Abstract
The incidence of chronic oral pain such as burning mouth syndrome is greater in perimenopausal females, and was postulated to be associated with gustatory nerve damage. We investigated if bilateral transection of the chorda tympani, with or without accompanying ovariectomy, affected oral capsaicin avoidance in rats. Female rats had restricted access to 2 bottles, one containing capsaicin (concentration range: 0.33–33 μM) and the other vehicle. Percent volume of capsaicin consumption and lick counts were measured. The concentration series was tested before and 0.5, 3, 6, 9 and 12 mo after the following surgical procedures: (a) bilateral transection of the chorda tympani (CTx), (b) ovariectomy (OVx), (3) CTx plus OVx, or (4) sham CT surgery. Presurgery there was a concentration- dependent decrease in licks and volume of capsaicin consumed with a threshold between 0.1–0.3 ppm. The majority of drink licks occurred during the first 9 min of access. Over the 12-month test period, the CTx group did not exhibit reduced capsaicin consumption, and consumed significantly more capsaicin at 6 and 9 months postsurgery. Rats in the OVx group consistently consumed significantly less capsaicin and exhibited significantly higher counts of capsaicin-evoked c-fos-like immunoreactivity in dorsomedial trigeminal subnucleus caudalis (Vc) compared to all other treatment groups. That CTx, with or without OVx, did not enhance capsaicin avoidance indicates that damage to the gustatory system does not disinhibit trigeminal nociceptive transmission.
Keywords: taste nerve, chorda tympani, capsaicin avoidance, ovariectomy, hyperalgesia, c-fos expression, trigeminal subnucleus caudalis, nucleus of solitary tract
Introduction
While trigeminal stimulation of the oral cavity can reduce gustatory transmission and the perceived intensity of certain tastants [11, 31, 33, 36, 40, 42, 47, 50, 54], it is less certain if oral gustatory stimulation affects trigeminal transmission including pain. Psychophysical studies have reported that certain tastants, particularly sucrose, reduce oral irritation elicited by capsaicin [46, 51, 52], and that sucrose can reduce pain in human infants [8, 12, 39] and nocifensive behavior in neonatal rats [2, 7, 43]. We recently reported that gustatory stimulation with NaCl and monosodium glutamate (MSG) acutely inhibits responses of neurons in trigeminal subnucleus caudalis to noxious lingual stimulation [10]. Inhibitory interactions between cranial nerves innervating the oral cavity have been previously reported [31], and anesthesia of the chorda tympani (CT) was reported to enhance lingual capsaicin irritation [53], prompting the hypothesis that damage to the gustatory system may relieve the trigeminal system of tonic inhibition [3]. Such interactions could potentially contribute to the etiology of chronic orofacial pain conditions such as burning mouth syndrome [3, 5, 32]. Indeed, burning mouth syndrome has been associated with dysgeusia [25, 28–30].
We presently tested the hypothesis that damage to gustatory nerves results in hyperalgesia within the oral cavity. We investigated if bilateral CT transection (CTx) in rats increases their sensitivity to the irritant capsaicin using a 2-bottle avoidance paradigm [9, 49]. As the prevalence of several disorders within the area innervated by the trigeminal nerve is increased in women [13, 20], we assessed the effect of ovariectomy (OVx) in our model. The trigeminal sensory complex is rich in neurons expressing estrogen receptors, and OVx has been reported to induce hyperalgesia in animal studies [1, 16, 17, 26, 27, 38, 41, 45]. Moreover, the incidence of burning mouth syndrome (BMS) and other chronic orofacial pain conditions is higher in perimenopausal females [20, 23, 28, 37, 55, 56]. We therefore further hypothesized that capsaicin avoidance would be enhanced in ovariectomized (OVx) rats as well as rats with both CTx and OVx. Finally, at the conclusion of the behavioral study we used c-fos immunohistochemistry to assess the numbers of neurons in trigeminal subnucleus caudalis (Vc) and the nucleus of the solitary tract (NTS) activated by lingual capsaicin. We hypothesized that groups exhibiting enhanced capsaicin avoidance in the behavioral study would exhibit increased c-fos expression in Vc.
Methods
Animals
Experiments were performed using 35 adult female Sprague-Dawley rats. The experimental protocol was approved by the UC Davis Institutional Animal Care and Use Committee. The animals were housed in polycarbonate cages in a vivarium with reversed 12:12 light cycle and were always tested at the same time each day during the dark phase. Rat chow was available ad libitum. Two water bottles were also available ad libitum during off-study periods. Presurgery behavioral testing commenced when all rats were 8 months old (mean weight >270 g; Fig. 1). On test days, the animals were water-restricted for 22 hr and had restricted 2-hr access to water as described below.
Fig. 1.
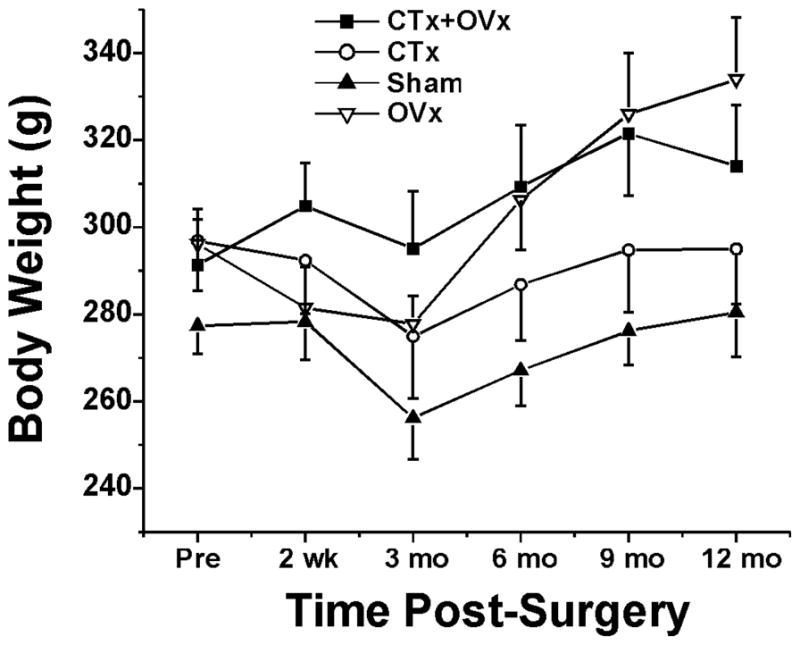
Body weights in each treatment group. Error bars: SEM.
Surgery
Following the completion of pre-surgery behavioral testing, the rats were assigned to one of four groups: (a) bilateral CTx, (b) bilateral CTx plus OVx, (c) OVx, and (d) and CT sham surgery controls. All surgical procedures were conducted approximately 6 weeks after the conclusion of pre-surgery behavioral testing. For CTx, rats were anesthetized with sodium pentobarbital (60 mg/kg ip) and the CT on each side accessed according to a procedure described previously [19]. Briefly, one skin incision was made ventrally and part of the mylohyoid muscle was resected. The medial pterygoid muscle was then carefully dissected to avoid bleeding of the adjacent venous sinuses. The CT was isolated central to its separation from the lingual nerve. A cotton thread was pulled loosely under the exposed CT, after which it was cut with ophthalmologic scissors. Removal of the intact thread confirmed transection. In sham-operated control rats the CT was exposed bilaterally in the same manner, but not transected. For OVx, the ovary was exposed through a lateral incision (approx. 1 cm) in the abdomen, ligated and transected. The same procedure was performed on the opposite side. In the CTx + OVx group, both surgical procedures were performed successively. All surgical incisions were sutured closed. Two wk following surgery, rats were retested behaviorally in the 2-bottle paradigm.
Two-bottle preference test
Prior to formal testing, the rats were accommodated to a metal box with access to two drinking bottles containing water in one 2-hr session per day for 3 successive days. During formal testing in the 2-bottle paired preference paradigm, rats that had been water-restricted for the previous 22 hr were transferred from the home cage to the metal box. The sipper tube of each bottle, and the floor of the metal box, were connected to a lickometer (DM-8; Columbus Instruments, Columbus OH) to simultaneously monitor licking from each of 32 bottles (2 per cage), thus allowing us to monitor drinking from 16 rats at once [9]. Each lickometer output channel was routed to computer via an interface (Columbus Instruments) and lick counts from each bottle were registered at 1-min intervals over the 2-hr access period. In addition, each bottle was weighed before and after the 2-hr drinking period to measure the volume of fluid consumption.
Prior to surgery, and at 0.5, 3, 6, 9 and 12 months post-surgery, rats were tested in the restricted access 2-bottle preference paradigm with an ascending series of capsaicin concentrations: 0.1 ppm (0.33 μM), 0.3 ppm (0.99 μM), 0.5 ppm (1.65 μM), 1 ppm (3.3 μM) and 10 ppm (33 μM). Capsaicin at a given concentration was provided in one bottle and the matching vehicle (0.005, 0.015, 0.025, 0.05 and 0.5% ethanol in distilled water) in the other. Each concentration of capsaicin was tested over 2 consecutive days, with the position of capsaicin- and vehicle-containing bottles switched each day to avoid positional preference. After the 2 test days at a given capsaicin concentration, water was provided in both bottles for the next 2 (or more) days prior to testing the next capsaicin concentration. Capsaicin was tested in an ascending concentration series. At the conclusion of the concentration series, rats received ad libitum water from 2 bottles in their home cages until the next test series.
c-fos immunohistochemistry
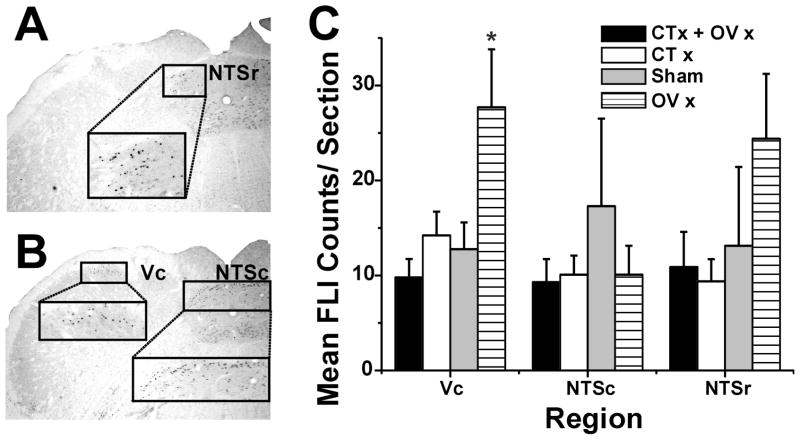
After behavioral testing was completed, rats were anesthetized with sodium pentobarbital (60 mg/kg ip). The mouth was gently held open and 0.1 ml of capsaicin (100 ppm; 330 μM) was placed on the anterior tongue bilaterally. This concentration was chosen because it, but not lower (10 ppm) concentrations, excited Vc neurons when applied to the tongue of barbiturate-anesthetized rats [14, 21]. Underlying gingiva was covered with Parafilm® to avoid stimulation of tissue other than the tongue. After 10 min the mouth was closed. Two hr after capsaicin application, the animal was perfused transcardially with 250 ml of phosphate-buffered saline followed immediately by 500 ml of 4% paraformaldehyde. The brainstem was removed, postfixed, and transferred to 30% sucrose for cryoprotection. Brainstems were cut in 50 μm frozen sections and immunohistochemically processed as described previously [15, 48]. Cell nuclei displaying black Fos-like immunoreactivity (FLI) were counted bilaterally in all sections at 150 μm intervals between the level of the pyramidal decussation caudally up to the rostral pole of the NTS. We specifically counted FLI in the dorsomedial aspect of Vc, as well as in the NTS at levels caudal (NTSc) and rostral to (NTSr) to the area postrema. The investigator counting FLI was blinded as to the experimental treatment. For each region of interest in each animal, the 5 sections with the highest counts of FLI were selected and imaged with a digital camera (MP-5, Q-Imaging, Technical Instruments, San Francisco CA) using Scion Image QCapture-Pro 6.0 software (Q-Imaging).
Data analysis
At each pre- and post-surgery time point, the volume of capsaicin solution consumed at each concentration was expressed as a % of total volume consumed from both bottles. The number of licks from the bottle containing capsaicin was also expressed as % of total licks from both bottles. Capsaicin consumption and lick counts were subjected to a repeated measures analysis of variance (ANOVA) with treatment group, capsaicin concentration and time as main effects. Post-hoc 2-way ANOVAs (group and capsaicin concentration as main effects) with Tukey’s test were subsequently conducted at each time point to directly assess group differences in consumption and licks as a function of time. Similarly, body weight was analyzed using repeated measures ANOVA with group and time as main effects. In addition, the microstructure of licking behavior was assessed for each capsaicin concentration presurgery. At each capsaicin concentration, the number of licks from the capsaicin- and vehicle-containing bottles were counted at 1-min intervals during the initial 30 min, averaged, and compared by repeated measures ANOVA and post-hoc Tukey’s tests with rat, time, replication and capsaicin concentration as main effects. Statistical analyses were done using Minitab 16 (Minitab Inc., State College, PA).
Counts of FLI in Vc, NTSrc and NTSr were averaged for each animal, and compared between treatment groups using one-way ANOVA followed by post hoc Tukey’s tests. P <0.05 was considered to be significant.
Results
Body weight
There was a significant effect of treatment on body weight (F3,206=69.41, p<0.001). Post-surgery, rats undergoing OVx weighed significantly more than rats in the sham group or group receiving CTx only. At 3 months post-surgery there was a small, but significant, drop in mean body weight in each group (F5,206=8.95; p<0.001), followed by weight gain over the ensuing 9 month period that was similar across treatment groups (group*time interaction F15,206=1.43, p=0.136; Fig. 1).
Microstructure of licking
We assessed lick counts, measured at 60 sec intervals, to determine the time course of drinking during the access period. As expected, as capsaicin concentration increased, the number of licks directed to the capsaicin-treated bottle decreased significantly (F5,11650=28.59, p<0.001). There was also a significant effect of time (F30,11650=24.93, p<0.001). Post-hoc analyses indicated that for all capsaicin concentrations, the majority of licks occurred within the first 9 minutes of the access period (Fig. 2). Interestingly, as capsaicin concentration increased, rats ceased drinking capsaicin solutions at progressively earlier time points as indicated by the significant concentration*time interaction (F150,11650=2.02, p<0.001). This trend can be seen in Fig. 2, where the peak number of licks occurred progressively earlier as capsaicin concentration increased. Finally, there was a significant difference in numbers of licks directed to the capsaicin-treated bottle between the first and second test days, with more licks occurring during the first compared to the second exposure (F1,11650=20.34, p<0.001). These results might reflect sensitization to capsaicin in contrast to a previous report [49], or a learning effect whereby rats used specific sensory cues from the initial exposure that allowed them to more quickly identify and avoid capsaicin in subsequent exposures.
Fig. 2.
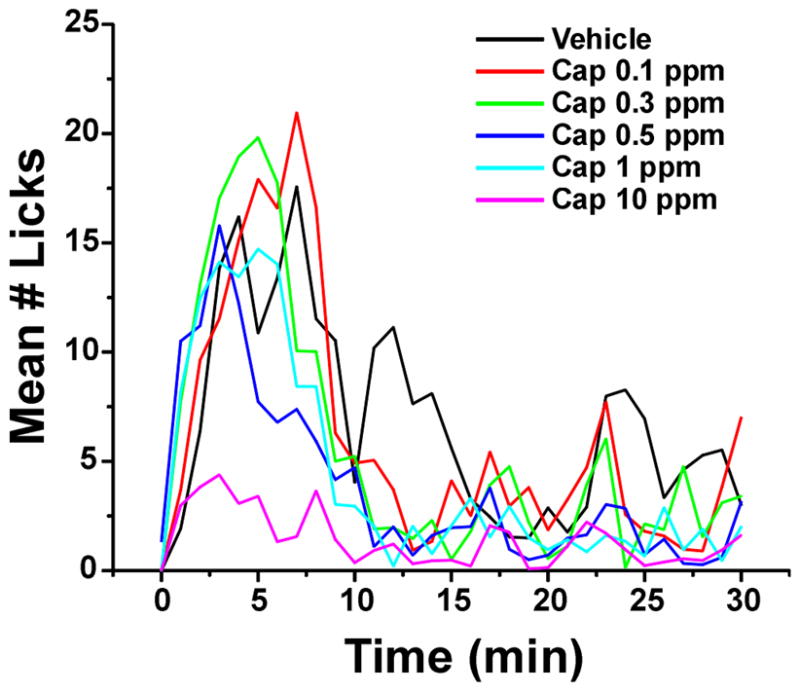
Pre-surgery microstructure of drinking bouts. Graph plots mean lick counts/ min during initial 30 min of exposure; each capsaicin concentration is indicated by a different color. Lick counts directed to the capsaicin-treated side were averaged (n= 35) over 2 days with bottle positions switched daily.
Postsurgery capsaicin consumption
Figs. 3A-E plot capsaicin consumption vs. capsaicin concentration for each treatment group at the indicated times postsurgery (●, CTx + OVx, n=10; ▲, CTx, n=9;▼, sham, n=9;◆, OVx, n=7). Fig. 3A additionally shows consumption data presurgery (■; all animals; n=35). Three-way, repeated measures ANOVA showed significant effects of group (F3,1949=12.50, p<0.001), time (F5,1949=3.85; p=0.002) and capsaicin concentration (F4,1949-171.00; p<0.001) on capsaicin consumption. Additionally, there were significant group*time (F15,1949=1.96; p=0.015), capsaicin concentration*time (F20,1949=1.79; p=0.017) and group*capsaicin concentration (F12,1949=1.96, p=0.024) interactions. To further explore the group differences at each capsaicin concentration, we subsequently performed separate two-way ANOVAs (group and capsaicin concentration as main effects) at each time point. These analyses allowed us to specify any between-group differences in capsaicin consumption for each capsaicin concentration tested, as detailed below.
Figure 3.
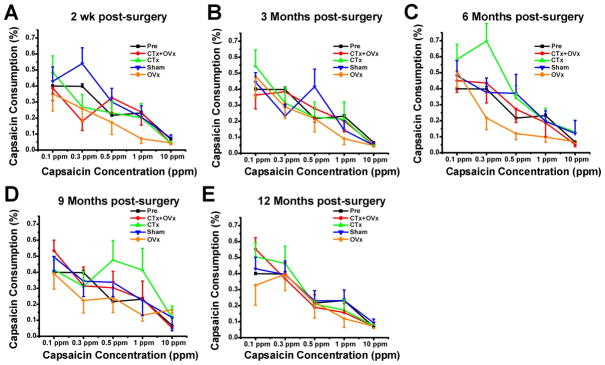
Time course of capsaicin avoidance pre- and post-surgery. Graphs plot % volume of capsaicin consumed vs. capsaicin concentration at indicated time points. A–E: 2 wk, 3 mo, 6 mo, 9 mo and 12 mo postsurgery, respectively. Error bars: SEM.
Pre-surgery, there were no significant differences in capsaicin consumption between groups (F=3,330=1.21, p=0.308). Consumption was significantly affected by capsaicin concentration (F4,330=30.68, p<0.001). Indeed, across all groups, capsaicin consumption generally decreased as the concentration increased. This trend is substantiated by a non-significant group*concentration interaction (F12,330=1.07, p=0.385).
At 2 wk postsurgery (Fig. 3A) the OVx group consumed significantly less capsaicin across all concentrations (F3,330=4.44, p=0.004). Not all groups showed a consistent concentration dependent decrease in capsaicin consumption as indicated by the significant group*concentration effect (F12,330=2.19, p=0.012). The OVx group drank less capsaicin as the concentration increased whereas all other groups showed some degree of inconsistency in this pattern (e.g. the sham group actually consumed more of the 0.3 ppm solution than the 0.1 ppm solution). At 3 months postsurgery there were no significant differences among groups in the amount of capsaicin consumed (F3,320=0.49; p=0.693; Fig. 3B) nor was there a significant group*concentration interaction (F12,320=1.59; p=0.094). At 6 months postsurgery, there was a significant group effect (F3,320=7.96, p<0.001) as well as a group*concentration effect (F12,320=1.99, p=0.025) with the OVx and CTx groups exhibiting the least and most capsaicin consumption, respectively (Fig. 3C). At 9 months postsurgery, the OVx group consumed the least amount of capsaicin (significant group effect F3,310=5.31, p= 0.001). However, consistent with the significant group*concentration effect, the CTx group consumed more capsaicin at intermediate concentrations (F12,310=2.58, p=0.003; Fig. 3D). At 12 months postsurgery there was no significant group effect(F3,310=1.03, p=0.310) or significant group*concentration interaction (F12,310=0.91, p=0.540) (Fig. 3E).
The lick count data were largely consistent with the consumption data. Three-way, repeated measures ANOVA showed significant effects of group (F3,1941=13.29, p<0.001), time (F5,1941=3.61; p=0.003) and capsaicin concentration (F4,1941=42.61; p<0.001) on the percentage of licks directed towards the capsaicin bottle. Additionally, there were significant group*time (F15,1941=1.89; p=0.021) and capsaicin concentration*time (F20,1941=1.79; p=0.017) interactions. To further explore the group differences at each capsaicin concentration, we subsequently performed separate two-way ANOVAs (group and capsaicin concentration as main effects) at each time point. These analyses allowed us to specify any between group differences in the percentage of licks directed towards the capsaicin bottle for each capsaicin concentration tested. At all time points, all groups showed a concentration-dependent decrease in licks on the capsaicin side as concentration increased. This was supported by significant concentration effects (F3,330=2.5–16.4; p<0.05 on all occasions,) but no significant group*concentration interaction (F12,330=1.0–1.45; p>0.1 on all occasions) ). Importantly, the OVx group consistently exhibited lower capsaicin lick counts at 0.5, 3 and 9 mo postsurgery (0.5 mo: F3,322=3.07, p= 0.028, vs. shams; 3 mo: F3,320=6.10, p<0.001, vs. CTx and CTx + OVx; 9 mo: F3,310=3.28, p= 0.021, vs. shams).
FLI
After behavioral testing was completed, FLI evoked by lingual application of 100 ppm capsaicin was assessed in all rats. The photomicrographs in Fig. 4A and B show examples of FLI in NTSr, Vc and NTSc, respectively. Counts of FLI are shown in Fig. 4C. There was a significant group effect with counts in Vc of the OVx group significantly greater compared to all other groups (F=4.30, p= 0.014). There were no significant between-group differences in Fos counts in the caudal or rostral NTS.
Fig. 4.
FLI. A: Upper box shows FLI in NTSr; lower box connected by dashed lines shows higher-power view. B: Upper boxes show FLI in Vc (left) and NTSc (right), connected by dashed lines to higher-power views in lower boxes. C: Bar graph plots mean counts of FLI (error bar: SEM) in Vc, NTSr and NTSc for each group. *: significant between-group difference (p < 0.05, ANOVA).
Discussion
The concentration-dependent avoidance of capsaicin observed presently in female rats is consistent with our previous studies with male rats [9, 49]. A salient finding is that CTx, with or without OVx, did not enhance capsaicin avoidance, arguing against the original hypothesis that gustatory nerve damage releases inhibition of trigeminal nociceptive pathways. Another salient finding is that OVx alone had a pronociceptive effect, enhancing capsaicin avoidance at several time points postsurgery and resulting in increased Fos expression by neurons in Vc. These findings are discussed, below.
Microstructure of drinking
Most drinking occurred within the first 9 min of exposure to the bottles. Rats initially sampled the capsaicin- and vehicle-treated sides in approximately equal proportions, and then exhibited a preference for the vehicle-treated side although the number of licks on the capsaicin-treated side also increased (for 0.1–1 ppm capsaicin concentrations). Presumably, the initial exposure to capsaicin was not so aversive that the rat permanently switched to the vehicle side; rather, the rat continued to sample capsaicin although with a lower probability. The rat may have continued to drink from the first bottle it encountered, even if it contained capsaicin, implying either that the motivation to drink outweighed aversion to the capsaicin burn or that the capsaicin burn required more time to develop. After its first encounter with capsaicin, the rat was more likely to select vehicle for the next lick bout presumably due to spatial memory (and avoidance) of the burn elicited by prior capsaicin. The probability of “correctly” choosing the vehicle-treated side may relate to the strength of aversive conditioning by prior capsaicin, consistent with concentration-dependent avoidance (Fig. 3). The licking pattern appeared to differ at the highest (10 ppm) capsaicin concentration (Fig. 2, magenta). There was a reduced probability of licking capsaicin during the first min of exposure, with very little increase over the next 6 min. This suggests that aversion to 10 ppm capsaicin developed rapidly, after which rats exhibited a preference for vehicle with a lower probability of returning to the capsaicin side. Nevertheless, some rats intermittently switched to the capsaicin side, suggesting that they forgot the association between position and capsaicin burn, or were unsure of the source of capsaicin due to the delayed onset of irritation.
CTx did not enhance capsaicin avoidance
A major outcome is that CTx rats did not exhibit increased sensitivity to capsaicin, and in fact exhibited reduced sensitivity to capsaicin at 6 and 9 mo postsurgery (Fig. 3C,D). This indicates that CTx does not result in chronic hyperalgesia to oral capsaicin due to the release of the trigeminal system from tonic gustatory inhibition. However, we cannot exclude the possibility of a short-lasting effect of CTx on trigeminal transmission that may have recovered by the time we began testing the rats 2-wk post-surgery. The reduced sensitivity at 6 and 9 mo after CTx was unexpected, but might be accounted for by the loss of capsaicin-sensitive CT afferents. We previously reported that while the majority of gustatory NTS neurons were unaffected by lingual capsaicin, 15% were excited [47] indicating that capsaicin may provide afferent drive via gustatory nerves, in addition to stimulating trigeminal afferents. Overall, the data do not support the idea that CT damage leads to oral hyperalgesia, at least within a 1 yr period.
Of interest was our observation that the OVx group consumed significantly less capsaicin over most of the 12 mo period of observation, consistent with enhanced sensitivity to oral irritation. Moreover, the OVx group exhibited significantly higher counts of FLI in Vc (Fig. 4C), suggesting peripheral and/or central sensitization of nociceptive Vc neurons. Many previous studies have reported that gonadal hormones modulate pain [1, 6, 18, 24]. Ovariectomized female rats showed significantly greater rubbing of the upper lip after formalin injection compared to males or sham-operated females [41], and several additional studies have reported pronociceptive effects of OVx in rat and mouse models of persistent pain [16, 17, 26, 27, 38, 45]. Importantly, we presently show that gustatory nerve damage alone, or in combination with OVx, did not result in oral hyperalgesia to capsaicin. Thus, if OVx induces a pro-nociceptive effect, this was presumably mitigated by an antinociceptive effect of CTx (as observed at 6 and 9 months in the CTx only group) to result in no overall change in capsaicin avoidance.
Model of burning mouth syndrome (BMS)
We attempted to mimic conditions of BMS in perimenopausal females, using aged (approaching 2 yr) ovariectomized female rats with damage to the gustatory nerves. The rationale is that the prevalence of BMS is greatly increased in oophorectomized [23] and hysterectomized [35] women. Moreover, it has been suggested that gustatory nerve damage contributes to BMS by disinhibiting trigeminal nociceptive transmission [3]. We presently assessed avoidance of orally ingested capsaicin solutions. BMS patients frequently avoid spicy food containing chili peppers, and Tobasco sauce elicited a strong burning sensation in a higher proportion of BMS patients compared to control subjects [22]. In seeming contradiction, the detection threshold for capsaicin was reported to be elevated in BMS patients [33]. This discrepancy might be explained by an overall reduction in lingual innervation, coupled with a greater proportion of capsaicin-sensitive TRPV1-expressing nerve fibers, in the lingual epithelium of BMS patients [57]. We presently observed that rats with OVx + CTx did not exhibit increased sensitivity to capsaicin, arguing against the idea that gustatory nerve damage enhances oral pain via disinhibition of trigeminal nociception. The CTx group exhibited reduced capsaicin sensitivity at certain time points postsurgery, consistent with a reduction in lingual innervation but not with increased expression of TRPV1. The age-matched, sham-operated control group did not exhibit any significant change in capsaicin sensitivity over time, arguing against age as a contributing factor. Only the OVx group exhibited a significant increase in capsaicin sensitivity, suggesting that a reduction in circulating gonadal hormones may contribute to the etiology of BMS. Overall, the present animal model incorporated several factors thought to contribute to BMS, some of which increased while others decreased capsaicin sensitivity. This supports the utility of the present model to assess bidirectional changes in oral nociception. However, more research is needed to determine if the aged female OVx rat represents a valid model for the human condition of BMS.
In conclusion, our data argue against the hypothesis that gustatory nerve damage disinhibits trigeminal nociceptive transmission to enhance orofacial pain and hyperalgesia, since CTx animals did not exhibit increased capsaicin avoidance. OVx animals did exhibit enhanced capsaicin avoidance and increased Fos expression in Vc, consistent with previous studies showing pronociceptive effects of gonadectomy. The present combination of CTx and OVx was intended to mimic conditions in perimenopausal female patients suffering from BMS. However, animals with CTx and OVx did not exhibit enhanced capsaicin avoidance, possibly because the two procedures induced opposing pro- and antinociceptive effects that cancelled each other out.
Summary.
Transection of gustatory nerves, with or without ovariectomy, did not affect capsaicin avoidance or trigeminal FOS expression. This argues against taste loss disinhibiting trigeminal nociception.
Acknowledgments
Supported by a grant from the National Institutes of Health (DE013685).
We thank Karen Zanotto, Austin Merrill and Katsuko Nishida for valuable technical assistance.
Footnotes
None of the authors declares a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amandusson Å. Blomqvist A Estrogenic influences in pain processing. 1. Front Neuroendocrinol. 2013;34(4):329–49. doi: 10.1016/j.yfrne.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Anseloni VCZ, Ren K, Dubner R, Ennis M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience. 2005;133:231–43. doi: 10.1016/j.neuroscience.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Bartoshuk LM, Snyder DJ, Grushka M, Berger AM, Duffy VB, Kveton JF. Taste damage: previously unsuspected consequences. Chem Senses. 2005;30 (Suppl 1):i218–i219. doi: 10.1093/chemse/bjh192. [DOI] [PubMed] [Google Scholar]
- 4.Bartoshuk LM. Comparing sensory experiences across individuals: recent psychophysical advances illuminate genetic variation in taste perception. Chem Senses. 2000;25(4):447–60. doi: 10.1093/chemse/25.4.447. [DOI] [PubMed] [Google Scholar]
- 5.Bartoshuk LM, Catalanotto F, Hoffman H, Logan H, Snyder DJ. Taste damage (otitis media, tonsillectomy and head and neck cancer), oral sensations and BMI. Physiol Behav. 2012;107(4):516–26. doi: 10.1016/j.physbeh.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Berkley KJ, Zalcman SS, Simon VR. Sex and gender differences in pain and inflammation: a rapidly maturing field. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R241–44. doi: 10.1152/ajpregu.00287.2006. [DOI] [PubMed] [Google Scholar]
- 7.Blass EM, Shide DJ. Some comparisons among the calming and pain-relieving effects of sucrose, glucose, fructose and lactose in infant rats. Chem Senses. 1994;19:239–249. doi: 10.1093/chemse/19.3.239. [DOI] [PubMed] [Google Scholar]
- 8.Blass EM, Watt LB. Suckling- and sucrose-induced analgesia in human newborn infants. Pain. 1999;83:611–23. doi: 10.1016/S0304-3959(99)00166-9. [DOI] [PubMed] [Google Scholar]
- 9.Boucher Y, Carstens MI, Sawyer CM, Zanotto KL, Merrill AW, Carstens E. Capsaicin avoidance as a measure of chemical hyperalgesia in orofacial nerve injury models. Neurosci Lett. 2013a;543:37–41. doi: 10.1016/j.neulet.2013.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucher Y, Felizardo R, Klein AH, Iodi Carstens M, Carstens E. Gustatory modulation of the responses of trigeminal subnucleus caudalis neurons to noxious stimulation of the tongue in rats. Europ J Neurosci. 2013b;38(6):2812–22. doi: 10.1111/ejn.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucher Y, Simons CT, Faurion A, Azerad J, Carstens E. Trigeminal modulation of gustatory neurons in the nucleus of the solitary tract. Brain Res. 2003;973:265–74. doi: 10.1016/s0006-8993(03)02526-5. [DOI] [PubMed] [Google Scholar]
- 12.Bucher HU, Moser T, Von Siebenthal K, Keel M, Wolf M, Duc G. Sucrose reduces pain reaction to heel lancing in preterm infants: A placebo-controlled, randomized and masked study. Pediatr Res. 1995;38:332–335. doi: 10.1203/00006450-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Cairns BE. The influence of gender and sex steroids on craniofacial nociception. Headache. 2007;47:319–24. doi: 10.1111/j.1526-4610.2006.00708.x. [DOI] [PubMed] [Google Scholar]
- 14.Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by application of different classes of irritant chemicals to the oral and ocular mucosa. J Neurophysiol. 1998;80:465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- 15.Carstens E, Saxe I, Ralph R. Brainstem neurons expressing c-fos immunoreactivity following irritant chemical stimulation of the rat’s tongue. Neuroscience. 1995;69:939–953. doi: 10.1016/0306-4522(95)00297-v. [DOI] [PubMed] [Google Scholar]
- 16.Ceccarelli I, Fiorenzani P, Massafra C, Aloisi AM. Long-term ovariectomy changes formalin-induced licking in female rats: the role of estrogens. Reprod Biol Endocrinol. 2003;1:24. doi: 10.1186/1477-7827-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen BL, Li YQ, Xie DH, He QL, Yang XX. Blocking TNF-α with infliximab alleviates ovariectomy induced mechanical and thermal hyperalgesia in rats. Neurol Sci. 2012;33(3):527–33. doi: 10.1007/s10072-011-0743-9. [DOI] [PubMed] [Google Scholar]
- 18.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8(5):397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Danilova V, Danilov Y, Roberts T, Elmer D, Hellekant G. Electrophysiological recordings of mammalian taste. In: Simon SA, Nicolelis MAL, editors. Methods in chemosensory research. CRC Press; 2002. pp. 247–248. [Google Scholar]
- 20.Dao TT, LeResche L. Gender differences in pain. J Orofac Pain. 2000;14(3):169–184. [PubMed] [Google Scholar]
- 21.Dessirier JM, Simons CT, Sudo M, Sudo S, Carstens E. Sensitization, desensitization and stimulus-induced recovery of trigeminal neuronal responses to oral capsaicin and nicotine. J Neurophysiol. 2000;84(4):1851–1862. doi: 10.1152/jn.2000.84.4.1851. [DOI] [PubMed] [Google Scholar]
- 22.Felice F, Gombos F, Esposito V, Nunziata M, Scully C. Burning mouth syndrome (BMS): evaluation of thyroid and taste. Med Oral Patol Oral Cir Bucal. 2006;11:E22–5. [PubMed] [Google Scholar]
- 23.Ferguson M, Carter J, Boyle P, Hart, Lindsay R. Oral complaints related to climacteric symptoms in oophorectomized women. J R Soc Med. 1981;74:492–498. doi: 10.1177/014107688107400707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24(4):485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 25.Formaker BK, Frank ME. Taste function in patients with oral burning. Chem Senses. 2000;25(5):575–81. doi: 10.1093/chemse/25.5.575. [DOI] [PubMed] [Google Scholar]
- 26.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958(1):139–45. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- 27.Gaumond I, Arsenault P, Marchand S. Specificity of female and male sex hormones on excitatory and inhibitory phases of formalin-induced nociceptive responses. Brain Res. 2005;1052(1):105–11. doi: 10.1016/j.brainres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Grushka M. Clinical features of burning mouth syndrome. Oral Surg. 1987;63:30–36. doi: 10.1016/0030-4220(87)90336-7. [DOI] [PubMed] [Google Scholar]
- 29.Grushka M, Sessle BJ, Howley TP. Psychophysical evidence of taste dysfunction in burning mouth syndrome. Chem Senses. 1986;11:485–498. [Google Scholar]
- 30.Grushka M, Sessle BJ, Howley TP. Psychophysical assessment of tactile, pain and thermal sensory functions in burning mouth syndrome. Pain. 1987;28(2):169–184. doi: 10.1016/0304-3959(87)90114-X. [DOI] [PubMed] [Google Scholar]
- 31.Halpern BP, Nelson LM. Bulbar gustatory responses to anterior and to posterior tongue stimulation in the rat. Am J Physiol. 1965;209:105–110. doi: 10.1152/ajplegacy.1965.209.1.105. [DOI] [PubMed] [Google Scholar]
- 32.Jääskeläinen SK. Pathophysiology of primary burning mouth syndrome. Clin Neurophys. 2012;123:71–77. doi: 10.1016/j.clinph.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 33.Just T, Steiner S, Pau HW. Oral pain perception and taste in burning mouth syndrome. J Oral Pathol Med. 2010;39(1):22–7. doi: 10.1111/j.1600-0714.2009.00824.x. [DOI] [PubMed] [Google Scholar]
- 34.Karrer T, Bartoshuk L. Effects of capsaicin desensitization on taste in humans. Physiol Behav. 1995;57:421–429. doi: 10.1016/0031-9384(94)e0076-g. [DOI] [PubMed] [Google Scholar]
- 35.Lamey P-J, Freeman R, Eddie S-A, Pankhurst C, Rees T. Vulnerability and presenting symptoms in burning mouth syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol End. 2005;99:48–54. doi: 10.1016/j.tripleo.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Lawless HT, Stevens DA. Effects of oral chemical irritation on taste. Physiol Behav. 1984;32:995–998. doi: 10.1016/0031-9384(84)90291-9. [DOI] [PubMed] [Google Scholar]
- 37.Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV. Association between female hormonal factors and oro-facial pain: study in the community. Pain. 2002;97(1–2):5–10. doi: 10.1016/s0304-3959(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 38.Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8(4):334–42. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Miller A, Barr RG, Young SN. The cold pressor test in children: methodological aspects and the analgesic effect of intraoral sucrose. Pain. 1994;6:175–83. doi: 10.1016/0304-3959(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 40.Osada K, Komai M, Bryant BP, Suzuki H, Goto A, Tsunoda K, Kimura S, Furukawa Y. Capsaicin modifies responses of rat chorda tympani nerve fibers to NaCl. Chem Senses. 1997;22:249–55. doi: 10.1093/chemse/22.3.249. [DOI] [PubMed] [Google Scholar]
- 41.Pajot J, Ressot C, Ngom I, Woda A. Gonadectomy induces site-specific differences in nociception in rats. Pain. 2003;104(1–2):367–73. doi: 10.1016/s0304-3959(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 42.Prescott J, Stevenson RJ. Effects of oral chemical irritation on tastes and flavors in frequent and infrequent users of chili. Physiol Behav. 1995;58:1117–1127. doi: 10.1016/0031-9384(95)02052-7. [DOI] [PubMed] [Google Scholar]
- 43.Roane DS, Martin RJ. Continuous sucrose feeding decreases pain threshold and increases morphine potency. Pharm Biochem Behav. 1990;35:225–229. doi: 10.1016/0091-3057(90)90230-f. [DOI] [PubMed] [Google Scholar]
- 44.Ren K, Blass EM, Zhou Q, Dubner R. Suckling and sucrose ingestion suppress persistent hyperalgesia and spinal Fos expression after forepaw inflammation in infant rats. Proc Natl Acad Sci USA. 1997;94:1471–5. doi: 10.1073/pnas.94.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: a model of functional abdominal pain. Pain. 2005;118(1–2):243–53. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Schöbel N, Kyereme J, Minovi A, Dazert S, Bartoshuk L, Hatt H. Sweet taste and chorda tympani transection alter capsaicin-induced lingual pain perception in adult human subjects. Physiol Behav. 2012;107:368–73. doi: 10.1016/j.physbeh.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Simons CT, Boucher Y, Carstens E. Suppression of central taste transmission by oral capsaicin. J Neurosci. 2003a;23(3):978–85. doi: 10.1523/JNEUROSCI.23-03-00978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simons CT, Boucher Y, Carstens MI, Carstens E. Lack of quinine-evoked activity in rat trigeminal subnucleus caudalis. Chem Senses. 2003b;28(3):253–59. doi: 10.1093/chemse/28.3.253. [DOI] [PubMed] [Google Scholar]
- 49.Simons CT, Gogineni AG, Iodi Carstens M, Carstens E. Reduced aversion to oral capsaicin following neurotoxic destruction of superficial medullary neurons expressing NK-1 receptors. Brain Res. 2002a;945(1):139–43. doi: 10.1016/s0006-8993(02)02913-x. [DOI] [PubMed] [Google Scholar]
- 50.Simons CT, O’Mahony M, Carstens E. Taste suppression following lingual capsaicin pre-treatment in humans. Chem Senses. 2002b;27(4):353–65. doi: 10.1093/chemse/27.4.353. [DOI] [PubMed] [Google Scholar]
- 51.Sizer F, Harris N. The influence of food additives and temperature on threshold perception of capsaicin. Chem Sens. 1985;10:279–86. [Google Scholar]
- 52.Stevens DA, Lawless HT. Putting out the fire: effects of tastants on oral chemical irritation. Percept Psychophys. 1986;39(5):346–50. doi: 10.3758/bf03203002. [DOI] [PubMed] [Google Scholar]
- 53.Tie K, Fast K, Kveton J, Cohen Z, Duffy VB, Green B, Prutkin JM, Bartoshuk LM. Anesthesia of chorda tympani nerve and effect on oral pain. Chem Sens. 1999;24:609. [Google Scholar]
- 54.Wang Y, Erickson RP, Simon SA. Modulation of rat chorda tympani nerve activity by lingual nerve stimulation. J Neurophysiol. 1995;73:1468–83. doi: 10.1152/jn.1995.73.4.1468. [DOI] [PubMed] [Google Scholar]
- 55.Woda A, Dao T, Gremeau-Richard C. Steroid dysregulation and stomatodynia (burning mouth syndrome) J Orofac Pain. 2009;23(3):202–10. [PubMed] [Google Scholar]
- 56.Woda A, Pionchon P. A unified concept of idiopathic orofacial pain: clinical features. J Orofac Pain. 1999;13(3):172–84. [PubMed] [Google Scholar]
- 57.Yilmaz Z, Renton T, Yiangou Y, Zakrzewska J, Chessell IP, Bountra C, Anand P. Burning mouth syndrome as a trigeminal small fibre neuropathy: Increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci. 2007;14:864–71. doi: 10.1016/j.jocn.2006.09.002. [DOI] [PubMed] [Google Scholar]