Summary
The aetiology of marginal zone lymphoma (MZL) is purported to differ by anatomic site. While this is supported by clinical series of single MZL sites, no population-based study has comprehensively assessed incidence patterns across sites. To gain insight into disease aetiology, we assessed MZL incidence by site using data from 18 U.S. Surveillance, Epidemiology and End Results (SEER) Program population-based registries. We calculated age-adjusted incidence rates (IRs) by sex, race, and calendar year. During 2001–2009, 4,081 (IR=5.7/1,000,000 person-years) and 8,821 (IR=12.3) individuals were diagnosed with nodal MZL and extranodal MZL, respectively. The most common extranodal sites were stomach (IR=3.8), spleen (IR=1.6), eye/adnexa (IR=1.4), and lung, skin, and salivary glands (IRs=0.9–1.0). We observed distinct age-specific patterns by MZL site, with IRs increasing steeply at younger ages and less prominently after mid-life at several sites, except skin. Gender and racial/ethnic disparities were also apparent across sites. Between 2001–2005 and 2006–2009, MZL IRs decreased significantly for gastric (-15%) and soft tissue (-28%) sites, whereas IRs increased significantly for lung (18%), skin (43%), and kidney/renal pelvis (116%). In combination, our findings support the contention that MZL is characterized by aetiological heterogeneity across sites and susceptibility is probably influenced by intrinsic characteristics and environmental exposures.
Keywords: Marginal zone lymphoma, Incidence, Population-based study, Anatomic site, Joinpoint analysis
Introduction
Marginal zone lymphoma (MZL) is a relatively uncommon disease, comprising approximately 3% of all lymphoid neoplasms (Morton, et al 2006). According to the 2008 World Health Organization (WHO) Classification of Tumours of the Haematopoietic and Lymphoid Tissues (Swerdlow, et al 2008), MZL is subclassified into three different entities, namely extranodal MZL of mucosa-associated lymphoid tissue (MALT), nodal MZL, and splenic MZL. Although this classification implies similarities of MZL cases within a given group, there is growing evidence of significant clinical, pathological and aetiological heterogeneity among MZLs occurring at different anatomic sites (Rawal, et al 2007, Remstein, et al 2006, Streubel, et al 2004, Traverse-Glehen, et al 2011, Ye, et al 2003).
MZL has been associated with immune system dysregulation as a result of sustained immune stimulation from chronic infections or autoimmune disorders (Bende, et al 2009, Suarez, et al 2006). For example, individuals with Sjogren syndrome have a more than 40-fold increased risk of MZL involving the salivary glands (Mellemkjaer, et al 2008, Smedby, et al 2006). Specific organisms have been implicated in the aetiology of MZL involving particular sites: Helicobacter pylori (stomach), Chlamydia psittaci (ocular adnexa), Campylobacter jejuni (small intestine) and Borrelia burgdorferi (skin) (Suarez, et al 2006). With suspected aetiological exposures differing by anatomic site, incidence patterns might also be expected to vary by anatomic site. However, data on site-specific MZL are largely based on clinical series, which are limited by small numbers of cases (Rawal, et al 2007, Thieblemont, et al 2000, Ye, et al 2003). Most population-based data describing the epidemiology of MZL have not considered site or have focused on selected sites (Hwang, et al 2009, Luminari, et al 2010, Rasmussen, et al 2011, Wu, et al 2009), and none have considered age- and gender-specific patterns by site. To gain insight into disease aetiology, we comprehensively assessed the incidence of MZL by site and patient demographic characteristics using data from the U.S. Surveillance, Epidemiology and End Results (SEER) Program for individuals diagnosed during 2001–2009.
Materials and Methods
Data from 18 population-based cancer registry areas of the SEER Program (SEER-18, released April 2012) are available for cases diagnosed since 2000. SEER-18 encompasses approximately 28% of the U.S. population and includes cancer registries in eight states (Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico and Utah), six metropolitan areas (Atlanta, Georgia; Detroit, Michigan; Los Angeles, San Francisco-Oakland, and San Jose-Monterey, California; Seattle-Puget Sound, Washington), and the areas of greater California, greater Georgia and rural Georgia, as well as the Alaska Native Tumor Registry.
The third edition of the International Classification of Diseases for Oncology (ICD-O-3) was published in 2000 and adopted for use by the SEER Program for cases diagnosed in 2001 onwards (Fritz, et al 2000). Morphology codes for MZL (ICD-O-3 morphology code M-9699) and splenic MZL (M-9689) were newly introduced with ICD-O-3, therefore our analysis includes cases diagnosed during 2001–2009, the years for which data were available. We also included cases of immunoproliferative small intestinal disease (M-9764) based on the InterLymph hierarchical classification of lymphoid neoplasms (Turner, et al 2010) (n<16 cases). With the exception of oral cavity/oropharynx, primary site was defined according to SEER site groups (Howlader, et al 2012) using ICD-O-3 topography codes specified in Table I. Given the important aetiological role of human papillomavirus (HPV) in some cancers of the oral cavity/oropharynx, we considered oropharyngeal sites according to their potential association with HPV infection, as previously described (Chaturvedi, et al 2008). MZL presenting at multiple primary sites simultaneously is coded to unknown primary site (C80.9) in the SEER Program.
Table I.
Age-adjusted incidence rates and incidence rate ratios of marginal zone lymphoma according to site and gender, SEER-18, 2001–2009*
| ICD-O-3 | Total | Median age (years) |
Male | Female | Male-to-female | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| topography codes | n | IR | n | IR | n | IR | IRR | (95% CI) | ||
| All sites† | C00.0–80.9 | 13,096 | 18.3 | 67 | 5,996 | 19.0 | 7,100 | 17.9 | 1.06 | (1.02–1.10)‡ |
| All nodal MZL | C77.0–77.9 | 4,081 | 5.7 | 69 | 1,933 | 6.2 | 2,148 | 5.4 | 1.15 | (1.08–1.22)‡ |
| All extranodal MZL | C00.0–41.9, 42.2, 44.0–76.8 | 8,821 | 12.3 | 66 | 3,962 | 12.5 | 4,859 | 12.3 | 1.01 | (0.97–1.06) |
| Oral cavity/oropharynx | ||||||||||
| HPV-related sites | C01.9, 02.4, 09.0–09.9,10.0–10.9, 14.2 | 106 | 0.1 | 69 | 50 | 0.2 | 56 | 0.1 | 1.12 | (0.74–1.67) |
| HPV-unrelated sites | C02.0–02.3, 02.8–06.9 | 89 | 0.1 | 67 | 30 | 0.1 | 59 | 0.1 | 0.68 | (0.42–1.07) |
| Salivary glands | C07.9–08.9 | 638 | 0.9 | 62 | 172 | 0.5 | 466 | 1.2 | 0.43 | (0.36–0.52)‡ |
| Nasopharynx | C11.0–11.9 | 63 | 0.1 | 59 | 34 | 0.1 | 29 | 0.1 | 1.32 | (0.77–2.25) |
| Stomach | C16.0–16.9 | 2,702 | 3.8 | 68 | 1,347 | 4.3 | 1,355 | 3.4 | 1.27 | (1.18–1.37)‡ |
| Small intestine | C17.0–17.9 | 258 | 0.4 | 66 | 134 | 0.4 | 124 | 0.3 | 1.33 | (1.03–1.72)‡ |
| Colon/rectum | C18.0–20.9 | 399 | 0.6 | 68 | 182 | 0.6 | 217 | 0.5 | 1.06 | (0.87–1.30) |
| Lung | C34.0–34.9 | 680 | 1.0 | 68 | 303 | 1.0 | 377 | 1.0 | 1.00 | (0.86–1.17) |
| Spleen | C42.2 | 1,125 | 1.6 | 68 | 531 | 1.7 | 594 | 1.5 | 1.12 | (1.00–1.27) |
| Skin | C44.0–44.9 | 692 | 0.9 | 58 | 402 | 1.2 | 290 | 0.7 | 1.60 | (1.37–1.86)‡ |
| Soft tissue, including heart | C38.0, 47.0–47.9, 49.0–49.9 | 170 | 0.2 | 67 | 63 | 0.2 | 107 | 0.3 | 0.74 | (0.53–1.02) |
| Breast | C50.0–50.9 | 263 | 0.4 | 67 | <16 | ~ | 252 | 0.6 | ~ | |
| Kidney/renal pelvis | C64.9, 65.9 | 51 | 0.1 | 70 | 32 | 0.1 | 19 | 0.05 | 2.18 | (1.19–4.07)‡ |
| Bladder | C67.0–67.9 | 40 | 0.1 | 72 | <16 | ~ | 26 | 0.1 | ~ | |
| Eye/adnexa | C69.0–69.9 | 1,026 | 1.4 | 65 | 447 | 1.4 | 579 | 1.5 | 0.96 | (0.84–1.09) |
| Thyroid | C73.9 | 174 | 0.2 | 62 | 57 | 0.2 | 117 | 0.3 | 0.58 | (0.41–0.80)‡ |
Abbreviations: SEER-18, 18 cancer registry areas of the Surveillance, Epidemiology and End Results Program; ICD-O-3, International Classification of Diseases for Oncology, 3rd edition; n, number; IR, incidence rate; IRR, incidence rate ratio; CI, confidence interval; ~ IRs and IRRs not calculated for fewer than 16 cases; MZL, marginal zone lymphoma; HPV, human papillomavirus.
All incidence rates are age-adjusted to the 2000 US standard population and expressed per 1,000,000 person-years. Incidence rate ratios are based on unrounded rates. The table enumerates extranodal sites with ≥40 total cases. Extranodal sites (and ICD-O-3 topography) excluded from the table: lip (C00.0–00.9; n<16), hypopharynx (C12.9–13.9; n<16), other oral cavity/oropharynx (C14.0, 14.8; n<16), esophagus (C15.0–15.9; n<16), anus (C21.0–21.8; n<16), liver/intrahepatic bile ducts (C22.0–22.1; n=32; IR=0.05), gallbladder (C23.9; n<16), pancreas (C25.0–25.9; n<16), other digestive organs (C26.0–26.9; n=27; IR=0.4), nose/nasal cavity/middle ear (C30.0–30.1, 31.0–31.9; n=36; IR=0.05), larynx (C32.0–32.9; n<16), pleura (C38.4; n=20; IR=0.03); trachea/mediastinum/other respiratory (C33.9, 38.1–38.3, 38.8, 39.0, 39.8, 39.9; n<16), bones and joints (C40.0–41.9; n=24; IR=0.03), peritoneum/retroperitoneum (C48.0–48.8; n<16), vulva (C51.0–51.9; n<16), vagina (C52.9; n<16), cervix (C53.0–53.9; n<16), uterus (C54.0–54.9, 55.9; n<16), ovary (C56.9; n<16), prostate (C61.9; n=17; IR=0.02), testis (C62.0–62.9; n<16), other male genital (C63.0–63.9; n<16), ureter (C66.9; n<16), other urinary (C68.0–68.9; n<16), brain (C71.0–71.9; n=21; IR=0.03), cranial nerves/other nervous system (C70.0–70.9, 72.0–72.9; n=23; IR=0.03), other endocrine and thymus (C37.9, 74.0–75.9; n=20; IR=0.03), ill-defined sites (C76.0–76.9, n<16)
Cases of MZL coded to ICD-O-3 topography sites of blood (C42.0); bone marrow (C42.1); reticuloendothelial system, not otherwise specified (C42.3); haematopoietic system, not otherwise specified (C42.4); or unknown primary site (C80.9) (total n=194) are excluded from the “All nodal MZL” and “All extranodal MZL” categories.
95% CI excludes 1.00 (based on unrounded upper and lower CI), and IRR is significant (P<0.05).
We calculated age-adjusted incidence rates (IRs), IR ratios (IRRs), and 95% confidence intervals (CIs) using the Incidence Rate Session in SEER*Stat software version 8.0.2 (seer.cancer.gov/seerstat). Rates were age-adjusted to the 2000 U.S. standard population using age groups <1, 1–4, 5–9, …, 80–84, and 85+ years, and expressed per 1,000,000 person-years (PY). We included all cases of MZL that were microscopically confirmed. IRs were calculated overall and according to primary site, gender (male, female), race/ethnicity (non-Hispanic whites (NHWs); Hispanic whites (HWs); blacks; Asians/Pacific Islanders (APIs); American Indians/Alaskan Natives; and other specified or unspecified race/ethnicity).
Using SEER*Stat we also calculated incidence rates by single year of age, 0–84 years, for both sexes combined overall and for each major MZL site. For the overall age-specific analysis, we used the Joinpoint Regression Program (Joinpoint Regression Program, Version 4.0.0, December 2012; Statistical Research and Applications Branch, National Cancer Institute), which fits a weighted log-linear model and utilizes a statistical algorithm to find the optimal number and location(s) of changes in slope (percent change in rate per year of advancing age), and tests whether the apparent change in slope is statistically significant (Kim, et al 2000). Joinpoint analysis represents a simplification of trends that is influenced by the number of point estimates and their standard errors. The analysis first assumes no joinpoints (e.g., a straight log-linear line is fit) and then adds one joinpoint at a time, up to three, to determine if a change in slope is statistically significant. In the final model, each joinpoint identifies a significant change in slope. Because MZL rarely occurs at younger ages (Supplementary Table I), we limited the joinpoint analyses to ages 25–84 years. For colon/rectum, lung and spleen, there were too few cases diagnosed between ages 24–44 years and analyses were limited to ages 45–84 years.
For age-specific analyses by gender, in an effort to evaluate the entire age spectrum, we calculated age-adjusted IRs in nine age groups (<15, 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and 85+ years) when the number of cases allowed. Incidence rates were plotted on a log-linear scale using the midpoint of each specified age group, as previously described (Devesa, et al 1995). IRs based on fewer than 16 cases are not shown in the tables, following SEER Program convention. Single (isolated) data points were excluded when evaluating age-specific patterns of the MZL sites by gender.
To provide a basis for comparison of site-specific MZL incidence patterns, we calculated age-adjusted IRs for all microscopically confirmed solid tumours (ICD-O-3 morphology codes 8000–9581) with malignant behavior diagnosed in SEER-18 during 2001–2009 using the ICD-O-3 topography codes specified in Table I. We calculated solid tumour IRs and IRRs for each site specified for MZL (except spleen) according to gender (Supplementary Table II), race/ethnicity (Supplementary Table III), and calendar year (Supplementary Table IV). IRs were age-adjusted to the 2000 U.S. standard population and expressed per 100,000 PY.
Results
Age
During 2001–2009, 13,096 individuals (IR=18.3 per 1,000,000 PY) were diagnosed with MZL in SEER-18, inclusive of all sites, with a median age of 67 years (Table I). Accounting for nearly one third of cases, nodal MZL (n=4,081; IR=5.7) occurred at a slightly older median age (69 years) than extranodal MZL (66 years), which accounted for most of the remaining cases (n=8,821; IR=12.3). The most common extranodal site was the stomach (IR=3.8), followed by the spleen (IR=1.6), eye/adnexa (IR=1.4) and lung, skin, and salivary glands (IRs=0.9–1.0). Most skin MZL occurred on the head/neck (34.8%), upper limb/shoulder (24.4%) and trunk (21.5%), with only 4.8% occurring on the lower extremity/hip. MZL of the skin and nasopharynx were associated with the youngest median age at diagnosis (<60 years), whereas MZL of the kidney/renal pelvis and bladder were associated with the oldest median ages (≥70 years).
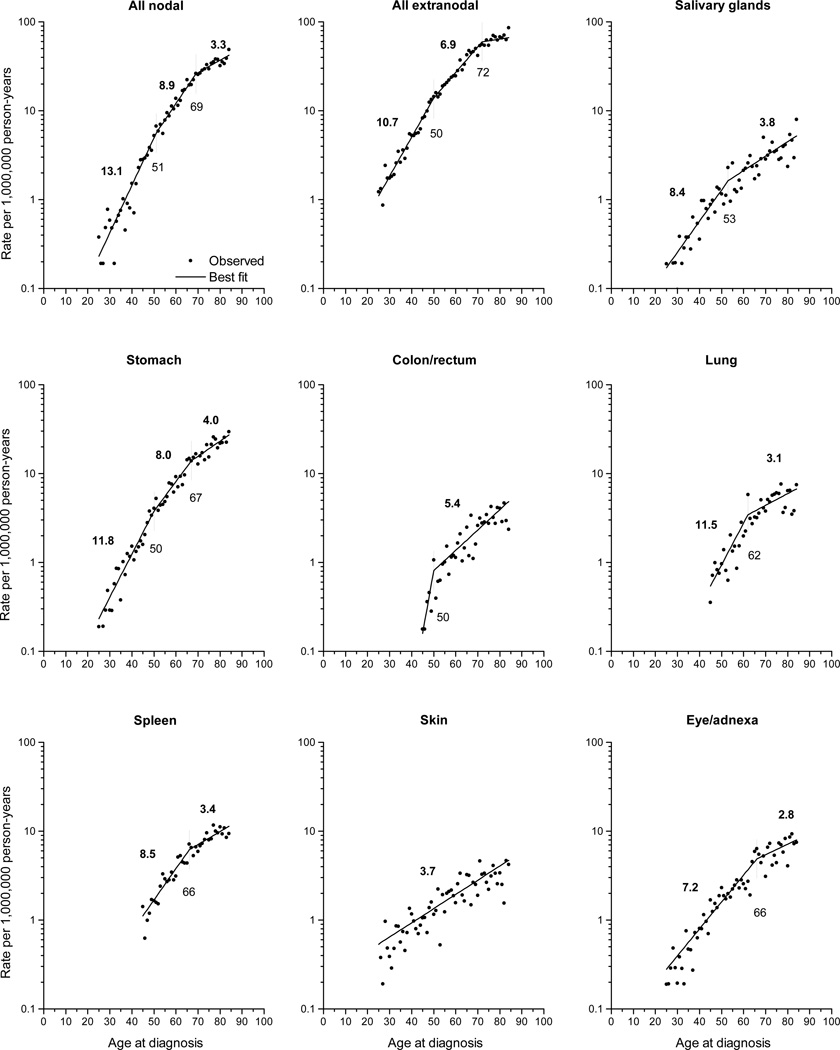
For sites with more than 350 cases, we evaluated age-specific IRs (Fig 1) and observed three distinct patterns: sites that were best described by a) two joinpoints (nodal, stomach); b) one joinpoint (salivary glands, colon/rectum, lung, spleen and eye/adnexa); and c) no joinpoints (skin). Among the sites with 2 joinpoints, there was consistency in the initial joinpoint occurring at ages 50–51 years and the second joinpoint at ages 67–69 years. Sites characterized by one joinpoint varied at the age where the joinpoint occurred, ranging from 50–53 years (salivary glands, colon/rectum) to 62 years (lung) and 66 years (spleen, eye/adnexa). Among sites with joinpoints, the steepest slopes occurred among the youngest age group, particularly all nodal (13.1% change in rate per year of advancing age), stomach (11.8%) and lung (11.5%). The least prominent rise with age occurred among the oldest ages, with changes ≤4.0% per year.
Fig 1.
Observed age-specific incidence rates of marginal zone lymphoma diagnosed in SEER-18 during 2001–2009 according to site by single year of age and predicted curve from the best fitting joinpoint model. Each significant slope (measured by the percent change per year of advancing age) is indicated above each fitted line segment, and age at each inflection point (joinpoint) is indicated below the respective vertical line.
Gender
The incidence of nodal MZL was significantly higher among males than females (male-to-female (M:F) IRR=1.15), whereas extranodal MZL occurred equally among men and women (M:F IRR 1.01) (Table I). However, gender disparities were evident when extranodal MZL was assessed by site. MZL IRs were significantly higher among males than females for the stomach (M:F IRR=1.27), small intestine (M:F IRR=1.33), skin (M:F IRR=1.60) and kidney/renal pelvis (M:F IRR=2.18), whereas they were significantly lower for the salivary glands (M:F IRR=0.43), soft tissue (M:F IRR=0.74) and thyroid (M:F IRR=0.58). MZL of the colon/rectum, lung, and eye/adnexa occurred nearly equally among the sexes.
Solid tumours at most sites predominated among males (Supplementary Table II). In contrast to MZL, IRs of solid tumours of the salivary glands (M:F IRR=1.73) and soft tissues (M:F IRR=1.44) were significantly higher among males than females. However, similar to MZL, a female predominance was evident for thyroid cancer (M:F IRR=0.34).
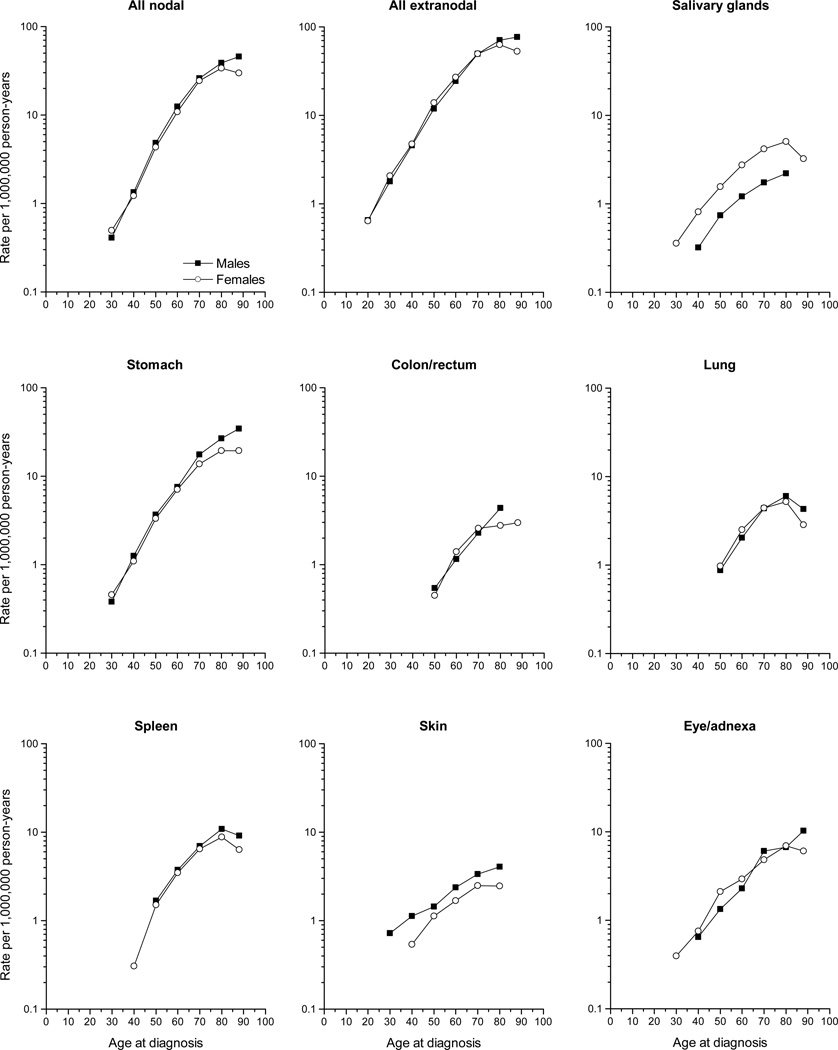
To determine if gender differences were evident across the entire age spectrum, we evaluated age-specific patterns of the MZL sites by gender (Fig 2). Notably, the IRs of MZL of skin were consistently higher among males across all ages, whereas salivary gland MZL IRs were higher among females across all ages. Except for the eye/adnexa, for all other sites evaluated, the gender disparity was largely accounted for by higher male IRs among the oldest age groups.
Fig 2.
Ten-year age-specific incidence rates of marginal zone lymphoma diagnosed in SEER-18 during 2001–2009 according to gender and site.
Race
MZL IRs overall were highest among NHWs (IR=19.0) and lowest among blacks (IR=13.1; Table II). A persistent NHW predominance was observed for nodal MZL and most extranodal sites, with several key exceptions. Compared to NHWs, HWs had significantly higher IRs of salivary gland MZL (IRR=1.56, 95% CI 1.23–1.96), and APIs had significantly higher MZL IRs in the colon/rectum (IRR=2.15, 95% CI 1.57–2.89) and eye/adnexa (IRR=1.45, 95% CI 1.18–1.76). The salivary MZL IR was significantly higher among HW females than NHW females (IRR=1.64, 95% CI 1.26–2.11) but not among males (IRR=1.23, 95% CI 0.70–2.00) (data not shown). The incidence of stomach MZL was generally similar across all racial/ethnic groups, with no significant difference in incidence among HWs, blacks or APIs compared to NHWs. MZL IRs of the colon/rectum and thyroid were lower among NHWs than other racial/ethnic groups, although only the elevated colon/rectum rate among APIs was significant. The higher IR of colon/rectum MZL among APIs was significant among females (API:NHW IRR=2.88, 95% CI 1.95–4.17) but not males (API:NHW IRR=1.40, 95% CI 0.78–2.33).
Table II.
Age-adjusted incidence rates and incidence rate ratios of marginal zone lymphoma according to site and race/ethnicity, SEER-18, 2001–2009*
| NHWs |
HWs |
Blacks |
APIs |
HW-to-NHW |
Black-to-NHW |
API-to-NHW |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IR | n | IR | n | IR | n | IR | IRR | (95% CI) | IRR | (95% CI) | IRR | (95% CI) | |
| All sites | 9,730 | 19.0 | 1,247 | 16.7 | 915 | 13.1 | 874 | 14.9 | 0.88 | (0.82–0.93)† | 0.69 | (0.64–0.74)† | 0.78 | (0.73–0.84)† |
| All nodal MZL | 3,163 | 6.2 | 355 | 4.9 | 276 | 4.0 | 197 | 3.4 | 0.80 | (0.71–0.90)† | 0.65 | (0.57–0.74)† | 0.56 | (0.48–0.64)† |
| All extranodal MZL | 6,422 | 12.6 | 880 | 11.5 | 623 | 8.8 | 668 | 11.3 | 0.92 | (0.85–0.99)† | 0.70 | (0.64–0.76)† | 0.90 | (0.83–0.98)† |
| Salivary glands | 420 | 0.8 | 103 | 1.3 | 42 | 0.6 | 56 | 0.9 | 1.56 | (1.23–1.96)† | 0.71 | (0.50–0.99)† | 1.10 | (0.81–1.46) |
| Stomach | 1,916 | 3.7 | 285 | 3.8 | 224 | 3.3 | 210 | 3.6 | 1.03 | (0.90–1.18) | 0.88 | (0.76–1.01) | 0.97 | (0.83–1.12) |
| Small intestine | 196 | 0.4 | 24 | 0.3 | <16 | ~ | 23 | 0.4 | 0.75 | (0.45–1.17) | ~ | 1.04 | (0.64–1.61) | |
| Colon/rectum | 242 | 0.5 | 45 | 0.6 | 39 | 0.6 | 56 | 1.0 | 1.31 | (0.91–1.81) | 1.25 | (0.86–1.77) | 2.15 | (1.57–2.89)† |
| Lung | 536 | 1.0 | 42 | 0.6 | 58 | 0.8 | 34 | 0.6 | 0.54 | (0.38–0.75)† | 0.75 | (0.56–0.99)† | 0.56 | (0.38–0.79)† |
| Spleen | 926 | 1.8 | 83 | 1.2 | 55 | 0.8 | 40 | 0.7 | 0.66 | (0.52–0.83)† | 0.43 | (0.32–0.57)† | 0.40 | (0.28–0.54)† |
| Skin | 533 | 1.1 | 62 | 0.7 | 28 | 0.4 | 38 | 0.6 | 0.65 | (0.48–0.85)† | 0.33 | (0.22–0.49)† | 0.54 | (0.37–0.75)† |
| Soft tissue, including heart | 130 | 0.3 | 16 | 0.2 | <16 | ~ | <16 | ~ | 0.90 | (0.49–1.51) | ~ | ~ | ||
| Female breast | 194 | 0.7 | 17 | 0.4 | 27 | 0.7 | <16 | ~ | 0.61 | (0.34–1.00) | 0.97 | (0.62–1.46) | ~ | |
| Eye/adnexa | 694 | 1.4 | 105 | 1.3 | 73 | 1.0 | 121 | 2.0 | 0.98 | (0.78–1.21) | 0.73 | (0.56–0.93)† | 1.45 | (1.18–1.76)† |
| Thyroid | 124 | 0.2 | 24 | 0.3 | <16 | ~ | 17 | 0.3 | 1.25 | (0.75–1.97) | ~ | 1.19 | (0.67–1.99) | |
Abbreviations: SEER-18, 18 cancer registry areas of the Surveillance, Epidemiology and End Results Program; NHWs, non-Hispanic whites; HWs, Hispanic whites; APIs, Asians/Pacific Islanders; n, Number of cases; IR, incidence rate; IRR, incidence rate ratio; CI, confidence interval; & IRs and IRRs not calculated for fewer than 16 cases; MZL, marginal zone lymphoma
Table excludes American Indians/Alaskan natives (n=60) and individuals with unspecified race/ethnicity (n=270). Extranodal sites included in the table are those for which ≥16 cases were diagnosed among at least two of the racial/ethnic groups. All incidence rates are age-adjusted to the 2000 US standard population and expressed per 1,000,000 person-years. Incidence rate ratios are based on unrounded rates.
95% CI excludes 1.00 (based on unrounded upper and lower CI), and IRR is significant (P<0.05).
In contrast to MZL, IRs of solid tumours of the salivary glands and thyroid were significantly lower among HWs, blacks, and APIs compared to NHWs, whereas stomach cancer IRs among each racial/ethnic group were about twice those among NHWs (Supplementary Table III). IRs of solid tumours of the colon/rectum were 20% higher only among blacks and approximately 20% lower among HWs and APIs compared to NHWs.
Calendar year
Overall incidence of MZL rose significantly by 10% from 2001–2005 to 2006–2009 (2006–2009:2001–2005 IRR), with an increase of 25% for nodal MZL in contrast to virtually no change for extranodal MZL (2006–2009:2001–2005 IRR=1.02) (Table III). However, among the extranodal sites, IRs significantly increased for MZL of the lung, skin, kidney/renal pelvis, and thyroid (2006–2009:2001–2005 IRR=1.18, 1.43, 2.16, and 1.42, respectively). The only MZL sites for which the IR decreased significantly was the stomach (2006–2009:2001–2005 IRR=0.85) and soft tissues (2006–2009:2001–2005 IRR=0.72).
Table III.
Age-adjusted incidence rates and incidence rate ratios of marginal zone lymphoma according to site and calendar year of diagnosis, SEER-18, 2001–2009*
| 2001–2005 | 2006-–2009 | (2006–2009)-to-(2001–2005) | ||||
|---|---|---|---|---|---|---|
| n | IR | n | IR | IRR | (95% CI) | |
| All sites | 6,679 | 17.4 | 6,417 | 19.3 | 1.10 | (1.07–1.14)† |
| All nodal MZL | 1,958 | 5.1 | 2,123 | 6.4 | 1.25 | (1.17–1.33)† |
| All extranodal MZL | 4,681 | 12.2 | 4,140 | 12.4 | 1.02 | (0.97–1.06) |
| Oral cavity/oropharynx | ||||||
| HPV-related sites | 54 | 0.1 | 52 | 0.2 | 1.11 | (0.74–1.66) |
| HPV-unrelated sites | 54 | 0.1 | 35 | 0.1 | 0.75 | (0.47–1.17) |
| Salivary glands | 349 | 0.9 | 289 | 0.9 | 0.96 | (0.82–1.13) |
| Nasopharynx | 40 | 0.1 | 23 | 0.1 | 0.66 | (0.38–1.14) |
| Stomach | 1,552 | 4.1 | 1,150 | 3.5 | 0.85 | (0.79–0.92)† |
| Small intestine | 135 | 0.3 | 123 | 0.4 | 1.07 | (0.83–1.37) |
| Colon/rectum | 203 | 0.5 | 196 | 0.6 | 1.09 | (0.89–1.33) |
| Lung | 336 | 0.9 | 344 | 1.0 | 1.18 | (1.01–1.38)† |
| Spleen | 593 | 1.6 | 532 | 1.6 | 1.03 | (0.91–1.16) |
| Skin | 309 | 0.8 | 383 | 1.1 | 1.43 | (1.23–1.67)† |
| Soft tissue, including heart | 103 | 0.3 | 67 | 0.2 | 0.72 | (0.52–0.99)† |
| Female breast | 137 | 0.7 | 115 | 0.6 | 0.95 | (0.74–1.23) |
| Kidney/renal pelvis | 18 | 0.05 | 33 | 0.1 | 2.16 | (1.18–4.09)† |
| Bladder | 17 | 0.05 | 23 | 0.1 | 1.53 | (0.78–3.05) |
| Eye/adnexa | 521 | 1.4 | 505 | 1.5 | 1.12 | (0.99–1.27) |
| Thyroid | 78 | 0.2 | 96 | 0.3 | 1.42 | (1.04–1.95)† |
Abbreviations: SEER-18, 18 cancer registry areas of the Surveillance, Epidemiology and End Results Program; n, number; IR, incidence rate; IRR, incidence rate ratio; CI, confidence interval; MZL, marginal zone lymphoma; HPV, human papillomavirus.
All incidence rates are age-adjusted to the 2000 US standard population and expressed per 1,000,000 person-years. Incidence rate ratios are based on unrounded rates.
95% CI excludes 1.00 (based on unrounded upper and lower CI), and IRR is significant (P<0.05).
Similar to MZL, incidence of solid tumours of skin, kidney/renal pelvis, and thyroid increased in 2006–2009 compared to 2001–2005 (2006–2009:2001–2005 IRR 1.07, 1.14, 1.33, respectively) and stomach cancer IRs decreased, although less prominently (6%) than the decrease (15%) observed for MZL (Supplementary Table IV). Unlike MZL, lung cancer IRs decreased significantly during the study time period (2006–2009:2001–2005 IRR=0.95), with significant decreases also noted for cancers of colon/rectum (2006–2009:2001–2005 IRR=0.89) and eye/adnexa (2006–2009:2001–2005 IRR=0.91). In contrast to the decline in soft tissue MZL, a 4% rise in incidence was noted for solid tumours at this site.
Discussion
Aetiological heterogeneity in lymphoma subtypes is suggested by varying lymphoma incidence patterns (Morton, et al 2006). With careful assessment of MZL incidence by patient subgroup, our study is the first population-based incidence study to support the aetiological heterogeneity in MZL occurring in different primary sites. Differences in the latency periods and/or timing of exposures are suggested by sites with early-onset (e.g., salivary glands, stomach, skin, eye/adnexa) and late-onset (colon/rectum, lung, spleen) incidence patterns. Differences in susceptibility or environmental exposures are supported by gender and racial/ethnic disparities observed across primary sites. The most notable disparity was found in MZL of the salivary glands, which predominated among females across the entire age spectrum, whereas MZL of the skin predominated among males across all age groups. Despite the fact that all nodal and all extranodal MZL had the highest incidence among NHWs, salivary gland MZL had a clear Hispanic predominance, and colon/rectum and eye/adnexa MZL predominated among APIs. Lastly, despite several infectious agents being implicated in the aetiology of MZL at specific sites (e.g., H. pylori, C. psittaci, C. jejuni, B. burgdorferi), the only site for which incidence rates decreased over time was stomach MZL, which was probably attributable to widespread recognition, treatment and eradication of H. pylori infection (Luminari, et al 2010).
As with other lymphoid neoplasms (Morton, et al 2006), the incidence of MZL increases with age, although the rate of rise and age at onset vary by primary site. Age-related increases in immune senescence, autoimmunity, and chronic inflammation probably contribute to the rising MZL incidence rates (Boren and Gershwin 2004, Coussens and Werb 2002). The onset in mid-life of MZL of the colon/rectum, lung, and spleen suggests that aetiological exposures are associated with long latency periods and/or occur later in life. These patterns contrast with the incidence of colon and lung carcinomas, which are characterized by early age at onset possibly related to an underlying genetic predisposition, younger age at relevant exposure(s) and/or a shorter latency period (Kamangar, et al 2006).
The incidence of MZL increased exponentially with age, reminiscent of the exponential rise in incidence with advancing age that characterizes the multi-step model of carcinogenesis described for solid tumours (Armitage and Doll 2004). Indeed, the multi-stage theory of carcinogenesis has been supported in epidemiological and molecular studies of gastric MZL (Seydel, et al 2003, Zucca, et al 1998). For gastric MZL, the declining rate of rise in incidence at older ages may reflect a change in susceptibility as H. pylori is treated. Similarly, MZL of salivary glands, lung, spleen and eye/adnexa were each characterized by a significant rise in IR among younger ages with slowing of the IR at older ages. The slowing in incidence rates with aging may reflect a change in disease susceptibility or different relevant exposures that vary with age. For example, the chronic inflammation and strong predilection for salivary gland involvement in Sjogren syndrome has been implicated in MZL occurring at this site. However, Sjogren syndrome is typically a disease of middle-aged women, thereby raising the question of whether it might disproportionately contribute to MZL occurring at older ages. Hepatitis C virus (HCV) has also been linked with non-Hodgkin lymphoma, including MZL of salivary glands and splenic lymphoma (De Vita, et al 1998, Peveling-Oberhag, et al 2013). Depending on the age at exposure, HCV might differentially contribute to MZL disease burden across the age spectrum. MZL of skin is the only evaluable site that was best characterized by a progressive rise in incidence with age (no joinpoints), suggesting less aetiological heterogeneity at this site.
Male gender is a known risk factor for most cancers, although reasons for this disparity are uncertain (Cook, et al 2009). With the exception of MZL of the salivary glands and thyroid, the overall incidence of nodal and extranodal MZL was significantly higher among males than females. The salivary glands, soft tissues and thyroid are sites known to harbour autoimmune and chronic inflammatory conditions that occur more commonly among females (Gleicher and Barad 2007). Notably, carcinoma of the salivary glands does not predominate among women (Cook, et al 2009). However, both MZL of the thyroid and thyroid carcinoma are associated with significantly higher rates among females than males, raising the possibility of shared risk factors for MZL and carcinomas at this site. Hashimoto thyroiditis, a female-predominant disease, has been associated with both thyroid lymphoma and papillary carcinoma (Ahmed, et al 2012). Sjogren syndrome also preferentially affects females (M:F ratio of 1 to 8–20) (Piram, et al 2012) and is associated with autoimmunity and chronic inflammation, both risk factors for salivary gland MZL (Smedby, et al 2006), and lymphomatous and non-lymphomatous thyroid cancer (Jara, et al 2007, Weng, et al 2012). However, Sjogren syndrome in men does not appear to increase the risk of any type of cancer (Weng, et al 2012). The gender disparity observed for salivary gland MZL was noted across all age groups, a pattern that is not observed for any of the major histological subtypes of salivary gland carcinoma (Boukheris, et al 2009).
In contrast to MZL of the salivary glands, MZL of skin predominated among males across all ages. B. burgdorferi infection has been associated with skin MZL in some cases in Europe, but not in the U.S., Asia and some parts of Europe, thereby challenging the aetiological role of this agent (Takino, et al 2008, Willemze, et al 2005). A role for autoimmune diseases is not suggested given that these occur uncommonly in association with MZL of skin (Willemze, et al 2005). The predominance of MZL of the skin occurring on the head/neck, trunk and upper extremities raises a possible link with ultraviolet radiation, given the similar distribution pattern seen in melanoma of skin, Merkel cell carcinoma and squamous cell and basal cell carcinomas (Karagas, et al 2006, Miller and Rabkin 1999). However, studies of ultraviolet radiation exposure and lymphoma have suggested an inverse relation with MALT lymphoma (Kricker, et al 2008, van Leeuwen, et al 2013). Similar to other skin cancers (Agelli and Clegg 2003, Karagas, et al 2006), MZL of the skin was associated with significantly lower incidence among all non-NHW racial/ethnic groups compared to NHWs, supporting a possible protective role of skin pigmentation.
For all nodal, stomach, colon/rectum, lung and spleen MZLs, IRs among males and females were remarkably similar across all except the oldest age groups. As such, the male predominance we report for overall rates is driven exclusively by the oldest age groups. This finding suggests a less prominent role for environmental exposures (including tobacco), occupational exposures and hormonal factors contributing to lymphomagenesis at these sites. Although male gender has been shown to be a significant risk factor for acquiring H. pylori infection (Replogle, et al 1995), we found nearly equal IRs of MZL of the stomach among men and women, except at older ages.
Our study is among the first to report a significantly increased incidence of salivary gland MZL among HWs compared to NHWs, with this finding limited to females. Data that describe Sjogren syndrome among different racial/ethnic groups are sparse, with one French study reporting twice the frequency among non-European than European residents, but with little representation of Hispanic populations (Piram, et al 2012). Few studies have evaluated HCV infection rates among Hispanics. However, in the U.S., Hispanic males have higher frequency of HCV positivity than Hispanic females (Tohme, et al 2013). As such, the reason for predominance of salivary gland MZL among Hispanic females remains uncertain. We confirm the previous finding of higher IRs of colon/rectum and eye/adnexa MZL among APIs than whites described in a population-based study covering 82% of the U.S. between 1999–2003 (Wu, et al 2009). We also found that the incidence of colon/rectum MZL among API females was almost triple that among NHW females, in contrast to a non-significant 40% higher incidence among API males compared to NHW males. We did not observe a similar API predominance for colorectal carcinoma, suggesting that alternate or additional factors probably contribute to MZL of the colon/rectum. A higher incidence of eye/adnexa MZL was also observed among male and female APIs (compared to NHWs), although risk factors for this racial/ethnic predominance are unknown. Future epidemiological studies of site-specific MZL should take racial/ethnic and gender differences into consideration.
Between 2001–2005 and 2006–2009, the overall incidence of MZL increased by 10%, which may, in part, reflect improved diagnosis. Cigarette smoking, a well-established risk factor for lung carcinoma, has not been convincingly linked with NHL (Castillo and Dalia 2012). Supporting this contention, we found a significant (18%) increase in MZL of the lung in 2006–2009 compared to 2001–2005, whereas lung cancer incidence decreased over this time period in keeping with the decline in smoking prevalence since the 1970s (Alberg, et al 2007). In contrast, the incidence of both stomach MZL and stomach carcinoma decreased during the study time period. Chronic gastritis due to H. pylori infection is a known predisposing condition for stomach MZL (Zucca, et al 1998), with epidemiological, clinical and experimental data supporting this strong association (Bayerdorffer, et al 1995, Doglioni, et al 1992, Hussell, et al 1993). The prevalence of H. pylori in the United States in the 1990s was estimated at 30–40% (Peterson, et al 2000) and is believed to be decreasing due to falling rates of infection during childhood (Fennerty 2005) and the introduction of effective eradication therapy. While HCV has been implicated in MZL, the percentage of individuals pursuing HCV treatment is smaller than those who do not pursue treatment (Tohme, et al 2013). Therefore, if barriers to HCV treatment can be overcome, IRs of HCV-related MZL should also decrease in the future. As additional MZL risk factors are identified, specific treatment or prevention efforts can similarly be applied.
The strengths of our study include the large numbers of cases available in a population-based setting, thus avoiding the biases associated with clinical series and allowing evaluation of MZL incidence by anatomic site. Despite the large number of cases, we were limited in the number of sites that could be fully evaluated by age, gender and race/ethnicity. Other limitations include the absence of centralized expert review, which may have detected significant misclassification of other B-cell lymphomas as MZL and the possibility of misclassification of MZL by site, particularly when multiple anatomic sites may be involved by MZL. As an example, we defined nodal MZL according to primary site of involvement and cannot be certain that these cases did not have evidence of extranodal or splenic involvement, according to the WHO definition of nodal MZL (Swerdlow, et al 2008). Additional studies will be needed to confirm the incidence patterns we describe by primary site as well as the age-specific patterns, perhaps with larger number of cases and/or alternate statistical methods.
In summary, our population-based study identifies distinctive MZL incidence patterns by primary site of disease and supports the aetiological diversity that has been suggested in smaller clinical series of MZL (Remstein, et al 2006, Streubel, et al 2004, Ye, et al 2003). Significant differences in incidence patterns by site highlight potential susceptibility variations by age, gender, and race/ethnicity, and temporal patterns suggest a possible role for environmental factors. For MZL sites with rising incidence (e.g., lung, skin, kidney/renal pelvis), consideration of increasing infectious or inflammatory diseases or changing environmental exposures should be pursued in future aetiological research. The findings from our study serve as a foundation to inform future epidemiological studies of MZL and support simultaneous consideration of age, gender, and race/ethnicity in addition to environmental exposures and co-existing health conditions.
Supplementary Material
Acknowledgments
This work was supported by the Department of Veterans Affairs Medical Center, Oklahoma City, OK and the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD. The authors thank Marth a S. Linet, M.D., M.P.H. for important contributions to the design of this research.
Footnotes
Authorship Contributions: MOK, LMM, SSD, REC, GMD designed the research; MOK, DPC, GMD conducted the analysis; MOK, LMM, SSD, REC, DDW, GMD analysed and interpreted the data; MOK and GMD wrote the manuscript; MOK, LMM, SSD, DPC, REC, DDW, GMD critically reviewed the manuscript and contributed important intellectual content.
Conflict of Interest Disclosures: The authors have no conflict of interest to declare.
References
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–841. doi: 10.1016/s0190-9622(03)02108-x. [DOI] [PubMed] [Google Scholar]
- Ahmed R, Al-Shaikh S, Akhtar M. Hashimoto thyroiditis: a century later. Adv Anat Pathol. 2012;19:181–186. doi: 10.1097/PAP.0b013e3182534868. [DOI] [PubMed] [Google Scholar]
- Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines. Chest. (2nd edition) 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. 1954. Int J Epidemiol. 2004;33:1174–1179. doi: 10.1093/ije/dyh216. [DOI] [PubMed] [Google Scholar]
- Bayerdorffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- Bende RJ, Maldegem van F, Noesel van CJ. Chronic inflammatory disease, lymphoid tissue neogenesis and extranodal marginal zone B-cell lymphomas. Haematologica. 2009;94:1109–1123. doi: 10.3324/haematol.2009.005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren E, Gershwin ME. Inflamm-aging: autoimmunity, and the immune-risk phenotype. Autoimmun Rev. 2004;3:401–406. doi: 10.1016/j.autrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Boukheris H, Curtis RE, Land CE, Dores GM. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: a population-based study in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:2899–2906. doi: 10.1158/1055-9965.EPI-09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo JJ, Dalia S. Cigarette smoking is associated with a small increase in the incidence of non-Hodgkin lymphoma: a meta-analysis of 24 observational studies. Leuk Lymphoma. 2012;53:1911–1919. doi: 10.3109/10428194.2012.673225. [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, Devesa SS, McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita S, Zagonel V, Russo A, Rupolo M, Cannizzaro R, Chiara G, Boiocchi M, Carbone A, Franceschi S. Hepatitis C virus, non-Hodgkin's lymphomas and hepatocellular carcinoma. Br J Cancer. 1998;77:2032–2035. doi: 10.1038/bjc.1998.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- Doglioni C, Wotherspoon AC, Moschini A, Boni de M, Isaacson PG. High incidence of primary gastric lymphoma in northeastern Italy. Lancet. 1992;339:834–835. doi: 10.1016/0140-6736(92)90280-g. [DOI] [PubMed] [Google Scholar]
- Fennerty MB. Helicobacter pylori: why it still matters in 2005. Cleve Clin J Med. 2005;72(Suppl 2):S1–S7. doi: 10.3949/ccjm.72.suppl_2.s1. discussion S14–21. [DOI] [PubMed] [Google Scholar]
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology. World Health Organization, Geneva; Switzerland: 2000. [Google Scholar]
- Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun. 2007;28:1–6. doi: 10.1016/j.jaut.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda: 2012. [Google Scholar]
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- Hwang YC, Kim TY, Kim WB, Shong YK, Yi KH, Shong M, Jo YS, Kim WS, Chung JH. Clinical characteristics of primary thyroid lymphoma in Koreans. Endocr J. 2009;56:399–405. doi: 10.1507/endocrj.k08e-355. [DOI] [PubMed] [Google Scholar]
- Jara LJ, Navarro C, Mdel Brito-Zeron P, Garcia-Carrasco M, Escarcega RO, Ramos-Casals M. Thyroid disease in Sjogren's syndrome. Clin Rheumatol. 2007;26:1601–1606. doi: 10.1007/s10067-007-0638-6. [DOI] [PubMed] [Google Scholar]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Weinstock MA, Nelson HH. Keratinocyte carcinomas (basal and squamous cell carcinomas of the skin) In: by Schottenfeld D, Fraumeni JF Jr., editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. pp. 1230–1250. [Google Scholar]
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kricker A, Armstrong BK, Hughes AM, Goumas C, Smedby KE, Zheng T, Spinelli JJ, Sanjose De S, Hartge P, Melbye M, Willett EV, Becker N, Chiu BC, Cerhan JR, Maynadie M, Staines A, Cocco P, Boffeta P. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122:144–154. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- Luminari S, Cesaretti M, Marcheselli L, Rashid I, Madrigali S, Maiorana A, Federico M. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol. 2010;21:855–859. doi: 10.1093/annonc/mdp402. [DOI] [PubMed] [Google Scholar]
- Mellemkjaer L, Pfeiffer RM, Engels EA, Gridley G, Wheeler W, Hemminki K, Olsen JH, Dreyer L, Linet MS, Goldin LR, Landgren O. Autoimmune disease in individuals and close family members and susceptibility to non-Hodgkin's lymphoma. Arthritis Rheum. 2008;58:657–666. doi: 10.1002/art.23267. [DOI] [PubMed] [Google Scholar]
- Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999;8:153–158. [PubMed] [Google Scholar]
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson WL, Fendrick AM, Cave DR, Peura DA, Garabedian-Ruffalo SM, Laine L. Helicobacter pylori-related disease - Guidelines for testing and treatment. Archives of Internal Medicine. 2000;160:1285–1291. doi: 10.1001/archinte.160.9.1285. [DOI] [PubMed] [Google Scholar]
- Peveling-Oberhag J, Arcaini L, Hansmann ML, Zeuzem S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol. 2013;59:169–177. doi: 10.1016/j.jhep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Piram M, Maldini C, Mahr A. Effect of race/ethnicity on risk, presentation and course of connective tissue diseases and primary systemic vasculitides. Curr Opin Rheumatol. 2012;24:193–200. doi: 10.1097/BOR.0b013e32835059e5. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Ralfkiaer E, Prause JU, Sjo LD, Siersma VD, Heegaard S. Malignant lymphoma of the lacrimal gland: a nation-based study. Arch Ophthalmol. 2011;129:1275–1280. doi: 10.1001/archophthalmol.2011.270. [DOI] [PubMed] [Google Scholar]
- Rawal A, Finn WG, Schnitzer B, Valdez R. Site-specific morphologic differences in extranodal marginal zone B-cell lymphomas. Arch Pathol Lab Med. 2007;131:1673–1678. doi: 10.5858/2007-131-1673-SMDIEM. [DOI] [PubMed] [Google Scholar]
- Remstein ED, Dogan A, Einerson RR, Paternoster SF, Fink SR, Law M, Dewald GW, Kurtin PJ. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol. 2006;30:1546–1553. doi: 10.1097/01.pas.0000213275.60962.2a. [DOI] [PubMed] [Google Scholar]
- Replogle ML, Glaser SL, Hiatt RA, Parsonnet J. Biologic sex as a risk factor for Helicobacter pylori infection in healthy young adults. Am J Epidemiol. 1995;142:856–863. doi: 10.1093/oxfordjournals.aje.a117725. [DOI] [PubMed] [Google Scholar]
- SEER-18 (2012) Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2011 Sub, Vintage 2009 Pops (2000–2009) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012, based on the November 2011 submission.
- Seydel J, Ullrich A, Bender R, Fischbach W, Blettner M. Helicobacter pylori and carcinogenesis of gastric B-cell lymphomas. Int J Cancer. 2003;104:646–649. doi: 10.1002/ijc.10994. [DOI] [PubMed] [Google Scholar]
- Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, Sundstrom C, Akerman M, Melbye M, Glimelius B, Adami HO. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- Streubel B, Simonitsch-Klupp I, Mullauer L, Lamprecht A, Huber D, Siebert R, Stolte M, Trautinger F, Lukas J, Puspok A, Formanek M, Assanasen T, Muller-Hermelink HK, Cerroni L, Raderer M, Chott A. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722–1726. doi: 10.1038/sj.leu.2403501. [DOI] [PubMed] [Google Scholar]
- Suarez F, Lortholary O, Hermine O, Lecuit M. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107:3034–3044. doi: 10.1182/blood-2005-09-3679. [DOI] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- Takino H, Li C, Hu S, Kuo TT, Geissinger E, Muller-Hermelink HK, Kim B, Swerdlow SH, Inagaki H. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathological study of cases from Asia, Germany, and the United States. Mod Pathol. 2008;21:1517–1526. doi: 10.1038/modpathol.2008.159. [DOI] [PubMed] [Google Scholar]
- Thieblemont C, Berger F, Dumontet C, Moullet I, Bouafia F, Felman P, Salles G, Coiffier B. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000;95:802–806. [PubMed] [Google Scholar]
- Tohme RA, Xing J, Liao Y, Holmberg SD. Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009–2010. Am J Public Health. 2013;103:112–119. doi: 10.2105/AJPH.2012.300858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse-Glehen A, Baseggio L, Salles G, Felman P, Berger F. Splenic marginal zone B-cell lymphoma: a distinct clinicopathological and molecular entity. Recent advances in ontogeny and classification. Curr Opin Oncol. 2011;23:441–448. doi: 10.1097/CCO.0b013e328349ab8d. [DOI] [PubMed] [Google Scholar]
- Turner JJ, Morton LM, Linet MS, Clarke CA, Kadin ME, Vajdic CM, Monnereau A, Maynadie M, Chiu BC, Marcos-Gragera R, Costantini AS, Cerhan JR, Weisenburger DD. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116:e90–e98. doi: 10.1182/blood-2010-06-289561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen MT, Turner JJ, Falster MO, Meagher NS, Joske DJ, Grulich AE, Giles GG, Vajdic CM. Latitude gradients for lymphoid neoplasm subtypes in Australia support an association with ultraviolet radiation exposure. Int J Cancer. 2013 doi: 10.1002/ijc.28081. [DOI] [PubMed] [Google Scholar]
- Weng MY, Huang YT, Liu MF, Lu TH. Incidence of cancer in a nationwide population cohort of 7852 patients with primary Sjogren's syndrome in Taiwan. Ann Rheum Dis. 2012;71:524–527. doi: 10.1136/annrheumdis-2011-200402. [DOI] [PubMed] [Google Scholar]
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- Wu XC, Andrews P, Chen VW, Groves FD. Incidence of extranodal non-Hodgkin lymphomas among whites, blacks, and Asians/Pacific Islanders in the United States: anatomic site and histology differences. Cancer Epidemiol. 2009;33:337–346. doi: 10.1016/j.canep.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Ye H, Liu H, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F, Leblond V, Speight P, Goodlad J, Lavergne-Slove A, Martin-Subero JI, Siebert R, Dogan A, Isaacson PG, Du MQ. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012–1018. doi: 10.1182/blood-2002-11-3502. [DOI] [PubMed] [Google Scholar]
- Zucca E, Bertoni F, Roggero E, Bosshard G, Cazzaniga G, Pedrinis E, Biondi A, Cavalli F. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid-tissue lymphoma of the stomach. N Engl J Med. 1998;338:804–810. doi: 10.1056/NEJM199803193381205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.