05 each). In Porto, each higher SD TFA consumption was associated with 4% lower 5-min SDNN (95%CI=1–8%, p=0.04), and 7% lower 5-min rMSSD (95%CI=1–13%, p=0.04).
Conclusions
Trans-18:2 consumption is associated with specific, less favorable indices of HRV in both older and young adults. Trans-18:1 consumption is associated with more favorable HRV indices in older adults. Our results support the need to investigate potential HRV related mechanisms whereby trans-18:2 may increase arrhythmic risk.
Keywords: Electrophysiology, trans-fatty acids, heart rate variability, nutrition
INTRODUCTION
Increased dietary trans-fatty acids (TFA) adversely impacts cardiovascular risk factors, including markers of lipoprotein metabolism, inflammation, and endothelial function.1–3 The magnitude of the observed associations between TFA consumption and cardiovascular disease events cannot be explained simply by changes in circulating lipids.2, 4 Moreover, several different types of TFA exist, each with potentially different dietary sources and health effects. In particular, higher plasma phospholipid and erythrocyte membrane 18:2 TFA (trans-18:2), are associated with higher risks of fatal ischemic heart disease and sudden cardiac death (SCD)5, 6 however, the latter outcome is not strongly related to blood lipid abnormalities1. Potential mechanisms for the relationship between TFA and SCD remain uncertain. Some have suggested that TFA may modulate cardiac membrane ion channel function7 or have proarrhythmic properties, affecting cardiovascular electrophysiology.8, 9 However, relationships between TFA consumption and cardiac electrophysiologic measures are not well established.
Heart rate variability (HRV) indices are established measures of cardiac electrophysiology and autonomic function. The autonomic nervous system has a central role in maintaining normal cardiac rhythm.10 For example, lower indices of the standard-deviation-of-all-normal-to-normal-R-R-intervals (SDNN) and ultra-low-frequency power (ULF) are associated with increased risk of cardiovascular events such as myocardial infarction, cardiomyopathy, valvular heart disease, congestive heart failure and mortality.11 Moreover, studies have suggested a relationship between lower HRV and coronary heart disease, atrial fibrillation and heart failure.12 Furthermore, growing evidence has established heart rate (HR) as a marker of autonomic activity13 and a higher resting HR has been associated with increased all-cause mortality, death from cardiovascular disease and SCD.13
Relationships between TFA consumption and HRV or HR could elucidate novel potential mechanisms whereby TFA may influence coronary heart disease and SCD risk. Relatively little is known about this topic. We tested the hypothesis that habitual TFA consumption would be associated with less favorable indices of HRV in two separate cohorts, a population-based cohort of older US adults in the Cardiovascular Health Study (CHS) and a cohort of young adults in Portugal (Porto). Given prior work that trans-18:2 TFA are more strongly linked to cardiac death and inflammation than trans-18:1 TFA5, 6, 14–16, we hypothesized that trans-18:2 may be more strongly related to less favorable HRV. We therefore investigated estimated dietary consumption of trans-18:1 and trans-18:2 separately in CHS, and, in Porto in which only estimated total TFA consumption was available, we determined whether total consumption was more closely linked to biomarkers of trans-18:1 or trans-18:2.
METHODS
Design and Samples
The study design and recruitment of CHS have been described.17, 18 Briefly, 5,201 ambulatory, non-institutionalized men and women ≥65 years of age were randomly selected and enrolled from Medicare lists in 4 US communities (Forsyth County, North Carolina, Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania) in 1989–90. In 1992–93, 687 additional black participants were similarly recruited, but were not included in this analysis due to lack of baseline HRV measures. The Institutional review committee at each site approved the study, and subjects provided written informed consent. A 24-hour Holter recording was obtained at baseline (1989–90, N=1,361), and again 5 years later (1994–95) in the same subjects (N=787). We excluded participants with atrial fibrillation, flutter, or pacemakers (n=36); markedly irregular rhythms (e.g., wandering atrial pacemaker or other non-sinus rhythm) (n=48);19 or missing data on dietary TFA consumption (N=97). Data on plasma phospholipid TFA were available in a subset of participants, measured 3 years after baseline in 1992–93. Because HRV data were not collected during that year (1992–93), prospective relationships between plasma phospholipid TFA and HRV were determined in the smaller subset of those who had valid HRV measures obtained 2 years later in 1994–95 (N=461 for time-domain and 424 for frequency-domain and non-linear measures).
The Porto cohort was established in 2008 to assess associations between lifestyle and HRV among healthy young adults.20 We recruited university students aged 18–21 years at baseline to participate in the longitudinal study from 2008–10. After excluding 2 individuals with known cardiovascular illness or using any medication or supplements that could influence HRV or without information on TFA consumption (n=22), 160 subjects were included in the analyses. Written informed consent was obtained from participants. The study was approved by the local ethics committee and conducted in accordance to the declaration of Helsinki.
Dietary Assessment of TFA Consumption and Plasma Phospholipid
In CHS, diet was assessed at baseline (1989–90) by a validated picture-sort food frequency questionnaire (FFQ) that asked about usual dietary habits over the prior year21, and dietary TFA consumption, including of trans-18:2 and trans-18:1 TFA separately, were estimated using the Harvard food composition database.22 In a subset (N=146), we assessed correlations between each type of dietary TFA and plasma phospholipid levels of specific TFA measured at baseline (1989–90), as previously described.22
In Porto, we assessed total TFA consumption using an annual 24-hour dietary recall performed each year over 3 years. Portion sizes of food and drink consumed were estimated using food models and photos. Food consumption was converted to nutrient values, including total TFA consumption and energy intake, by Food Processor Plus®, (ESHA Research, Oregon, USA) which uses the United States Department of Agriculture database.23 Traditional Portuguese dishes were added using Portuguese food composition databases.24 Due to limited food composition data, estimates of TFA consumption in Porto were only available for total TFA and not for different types of TFA. We therefore evaluated correlations between total TFA consumption and specific plasma phospholipid TFA in a subset (N=40) to identify which type(s) of TFA was being captured by the Porto dietary estimates.
Assessment of HRV
HRV can be evaluated by time-domain, frequency-domain and non-linear methods25 and based on either short-term (e.g., 5 to 20-minutes) or long-term (e.g., 24-hour) recordings (Table 1). Short-term measures obtained at rest do not capture circadian or sleep-related changes and reflect mainly resting parasympathetic (respiratory) variation in HR. Long-term measures reflect a complex interaction of autonomic inputs, and can also capture longer-term circadian differences in HRV as well as daytime and night-time baroreceptor and respiratory autonomic variation.
Table 1.
Measures of heart rate variability (HRV) in the Porto and Cardiovascular Health Study cohorts.*
| Variable | Brief Physiologic Correlation |
|---|---|
| Analysis of Short-term recordings (5-min) | |
| Time-domain | |
| SDNN, ms | Combined sympathetic and parasympathetic modulation of the heart rate due to respiratory sinus arrhythmia, representing cardiac vagal control. When measured in a supine position with paced breathing, 5-min SDNN mostly reflects parasympathetic activity. Higher values reflect higher parasympathetic (vagal) influence. |
| rMSSD, ms | Mainly parasympathetic modulation of the heart rate due to respiratory sinus arrhythmia, representing cardiac vagal control. Higher values reflect higher parasympathetic (vagal) influence. |
| Frequency-domain | |
| HF, ms2 | Vagal modulation of heart rate in response to respiration. Higher values reflect higher parasympathetic (vagal) influence or greater beat-to-beat variability due to erratic rhythm. |
| Analysis of long-term recordings (24-hour) | |
| Time-domain | |
| SDNN, ms | Standard deviation of all N-N intervals (from the entire recording). Reflects longer-term circadian differences and total HRV. |
| SDANN, ms | Standard deviation of 5-min average of N-N intervals. Total circadian activity. Additionally, it is related with HR and coefficient of variation, which may reflect functional capacity26,27. |
| SDNN-index, ms | Averaged 5-min SDNN. Reflects combined sympathetic and vagal activity but independent of circadian rhythm. |
| rMSSD, ms† | Root mean square of successive differences between N-N intervals. Reflects the average of daytime and night-time parasympathetically-mediate respiratory variation. Higher values reflect higher parasympathetic (vagal) influence or greater degree of erratic rhythm19. |
| Frequency-domain | |
| LF/HF | Has been proposed has an index of sympathovagal balance. However interpretation of this index is controversial. |
| LFnu, %† | Precise interpretation of this index is controversial. However there is evidence that normalized LF can be a measure of sympathetic modulation of heart rate. LF band is between 0.04 and 0.15 Hz. |
| HFnu, %† | Relative vagal modulation of heart rate in response to respiration. Higher values reflect higher parasympathetic (vagal) influence or greater degree of erractic rhythm. HF band is between 0.15 and 0.4 Hz, |
| ULF, ms2 | Fluctuations in R-R intervals with underlying cycle length of >5-min and ≤24-hour. Predominantly circadian rhythm but other influences including activity and neuroendocrine rhythms. ULF band is below 0.003Hz. Additionally, it is related with HR and coefficient of variation, which may reflect functional capacity26,27. |
| TP, ms2 | Variance of all N-N intervals. |
| VLF, ms2 | VLF may reflect both vagal control of heart rate and also the effect of the renin-angiotensin system. |
| Non-linear | |
| DFA1 | Short-term fractal scaling exponent. Reflects randomness or correlatedness of the N-N intervals pattern. Totally random R-R intervals pattern has a value of 0.5, whereas a totally correlated pattern has a value of 1.5. |
| SD12, Poincare Ratio | Organization of heart rate patterns based on the ratio of the axes of an ellipse fitted to the scatter plot of N-N vs. N-N+1 intervals. Higher values can reflect a greater degree of erratic rhythm. |
DFA1= short-term fractal scaling exponent, HF= high-frequency power, LF/HF ratio, LFnu= normalized low-frequency power, HFnu =normalized high-frequency power; SD12= Poincaré plot ratio, rMSSD=square-root-of –the-mean-of-the-squares-of-successive-R-R-intervals differences, SDNN=standard-deviation-of-the-R-R-intervals, SDANN= standard-deviation-of-5-minutes-average-of-the-R-R-intervals, SDNN-index, TP= total power, ULF= ultra-low-frequency power, VLF= very low frequency power.
In Porto, frequency domain HRV was assessed using a traditional fast Fourier transform. Repeated HRV measures in Porto had high intracorrelation (Cronbach's Alpha: SDNN 0.83; rMSSD 0.84, HF 0.74; HF 0.82), suggesting good internal consistency. In CHS, the sampling frequency was 128 Hz, and ectopic beats were handled by linear spline, although ectopy was not highly prevalent in this population-based rather than clinical cohort.
Specific HRV indices including rMSSD, LFnu, and HFnu can be exaggerated by erratic HR patterns, i.e., sinus arrhythmia of non-respiratory origin, which is especially common in older adults. Therefore, to minimize confounding, by erratic rhythm in CHS, we assessed rMSSD, LFnu, and HFnu among individuals with lower erratic HRV (DFA1>1.04 for baseline analyses and DFA1>1.14 for longitudinal analyses), consistent with our prior methods for HRV analyses in this cohort.28 Sensitivity analyses evaluating rMSSD, LFnu, and HFnu among all participants were not significantly different.
HRV assessment in CHS28, 29 and Porto20 have been described previously. In CHS, a 2-channel 24-hour Holter recording was obtained (Del-Mar-Medical Systems, Irvine, California), and 24-hr HR and HRV were determined at the Washington University School of Medicine HRV laboratory. Recordings were acceptable for analysis with ≥18 hours of usable data, requiring for time-domain analyses ≥50% of each segment to be N-N (normal-to-normal) interbeat intervals (n=1076 in 1989–90; n=578 in 1994–95) and for frequency-domain and nonlinear analyses, which are more sensitive to missing data, ≥80% of each segment to consist of N-Ns interbeat intervals (n=1034 in 1989–90; n= 544 in 1994–95). Beat onset detection and classification were reviewed and edited by trained technicians and overread in detail by Dr. Stein (GE Marquette-Mars 800-Holter analyzer, Milwaukee, Wisconsin). In Porto, R-R intervals were recorded during 20-minutes in a quiet room using the Polar-Advantage NV Heart Monitor (Polar-Electro-OY, Finland), which in healthy subjects provides R-R interval measurements comparable to more conventional ECG devices.30, 31 During the last 5-minutes of recording, used to analyze HRV, participants were in the supine position and matched their breathing to a metronome-paced frequency of 12 breaths/min. R-R intervals were analyzed using Kubios-HRV Software-1.1.32
Covariates
In CHS, participants completed a standardized questionnaire on medical history, health status, and lifestyle habits and underwent a clinic examination including: blood pressure, ECG and anthropometric measures.17 Possible dietary confounders were estimated from responses to the FFQ. Usual leisure-time physical activity was assessed at baseline (1989–90) and at the third (1992–93) annual visit using a modified Minnesota Leisure-Time Activities questionnaire that evaluated frequency and duration of fifteen different activities during the prior two weeks.33
In Porto, anthropometric measures were obtained by standardized methods at each of three visits. Habitual alcohol intake (yes/no) and smoking habits (yes/no) were assessed annually by questionnaire. Potential dietary confounders, such as omega-3 polyunsaturated fatty acids, fiber, and total energy intake, were estimated from the multiple 24-hour recalls. Free-living physical activity was objectively monitored for seven days by uniaxial accelerometers (model-GT1M, Fort Walton Beach, Florida). Freedson's cutoffs were used to analyze accelerometer data via a Mahuffe-activity analyzer34, and daily-time spent in moderate-to-vigorous physical activity was calculated.
Statistical Analysis
HRV measures were tested for normality through numeric and graphical methods (swilk and qnorm commands in STATA), and natural log transformed as needed to facilitate parametric comparisons. In CHS, cross-sectional associations at baseline between estimated trans-18:1 and trans-18:2 consumption and HRV were evaluated by multivariate-adjusted linear regression. We also evaluated prospective associations between TFA consumption at baseline and HRV at the fifth visit. Additionally, we analyzed relationships between plasma phospholipid TFA measured at the third annual visit and HRV measured at the fifth annual visit by multivariate-adjusted linear regression. Dietary and plasma phospholipid TFA were normalized to one standard deviation (SD) differences for mutual comparison. Multivariate models were adjusted for: age, gender, race, education, income, clinical sites, smoking, body mass index (BMI), prevalent diabetes mellitus, coronary heart disease, hypertension, β-blocker use or anti-hypertensive medication, leisure-time physical activity, alcohol, total energy intake, energy adjusted eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), 16:1 TFA (trans-16:1), energy adjusted quintiles of fruit and energy adjusted quintiles of vegetable consumption. We additionally assessed fiber consumption; levels of fibrinogen, C-reactive protein, triglycerides, LDL cholesterol, and HDL cholesterol; and use of digitalis, anti-arrhythmic medication, and anti-depressants as potential confounders or mediators. Inclusion of these variables did not appreciably alter results, and thus these were not included in the final analyses. Missing covariate values (all<8%) were imputed by linear regression using age, race, gender, income, and prevalent cardiovascular disease.
In Porto, we took advantage of repeated measures of diet and HRV (2008-09-10) by evaluating random-effects linear regression models, simultaneously assessing the multiple measures and taking into account within-individual variability, and also by evaluating simple linear regression using the averages of the three measures of TFA consumption (g/day) and HRV to minimize within-person measurement error. Both methods provided similar results, and we present the results based on their averages. We used multivariate-adjusted linear regression to examine cross-sectional associations over three years between total TFA consumption and HRV as continuous variables, normalizing the TFA measure to one SD for comparability. Multivariate models were adjusted for: age, total energy intake, gender, smoking, physical activity, alcohol intake, and BMI. We examined other potential confounders or mediators, including fiber intake, blood glucose and triglycerides, but these were not included in the final model, as they did not alter results. Missing accelerometer values for physical activity (8%) were imputed with a linear regression using nonmissing data from the other visits, as well as age and gender. TFA consumption in each cohort was adjusted for total energy intake using the residual method.35 Potential nonlinear associations between TFA consumption and SDNN were assessed using restricted cubic splines.36 All p values were two-tailed (α=0.05). Analyses were performed using Stata-10.1 (College-Station, Texas).
RESULTS
Table 2 shows the descriptive characteristics of both cohorts. At baseline, CHS participants were 72±5 years old, with average total TFA consumption of 3.7±1.2 grams/day. In Porto, at baseline participants were 19±2 years old, with average total TFA consumption of 1.6±1.5 grams/day.
Table 2.
Baseline characteristics of younger adults in the Porto and older adults in the Cardiovascular Health Study.
| Characteristic | Porto | Cardiovascular Health Study |
|---|---|---|
| N | 160 | 1,076 |
| Age, years | 19±2 | 72±5 |
| Gender, % male | 48 | 46 |
| Race, % white | 100 | 95 |
| Education, % greater or equal to high school | 100 | 75 |
| Body mass index, kg/m2 | 23±3 | 27±4 |
| Prevalent diabetes mellitus, % | 0 | 15* |
| Prevalent coronary heart disease, % | 0 | 24* |
| Prevalence of hypertension, % | 0 | 40* |
| Current smoking, % | 6 | 10 |
| Leisure-time activity, kcal/wk | † | 1392±1747† |
| Physical activity, min/day | 61±29† | † |
| β blocker use, % | 0 | 14 |
| Anti-hypertensive medication use, %* | 0 | 43* |
| Estimated dietary intake | ||
|
| ||
| Total energy intake, g/d | 2169±622‡ | 2029±684§ |
| Total trans-fatty acids intake, g/d | 1.6±1.5‡ | 3.7±1.2§ |
| Trans-18:1, g/d | ‡ | 2.0±0.7§ |
| Trans -18:2, g/d | ‡ | 0.16±0.06§ |
| Trans -16:1, g/d | ‡ | 0.08±0.03§ |
| Total n-3 PUFAs, g/d | 0.66±0.4‡ | 0.28±0.2§ |
| HRV indices | ||
|
| ||
| SDNN, ms | 67.1±29.9|| | 121.6 ±34.8¶ |
| SDANN, ms | || | 110.8±33.4¶ |
| SDNN-índex, ms | || | 44.1 ±15.2¶ |
| rMSSD, ms | 72.1±40.7|| | 26.5±16.9¶ |
| TP, ln ms2 | || | 9.50±0.59¶ |
| ULF, ln ms2 | || | 9.37±0.61¶ |
| HF, ln ms2 | 7.84±0.85|| | 4.44±0.73¶ |
| DFA1 | || | 1.04±0.18¶ |
| SD12 | || | 0.27±0.1¶ |
| HR, bpm | 69.0±9.1|| | 73.3 ±9.0¶ |
Values are mean±standard deviation (continuous variables) or percentage (categorical variables). For more details on these HRV indices, see Table 1.
Diabetes =fasting glucose >140 mg/dl, two hour post-oral challenge glucose >200 mg/dl, or use of insulin or oral hypoglycemic medications. Coronary heart disease=history of myocardial infarction, angina, or coronary revascularization. Hypertension = three categories: (1) systolic <140 and diastolic <90 mm Hg; (2) systolic 140–159 or diastolic 90–94 mmHg; and (3) systolic 160+ or diastolic 95+ mm Hg or if the participant report physician-diagnosed hypertension and also taking antihypertensive medication. The prevalence of the third category is reported here. Anti-hypertensive medication use included calcium-channel blockers, diuretics, vasodilators, or angiotensin converting enzyme inhibitors.
Estimated in Porto by accelerometer and in CHS by the Minnesota Leisure-Time Activities questionnaire.
Estimated using the average of three 24-hour recalls. Individual trans fatty acids not separately estimated in Porto.
Estimated using a semi-quantitative food frequency questionnaire.
Estimated using short-term (5-min) measures; longer-term HRV indices not available in Porto
Estimated using long-term (24-hour) measures.
Cross-sectional Associations between TFA consumption and HRV at baseline in CHS
In CHS, after multivariate adjustment, increased trans-18:2 consumption was cross-sectionally associated with decreases in several indices of 24-hour HRV (Table 3). Among time-domain measures, each one SD (0.06 g/day) of higher trans-18:2 consumption was associated with 12% lower SDNN and lower standard-deviation-of-5-minute-average-N-N-intervals (SDANN) (both reflecting long-term circadian HRV), and 11% lower SDNN-index (average-of-the-5-min-standard-deviations-of-N-N-intervals, reflecting combined sympathetic and parasympathetic modulation of HR) (p<0.01 each). Consistent with this, among frequency-domain measures, each one SD higher trans-18:2 consumption was associated with lower circadian HRV and vagal modulation as reflected by 24% lower total power (TP) and ultra-low-frequency power (ULF), and 24% lower very-low ultra frequency power (VLF) (p<0.01 each). Trans-18:2 consumption was also associated with higher HR, including both 24-hour average HR (+3.20 bpm/SD of consumption, p=0.006) and resting HR (+3.26 bpm/SD of consumption, p=0.01).
Table 3.
Multivariate-adjusted cross-sectional differences in heart rate and heart rate variability (HRV) per each one standard deviation higher intake of trans-18:2 and trans-18:1 among older adults in the Cardiovascular Health Study.
| Trans-18:2 intake | Trans-18:1 intake | |||
|---|---|---|---|---|
| Multivariate-adjusted difference (95%CI) in each HRV index for each 1 SD (0.06 g/d) higher consumption of trans-18:2 fatty acids* | p | Multivariate-adjusted difference (95%CI) in each HRV index for each 1 SD (0.68 g/d) higher consumption of trans18:1 fatty acids * | p | |
| 24-hour Heart Rate, bpm† (N=1,076) | +3.20 (0.92, 5.48) | 0.006 | −2.85 (−5.17, −0.52) | 0.02 |
| Resting Heart Rate, bpm† (N=1,076) | +3.26 (0.68, 5.83) | 0.01 | −3.09 (−5.71, −0.46) | 0.02 |
|
| ||||
| Time-domain HRV (N=1,076) | ||||
| SDNN, ms | −12% (−19, −6) | 0.001 | +16% (7, 25) | <0.001 |
| SDANN, ms | −12% (−19, −5) | 0.001 | +16% (7, 26) | <0.001 |
| SDNNindex, ms | −11% (−19, −3) | 0.007 | +14% (4, 24) | 0.004 |
| rMSSD, ms‡ | −9% (−19, 4) | 0.17 | +7% (−4, 21) | 0.27 |
| Frequency-domain HRV (N=1034) | ||||
| TP, ms2 | −24% (−36, −11) | 0.001 | +37% (16, 60) | <0.001 |
| ULF, ms2 | −24% (−36, −11) | 0.001 | +37% (16, 61) | <0.001 |
| VLF, ms2 | −24% (−36, −9) | 0.003 | +33% (11, 60) | 0.002 |
| LF/HF, ratio | −1% (−14, 15) | 0.96 | +1% (−13, 17) | 0.90 |
| LFnu, % †‡ | +1.09 (−1.04, 3.24) | 0.32 | −0.4 (−2.56, 1.79) | 0.73 |
| HFnu, %†‡ | −1.16 (−2.81, 0.5) | 0.17 | +0.65 (−1.03, 2.33) | 0.45 |
| Non-linear HRV (N=1034) | ||||
| DFA1† | −0.01 (−0.05, 0.04) | 0.77 | +0.01 (−0.04, 0.06) | 0.73 |
| SD12 | 0% (−8, 8) | 0.91 | +1% (−8, 8) | 0.95 |
Because most HRV indices were log-transformed prior to analysis, values represent the percent difference in each HRV index according to each one unit (one SD) of higher TFA consumption.
Normally distributed and were not log-transformed prior to analysis. Values represent absolute difference in each HRV index according to each one unit (one SD) of higher plasma phospholipid TFA.
Analyses adjusted for: age (years), gender (male/female), race (white/nonwhite), education (< high school, high school, > high school), income (≤/> $ 25,000/yr), clinical site (four categories), smoking (never/former/current), BMI (kg/m2), diabetes mellitus (yes/no), coronary heart disease (yes/no), hypertension (three categories), β-blocker use (yes/no), other anti-hypertensive medication use (yes/no), leisure-time physical activity (kcal/week), alcohol (drinks/week), and consumption of total energy (kcal/d), trans-16:1 fatty acids (mg/day), EPA and DHA (quintiles), fruits (quintiles), and vegetables (quintiles). All models also mutually adjusted for consumption of trans-18:2 and trans-18:1 to investigate their independent effects.
Because erratic (abnormal sinus) HRV is common in older adults and can bias certain HRV indices, rMSSD, LFnu, and HFnu were evaluated among individuals with lower erratic HRV (DFA>median; n =625), consistent with our prior methods for HRV analyses in this cohort.28
In sensitivity analyses, results for pNN50 were generally similar to those for rMSSD; and we found no significant associations of TFA with heart rate turbulence (HRT).
R-squared values are the following: 0.21 (24-hour HR); 0.19 (resting HR); 0.15 (SDNN); 0.15 (SDANN); 0.14 (SDNNindex); 0.10(rMSSD); 0.14 (TP); 0.15 (ULF); 0.17 (VLF); 0.17 (LF/HF ratio); 0.13 (LFnu); 0.16 (HFnu); 0.17 (DFA1); 0.14 (SD12). nu = normalized units
In contrast to findings for trans-18:2, trans-18:1consumption at baseline was cross-sectionally associated with higher HRV (Table 3). One SD (0.68 g/day) of higher trans-18:1 consumption was associated with 16% higher SDNN and SDANN and 14% higher SDNN-index (p<0.01 each) and 37% higher TP and ULF, 33% higher VLF (p<0.01 each). trans-18:1 consumption was also associated with lower HR (24-hour HR: −2.85 bpm/SD, p=0.02; resting HR: −3.09 bpm/SD, p=0.02).
Neither trans-18:2 nor trans-18:1 consumption were associated with other HRV indices, including rMSSD (square-root-of-the-mean-of-the-squares-of-successive-N-N differences; reflecting mainly vagal modulation of HR), low-frequency high-frequency power ratio (LF/HF), normalized low-frequency power (LFnu), normalized high-frequency power (HFnu), Poincaré plot ratio (SD12), nor the short-term fractal scaling exponent (DFA1).
Longitudinal Associations between TFA consumption at baseline and HRV 5 years later in CHS
Longitudinal analyses in CHS (Table 4) were generally consistent with the cross-sectional analyses. Greater trans-18:2 consumption was associated with lower time-and frequency-domain circadian and vagal indices of HRV measured 5 years later, whereas higher trans-18:1 consumption was associated with several more favorable indices of HRV. Each one SD higher consumption of trans-18:2 at baseline was associated with lower time-domain measures 5 years later, including 15% lower SDNN and SDANN and 14% lower SDNN-index (p<0.05 each); and a trend toward higher resting HR (+3.88 bpm, p=0.06). Conversely, each one SD higher trans-18:1 consumption at baseline was associated with higher time-domain measures 5 years later, including: 19% higher SDNN and SDANN and 15% higher SDNN-index, (p<0.05 each); and a trend toward lower resting HR (−4.11 bpm, p=0.05). One SD higher trans-18:2 consumption was associated with 30 % lower TP and ULF (p<0.05 each). In contrast, one SD higher trans-18:1 consumption was associated with 44% higher TP and 45% higher ULF (p<0.05 each). Neither trans-18:2 nor trans-18:1 were prospectively associated with rMSSD, LF/HF, HFnu, LFnu, VLF, nor non-linear indices of HRV.
Table 4.
Multivariate-adjusted longitudinal differences in heart rate and heart rate variability (HRV), 5 years after dietary assessment, per each one standard deviation higher intake of trans-18:2 and trans-18:1 fatty acids among older adults in the Cardiovascular Health Study.
| Trans-18:2 intake | Trans-18:1 intake | |||
|---|---|---|---|---|
| Multivariate-adjusted difference (95%CI) in each HRV index for each 1 SD (0.06 g/d) higher consumption of trans-18:2 fatty acids* | p | Multivariate-adjusted difference (95%CI) in each HRV index for each 1 SD (0.68 g/d) higher consumption of trans-18:1 fatty acids* | p | |
| Resting Heart Rate, bpm (N=529) † | +3.88 (−0.10, 7.86) | 0.06 | −4.11 (−8.18, −0.44) | 0.05 |
|
| ||||
| Time-domain HRV (N=578) | ||||
| SDNN, ms | −15% (−25, −4) | 0.009 | +19% (5, 34) | 0.007 |
| SDANN, ms | −15% (−25, −3) | 0.02 | +19% (5, 36) | 0.009 |
| SDNNindex, ms | −14% (−25, −1) | 0.03 | +15% (1, 32) | 0.04 |
| rMSSD, ms‡ | −10% (−36, 11) | 0.34 | +12% (−9, 38) | 0.26 |
| Frequency-domain HRV (544) | ||||
| TP, ms2 | −30% (−46, −10) | 0.005 | +44% (11, 86) | 0.006 |
| ULF, ms2 | −30% (−47, −9) | 0.008 | +45% (11, 90) | 0.007 |
| VLF, ms2 | −23 (−41, 2) | 0.07 | +24% (−7, 64) | 0.14 |
| LF/HF, ratio | 10% (−12, 38) | 0.38 | −7% (−26, 17) | 0.56 |
| LFnu, %†‡ | 0.69 (−2.66, 4.04) | 0.68 | −0.04 (−3.46, 3.38) | 0.98 |
| HFnu, %†‡ | −1.37 (−4.22, 1.48) | 0.34 | +1.14 (−1.77, 4.04) | 0.77 |
| Non-linear HRV (N=544) | ||||
| DFA1† | +0.01 (−0.07, 0.09) | 0.79 | −0.005 (−0.09, 0.08) | 0.90 |
| SD12 | −3% (−17, 7) | 0.57 | +3% (−8, 18) | 0.60 |
See Legend Table 3.
rMSSD, nuLF, and nuHF were evaluated among individuals with lower erratic HRV (DFA> median; n=345). R-squared values are the following: 0.15 (resting HR); 0.12 (SDNN); 0.11 (SDANN); 0.12 (SDNNindex); 0.16(rMSSD); 0.13 (TP); 0.12 (ULF); 0.17 (VLF); 0.16 (LF/HF ratio); 0.15(LFnu); 0.18 (HFnu); 0.17 (DFA1); 0.13 (SD12).
In a subset of 146 CHS participants with phospholipid fatty acid measures at the same time as the dietary assessments, estimated dietary consumption of each TFA was moderately correlated with the respective plasma phospholipid levels (r=0.21 for trans-18:1; r=0.30 for trans-18:2). Based on linear regression between different reported foods and dietary trans-18:2 consumption in CHS, the unit of trans-18:2 consumption evaluated in the present analysis (one SD, 0.06 mg/d) corresponded to about 1 serving/day of baked foods (doughnuts, cookies, cakes, pastry) or 1 serving/day of commercial fried foods.
Longitudinal Associations between plasma phospholipid TFA levels in year 3 and HRV 2 years later in CHS
We investigated relationships between plasma phospholipid TFA levels at year three and HRV two years later in CHS (N=461) (Table 5). Higher levels of plasma phospholipid trans-18:1 were associated with higher time-domain HRV measures including 5% higher SDNN (p=0.006) and 6% higher SDANN (p=0.006); higher frequency-domain measures including 10% higher TP and 11% higher ULF (p=0.01 each); and lower resting HR (−1.49, p=0.02). Additionally plasma phospholipid trans-18:1 levels were associated with more favorable non-linear indices including higher DFA1 (+0.03 higher, p=0.01) and lower SD12 (4% lower, p=0.03). Levels of plasma phospholipid trans-18:2 were not significantly associated with HRV indices in the longitudinal analysis.
Table 5.
Multivariate-adjusted longitudinal differences in hear rate and heart rate variability (HRV) per each one standard deviation higher levels of plasma phospholipid trans-18:2 and trans-18:1 fatty acids assessed two years earlier in the Cardiovascular Health Study.
| Plasma phospholipid trans-18:2 | Plasma phospholipid trans-18:1 | |||
|---|---|---|---|---|
| Multivariate-adjusted difference (95%CI) in each HRV index for each 1 SD (0.08 g/d) higher of plasma phospholipid trans-18:2 fatty acids* | p | Multivariate-adjusted difference (95%CI) in each HRV index for each 1 SD (0.71 g/d) higher of plasma phospholipid trans-18:1 fatty acids* | p | |
| Resting Heart Rate, bpm (N=454)† | +0.87 (−0.26, 2.00) | 0.13 | −1.49 (−2.70, −0.28) | 0.02 |
|
| ||||
| Time-domain HRV (N=461) | ||||
| SDNN, ms | +1% (−3, 4) | 0.73 | +5% (2, 9) | 0.006 |
| SDANN, ms | +1% (−3, 5) | 0.62 | +6% (2, 10) | 0.006 |
| SDNNindex,ms | −1% (−5, 3) | 0.69 | +4% (−1, 8) | 0.10 |
| rMSSD, ms‡ | −1% (−7, 7) | 0.98 | −3% (−10, 4) | 0.44 |
| Frequency-domain HRV (N=424) | ||||
| TP, ms2 | +1% (−8, 8) | 0.99 | +10% (2, 19) | 0.01 |
| ULF, ms2 | +1% (−7, 8) | 0.98 | +11% (2, 21) | 0.01 |
| VLF, ms2 | −3% (−11, 5) | 0.48 | +9% (0, 19) | 0.05 |
| LF/HF, ratio | +1% (−5, 8) | 0.72 | +7% (−1, 15) | 0.06 |
| LFnu, %†‡ | −0.12 (−1.15, 0.93) | 0.83 | +0.60 (−0.44, 1.63) | 0.26 |
| HFnu, %†‡ | −0.39 (−1.26, 0.47) | 0.37 | −0.48 (−1.34, 0.39) | 0.28 |
| Non-linear HRV (N=424) | ||||
| DFA1† | −0.008 (−0.033, 0.017) | 0.51 | +0.03 (0.008, 0.06) | 0.01 |
| SD12 | 0% (−4, 4) | 0.87 | −4% (−8, 0) | 0.03 |
Because most HRV indices were log-transformed prior to analysis, values represent the percent difference in each HRV index according to each one unit (one SD) of higher plasma-phospholipid TFA.
Normally distributed and were not log-transformed prior to analysis. Values represent absolute difference in each HRV index according to each one unit (one SD) of higher plasma phospholipid TFA.
Analyses adjusted for: age (years), gender (male/female), race (white/nonwhite), education (< high school, high school, > high school), income (≤/> $ 25,000), smoking (never, former, current), BMI (kg/m2), diabetes mellitus (yes/no), coronary heart disease (yes/no), hypertension (three categories), β-blocker use (yes/no), anti-hypertensive medication use (yes/no), leisure-time physical activity (kcal/d), alcohol use (drinks/week), and plasma phospholipid levels of trans-16:1 fatty acids, trans-18:1, trans-18:2, EPA and DHA. Trans-18:1 and trans-18:2 fatty acids were mutually adjusted.
Because erratic (abnormal sinus) HRV is common in older adults and can bias certain HRV indices, rMSSD, nuLF, and nuHF were evaluated among individuals with lower erratic HRV (DFA> median; n =265), consistent with our prior methods for HRV analyses in this cohort.28
In sensitivity analyses, results for pNN50 were generally similar to those for rMSSD; and we found no significant associations of TFA with heart rate turbulence (HRT).
R-squared values are the following: 0.15 (resting HR); 0.09 (SDNN); 0.09 (SDANN); 0.09 (SDNNindex); 0.12(rMSSD); 0.10 (TP); 0.10 (ULF); 0.13 (VLF); 0.15 (LF/HF ratio); 0.18(LFnu); 0.25 (HFnu); 0.15 (DFA1); 0.09 (SD12). nu = normalized units
Sensitivity Analyses in CHS
Age-adjusted analyses were generally similar to the multivariate analyses (supplementary material). In sensitivity analysis, when excluding subjects with prevalent CHD at baseline in CHS or adjusting for waist circumference in place of BMI, results were also generally similar (data not shown). In post-hoc analyses, we investigated potential interaction between the TFA subtypes by evaluating the trans-18:2/trans-18:1 ratio. No significant independent association of this ratio with any HRV index was observed (data not shown).
Cross-sectional Associations between TFA consumption and HRV in Porto
Only total TFA consumption was measured in Porto, estimated from multiple 24-hour recalls. In a subset (N=40), total TFA consumption correlated with plasma phospholipid trans-18:2 (r=0.32, p=0.04), but not with trans-18:1 (r=0.02, p=0.80). This suggested that total TFA consumption in Porto, as estimated by the dietary recall and the food composition database in this cohort, largely reflected consumption of trans-18:2, rather than consumption of trans-18:1. After multivariate adjustment, total TFA consumption was cross-sectionally associated with lower 5-min HRV in Porto (Table 6). Each 1 SD (1.5 g/d) of higher TFA consumption was related to lower values of time-domain indices, including 4% lower SDNN and 7% decreased rMSSD (p=0.04 each). Consistent with these results, TFA consumption was also associated with a trend toward higher HR (+1.10 bpm, p=0.07), and lower HF (−11%, p=0.08) (also reflecting vagally-mediated HRV).
Table 6.
Multivariate-adjusted cross-sectional differences in heart rate and heart rate variability per each one standard deviation higher intake of total trans-fatty acids among younger adults in Porto.
| Total trans-fatty acids intake | ||
|---|---|---|
| Multivariate-adjusted difference (95%CI) in each HRV index for each 1 SD (1.5 g/d) higher consumption of total trans-fatty acids* | p | |
| Resting Heart rate, bpm (N=160)† | +1.1 (−0.7, 2.3) | 0.07 |
|
| ||
| Time-domain HRV (N=160) | ||
| SDNN, ms | − 4% (−8, −1) | 0.04 |
| rMSSD, ms | −7%(−13, −1) | 0.04 |
| Frequency-domain HRV (N=160) | ||
| HF, ms2 | −11% (−22, 2) | 0.08 |
Because most HRV indices were log-transformed prior to analysis, values represent the percent difference in each HRV index according to each one unit (one SD) of higher total TFA consumption.
Resting HR was normally distributed and not log-transformed prior to analysis. Thus, these values represent the absolute difference in resting HR according to each one unit (one SD) of higher total TFA consumption. Analysis adjusted for age: (years), gender, current smoking (yes/no) moderate to vigorous physical activity (min/day), alcohol use (yes/no), BMI (kg/m2), and consumption of total n-3 PUFA (mg/day), dietary fiber (g/day) and total energy (kcal/d).
R-squared values are the following: 0.36 (resting HR); 0.14 (SDNN); 0.15 (rMSSD); 0.13 (HF)
Linearity vs. non-linearity of relationships between TFA consumption and HRV
We found little evidence for nonlinearity of observed relationships. For example, in Porto higher total TFA consumption was monotonically associated with lower SDNN, and in CHS, higher trans-18:2 consumption was monotonically associated with lower SDNN (Figure 1).
Figure 1.
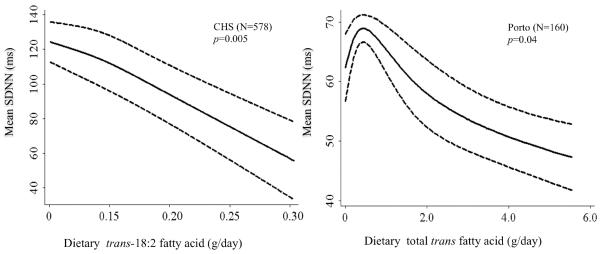
Multivariable-adjusted associations of trans-fatty acid intake and mean standard deviation of the N-N intervals (SDNN), as assessed nonparametically by means of restricted cubic splines. As expected due to differences between 24-hour vs. short-term (5-min) HRV, SDNN mean values were higher in CHS than in Porto.
Values in CHS are adjusted for age (years), gender (male/female), race (white/nonwhite), education (<high school, high school, >high school), income (≤/>$25,000), clinical sites (four categories), smoking (never/former/current), BMI (kg/m2), diabetes mellitus (yes/no), coronary heart disease (yes/no), hypertension (three categories), β-blocker use (yes/no), other anti-hypertensive medication (yes/no), leisure-time physical activity (kcal/week), alcohol use (drinks/week), and consumption of total energy (kcal/d), trans-16:1 fatty acids (mg/day), EPA and DHA (quintiles), fruits (quintiles), and vegetables (quintiles). Values in Porto are adjusted for age (years), gender, current smoking (yes/no) moderate to vigorous physical activity (min/day), alcohol use (yes/no), BMI (kg/m2), and consumption of total n-3 PUFA (mg/day), dietary fiber (g/day) and total energy (kcal/d).
DISCUSSION
Among older adults in CHS, higher trans-18:2 consumption was both cross-sectionally and prospectively associated with specific, less favorable indices of HRV. Consistent with these findings, in Porto, we observed cross-sectional associations of total TFA consumption, which correlated most strongly with circulating phospholipid trans-18:2, with short-term HRV indices assessed annually over 3 years. To our knowledge, this study is the first to identify, in two independent cohorts, a relationship between habitual dietary consumption of TFA, particularly trans-18:2, and unfavorable HRV measures. These results support emerging evidence that trans-18:2, in particular, may increase cardiac risk.1–3, 5, 6
TFA consumption was only associated with certain HRV indices. In CHS, trans-18:2 consumption was associated with less favorable indices that reflect 24-hour circadian activity as SDNN, SDANN, TP, and ULF, as well as with less favorable indices that mainly reflect vagal modulation, such as HR. Additionally, trans-18:2 consumption was associated with lower values of VLF power. Although the exact physiologic mechanisms responsible for VLF power are still a matter of discussion, evidence suggests that VLF may reflect both vagal control of heart rate; the activity of the renin-angiotensin system11; and it is also related with HR and coefficient of variation, which may reflect functional capacity.26, 27Trans-18:2 consumption was also associated with higher 24-hour and resting HR. In contrast, trans-18:2 consumption was not significantly associated with other HRV indices, including measures of erratic vs. more organized HRV patterns (DFA1, Poincaré ratio)19 or indices such as LFnu, HFnu, and LF/HF that can be interpreted as reflecting relative sympathetic modulation, relative parasympathetic modulation of HR or sympathovagal balance respectively.11 Similarly, in Porto, TFA consumption was inversely associated with specific supine short-term measures of HRV, including SDNN, rMSSD, and a trend toward higher HR, but not with other measures.
Lower SDNN and ULF are significant predictors of clinical events including: myocardial infarction, cardiomyopathy, mortality, and arrhythmic mortality.11 Moreover, lower rMSSD, VLF and higher HR may suggest a relative reduction in parasympathetic activity.11 Loss of protective vagal reflexes appears related to ventricular tachyarrhythmias.37, 38 Furthermore, some evidence suggests that vagal activity may contribute to immune responses and lowering of inflammation, e.g., the “nicotinic anti-inflammatory pathway”.39, 40 Higher resting HR is also an independent risk factor for SCD, fatal cardiovascular disease, and all-cause mortality.13 We recognize that while HRV indices are associated with risk of clinical events and specifically with SCD, their sensitivity and specificity for risk of malignant arrhythmias are not high. Relatively few clinical characteristics or diagnostic tests have high sensitivity or specificity for risk of significant arrhythmias, including most traditional cardiac risk factors for many of which associations with life-threatening arrhythmias are weaker than for HR and HRV. For example, among 8,917 middle aged (35 to 69 years) Japanese adults, lower HRV was more strongly associated with risk of SCD (RR=2.01, 95% CI=1.17, 3.44, for higher vs. lower HRV evaluated as a binary variable) than several traditional cardiovascular risk factors including: total cholesterol (RR=0.85, 95% CI= 0.50, 1.44, per 10 mg/dl), triglycerides (RR=0.99, 95%CI= 0.94, 1.05, per 10 mg/dl), and BMI (RR=1.01, 95%CI= 0.93, 1.09, per 1kg/m2)41. In the present analysis, the associations of dietary TFA with HRV may help elucidate potential pathways of effects of TFA consumption on the heart.
Our findings support further experimental investigation of how fatty acids in general, and TFA in particular, might affect cell membrane and ion channel functions. In vitro and animal studies suggest that dietary fatty acids can alter the function of trans-membrane cell proteins, including cardiac ion channels.7 Specific individual fatty acids appear to be preferentially incorporated into lipid rafts or caveolae that modulate membrane receptor function.7 In a small (N=79) 8-week intervention study, where a diet rich in TFA was given to one group of men42, a post hoc analysis suggested that daily 20g TFA dietary supplementation tended to reduce rMSSD and increase HR, but conclusions were limited by the small sample size, the exclusion of several subjects from the final analysis, and the relatively high HRV in these healthy men. Also, TFA consumption in this study (6.8% of total energy) was from bakery products and comprised both trans-18:1 and trans-18:2 (55% and 5% of total fatty acids, respectively), limiting conclusions for specific effects of different TFAs. Our findings support the possibility of adverse HRV effects of trans-18:2 consumption at usual dietary levels of intake in free-living populations.
The associations of dietary trans-18:2 with HRV in CHS could not be confirmed using plasma phospholipid TFA. Reasons for this inconsistency are unclear. Biomarker levels were available in fewer subjects, which could have limited statistical power to detect associations. Dietary questionnaire and circulating biomarker values of TFA are also each imperfect estimates of true habitual TFA consumption. Circulating levels reflect the in vivo balance of both diet and metabolism, rather than dietary consumption alone; and also reflect relatively short-term (several weeks) exposure. Conversely, dietary questionnaires estimate TFA consumption with errors due to variability in TFA content of otherwise very similar appearing foods, product formulations over time, and relative lack of comprehensive nutrient databases on TFA levels, especially trans-18:2 levels, in multiple categories of foods. Thus, given their different sources of errors, the observed correlations between the TFA dietary estimates and biomarker levels in our cohorts represent underestimates of their association with true long-term TFA consumption. Notably, because each of these sources of error are unlikely to be related to HRV, these errors limit the ability to detect associations with HRV. Consequently, the actual associations of TFA consumption with specific HRV indices may be stronger than we observed, and the other null findings we observed, both for dietary TFA and TFA biomarkers, should be interpreted with caution.
We estimated that one SD of trans-18:2 consumption in CHS corresponded to about 1 servings/day of bakery foods or 1 serving/day of commercial fried foods. Trans-18:1 is the most abundant type of TFA in foods made with partially hydrogenated vegetable oils.22 In contrast, many known food sources of partially hydrogenated vegetable oils do not correlate with trans-18:2 blood levels, suggesting that blood levels may also be determined by exposure from other potential dietary sources, e.g. possibly related to oil deodorization or high temperature cooking processes.22 Our findings support the need for further investigation of potential electrophysiologic effects of dietary trans-18:2 and determinants of both their dietary exposure and circulating blood levels.
We found dietary and plasma phospholipid trans-18:1 to be associated with more favorable HRV measures in CHS, including indices of abnormal HR patterns (DFA1 and SD12), circadian modulation (SDNN, SDANN, ULF), vagal activity (VLF, HR). The divergent associations with HRV of trans-18:2 versus trans-18:1 are consistent with emerging evidence that these fatty acids may have different effects on some health outcomes. In our own and others' previous work, trans-18:2 has had generally positive associations, while trans-18:1 has had generally null or inverse associations, with risk of coronary heart disease and sudden cardiac arrest.5, 6, 8, 16, 43 A recent animal study, focusing on atherosclerosis, found that trans-18:2 consumption increased biomarkers of endothelial dysfunction (ICAM-1) and oxidative stress.9 However, the potentially greater harm of trans-18:2 versus trans-18:1 cannot be stated with certainty. Even though some studies support this concept5, 6, 8, 16, 43, others have not found consistent differences in associations with cardiac risk of trans-18:1 versus trans-18:2 fatty acids.8, 14, 15 Differences in health effects of trans-18:1 and trans-18:2 deserve further attention, especially as most policy focus to-date has been on partially hydrogenated vegetable oils that contain mostly trans-18:1.
Our analysis had several strengths. We evaluated the relationships between TFA consumption and HRV, including both short-term and 24-hour indices, in two separate cohorts. Information on dietary habits, HRV measures, and others risks were collected prospectively by standardized methods. We evaluated several HRV using several classes of measures including time-domain, frequency-domain and non-linear. We adjusted for several relevant confounders characterized prospectively using standardized methods, minimizing residual confounding. We found generally similar findings for trans-18:2 consumption in older and younger adults in two distinct cohorts employing different methods for dietary and HRV assessments, which increases confidence in the validity and generalizability of our findings.
Potential limitations are acknowledged. In both cohorts, residual confounding due to unknown or incompletely measured factors cannot be excluded, even though a range of covariates were available and evaluated as potential confounders. Inconsistency between CHS results for dietary and plasma phospholipid trans-18:2 was present. The 2 cohorts were different in many ways, including country, subject age, time period of evaluation, and dietary and HRV assessment methods. For example, given the age differences in these cohorts, the participants in CHS may have been exposed to TFA for a longer period of time than those in Porto. Similarly, types and sources of TFA exposure may not have been the same in these two cohorts. Nevertheless, in light of these many differences, we found generally concordant results in these two independent cohorts. Our findings cannot distinguish potential acute vs. chronic relationships between TFA and HRV, and further investigation of time courses of potential effects is needed. We evaluated multiple HRV indices, increasing the possibility of a chance finding. However, the outcomes of the analyses were generally consistent in the two cohorts and with our prespecified hypotheses.44 The Porto cohort had a smaller sample size and potential for measurement error from using 24-hour dietary recalls to assess TFA consumption, which could have attenuated findings toward the null. Conversely, the serial assessments over three years increased statistical power and would reduce error inherent in 24-hour recalls. Most participants were Caucasian, potentially limiting generalizability if effects of TFA consumption on HRV vary by race.
In conclusion, estimated trans-18:2 consumption was associated with specific and less favorable indices of HRV in both young and older adults, whereas trans-18:1 consumption and blood levels were positively associated with some HRV indices in older adults. Even with broad differences in age ranges, other cohort characteristics, and diet and HRV assessment methods, results were generally consistent in both studies. Because HRV is a measure of autonomic function, which is a potential mediator for heart disease and especially SCD, our results indicate the need for further studies to characterize the dietary sources and potential electrophysiological roles of different TFA subtypes.
Supplementary Material
Acknowledgments
The authors express their gratitude to the CHS participants. A full list of participating CHS investigators and institutions is at http://www.chs-nhlbi.org.
Sources of Funding The National Institutes of Health (NHLBI, NIDDK) provided support for this research (R01-HL085710-01) and for CHS (N01-HC-85239,N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222,N01-HC-75150, N01-HC-45133; HL080295, HL-075366; NIA Grant/Contract numbersAG-023269, AG-15928, AG-20098, and AG-027058); University of Pittsburgh Claude.D. Pepper Older Americans Independence Center grant number P30-AG-024827; with additional contribution from with additional contribution from the NIH Office of Dietary Supplements and National Institute of Neurological Disorders and Stroke. See also http://www.chs-nhlbi.org/pi.htm. Luisa Soares-Miranda was supported by Portuguese Foundation for Science and Technology (FCT) grant BD/38502/2007. The Porto study was supported by FCT Portugal grant PTDC/DES/101333/2008. The funders had no role in study design or conduct; data collection, management, analysis, or interpretation; or manuscript preparation, review, or approval.
Footnotes
Disclosures Harvard University has filed a provisional patent application, that has been assigned to Harvard University, listing Dr. Mozaffarian as a co-inventor to the US Patent and Trademark Office for use of trans-palmitoleic acid to prevent and treat insulin resistance, type 2 diabetes, and related conditions. Dr. Mozaffarian also reports being on the Scientific Advisory Board of Unilever North America.
References
- 1.Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: Experimental and observational evidence. Eur J Clin Nutr. 2009;63(Suppl 2):S5–21. doi: 10.1038/sj.ejcn.1602973. [DOI] [PubMed] [Google Scholar]
- 2.Micha R, Mozaffarian D. Trans fatty acids: Effects on cardiometabolic health and implications for policy. Prostaglandins Leukot Essent Fatty Acids. 2008;79:147–152. doi: 10.1016/j.plefa.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr. 2009;63(Suppl 2):S22–33. doi: 10.1038/sj.ejcn.1602976. [DOI] [PubMed] [Google Scholar]
- 5.Lemaitre RN, King IB, Mozaffarian D, Sotoodehnia N, Rea TD, Kuller LH, Tracy RP, Siscovick DS. Plasma phospholipid trans fatty acids, fatal ischemic heart disease, and sudden cardiac death in older adults: The cardiovascular health study. Circulation. 2006;114:209–215. doi: 10.1161/CIRCULATIONAHA.106.620336. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre RN, King IB, Raghunathan TE, Pearce RM, Weinmann S, Knopp RH, Copass MK, Cobb LA, Siscovick DS. Cell membrane trans-fatty acids and the risk of primary cardiac arrest. Circulation. 2002;105:697–701. doi: 10.1161/hc0602.103583. [DOI] [PubMed] [Google Scholar]
- 7.Katz AM. Trans-fatty acids and sudden cardiac death. Circulation. 2002;105:669–671. [PubMed] [Google Scholar]
- 8.Chiuve SE, Rimm EB, Manson JE, Whang W, Mozaffarian D, Stampfer MJ, Willett WC, Albert CM. Intake of total trans, trans-18:1, and trans-18:2 fatty acids and risk of sudden cardiac death in women. Am Heart J. 2009;158:761–767. doi: 10.1016/j.ahj.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui RA, Harvey KA, Ruzmetov N, Miller SJ, Zaloga GP. N-3 fatty acids prevent whereas trans-fatty acids induce vascular inflammation and sudden cardiac death. Br J Nutr. 2009;102:1811–1819. doi: 10.1017/S0007114509992030. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Mazgalev TN. Arrhythmias and vagus nerve stimulation. Heart Fail Rev. 2011;16:147–161. doi: 10.1007/s10741-010-9178-2. [DOI] [PubMed] [Google Scholar]
- 11.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: Measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malpas SC. Neural influences on cardiovascular variability: Possibilities and pitfalls. Am J Physiol Heart Circ Physiol. 2002;282:H6–20. doi: 10.1152/ajpheart.2002.282.1.H6. [DOI] [PubMed] [Google Scholar]
- 13.Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. Trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004;80:1521–1525. doi: 10.1093/ajcn/80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr. 2004;79:606–612. doi: 10.1093/ajcn/79.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baylin A, Kabagambe EK, Ascherio A, Spiegelman D, Campos H. High 18:2 trans-fatty acids in adipose tissue are associated with increased risk of nonfatal acute myocardial infarction in costa rican adults. J Nutr. 2003;133:1186–1191. doi: 10.1093/jn/133.4.1186. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The cardiovascular health study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the cardiovascular health study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 19.Stein PK, Domitrovich PP, Hui N, Rautaharju P, Gottdiener J. Sometimes higher heart rate variability is not better heart rate variability: Results of graphical and nonlinear analyses. J Cardiovasc Electrophysiol. 2005;16:954–959. doi: 10.1111/j.1540-8167.2005.40788.x. [DOI] [PubMed] [Google Scholar]
- 20.Soares-Miranda L, Negrao CE, Antunes-Correa LM, Nobre TS, Silva P, Santos R, Vale S, Mota J. High levels of c-reactive protein are associated with reduced vagal modulation and low physical activity in young adults. Scand J Med Sci Sports. 2012;22:278–284. doi: 10.1111/j.1600-0838.2010.01163.x. [DOI] [PubMed] [Google Scholar]
- 21.Kumanyika SK, Tell GS, Shemanski L, Martel J, Chinchilli VM. Dietary assessment using a picture-sort approach. Am J Clin Nutr. 1997;65:1123S–1129S. doi: 10.1093/ajcn/65.4.1123S. [DOI] [PubMed] [Google Scholar]
- 22.Micha R, King IB, Lemaitre RN, Rimm EB, Sacks F, Song X, Siscovick DS, Mozaffarian D. Food sources of individual plasma phospholipid trans fatty acid isomers: The cardiovascular health study. Am J Clin Nutr. 2010;91:883–893. doi: 10.3945/ajcn.2009.28877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Food processor . Nutrition analysis & fitness software. [Google Scholar]
- 24.Martins I, Porto A, Oliveira L. Tabela da composição de alimentos. 2006. [Google Scholar]
- 25.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 26.Roach D, Wilson W, Ritchie D, Sheldon R. Dissection of long-range heart rate variability: Controlled induction of prognostic measures by activity in the laboratory. J Am Coll Cardiol. 2004;43:2271–2277. doi: 10.1016/j.jacc.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 27.Raj SR, Roach DE, Koshman ML, Sheldon RS. Activity-responsive pacing produces long-term heart rate variability. J Cardiovasc Electrophysiol. 2004;15:179–183. doi: 10.1111/j.1540-8167.2004.03342.x. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in us adults. Circulation. 2008;117:1130–1137. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 29.Furberg CD, Manolio TA, Psaty BM, Bild DE, Borhani NO, Newman A, Tabatznik B, Rautaharju PM. Major electrocardiographic abnormalities in persons aged 65 years and older (the cardiovascular health study). Cardiovascular health study collaborative research group. Am J Cardiol. 1992;69:1329–1335. doi: 10.1016/0002-9149(92)91231-r. [DOI] [PubMed] [Google Scholar]
- 30.Radespiel-Troger M, Rauh R, Mahlke C, Gottschalk T, Muck-Weymann M. Agreement of two different methods for measurement of heart rate variability. Clin Auton Res. 2003;13:99–102. doi: 10.1007/s10286-003-0085-7. [DOI] [PubMed] [Google Scholar]
- 31.Nunan D, Donovan G, Jakovljevic DG, Hodges LD, Sandercock GR, Brodie DA. Validity and reliability of short-term heart-rate variability from the polar s810. Med Sci Sports Exerc. 2009;41:243–250. doi: 10.1249/MSS.0b013e318184a4b1. [DOI] [PubMed] [Google Scholar]
- 32.Niskanen JP, Tarvainen MP, Ranta-Aho PO, Karjalainen PA. Software for advanced hrv analysis. Comput Methods Programs Biomed. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Siscovick DS, Fried L, Mittelmark M, Rutan G, Bild D, O'Leary DH. Exercise intensity and subclinical cardiovascular disease in the elderly. The cardiovascular health study. Am J Epidemiol. 1997;145:977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 34.Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, inc. Accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Willett W, Stampfer MJ. Total energy intake: Implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 36.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 37.Billman GE. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: Effect of endurance exercise training. Am J Physiol Heart Circ Physiol. 2009;297:H1171–1193. doi: 10.1152/ajpheart.00534.2009. [DOI] [PubMed] [Google Scholar]
- 38.Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis. 2008;50:404–419. doi: 10.1016/j.pcad.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 40.Huston JM, Tracey KJ. The pulse of inflammation: Heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kataoka M, Ito C, Sasaki H, Yamane K, Kohno N. Low heart rate variability is a risk factor for sudden cardiac death in type 2 diabetes. Diabetes Res Clin Pract. 2004;64:51–58. doi: 10.1016/j.diabres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Dyerberg J, Eskesen DC, Andersen PW, Astrup A, Buemann B, Christensen JH, Clausen P, Rasmussen BF, Schmidt EB, Tholstrup T, Toft E, Toubro S, Stender S. Effects of trans- and n-3 unsaturated fatty acids on cardiovascular risk markers in healthy males. An 8 weeks dietary intervention study. Eur J Clin Nutr. 2004;58:1062–1070. doi: 10.1038/sj.ejcn.1601934. [DOI] [PubMed] [Google Scholar]
- 43.Roberts TL, Wood DA, Riemersma RA, Gallagher PJ, Lampe FC. Trans isomers of oleic and linoleic acids in adipose tissue and sudden cardiac death. Lancet. 1995;345:278–282. doi: 10.1016/s0140-6736(95)90274-0. [DOI] [PubMed] [Google Scholar]
- 44.Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol. 1995;142:904–908. doi: 10.1093/oxfordjournals.aje.a117737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


