Abstract
Background/Aims
We have compared dendritic cell (DC) function derived from the alcoholic liver disease (ALD) sensitive Long–Evans (LE) and resistant Fischer rat strains to determine if the influence of ethanol on DCs was dependent on ALD.
Methods
The LE and Fischer rats were fed an ethanol-containing or isocaloric control liquid diet for 8 weeks and comparisons were made to LE rats injected with thioacetamide as a liver disease control. DCs were isolated from the spleen after expansion with human Fms-like tyrosine kinase receptor 3 ligand plasmid. Maturation markers CD86, CD80, CD40 and MHC-II were analysed by flow cytometry with or without lipopolysaccharide and poly I:C stimulation. Production of tumour necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-12p40 and IL-10 cytokines and the antigen presentation ability of DCs was determined.
Results
Only LE rats developed ALD characterized by liver injury, elevated alanine aminotransferase levels and steatosis; CD86 and CD40 expression was decreased in LE but not Fischer rats. Reduced TNF-α, IFN-γ, IL-12, proinflammatory and enhanced IL-10 cytokine production was found in DCs isolated from ethanol-fed LE but not Fischer rats. Allostimulatory activity was reduced in LE compared with the Fischer strain. In contrast, DCs isolated from thioacetamide-induced liver damage displayed a reduction only in IL-12p40; TNF-α, IL-10 and IFN-α production as well as antigen presenting ability remained intact compared with controls.
Conclusions
ALD sensitive LE rats exhibited characteristics of a suppressed DC phenotype that was not observed following thioacetamide-induced liver disease, which suggests an important role for ALD in altering the host cellular and humoral immune responses.
Keywords: dendritic cells, ethanol, immune function, liver disease, thioacetamide
Alcohol-induced disorders have become a serious health problem in USA. About 7.1% of Americans older than 18 years meet the criteria for alcohol abuse (1). All cell types within the liver including hepatocytes, immune cells and hepatic stellate cells may be adversely influenced by ethanol because it affects metabolic, immunological and inflammatory cellular processes.
Ethanol consumption is closely related to the progression of HCV infection. Excessive ethanol intake increases HCV RNA levels in serum, which is accompanied by impairment of cellular immunity (2, 3). Heavy ethanol abuse greatly exacerbates the risk of cirrhosis among patients with persistent HCV infection (3, 4). The increased risk of HCV progression in alcoholic patients may be attributed to impaired cellular immunity to viral infection induced by chronic ethanol abuse (2). Long-term ethanol ingestion may broadly influence immunity (5, 6). Previous experimental studies suggest that CD4+ T cell proliferation and cytotoxic T-lymphocyte (CTL) activity responses to NS5 or hepatitis C virus (HCV) core proteins were suppressed by chronic ethanol consumption (3, 7, 8). Further investigations reveal that viral antigen specific impairment of CD4+ T cell proliferation and CTL activity may be restored by interleukin (IL)-2 and granulocyte-macrophage colony-stimulating factor additions (7, 8). These findings imply the potential involvement of antigen presenting cells or dendritic cell (DC) dysfunction in altering the host cellular immune response induced by ethanol.
The influence of chronic ethanol exposure on DC function was further explored using a murine model (9). DCs derived from mice fed ethanol for 8-weeks exhibited decreased maturation marker expression such as CD40 and CD80 necessary for antigen presentation although endocytic uptake of proteins exhibited by immature DCs was not affected. Further studies confirmed that allostimulatory activity of DCs derived from ethanol-fed mice was impaired compared with cells isolated from isocaloric pair-fed control animals. Ethanol feeding was shown to suppress pro-inflammatory cytokine [tumour necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-12 and IL-6] production and secretion by DCs when stimulated with lipopolysaccharide (LPS) or poly I:C; in contrast, IL-10 levels were increased. More importantly, impaired CTL responses to HCV NS5 protein observed in ethanol-fed mice could be restored by syngeneic transfer of DCs derived from isocaloric pair-fed controls but not ethanol-fed animals (9).
The results from this murine model establish the inhibitory effects of ethanol on DC function and illustrates how it impairs anti-viral immune response (9–13). Similar inhibitory actions of ethanol on human DCs have been observed by others (14–16). However, it is unknown whether the alteration of DC function is due entirely to ethanol alone (without liver disease) or ethanol plus alcoholic liver disease (ALD) using this murine model because there was lack of progressive hepatocyte injury, oxidative stress, mitochondrial dysfunction, severe steatosis and fibrosis upon continued alcohol abuse. Recently, it was observed, unexpectedly, that an outbred Long–Evans (LE) rat strain exhibited many of the pathological, biochemical and molecular features of ethanol-induced hepatotoxicity similar to human disease while on the Lieber–DeCarli liquid diet, which have not been previously observed in any other rodent model (17–20). In this context, we compared the features of ethanol-induced liver injury in the susceptible LE rats with two other inbred strains i.e., Fischer and Sprague–Dawley (18–21); other than mild steatosis, Fischer rats did not develop liver disease whereas Sprague–Dawley had an intermediate phenotype. We hypothesized that DC function may be differentially affected by ALD which could be ideally studied in experimentally susceptible and resistant rat strains. To test this idea, we fed both the LE (sensitive) and Fischer (resistant) rats with the Lieber–DeCarli liquid diet for 8 weeks and compared results to the isocaloric pair-fed controls with respect to the characteristics and downstream effects of ethanol on ALD and DC function because DCs are a critical cellular component in generating robust adaptive immune responses to viral and bacterial pathogens to which alcoholics are highly susceptible. Finally, to determine if alterations in DC functions were directly related to ALD and not to necroinflammatory liver diseae per se, we utilized a liver disease control produced in LE rats. In this model, liver injury was induced in LE rats by treatment with thioacetamide, which has been previously shown to produce severe acute and chronic hepatocyte injury followed by the development of cirrhosis (22, 23).
Materials and methods
Media and reagents
The DCs were cultured in HEPES-buffered RPMI-1640 medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 2mM l-Glutamine, 1% non-essential amino acid 5 × 10−5M β-2-mercaptoethanol, 1mM sodium pyruvate, 100 U/ml penicillin, 100 µg/ml streptomycin and heat inactivated 10% fetal bovine serum. Other chemicals (LPS, poly I:C) were purchased from Sigma-Aldrich.
Rats and ethanol feeding regimen
The 150–230 g, male Fischer and LE rats (Harlan Laboratories, Indianapolis, IN, USA) were fed ad libitum with ethanol containing or isocaloric control liquid diet (Bioserv, Frenchtown, NJ, USA) for 8 weeks. After 8 weeks, the animals were injected rapidly intravenously (X2) via the tail vein on day 0 and 6 with a plasmid for DCs generation in the spleen and then sacrificed. Blood was collected by cardiac puncture. Liver tissue was excised and processed for histological analysis and biochemical assays. For the liver injury model, chow-fed LE rats were administered 200 mg/kg body weight of thioacetamide (Sigma-Aldrich) dissolved in saline or saline alone (control) intraperitoneally (i.p.) three times a week for 1 month before expansion of DCs in the spleen by human Fms-like tyrosine kinase receptor 3 ligand (hFlt3L) plasmid injection. All animal protocols have been reviewed and approved by the Lifespan Animal Care and Use Committee.
Histology
Histological changes in the liver were assessed by hematoxylin– eosin (H&E), Oil Red O and Trichrome staining. Slides were scanned with Aperio Scancope CS (Aperio Technologies Inc., Vista, CA, USA).
Immunohistochemistry
Liver samples were fixed using histofix and frozen using optimal cutting temperature compound (Tissue-Tek Fisher Scientific, Pittsburgh, PA, USA). Then, 5 µm thick sections were cut using a microtome (Reichert-Jung model 2030) and placed on microscope slides. The slides were submerged in a pressure cooker containing distilled water to allow for rehydration. Antigen Unmasking Solution (Vector Laboratory, Burlingame, CA, USA) was then added to the pressure cooker, and the samples were allowed to boil and increase in pressure in order for antigen retrieval. The sections were washed with phosphate-buffered saline and incubated with 3% blocking serum in fluorescent treponema antigen for 30 min in humidified chamber at 20 °C. A 1:1 mixture containing both anti-rat cytokeratin 8 and 18 antibodies (Progene Biotechnik, Heidelberg, Germany) was added onto the sections and allowed to incubate for 1 h in a humidified chamber at 20 °C. The sections were followed by an incubation with biotinylatetd secondary antibody (Vector laboratory) for 30 min. Tissue sections were incubated in ABC solution (Vector Laboratory) for 45 min. Finally, the slides were immersed in 0.5% diaminobenzidine tetrahydrochloride (Sigma-Aldrich) for 3–5 min at 20 °C for development, rinsed with distilled water, stained with hematoxylin (Surgipath, Richmond, IL, USA), rinsed and mounted on cover slips using Micromount Mounting Medium (Surgipath).
Biochemical assays
Serum alanine aminotransferase (ALT) levels were measured by using a commercially available kit (Thermo Fischer Scientific Inc., Waltham, MA, USA). Serum ethanol levels were measured by Analox GM7 analyser (Analox Instruments, Lunenburg, MA, USA). Liver lysates were used to measure triglyceride concentrations according to manufacturer’s instructions (BioVision Inc., Mountain View, CA, USA).
Generation of dendritic cells
To increase the rat DC population in vivo, 300 µg of the plasmid pUMVC3-hFLex, expressing the secreted portion of the hFlt3L (Vector Core Laboratory, University of Michigan, Ann Arbor, MI, USA), was dissolved in 25 ml Ringer’s solution and injected directly into the tail vein of rats within 15 s using the hydrodynamic gene delivery protocol (24). The plasmid was injected twice (days 0 and 6), and spleens were harvested on day 12 for preparation of single spleen cell suspensions in serum-free medium after red blood cells were lysed. The OX62+DCs were isolated with magnetic beads coated with monoclonal antibodies to DC (OX62) after 14.5% Nycodenz gradient centrifugation enrichment according to the to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA, USA). The DCs obtained by this method from both the ethanol and control diet-fed Fischer and LE rats were used for subsequent assays.
Cytokine production
To evaluate cytokine production, DCs derived from ethanol and control diet-fed rats were cultured at 24-well flat-bottom plates at 1 × 106 cells in 1ml of medium. Cells were stimulated with 0.2 µg/ml, or 1 µg/ml of LPS or 1 µg/ml, or 10 µg/ml of poly I:C. After 24 h, the supernatants were collected and the levels of IFN-γ, TNF-α, IL-12p40 and IL-10 were quantified using enzyme- linked immunosorbent assay (ELISA) kits purchased from eBioscience (San Diego, CA, USA), Invitrogen (Carlsbad, CA, USA) or BD Biosciences (San Jose, CA, USA) and performed according to the manufacturer’s instructions.
Allostimulatory activity
T cells were isolated from the spleens Fischer and LE rats by a commercial available pan T-cell isolation kit that uses immunomagnetic beads according to the manufacturer’s protocol (Miltenyi Biotec). The DCs isolated from ethanol and control pair-fed Fischer and LE rats were added in triplicate at various ratios to 1 × 105 T lymphocytes in 96-well flat-bottom plates (T cells from Fischer rats added to DCs from LE rats, as well as T cells from LE rats added to DCs from Fischer rats). Cultures were maintained in IMDM (Sigma-Aldrich) supplemented with 2mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 1mM sodium pyruvate, 1mM HEPES, 5 × 10M β-2-mercaptoethanol and 5% normal rat serum for 3 days. The secretion of IL-2 was measured by ELISA kits purchased from R&D systems (Minneapolis, MN, USA). This set of experiments evaluated antigen presentation ability before and after maturation of DCs by 0.2 µg/ml of LPS for 24 h.
Flow cytometry analysis
Cells (5 × 105) were incubated with purified anti-rat CD16/32 to block Fc receptors for 5 min and then incubated with phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-labelled antibodies. Antibodies were diluted in Hank’s balanced salt solution containing 2% fetal bovine serum, which also served as the wash medium. Samples were analysed with a FACScalibur (BD Biosciences). The following antibodies were used for cell surface staining: PE-anti-rat CD103 (OX62, eBioscience), FITC-anti-rat CD80 (AbD Serotec, Kidlington, UK), FITC-anti-rat CD4 (BD Biosciences), FITC-anti-rat CD86 (BD Biosciences), FITC-anti-rat CD40 (BD Biosciences), FITC-anti-rat MHC-II (BD Biosciences) and along with the recommended isotype controls.
Statistical analysis
Results were analysed using the sigmastat statistics program (Jandel Scientific, San Rafael, CA, USA). Individual means were compared using a non-paired Student’s t-test. Data derived from >2 groups were compared by one-way analysis of variance followed by a Tukey Dunn test to identify the groups that differed. Differences at P<0.05 were considered significant.
Results
Long–Evans rats develop liver injury and steatosis whereas Fischer rats do not
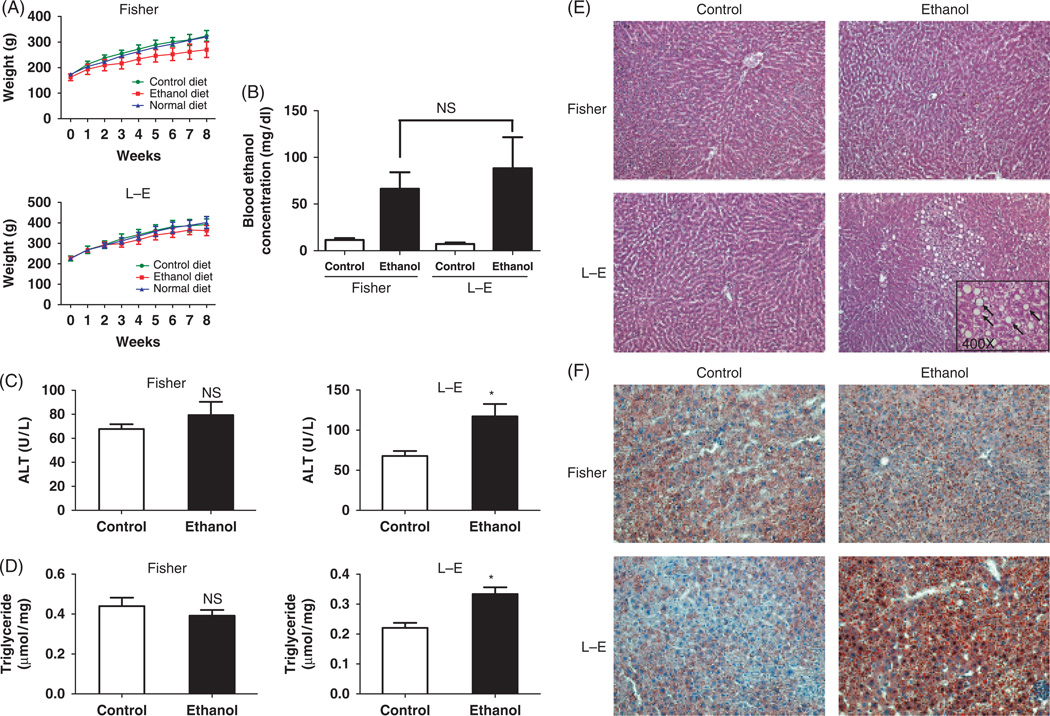
We fed Fischer and LE rats with the ethanol containing diet for 8 weeks. The body weight was similar in animals consuming the control liquid diet, compared with those given the normal diet (chow fed). Rats consuming the ethanol liquid diet showed a slightly reduced (P = NS) bodyweight compared with matched animals fed the isocaloric control diet in both rat strains (Fig. 1A). Blood ethanol concentration was measured; no difference in blood levels was found in ethanol-fed Fischer compared with LE rats (Fig. 1B), suggesting that both strains had similar ethanol intake. Liver triglyceride content and ALT levels were comparable in ethanol and isocaloric pair-fed control Fischer rats. However, liver triglycerides and ALT levels were significantly increased in the chronic ethanol-fed LE rats compared with animals that received the isocaloric pair-fed control diet (Fig. 1C and D). These observations, confirmed by histology, suggest that liver injury and steatosis were present in LE compared with Fischer rats during chronic ethanol feeding; indeed H&E staining demonstrated striking micro-and macrovesicular steatosis (Fig. 1E and F), foci of inflammation and hepatocyte degeneration (Fig. 2A) in ethanol-fed LE but not in Fischer rats (Figs 1E and 2B). There was no evidence of cirrhosis by trichrome staining (data not shown) but prolonged administration of alcohol of greater than one year in the LE rat produces marked fibrosis and cirrhosis (25). Oil red staining revealed substantial hepatic micro- and macrosteatosis in LE compared with Fischer rats (Figs 1F, 2A and B). It was of interest that intermediate filament cytokeratin 8/18 intracellular levels were decreased in ethanol-fed LE rat steatotic hepatocytes undergoing degeneration (Fig. 2A and B) as has been observed in human steatohepatitis (26). Taken together, LE but not Fischer rats developed histological features of ALD after 8-week of ethanol feeding, which further substantiates previous findings (17–21). In addition, LE rats develop metabolic and hepatic injury characteristic of ALD illustrated by oxidative stress, mitochondrial DNA damage, p53 activation, insulin resistance, decreased survival signalling and enhanced apoptosis of hepatocytes as described (21); such features are similar to observations in humans with ALD.
Fig. 1.
Long–Evans (LE) but not Fischer rats developed alcoholic liver disease (ALD). (A) Changes in body weight of Fischer and LE rats on ethanol and isocaloric pair-fed control liquid or chow-fed normal diets for 8 weeks (B). Blood ethanol concentrations of Fischer and LE rats on isocaloric control or ethanol liquid diets. Measurement of liver triglyceride (C) or serum alanine aminotransferase (ALT) levels (D) derived from Fischer and LE rats on control or ethanol liquid diets for 8 weeks, *P < 0.05. Hematoxylin–eosin (H&E) (E) and Oil Red O staining (F) of Fischer and LE rat liver. Arrows (inset) indicate robust macro- and microsteatosis (magnification, × 100 for H&E, × 200 for Oil Red O staining, and × 400 for the inset). There were six animals in the ethanol and pair-fed groups.
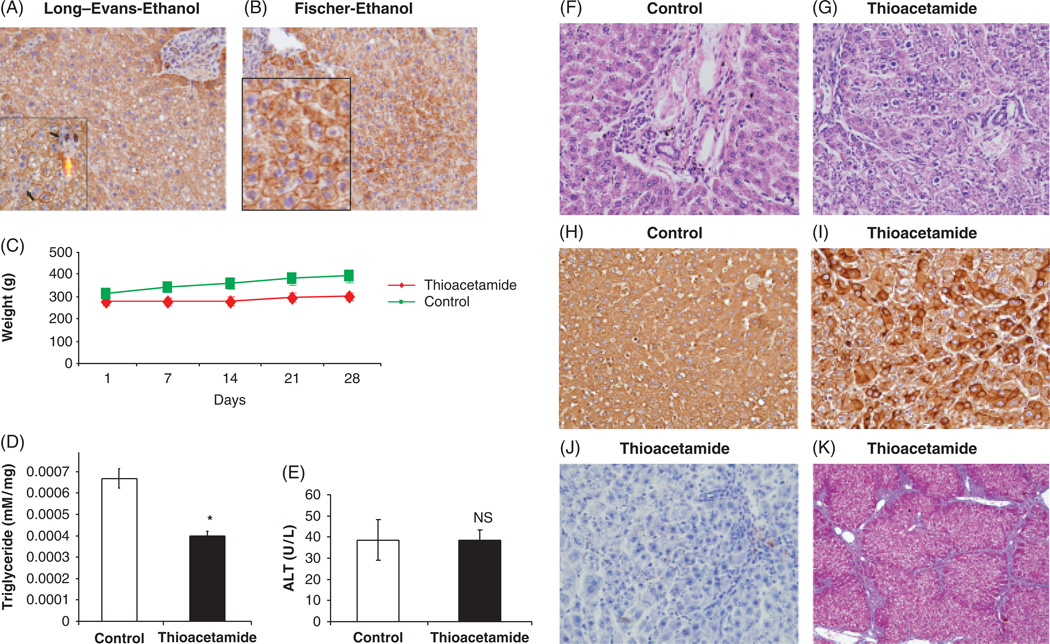
Fig. 2.
Characteristics of liver injury. Immunohistochemistry of cytokeratin 8 and 18 staining (× 100) of liver derived from Long–Evans (LE) (A) and Fischer (B) rats. In the LE strain (inset × 400), there was macro- and microsteatosis, hepatocyte degeneration (large arrow) and foci of inflammation (small arrows) (C) Body weight of thioacetamide-treated LE rats compared with control. (D) Triglyceride levels (TG) of thioacetamide or saline-treated LE rats, *P < 0.05. Significantly less IG deposition in the thioacetamide-treated group was observed. (E) Serum alanine aminotransferase (ALT) levels in thioacetamide and control LE rats. H&E staining of control (F) and thioacetamide-treated (G) rat livers illustrating hepatocyte necrosis and cell drop out as well as lobular disarray (× 100). Cytokeratin 8 and 18 immunostaining of (H) control and thioacetamide-treated (I) rat liver demonstrate reduced staining in degenerating hepatocytes and lesser degree of immunoreactivity in severely steatotic hepatocytes of ethanol-treated LE rats (A and B; × 200). (J) Represents Oil Red-O staining of thioacetamide-treated rat liver demonstrating strikingly lower lipid accumulation compared with alcoholic liver disease (ALD) (see Fig. 1E; × 200). (K) Trichrome staining of thioacetamide-treated rat liver demonstrating the presence of cirrhosis in addition to severe liver injury (× 10).
To be able distinguish whether the adverse effects on DCs are because of ethanol-induced liver disease rather than hepatic necroinflammation and cirrhosis as produced by another agent, we treated LE rats with thioacetamide to induce severe acute and chronic hepatocyte injury in the absence of ethanol. Indeed, body weight was significantly higher for the rats injected with saline as compared with those receiving thioacetamide (Fig. 2C). Biochemical and histological analyses of hepatic lipids and triglycerides (Fig. 2D and J) revealed lower amounts of accumulation in thioacetamide-treated LE rat liver which was in contrast to finding of ALD produced in the same species (Fig. 1D and F). Staining by H&E demonstrated extensive hepatocyte necrosis, cell drop out and lobular disarray (Fig. 2G and I) as well as prominent cirrhosis by trichrome staining (Fig. 2K). There was reduced cytokeratin 8/18 staining in many hepatocytes undergoing necrosis of the thiocatamide-treated LE rat compared with saline injected controls (Fig. 2H–I).
Dendritic cell expansion by hydrodynamic delivery of the human Fms-like tyrosine kinase receptor 3 ligand plasmid
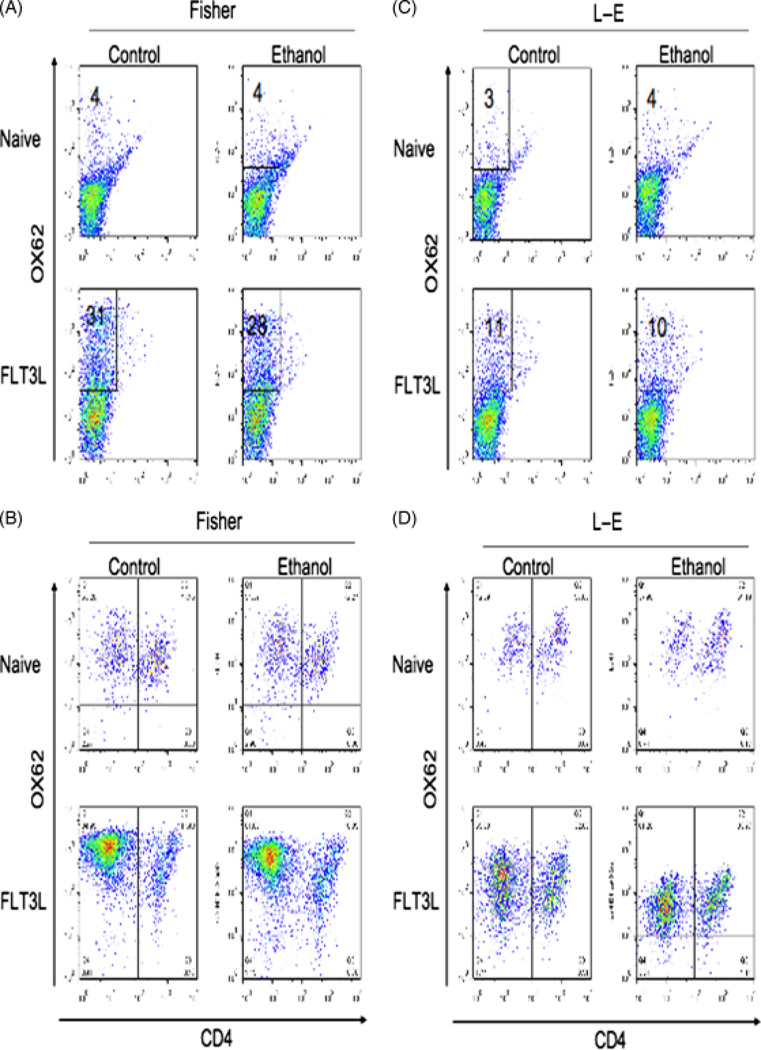
To obtain sufficient DCs for subsequent experiments, we employed hydrodynamic delivery of the hFlt3L encoding plasmid (pUMVC3-hFlex to expand this population in the spleen. Using this procedure, the total number of splenocytes was increased from 3–4 × 108 to 8–10 × 108 in Fischer rats and from 5–7 × 108 to 8.5–10.5 × 108 in LE rats on the chronic ethanol diet (P = NS). About 30% splenocytes in Fischer rats and 10% splenocytes in LE rats were OX62 positive after hFlt3L plasmid injection compared with about 4% OX62 positive cells in both Fischer and LE rat spleens before injection of this plasmid. There were no significant differences in the efficacy of expansion in animals on the isocaloric pair-fed diet compared with the ethanol-fed group (Fig. 3A and C). Two different subset of rat DCs were characterized by the expression of the surface CD4 marker as described previously (27, 28). DCs were isolated from control and ethanol-fed Fischer and LE rats by magnetic-activated cell sorting, then stained them for OX62 and CD4+ expression. As shown in Fig. 3B and D, the percent of OX62+CD4− DCs was strikingly increased while the percent of OX62+CD4+ cells decreased, suggesting that hFlt3L expression principally expanded the OX62+CD4− subpopulation.
Fig. 3.
Isolation and characterization of dendritic cells (DCs) from the spleen following expansion by hydrodynamic delivery of human Fms-like tyrosine kinase receptor 3 ligand (hFlt3L) encoding plasmid. Flow cytometry analysis of splenocytes isolated from Fischer (A) or Long–Evans (LE)rats (C) before and after hFlt3L plasmid administration. Flow cytometry analysis of DCs purified from Fischer (B) and LE rats (D) by magnetic beads before or after hFlt3L plasmid administration. Thus, hydrodynamic delivery and expression of the hFlt3L plasmid greatly expands the DC population in rats. The experiment was repeated three times with similar results.
Ethanol decreased expression of CD86 and CD40 in dendritic cells from Long–Evans rats
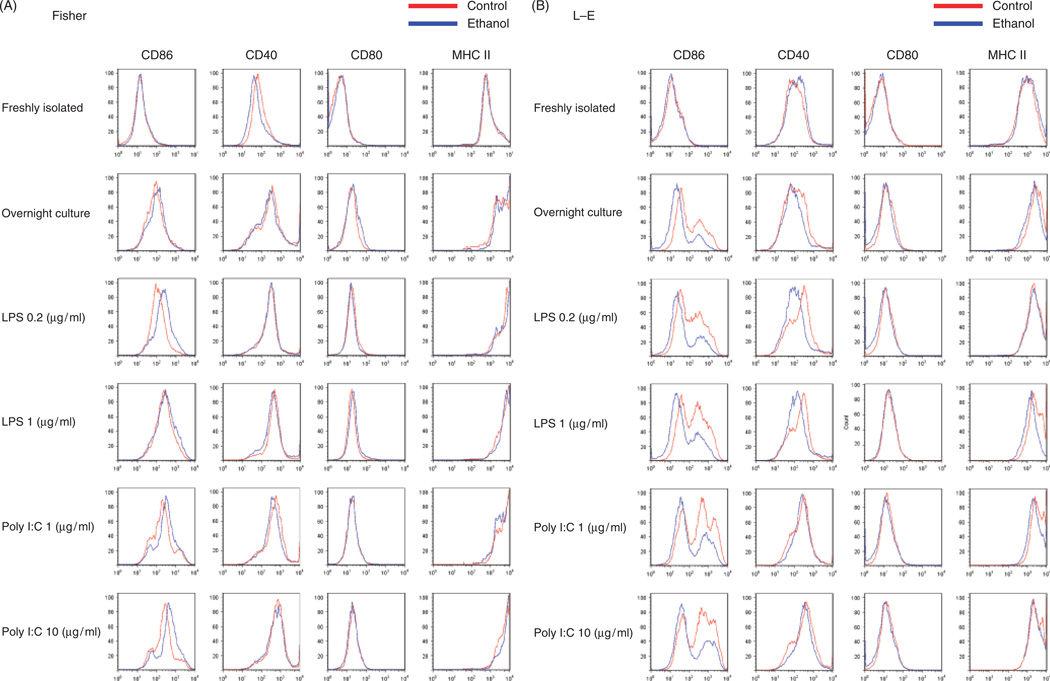
We examined cell surface maturation marker expression. Freshly isolated DCs expressed relatively low levels of CD86, CD40, CD80, which is consistent with previous results demonstrating that Flt3L treatment induced equal expansion of immature DCs (29, 30). There were no differences in the expression of these maturation markers in control and ethanol-fed Fischer and LE rats except for a slightly lower CD40 in DCs isolated from Fischer control diet-fed animals compared with the ethanol group (Fig. 4A and B). DCs were cultured overnight with or without LPS/poly I:C additions. In Fischer rats, we observed higher CD86 expression in ethanol-fed rats stimulated with LPS low (0.2 µg/ml), poly I:C low (1 µg/ml) and high (10 µg/ml) groups; all other maturation markers were unchanged with ethanol feeding (Fig. 4A). In contrast, DCs derived from chronic ethanol-fed LE rats revealed significantly lower expression of CD86 in overnight culture with LPS/poly I:C (Fig. 4B). We observed substantially lower CD40 expression in overnight cultures with LPS in ethanol-fed LE rats as well (Fig. 4B). These results demonstrated that chronic ethanol feeding was associated with decreased expression of key cell surface molecules such as CD86 and CD40 required for activating T cells in the ALD-sensitive LE but not the resistant Fischer rat strain. To determine the effect of thiocatamide on DC phenotype, we analysed CD40 and CD86 expression on DCs derived from treated LE rats (data not shown). CD40 expression levels were comparable to the saline control on the DCs surface in both the immature and mature state after LPS (0.2 µg/ml) and poly I:C (1 µg/ml) stimulation. However, CD86 expression was increased in mature DCs derived from thioacetamide-treated LE rats indicating that this liver injury model does not adversely effect costimulatory molecule expression when compared with DCs isolated from ALD.
Fig. 4.
Flow cytometry analysis of maturation marker expression. Dendritic cells (DCs) were purified by magnetic beads from control or ethanol-fed Fischer and Long–Evans (LE) rats after human Fms-like tyrosine kinase receptor 3 ligand (hFlt3L) expansion. These isolated DCs were studied both fresh and after overnight culture with or without lipopolysaccharide (LPS), and poly I:C additions. DCs were evaluated for CD86, CD40, CD80 and MHC-II expression. (A) Results from DCs derived from Fischer rats on control and ethanol diet and (B) LE rats. Note that chronic ethanol feeding decreases CD86 and CD40 expression on DCs derived from LE but not in Fischer rats. The experiment was repeated three times with similar results.
Ethanol reduced pro-inflammatory and increased anti-inflammatory cytokine production in dendritic cells derived from Long–Evans rats
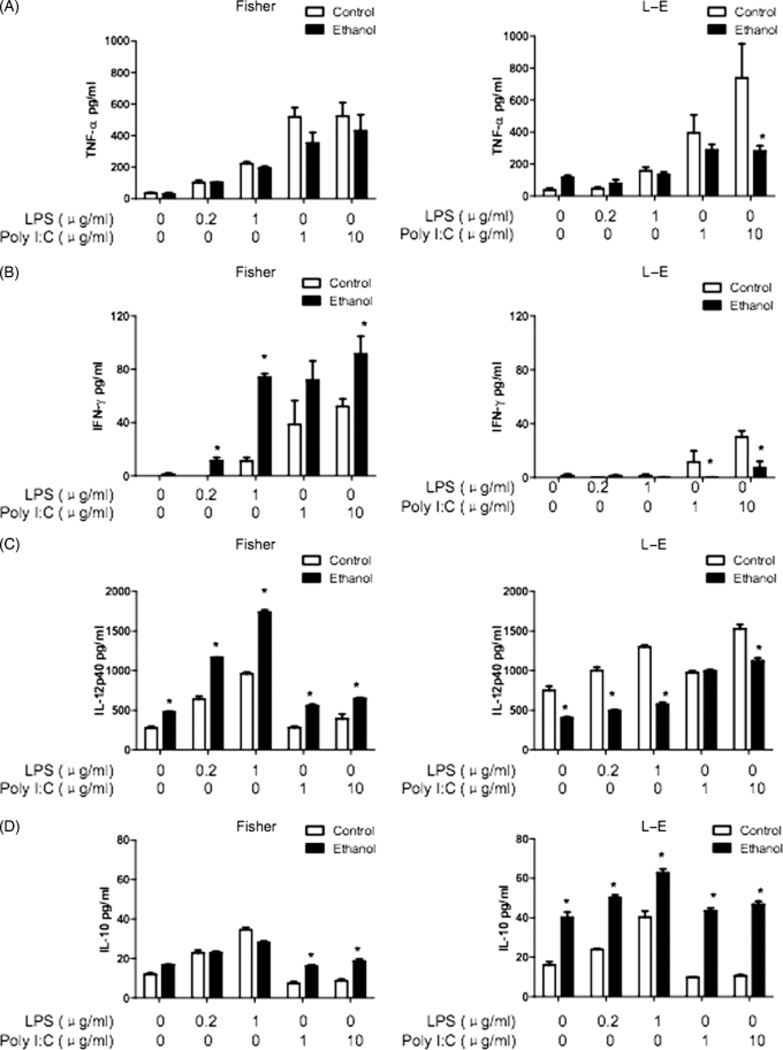
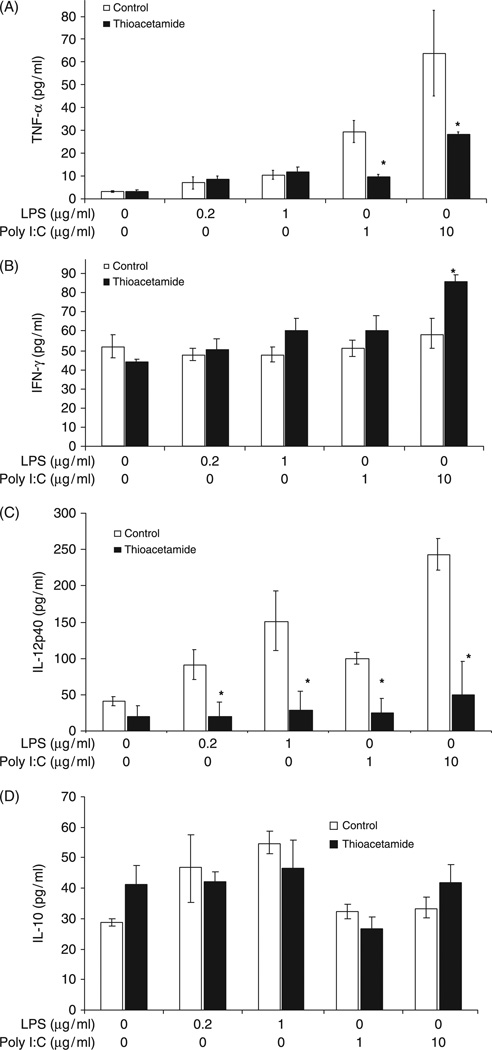
Several important cytokines (TNF-α, IFN-γ, IL-12 and IL-10) secreted by DCs were measured; these molecules are considered critical for determining the Th1/Th2 balance and subsequent anti-viral and anti-bacterial immune responses. Cytokine production by DCs after LPS or poly I:C stimulation were determined by ELISA. All pro-inflammatory cytokines were significantly suppressed in DCs derived from chronic ethanol-fed LE rats compared with isocaloric pair-fed controls after LPS or poly I:C stimulation (Fig. 5). In Fischer rats, the TNF-α levels were similar in DCs isolated from ethanol and control diet-fed groups after LPS and poly I:C stimulation whereas IFN-γ and IL-12 were found to be surprisingly higher in the chronic ethanol-fed group (Fig. 5). In contrast, IL-10 levels were similar in LPS-stimulated DCs isolated from isocaloric pair-fed controls and ethanol-fed Fischer rats; IL-10 levels were slightly higher after poly I:C stimulation in DCs derived from these animals. However, there was a substantial increase in IL-10 production in both LPS and poly I:C stimulated DCs derived from ethanol-fed LE rats compared with isocaloric pair-fed controls (Fig. 5). Therefore, major alterations were observed (suppression of pro-inflammatory and upregulation of anti-inflammatory cytokines) in the production and secretion patterns exhibited by DCs when comparing the ALD-sensitive LE to resistant Fischer rat strains after consuming the chronic ethanol and isocaloric pair-fed control diets for 8 weeks. Cytokine expression profiling of mature and immature DCs were extended to the thioacetamide LE rat model as well (Fig. 6). The major finding was that IL-12p40 levles were much lower in both immature and mature DCs. However, IL-10 levels were no different than controls, which was in striking contrast to DCs isolated from ALD (compare Figs 5D to 6D). There were also substantial differences in IFN-γ secretion as indicated by higher levels produced from DCs isolated from thioacetamide-treated LE rats (Figs 5B and 6B). Finally, these were substantially reduced secretion of TNF-α by DCs isolated from thioacetamide LE rats compared with ALD (Figs 5A and 6A).
Fig. 5.
Cytokine production by dendritic cells (DCs) after LPS or poly I:C stimulation. DCs were purified by magnetic beads from the spleens of control or ethanol-fed Fischer and LE rats after human Fms-like tyrosine kinase receptor 3 ligand (hFlt3L) expansion. These DCs were cultured for 24 h with or without LPS or poly I:C. Cell culture supernatants were collected for cytokine measurements by enzyme-linked immunosorbent assay. (A) Tumour necrosis factor (TNF)-α levels, *P < 0.05, (B) interferon (IFN)-γ levels, *P < 0.01, (C) interleukin (IL)-12p40 levels, *P < 0.01. (D) IL-10 levels, *P < 0.01. It is apparent that chronic ethanol feeding inhibits pro-inflammatory and increases anti-inflammatory cytokine production in the alcoholic liver disease-sensitive LE rat. The experiment was repeated three times with similar results.
Fig. 6.
Cytokine secretion by dendritic cells (DCs) derived from thioacetamide-treated rats upon LPS or poly I:C stimulation compared with saline administered controls. DCs were isolated after human Fms-like tyrosine kinase receptor 3 ligand (hFlt3L) expansion, treated and cytokines measured as described in Fig. 5. (A) Tumour necrosis factor (TNF)-α, *P < 0.05, (B) interferon (IFN)-γ, *P < 0.01, (C) interleukin (IL)-12p40, *P < 0.01 decrease in thioacetamide-treated rats compared with the control group, while (D) IL-10 levels are not significantly different from control DCs.
Allostimulatory activity exhibited by dendritic cells
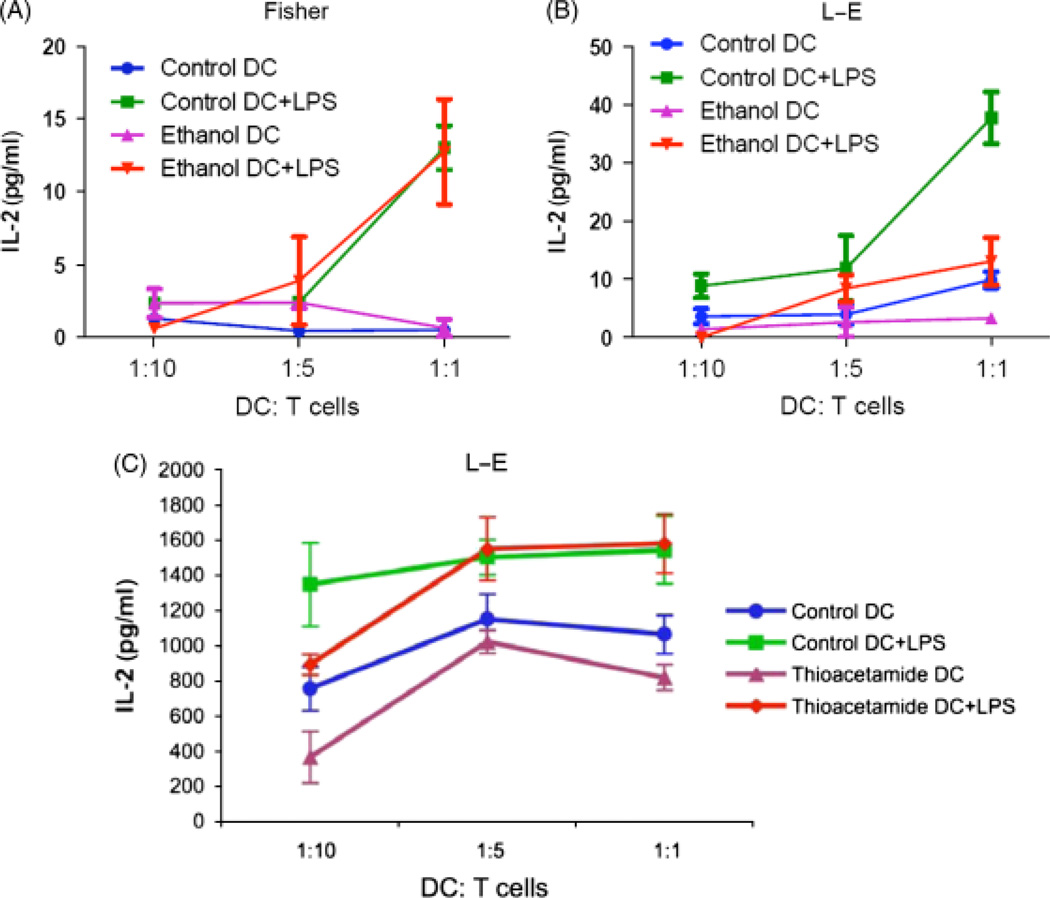
The capability of antigen presentation by DCs was evaluated by measuring allostimulatory activity. Before maturation induced by LPS, DCs from all groups had a very low ability to initiate proliferation of naïve allogeneic T cells (Fig. 7). DCs matured by LPS showed a higher capacity to initiate proliferation. In LE rats, ethanol feeding significantly decreased DCs allostimulatory activity as indicated by low IL-2 production when compared with DCs isolated from controls and cocultured with naïve allogeneic T cells. However, in the ALD resistant Fischer rats, no significant difference in IL-2 production was observed by chronic ethanol feeding as compared with animals on the control diet when their DCs were cocultured with naïve allogeneic T cells (Fig. 7). Comparison of alloreactivity of both immature and mature DCs derived from thioacetamide-treated LE rats to control revealed no difference in antigen presentation ability suggesting that the functional impairment in DCs is associated with ALD and not the severe hepatocyte injury and cirrhosis produced by thioacetamide (Fig. 7C).
Fig. 7.
Allostimulatory activity exhibited by dendritic cells (DCs) before or after lipopolysaccharide (LPS) maturation. DCs were purified by magnetic beads from the spleen of control and ethanol-fed Fischer and LE rats or thioacetamide-treated LE rats after human Fms-like tyrosine kinase receptor 3 ligand (hFlt3L) expansion. DCs were cultured with/without 0.2 µg/ml LPS as a maturation stimulus. After 24 h of culture, DCs were collected and cocultured with naïve allogeneic T cell purified by magnetic beads from Long–Evans (LE) or Fischer rats as indicated ratio. Supernatants were collected for detection of interferon (IL)-2 levels as measured by enzyme-linked immunosorbent assay after 3 days culture. (A) IL-2 levels of Fischer DCs cocultured with LE T cells, and (B) IL-2 levels of LE DCs cocultured with Fischer T cells. (C) IL-2 levels of thioacetamide-treated LE DCs cocultured (allostimulation) with Fischer T cells. Similar to DCs purified from ethanol-treated Fischer rats, thioacetamide does not impair DC function in activating T cells. The experiment was repeated three times with similar results.
Discussion
In the present study, DCs were expanded for functional analysis by using the hydrodynamic delivery of hFlt3L plasmid, which has not previously been established in various rat strains (31–34). Comparisons were made with respect to DC phenotype and function in ALD sensitive and resistant rat strains to determine the possible role of liver disease in the aetiology of DC functional impairment in the context of chronic ethanol exposure. Thioacetamide-induced hepatocyte injury was used as a liver disease control (7–9).
The DCs have been used for immunotherapy against chronic viral infection like HCV and advanced tumour growth because of their unique properties of antigen processing, presentation and stimulation of CD4+ and CD8+ immune responses (9, 31–33). It is difficult to generate sufficient numbers of DCs for characterization and subsequent vaccine studies in rats (9, 33). We have previously developed protocols to expand large numbers of DCs in mice by using hydrodynamic gene delivery of the hFlt3L expression plasmid pUMVC3-hFLex (9, 31, 32), which increased DC populations about five- to 10-fold. There has been no reports regarding DC expansion in rats. In this study, we developed an effective protocol. In these investigations, the plasmid dose was increased from 10 µg in 2 ml saline (mouse) to 300 µg in 25ml Ringer’s solution for the rat based on established criteria (24, 35). The DC expansion efficiency was similar to mice in the Fischer rats where more than 25% of the splenocytes were OX62 positive DCs compared with 4% in non-treated animals. Although the expansion efficiency was lower in LE rats (about 10% splenocytes were OX62 positive DCs), the hydrodynamic plasmid delivery greatly increased the splenic DC numbers (Fig. 3). The reason for this phenomenon may be due, in part, to the presence of ALD in the LE rat as expression of the hFlt3L plasmid depends on transfection of functional hepatocytes following hydrodynamic injection (24). However, unlike mice, we did not find an adverse influence of ethanol feeding on DC expansion efficiency in both Fischer and LE rat strains (Fig. 3). The mechanisms for lack of an ethanol effect in this regard are unknown but may relate, in part, to species variation or genetic differences. The hFlt3L injection approach was applied to thioacetamide-treated LE rats. These rats had hypersplenism as previously reported (36), but the DC number retrieved from the spleen was slightly lower in the thioacetamide-treated animals compared with the saline- treated controls (data not shown).
The enriched DC population was characterized by staining for CD8+ and CD4+ cell surface markers. Consistent with previous observations, all OX62 positive cells were CD8 negative (37). Two DC subsets were identified based on whether they expressed CD4 or not as described previously (27, 28, 37). Although the ontogeny of DC subsets in rats has not been extensively studied, CD4−DCs appeared to be a homogenous population of cells with myeloid-like morphological features and CD4+DCs appeared much more heterogeneous regarding their morphology and to a lesser extent their phenotype (28). After the hydrodynamic delivery of the hFlt3L expression plasmid, CD4−DCs were preferentially expanded in both Fischer and LE rats (Fig. 3). These findings were consistent with previous studies in mice, where CD11c+CD11b+ myeloid DCs comprise more than 80% of total CD11c+DCs population following Flt3L expansion (32). Such observations suggest that myeloid DCs are also the main target of Flt3L-induced expansion in the rat.
There is a wide spectrum of alcohol-related liver pathology in humans. Alcohol is not only a direct hepatotoxin but is also considered to be a ‘permissive’ agent that causes liver injury (38, 39). Several rodent models have been established using the Lieber–DeCarli ethanol-containing liquid diet as well as the Tsukamoto–French intragastric feeding approach with or without lipid additions to the ethanol-containing diet (40). The use of such animal models has contributed to a better understanding of how the severity of liver injury may be influenced by factors other than ethanol, such as nutrition (fat intake), oxygen deprivation, gene regulation and immune dysfunction. However, small animal models that recapitulate the hepatic histological, biochemical and molecular changes indicative of human ALD have been difficult to develop (40).
There is a need to develop an ALD rodent model that has histological and biochemical features of human disease. Most murine and rat strains develop very mild liver injury and steatosis. However, recent studies revealed that a certain outbred rat strain was highly susceptible whereas inbred strains were resistant to ALD when fed the Lieber–DeCarli liquid diet (18–21). Indeed, LE rats remarkably reveal ALD features such as insulin resistance, decreased cell survival signalling, hepatic steatotis, hepatocellular injury, hepatocyte apoptosis following degeneration, increased oxidative stress and enhanced collagen gene and TNF-α expression when placed on an ethanol diet 8 weeks or longer (17–21). In contrast, Fischer rats do not develop liver disease or any of the above features found in the LE strain (Fig. 1). Thus, these animal models provide an experimental system to not only better characterize the liver disease related to continued alcohol consumption but to know precisely the state of chronic liver disease by histological and biochemical analysis at the time of immunological study to access DC function.
Thus, to determine how ALD affected DC functional properties, such cells were isolated from control or ethanol-fed Fischer and LE rats after hFlt3L expansion. Maturation marker expression including CD86, CD40, CD80, MHC-II were analysed by flow cytometry in freshly isolated DCs and those cultured with/without different concentrations of maturation stimuli such as LPS/poly I:C for 24 h. The CD86, CD40 and CD80 expression levels were relatively low in freshly isolated DCs from both control and ethanol-fed Fischer or LE rats; after overnight culture, expression levels were significantly increased. Furthermore, expression of these maturation markers were increased in the presence of LPS/poly I:C in a dose dependent manner (Fig. 4A and B). MHC-II expression was found to be high in freshly isolated DCs and significantly increased following overnight culture with or without LPS/poly I:C stimulation.
In Fischer rats, expression levels of these markers were found to be similar in freshly isolated or cultured DCs. The CD40 levels were marginally lower (P = NS) in freshly isolated DCs from ethanol-fed rats, but this difference disappeared after overnight culture with/without LPS/poly I:C additions. Surprisingly, CD86 levels were slightly higher (P = NS) in ethanol-fed Fischer rats compared with animals that received the control diet.
In contrast, CD86 and CD40 expression was significantly lower (P < 0.01) in ethanol-fed LE rats compared with controls after overnight culture with/without LPS/poly I:C stimulation. Consistent with cell surface maturation marker expression, it was found that pro-inflammatory cytokines production and secretion including TNF-α, IFN-γ, IL-12p40 was substantially suppressed while IL-10 was greatly increased in ethanol-fed LE rats compared with controls following LPS/poly I:C stimulation, whereas in Fischer rats, the TNF-α production was comparable in DCs isolated from both ethanol fed and controls (i.e., animals fed the isocaloric control diet sans ethanol). Moreover, IFN-γ and IL-12p40 concentrations were higher in ethanol-fed Fischer rats than controls (Fig. 5A and B). Finally, no difference in TNF-α levels was observed in ethanol and control diet-fed DCs isolated from the ethanol and isocaloric pair-fed Fischer strain. Overall there is a propensity to generate Th2-type immune responses because the secretion of three critical cytokines (namely TNF-α, IFN-γ and IL-12) promote the Th1 differentiation pathway of CD4+ and CD8+ into antigen-specific functional cells is strikingly impaired. In addition, IL-10 is increased in ethanol-fed animals that provides another boost towards a robust Th2-type response observed only in the ALD sensitive LE rat.
Parallel to ethanol-fed LE and Fischer rats, DCs derived from thioacetamide-treated LE rats were characterized with respect to costimulatory molecule expression, cytokine production and antigen presenting capacity to determine if the phenotypic and functional alterations in DCs observed in ALD could also be found with severe liver injury and cirrhosis in the absence of chronic alcohol. Thioacetamide is a hepatotoxin known to cause centrilobular necrosis after a single dose, and to develop cirrhosis and hepatocellular carcinoma upon its chronic administration (22, 23). After its activation by FAD and CYP2E1 monooxygenases, thioacetamide forms reactive oxygen species and thioacetamide-S-oxide-derived radicals capable of inducing oxidative stress and covalent interactions with macromolecules (23, 41). Such cellular actions lead to hepatocyte damage and depletion proliferation as well as a propensity for the disruption of mitochondrial membranes and glutathione reduction (22); all of which will contribute to cell death. Thus, thioacetamide is a suitable agent to cause non-alcoholic liver damage. Indeed i.p. thioacetamide injection resulted in extensive damage to the livers of LE rats as characterized by necrotic cells, cell drop out, loss of cytokeratin 8/18 staining in damaged hepatocytes, lobular disarray and fibrosis as shown in Fig. 2. In contrast to ALD, analyses of costimulatory molecules revealed comparable CD40 and CD86 increased expression by thioacetamide and treated LE rat DCs compared with controls in both the mature and immature state. More important, allostimulatory capacity of DCs derived from thioacetamide-treated LE rats with severe liver injury and cirrhosis was not impaired (Fig. 7). In the DCs derived from thioacetamide-treated rats compared with saline administered controls, the major abnormality appeared to be reduced IL-12p40 production. However, DCs isolated from ALD were characterized by (1) high levels of IL-10 expression, (2) striking reduced IFN-γ production, as well as (3) elevated TNF-α secretion. Such findings illustrate the substantial phenotypic and functional defects produced during chronic ethanol feeding in the LE rats with ALD that are not present in another LE rat model of severe liver injury. Previous studies revealed that ethanol feeding may also reduce allostimulatory activity of DCs in mice and humans (9, 12, 14–16). We explored the influence of ethanol feeding on allostimulatory activity in Fischer and LE rats. As expected, ethanol feeding greatly suppressed the allostimulatory activity of DCs derived from LE rats after LPS maturation (Fig. 7B). However, in the Fischer rat strain, no difference was observed in the allostimulatory activity of DCs (Fig. 7A). Interestingly, results from a human study were similar to our current observations in Fischer rats i.e., increased secretion of IL-1β, IL-6, IL-12 and TNF-α was found in peripheral blood DCs derived from chronic alcoholic patients without liver disease. In contrast, DCs prepared from the blood of patients with chronic ethanol-induced cirrhosis and continued active ethanol intake had abnormally low production of IL-1β and TNF-α by peripheral blood derived DCs which may suggest an important role for ALD in the pathogenesis of DC dysfunction (42).
In summary, our results revealed that the ALD sensitive LE rat, with confirmation of the degree of chronic liver injury at the time of study, exhibited substantial suppression of DC function after chronic ethanol feeding compared with the ALD resistant Fischer strain and thioacetamide-treated LE rats employed as a liver disease control. These findings provide strong experimental evidence for an important role of ALD in altering antigen-presenting capacity of DCs to T-cells.
Acknowledgments
Supported by AA-008169 and AA-008169-20S1 from the National Institutes of Health.
References
- 1.Osna N. Alcohol and liver disease. Semin Liver Dis. 2009;29:139. doi: 10.1055/s-0029-1214369. [DOI] [PubMed] [Google Scholar]
- 2.Oshita M, Hayashi N, Kasahara A, et al. Increased serum hepatitis C virus RNA levels among alcoholic patients with chronic hepatitis C. Hepatology. 1994;20:1115–1120. [PubMed] [Google Scholar]
- 3.Szabo G, Wands JR, Eken A, et al. Alcohol and hepatitis C virus-interactions in immune dysfunctions and liver damage. Alcohol: Clin Exp Res. 2010;34:1675–1686. doi: 10.1111/j.1530-0277.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris DR, Gonin R, Alter HJ, et al. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med. 2001;134:120–124. doi: 10.7326/0003-4819-134-2-200101160-00012. [DOI] [PubMed] [Google Scholar]
- 5.Nath B, Szabo G. Alcohol-induced modulation of signaling pathways in liver parenchymal and nonparenchymal cells: implications for immunity. Semin Liver Dis. 2009;29:166–177. doi: 10.1055/s-0029-1214372. [DOI] [PubMed] [Google Scholar]
- 6.Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- 7.Encke J, Wands JR. Ethanol inhibition: the humoral and cellular immune response to hepatitis C virus NS5 protein after genetic immunization. Alcohol Clin Exp Res. 2000;24:1063–1069. [PubMed] [Google Scholar]
- 8.Geissler M, Gesien A, Wands JR. Inhibitory effects of chronic ethanol consumption on cellular immune responses to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmids. J Immunol. 1997;159:5107–5113. [PubMed] [Google Scholar]
- 9.Aloman C, Gehring S, Wintermeyer P, Kuzushita N, Wands JR. Chronic ethanol consumption impairs cellular immune responses against HCV NS5 protein due to dendritic cell dysfunction. Gastroenterology. 2007;132:698–708. doi: 10.1053/j.gastro.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Edsen-Moore MR, Fan J, Ness KJ, et al. Effects of chronic ethanol feeding on murine dendritic cell numbers, turnover rate, and dendropoiesis. Alcohol Clin Exp Res. 2008;32:1309–1320. doi: 10.1111/j.1530-0277.2008.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinz R, Waltenbaugh C. Ethanol consumption modifies dendritic cell antigen presentation in mice. Alcohol Clin Exp Res. 2007;31:1759–1771. doi: 10.1111/j.1530-0277.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- 12.Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79:941–953. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- 13.Lau AH, Szabo G, Thomson AW. Antigen-presenting cells under the influence of alcohol. Trends Immunol. 2009;30:13–22. doi: 10.1016/j.it.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin Exp Res. 2004;28:824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- 15.Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- 16.Szabo G, Dolganiuc A, Mandrekar P, White B. Inhibition of antigen-presenting cell functions by alcohol: implications for hepatitis C virus infection. Alcohol. 2004;33:241–249. doi: 10.1016/j.alcohol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 17.De La Monte SM, Yeon JE, Tong M, et al. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol. 2008;23(Part 2):e477–e486. doi: 10.1111/j.1440-1746.2008.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denucci SM, Longato L, Lawton M, et al. Rat strain differences in susceptibility to alcohol-induced chronic liver injury and hepatic insulin resistance. Gastro Res Pract. 2010 doi: 10.1155/2010/312790. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronis MJ, Wands JR, Badger TM, et al. Alcohol-induced disruption of endocrine signaling. Alcohol Clin Exp Res. 2007;31:1269–1285. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 20.Yeon JE, Califano S, Xu J, Wands JR, De La Monte SM. Potential role of PTEN phosphatase in ethanol-impaired survival signaling in the liver. Hepatology. 2003;38:703–714. doi: 10.1053/jhep.2003.50368. [DOI] [PubMed] [Google Scholar]
- 21.Derdak Z, Lang CH, Villegas KA, et al. Activation of p53 enhances apoptosis and insulin resistance in a rat model of alcoholic liver disease. J Hepatol. 2010;54:164–172. doi: 10.1016/j.jhep.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stankova P, Kucera O, Lotkova H, et al. The toxic effect of thioacetamide on rat liver in vitro. Toxicol In Vitro. 24:2097–2103. doi: 10.1016/j.tiv.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Waters NJ, Waterfield CJ, Farrant RD, Holmes E, Nicholson JK. Metabonomic deconvolution of embedded toxicity: application to thioacetamide hepato- and nephrotoxicity. Chem Res Toxicol. 2005;18:639–654. doi: 10.1021/tx049869b. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama H, Higuchi N, Nishikawa Y, et al. High-level expression of naked DNA delivered to rat liver via tail vein injection. J Gene Med. 2002;4:333–341. doi: 10.1002/jgm.281. [DOI] [PubMed] [Google Scholar]
- 25.Derdak Z, Villegas KA, Wands JR. Fibrosis-related transcription factor early grwoth response-1 (EGR1) promotes steatosis in the liver of ethanol-fed Long–vans rats by activating sterol regulatory element binding protein-1 (SREBP1) Hepatology. 2010;52:1112A. [Google Scholar]
- 26.Lackner C, Gogg-Kamerer M, Zatloukal K. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol. 2008;48:821–828. doi: 10.1016/j.jhep.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Zhang M, Jenkins C, Macpherson GG. Dendritic cell heterogeneity in vivo: two functionally different dendritic cell populations in rat intestinal lymph can be distinguished by CD4 expression. J Immunol. 1998;161:1146–1155. [PubMed] [Google Scholar]
- 28.Voisine C, Hubert FX, Trinite B, Heslan M, Josien R. Two phenotypically distinct subsets of spleen dendritic cells in rats exhibit different cytokine production and T cell stimulatory activity. J Immunol. 2002;169:2284–2291. doi: 10.4049/jimmunol.169.5.2284. [DOI] [PubMed] [Google Scholar]
- 29.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maraskovsky E, Pulendran B, Brasel K, et al. Dramatic numerical increase of functionally mature dendritic cells in FLT3 ligand-treated mice. Adv Exp Med Biol. 1997;417:33–40. doi: 10.1007/978-1-4757-9966-8_6. [DOI] [PubMed] [Google Scholar]
- 31.Gehring S, Gregory SH, Wintermeyer P, Aloman C, Wands JR. Generation of immune responses against hepatitis C virus by dendritic cells containing NS5 protein-coated microparticles. Clin Vaccine Immunol. 2009;16:163–171. doi: 10.1128/CVI.00287-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gehring S, Gregory SH, Wintermeyer P, et al. Generation and characterization of an immunogenic dendritic cell population. J Immunol Methods. 2008;332:18–30. doi: 10.1016/j.jim.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Kuzushita N, Gregory SH, Monti NA. Vaccination with protein-transduced dendritic cells elicits a sustained response to hepatitis C viral antigens. Gastroenterology. 2006;130:453–464. doi: 10.1053/j.gastro.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Wintermeyer P, Gehring S, Eken A, Wands JR. Generation of cellular immune responses to HCV NS5 protein through in vivo activation of dendritic cells. J Viral Hepat. 2009;17:705–713. doi: 10.1111/j.1365-2893.2009.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007;15:2063–2069. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 36.Zuchini R, Huang CH, Tsai HW, et al. Electromagnetic thermoablation to treat thrombocytopenia in cirrhotic and hypersplenic rats. J Gastroenterol Hepatol. 25:1578–1586. doi: 10.1111/j.1440-1746.2010.06266.x. [DOI] [PubMed] [Google Scholar]
- 37.Trinite B, Voisine C, Yagita H, Josien R. A subset of cytolytic dendritic cells in rat. J Immunol. 2000;165:4202–4208. doi: 10.4049/jimmunol.165.8.4202. [DOI] [PubMed] [Google Scholar]
- 38.Sorensen TI. Alcohol and liver injury: dose-related or permissive effect? Liver. 1989;9:189–197. doi: 10.1111/j.1600-0676.1989.tb00399.x. [DOI] [PubMed] [Google Scholar]
- 39.Hall PM. Genetic and acquired factors that influence individual susceptibility to alcohol-associated liver disease. J Gastroenterol Hepatol. 1992;7:417–426. doi: 10.1111/j.1440-1746.1992.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 40.De La MHP, Lieber CS, Decarli LM, et al. Models of alcoholic liver disease in rodents: a critical evaluation. Alcohol Clin Exp Res. 2001;25(Suppl ISBRA):254S–2561. doi: 10.1097/00000374-200105051-00041. [DOI] [PubMed] [Google Scholar]
- 41.Zaragoza A, Andres D, Sarrion D, Cascales M. Potentiation of thioacetamide hepatotoxicity by phenobarbital pretreatment in rats. Inducibility of FAD monooxygenase system and age effect. Chem Biol Interact. 2000;124:87–101. doi: 10.1016/s0009-2797(99)00147-7. [DOI] [PubMed] [Google Scholar]
- 42.Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res. 2007;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]