Abstract
Older adults are among the most vulnerable to adverse cognitive effects of psychotropic medications and, therefore, the personalization of psychotropic treatment based on adverse drug reactions in this demographic is of great importance. We examined changes on neuropsychological tests of attention attributable to selective serotonin reuptake inhibitor (SSRI) treatment in anxious older adults. We also examined whether variation in serotonin receptor genes was associated with reduced attentional performance with SSRIs. We examined change from pre- to post-treatment in two attention measures – digit span and coding – in 133 adults aged ≥60 yr with generalized anxiety disorder in a 12-wk trial of escitalopram vs. placebo. We also examined attentional change in relation to genetic variability in four central serotonin receptors: the serotonin transporter and serotonin 1A, 2A and 1B receptors. Digit span scores were significantly lowered in patients receiving escitalopram relative to placebo, indicating reduced attentional performance attributable to the SSRI. Individuals with high-transcription variants in the receptors 5-HTR2A rs6311 and 5-HTR1B rs11568817 had greater reductions in attention with SSRI treatment compared to placebo. We conclude that SSRIs reduce attention in older adults, particularly in those with high-expression genetic variants at the serotonin 2A and 1B receptors. Analysing neuropsychological changes with SSRIs in relation to genetic variation in the serotonin system may be a useful strategy for detecting subgroups of older adults who are more susceptible to side-effects of SSRIs. These results, if confirmed, could lead to the personalization of SSRI use to reduce adverse neurocognitive effects.
Keywords: Anxiety, antidepressant, elderly, neuropsychological functioning, pharmacogenomics
Introduction
Roughly one in seven older adults in the US is taking an antidepressant and the most commonly prescribed are serotonin specific reuptake inhibitors (SSRIs; Chung, 2005; Olfson & Marcus, 2009; Pagura et al. 2011). Yet little research has examined in a controlled or experimental fashion the neurocognitive effects of SSRIs in this demographic. A placebo-controlled study of the serotonin–norepinephrine reuptake inhibitor duloxetine in late-life depression (LLD) showed significant improvement in verbal learning and memory but also a seeming trend towards worse attention (Raskin et al. 2007). Other research has been mixed regarding improvement of cognitive impairment in LLD with SSRIs, with one report showing significant improvement in memory with escitalopram (Savaskan et al. 2008) and others showing a lack of significant improvement (Butters et al. 2000; Nebes et al. 2003). Similarly, in young adults with depression, there have been mixed findings, with some evidence of improved cognitive functioning in SSRI responders but not non-responders (Mandelli et al. 2006) and in post-menopausal women, potentially modulated by oestradiol levels (Pae et al. 2008). We recently demonstrated in late-life generalized anxiety disorder (LLGAD) that the SSRI escitalopram produced no memory or other cognitive improvements aside from a single executive task (Butters et al. 2011). Given that LLD and LLGAD cause neurocognitive impairment in older adults (Bhalla et al. 2006; Butters et al. 2000), the failure of SSRIs to improve neurocognitive function in these disorders raises the possibility that SSRIs, while helping neurocognition in some ways (for example, by reducing the effects of depression and anxiety on certain cognitive biases; Mogg et al. 2004; Savaskan et al. 2008) may be impairing other aspects of neurocognition. SSRIs have been implicated in sedation, cognitive impairment, increased body sway and falls (Drueke et al. 2009; Dumont et al. 2005; Fava et al. 2006; Gagne et al. 2011; Joo et al. 2002; Laghrissi-Thode et al. 1995; Pollock, 1999; Wroolie et al. 2006), suggesting that they may cause neurocognitive impairment at least in some older adults.
The proximal target of all SSRIs is the serotonin transporter, with consequent altered serotonergic transmission at the serotonin 1A, 2A, 1B and other receptors (Lotrich & Pollock, 2005). These serotonin receptors are found throughout cortical areas involved in cognition, with significant inter-individual variation (Saulin et al. 2011). There is known functional genetic variability in these receptors, leading to genetic variants in which higher or lower numbers of the receptors are expressed (Lotrich & Pollock, 2005). This generates the hypothesis that genetic variability leading to functional genetic effects – that is, differential expression of these serotonin receptors and thus greater or fewer serotonin receptors – may predict an individual's risk of neurocognitive side effects.
We completed a placebo-controlled evaluation of escitalopram, a first-line treatment for GAD (Baldwin et al. 2011), in LLGAD (Lenze et al. 2009). The study included pre- and post-treatment assessment with a neuropsychological test battery and also genetic and pharmacokinetic (drug level) tests. Therefore, we were able to examine neurocognitive changes attributable to escitalopram and their genetic and pharmacokinetic predictors. We hypothesized that patients receiving SSRI would exhibit a reduction in attentional performance compared to those receiving placebo. Further, we examined the relationship of attentional change with functional genetic polymorphisms in key central serotonin receptors and with drug concentration.
Method
The study was a 12-wk, double-blind, randomized controlled trial comparing escitalopram and placebo (Lenze et al. 2009). Subjects were aged ≥60 yr, with a principal diagnosis of GAD (according to the Structured Diagnostic Interview for DSM-IV axis I diagnoses; First et al. 1996) and a score of ≥17 on the Hamilton Anxiety Rating Scale (Hamilton, 1959) Subjects were randomized to 10 mg escitalopram (increased to 20 mg after 4 wk if tolerated and as needed) or placebo. The University of Pittsburgh Institutional Review Board approved the study. Recruitment sources included primary care sites, speciality mental health practices and advertisements. Co-morbid unipolar depression and other anxiety disorders were allowed as was mild cognitive impairment (i.e. not dementia). Exclusion criteria included lifetime psychosis or bipolar disorder, dementia, medical instability, exogenous steroid use (including inhaled steroids) and antidepressant or anxiolytic co-prescription (with the exception of continuing low-dose benzodiazepines if already in use for at least 2 months). Severity of symptoms was assessed using the Hamilton scales for depression and anxiety (Hamilton, 1959, 1960) and the Penn State Worry Questionnaire, which measures worry severity (Meyer et al. 1990).
Neuropsychological assessment included pre- and post-treatment testing with the Repeatable Battery for the Assessment of Neuropsychological Status (Randolph, 1998). Forms A and B were administered in a counterbalanced manner. The present analysis focused on the two tests of attention: digit span and coding. The digit span is a test of basic attention that requires the subject to repeat sequences of single-digit numbers in the same order as read aloud by the examiner. The length of the sequences increases progressively, to a maximum of nine digits. An individual's ability to register a sequence of numbers in immediate memory depends greatly on his or her degree of focused attention. The coding test is a more multi-factorial visuoperceptual-motor decoding task that requires the subject to associate single-digit numbers with unfamiliar symbols. A stimulus set of nine printed digit-symbol pairs is presented above rows of numbers without the appropriate symbols. The subject is instructed to draw the correct symbol below each of the numbers using the digit-symbol code presented above. The score is based on the number of substitutions completed within a 90-s time limit.
DNA was extracted from blood using standard procedures. For all serotonin receptor polymorphisms other than the serotonin transporter, we used the Sequenom™ (USA) technology. For the serotonin transporter polymorphisms we followed a protocol that genotypes the SLC6A4 promoter haplotype (Wendland et al. 2006). In short, it is a triplex polymerase chain reaction protocol followed by double restriction endonuclease digestion, determining the phase-certain 5-HTTLPR and rs25531 genotypes, which are then combined as Sa, Sg, La and Lg haplotypes. All haplotypes other than La are considered low-expressing, consistent with other research (Beevers et al. 2011; Dombrovski et al. 2010; Frodl et al. 2008; Steiger et al. 2007; Thakur et al. 2010; Wankerl et al. 2010). For genetic analyses, we initially examined only Caucasian subjects, although we re-ran results with the entire sample, which included 20 African–Americans; results did not change in effect size or significance when the entire sample was used. No genotypes violated Hardy–Weinberg equilibrium (HWE) in the Caucasian-only sample, although rs25531 violated HWE in the entire sample because of a greater-than-expected number of g/g homozygotes (n = 3) among African–Americans.
Plasma samples for escitalopram levels were obtained at weeks 2, 8 and 12. We assessed escitalopram concentrations using high performance liquid chromatography with ultraviolet detection (Foglia et al. 1997). Nonlinear mixed effects modelling was used for the population pharmacokinetic analysis using the NONMEM computer program (version 5, level 1.1; University of California at San Francisco, USA; Beal & Sheiner, 1992) to calculate normalized average daily exposure for each subject at a given dose. From this model, the variable utilized in this study was average escitalopram concentration at the post-treatment time-point (Jin et al. 2009). We used only parent compound levels, as metabolites appear to have little to no pharmacological effect in vivo.
Statistical analysis
We examined the relationship between treatment group and attentional change in 133 GAD subjects for whom both pre- and post-treatment neuropsychological data were available. We tested the hypothesis that change in escitalopram-treated subjects was more negative (deleterious) than placebo-treated subjects, using mixed effect models (PROC MIXED in SAS). We also examined the same models divided by genetic group. The focus in these mixed effect analyses was the treatment group × time interaction, which showed the relative degree of change in attention in the escitalopram and placebo groups in the overall sample and in each genetic subgroup. We retested the significant results with alternative, equally appropriate statistical techniques (repeated measures analysis of variance, analysis of covariance on the change controlling for baseline and Wilcoxon); all provided the same findings and level of significance. To maximize power, we dichotomized subjects within each genotype into ‘high transcription’ or ‘low transcription’ groups based on the presence or absence of at least one high-transcription allele (Conner et al. 2010; Lemonde et al. 2004; McMahon et al. 2006; Villafuerte et al. 2009; Wilkie et al. 2008). We also examined the same models with haplotypes composed of the three serotonin 1B polymorphisms. Finally, we also examined pharmacokinetic effects by testing the correlation between average escitalopram concentration (derived from the NONMEM model) with change in attention.
Results
From 2005–2007, we randomized 177 subjects to escitalopram or placebo. Of these, 44 dropped out of the study or did not provide pre- and post-treatment neuropsychological data, leaving 133 subjects for the present analysis. Table 1 shows demographic and clinical features of these 133 subjects. Because the table shows a small over-representation of males in the placebo group, all analyses below were re-run controlling for gender; results did not change in significance.
Table 1. Baseline characteristics of the sample.
| All patients (n = 133) | Placebo patients (n = 69) | Escitalopram patients (n = 64) | χ2/t/ Wilcoxon value | p value | |
|---|---|---|---|---|---|
| Age | 71.6 (s.d. =8.0) | 72.4 (s.d. = 8.6) | 70.7 (s.d. = 7.3) | 1.19 | 0.2352 |
| Gender | |||||
| Male | n = 46 (35%) | n = 28 (41%) | n = 18 (28%) | 2.28 | 0.1313 |
| Female | n =87 (65%) | n = 41 (59%) | n = 46 (72%) | ||
| Geographic ancestry | |||||
| White | n = 113 (85%) | n = 60 (87%) | n = 53 (83%) | 0.45 | 0.5041 |
| Black (n = 19) or Asian Pacific (n = 1) | n =20 (15%) | n =9 (13%) | n =11 (17%) | ||
| Taking benzodiazepines | |||||
| Yes | n = 19 (14%) | n =11 (16%) | n = 8 (12%) | 0.32 | 0.5709 |
| No | n = 114 (86%) | n = 58 (84%) | n = 56 (88%) | ||
| Hamilton Anxiety Scale | 22.7 (s.d. =4.3) | 22.5 (s.d. = 4.5) | 22.9 (s.d. =4.2) | −0.64 | 0.5255 |
| Penn State Worry Questionnaire | 56.3 (s.d. = 12.7) | 57.9 (s.d. = 12.6) | 54.6 (s.d. = 12.6) | 1.48 | 0.1403 |
| Hamilton Depression Scale | 11.8 (s.d. = 3.8) | 12.0 (s.d. = 4.2) | 11.6 (s.d. = 3.4) | 0.70 | 0.4863 |
| RBANS total index score | 94.7 (s.d. = 15.7) | 93.7 (s.d. = 16.1) | 95.8 (s.d. = 15.3) | −0.77 | 0.4422 |
| RBANS digit span score | 10.4 (s.d. = 2.5) | 10.4 (s.d. = 2.5) | 10.5 (s.d. = 2.5) | −0.28 | 0.7791 |
| RBANS coding score | 39.4 (s.d. = 10.1) | 38.5 (s.d. = 10.7) | 40.3 (s.d. = 9.3) | −1.01 | 0.3161 |
| CIRSG (medical co-morbidity) score | 9.0 (s.d. = 3.9) | 8.6 (s.d. = 4.0) | 9.6 (s.d. = 3.9) | −1.51 | 0.1330 |
| Education, years | 14.1 (s.d. = 2.8) | 14.1 (s.d. = 3.1) | 14.1 (s.d. = 2.5) | 0.05 | 0.9599 |
| Age of onset | 38.7 (s.d. = 27.2) | 35.9 (s.d. = 27.8) | 41.7 (s.d. = 26.4) | −1.23 | 0.2215 |
| Duration, months | 383 (s.d. = 332) | 426 (s.d. = 344) | 337 (s.d. = 314) | 1.55 | 0.1224 |
RBANS, Repeatable Battery for the Assessment of Neuropsychological Function; CIRSG, Cumulative Illness Rating Scale for Geriatrics.
The RBANS total index has a mean of 100 (s.d. = 15) in healthy adults.
Figure 1 shows the effects of escitalopram vs. placebo on the two tests of attention over the 12-wk clinical trial. We found a significant reduction in the score of one – digit span – with escitalopram, compared to placebo. The effect size of this reduction was Cohen's d = 0.33 (between a small and medium effect size). The other test – coding – showed no different between escitalopram vs. placebo. All subsequent analyses focused on digit span score as an outcome. We re-ran this test controlling for possible confounds: baseline Hamilton or Penn State Worry Questionnaire scores, demographics and co-morbidities. The significance of the effect on digit span did not change.
Fig. 1.
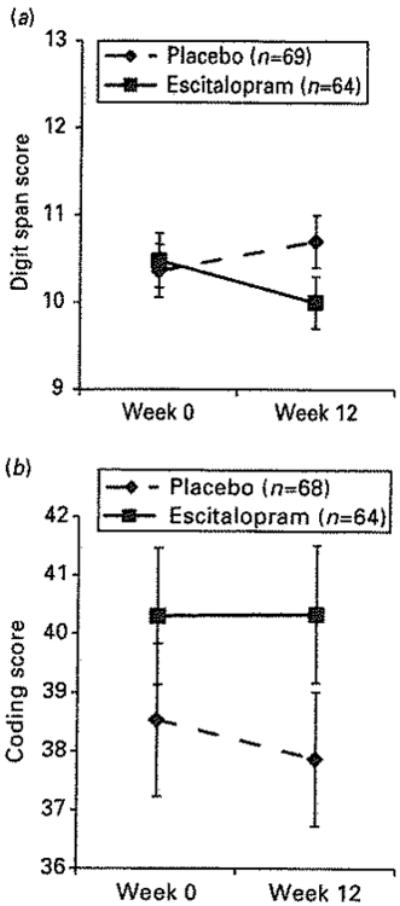
Decrement in attention in escitalopram-treated vs. placebo-treated patients: effects seen with digit span task (a) but not with coding task (b).
We chose six polymorphisms in the serotonin system – in the serotonin transporter and 1A, 2A and 1B receptors – based on biological plausibility and published data demonstrating their functional effects on transcription, as well as sufficiently high minor allele frequency (see Table 2). Because of the multiple genotypes, we used Bonferroni's corrected p-value of 0.008 (=0.05/6). Table 3 shows associations of the six genetic polymorphisms with digit span changes from pre-to post-treatment [Supplementary Table S1 (online) contains full treatment group, time and group × time interaction statistics]. As Table 3 shows, two polymorphisms were significant with a corrected p<0.008: rs6311 (serotonin receptor 2A G-1438A, where A is the high-transcribing allele) and rs11568817 (serotonin receptor 1B T-161G, where G is the high-transcribing allele). In both cases, the high-transcription group (A/A + A/G for rs6311, G/G + G/T for rs11568817) showed the significant decrease in the digit span test with escitalopram vs. placebo, while the low-transcription homozygote group did not. These contrasts are displayed in Fig. 2. The effect sizes of these decreases were d =0.64 for rs6311 and d =0.55 for rs11568817, indicating that, within these specific genotype groups, the magnitude of attention change was in the medium to medium-large effect size range.
Table 2. Candidate genotypes for attention changes with escitalopram vs. placebo.
| Chromosome | Polymorphism | rs number | Alleles | n | High-expressing allele | High-expressing allele frequency |
|---|---|---|---|---|---|---|
| 5 | HTR1A (C-1019G) | rs6295 | C, G | 132 | G | 0.50 |
| 6 | HTR1B (T-261G) | rs11568817 | T, G | 130 | G | 0.48 |
| 6 | HTR1B (A-161T) | rs130058 | A, T | 127 | A | 0.65 |
| 6 | HTR1B (G861C) | rs6296 | G, C | 128 | G | 0.74 |
| 13 | HTR2A (G-1438A) | rs6311 | G, A | 132 | A | 0.41 |
| 17 | 5-HTT triallelic haplotype | 5-HTTLPR + rs25531 | S or L + G, L + A | 133 | L + A | 0.47 |
Table 3. Relationship of serotonin receptor genotypes with digit span change.
| Polymorphism | Genotype | n | Digit span scores | Analysis (treatment group × time interaction) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Escitalopram | Placebo |
|
|||||||||
|
|
|
||||||||||
| Pre | Post | Δ | Pre | Post | Δ | F | p | Estimate (s.e.) | |||
| rs6295 (HTRlA) | G/G + G/C | 86 | 10.64 | 9.93 | −0.71 | 10.50 | 10.86 | 0.36 | 6.3 | 0.0143 | 0.09 (0.04) |
| C/C | 26 | 11.90 | 11.30 | −0.60 | 11.19 | 11.25 | 0.06 | 0.43 | 0.5163 | 0.06 (0.08) | |
| rs11568817 (HTR1B) | G/G + G/T | 88 | 10.85 | 9.92 | −0.93 | 10.57 | 11.00 | 0.43 | 9.3 | 0.0031* | 0.11 (0.04) |
| T/T | 22 | 10.73 | 10.64 | −0.09 | 11.18 | 10.82 | −0.36 | 0.08 | 0.7793 | −0.02 (0.08) | |
| rs130058 (HTR1B) | A/A + A/T | 92 | 10.82 | 10.13 | −0.69 | 10.74 | 10.94 | 0.20 | 4.17 | 0.0442 | 0.07 (0.04) |
| T/T | 15 | 10.50 | 10.30 | −0.20 | 10.20 | 11.20 | 1.00 | 0.90 | 0.3610 | 0.10 (0.11) | |
| rs6296 (HTR1B) | G/G + G/C | 100 | 10.78 | 10.02 | −0.76 | 10.67 | 10.95 | 0.28 | 5.98 | 0.0163 | 0.09 (0.04) |
| C/C | 8 | 10.80 | 11.20 | 0.40 | 11.33 | 11.00 | −0.33 | 0.18 | 0.6873 | −0.06 (0.14) | |
| rs6311 (HTR2A) | A/A + A/G | 70 | 10.75 | 9.81 | −0.94 | 10.13 | 10.71 | 0.58 | 9.1 | 0.0035* | 0.13 (0.04) |
| G/G | 42 | 11.10 | 10.80 | −0.30 | 11.64 | 11.41 | −0.23 | 0.01 | 0.9107 | 0.01 (0.05) | |
| 5-HTTLPR + | LA + | 78 | 11.06 | 10.36 | −0.70 | 10.57 | 10.81 | 0.24 | 4.1 | 0.0455 | 0.08 (0.04) |
| rs25531 | LA− | 35 | 10.53 | 10.00 | −0.53 | 10.94 | 11.28 | 0.34 | 1.3 | 0.2639 | 0.08 (0.06) |
Met significance at corrected p = 0.008.
Data are in Caucasians only. Within each genotype, treatment group × time interaction tests the relative change in digit span in the escitalopram and placebo groups. Full statistical data (group, time effects) can be found in Supplementary Table S1.
Fig. 2.
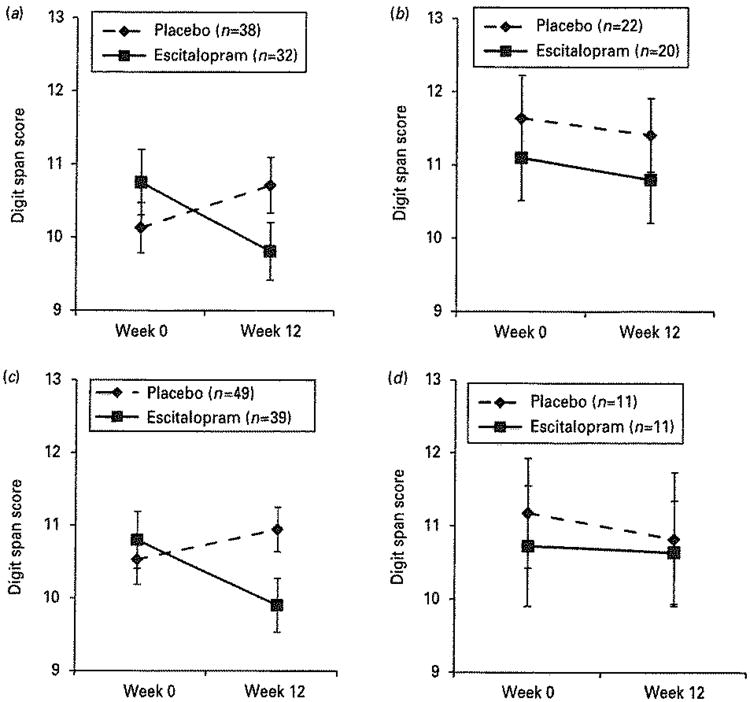
Digit span decrement in genetic subgroups of the serotonin 2A receptor (5-HTR2A rs6311) and the serotonin 1B receptor (5-HTR1B rs11568817).
Further analyses of these two genotypes suggest that the effect of escitalopram vs. placebo on attentional change was similar in both the high-transcription homozygote subjects and the heterozygote subjects (Supplementary Figs S1 and S2). For rs6311, the A/A group estimate for the group × time interaction was 0.16 (s.e. 0.09) and the A/G estimate was 0.11 (s.e. 0.05), vs. 0.01 (s.e. 0.05) for the low-transcription homozygotes (G/G). Similarly for rs11568817, the G/G estimate was 0.08 (s.e. 0.08) and the G/C estimate was 0.13 (0.04), vs. −0.02 (s.e. 0.08) for the low-transcription homozygotes (T/T). Formal statistical tests were not run because of the small sample sizes; estimates are provided here only to portray the extent of similarity between the genotype subgroups.
As Table 3 also shows, in all of other genotypes, the high-transcription subgroup also showed a decrease in the digit span test in the escitalopram group compared to the placebo group, although the trend was in the same direction, the significance levels only reached p <0.05 and did not survive correction for multiple testing. Therefore, no further analyses were carried out with these genetic subgroups.
We tested linkage disequilibrium between the three serotonin 1B receptor polymorphisms using PLINK software (http://pngu.mgh.harvard.edu/purcell/plink/; Purcell et al. 2007). The three genotypes were in linkage disequilibrium (D′=1 for all three associations), with R2=0.54 between rs11568817 and rs130058; R2 = 0.36 between rs11568817 and rs6296 and R2=0.20 between rs130058 and rs6296. These values are similar to what is observed in HapMap data (www.hapmag.org). We also carried out haplotype analyses on the three serotonin 1B genotypes. The data are included in Supplementary Table S2, which sorts all diplotypes (haplotype pairs) in order of ‘risk’, defined as the treatment group × time estimate for digit span within that diplotype. Given the small sample size for many of the diplotypes, we cannot draw strong conclusions about the potential for multiple genotypic effects from the linked single nucleotide polymorphisms (SNPs). However, individuals who are ‘risk’ homozygotes at rs130058 (i.e. A allele), and rs6296 (i.e. G allele) have the lowest risk when they also have the low-expressing allele at rs11568817 (i.e. the T allele). This suggests that the rs11568817 locus is either the only genetic effect of these three serotonin 1B SNPs or it dominates other effects.
To ensure that differences in digit span change were not due to different treatment outcomes, we examined the correlations of change in Penn State Worry Questionnaire and in Hamilton Anxiety score with change in digit span. There was a small though significant relationship (r=0.18, p=0.045) of Hamilton Anxiety change with digit span change: those with a greater drop in anxiety symptoms also had a greater drop in digit span. This relationship was not seen with Penn State Worry Questionnaire change. The correlation was no different in the escitalopram and placebo groups and was no different among genotypes (data not shown). Therefore, treatment outcome did not appear to account for the digit span findings. Additionally, to ensure that differences in digit span change were not due to differential benzodiazepine use, we examined proportions of each genotype who were taking benzodiazepines; the proportion was small (<20% in all groups) and did not significantly differ between any genotype on exact tests (exact p range 0.25–0.80).
We also carried out pharmacokinetic analyses in the escitalopram group. Escitalopram average drug concentration was not correlated with digit span changes in the overall group nor in any subgroup at rs6311 or rs11568817 (see Supplementary Table S3).
Discussion
Antidepressant medications are widely and increasingly prescribed in older adults, the demographic at highest risk for adverse neurocognitive changes from psychotropic medications, owing to pharmacokinetic changes and reduced physiologic and cognitive homeostatic capacity (Pollock, 1999). In this study we found that the SSRI escitalopram resulted in reductions in attention as measured by the digit span in older adults during acute treatment for anxiety. This effect appeared isolated to subjects with high-transcription serotonin receptor genotypes. The randomized design with placebo control is a strength of this study, because it allows us to control for expectancy, practice and time effects.
Reduction in attentional performance from SSRIs is not surprising. SSRIs affect cortical and subcortical regions involved in attention and other aspects of neurocognition (Lotrich & Pollock, 2005; Smith et al. 2011). In geriatric depression, SSRI administration has been shown to alter cerebral glucose metabolism in many of these areas, although the behavioural or clinical significance of these changes remains unclear (Diaconescu et al. 2010).
There are some caveats to this finding of reduced attentional performance with SSRI use. First, escitalopram reduced performance in the digit span, which measures the basic ability to pay attention for a few seconds, but it did not reduce performance on the more multifactorial coding task. The effect size of this reduction in the overall group was in the small–medium range, although it was higher (medium to medium-large) in the two significant genotype-defined subgroups. It is unclear whether this reduction represents a clinically relevant impairment or a subclinical change. We are not aware of any study that has determined the clinical significance of changes on the digit span. However, digit span measures a very basic cognitive function (the ability to maintain focus for a few seconds), upon which most other cognitive and everyday functions depend. For example, the ability to learn new information is highly dependent upon the amount of information one is able to encode, which, in turn, is highly dependent upon how well one is able to focus his/her attention. It is also important for understanding long sentences and performing everyday tasks. As such, it would seem the decrement in attention that we detected (which was about 0.5 for the overall escitalopram group and around 1 point drop, meaning an entire digit lost, in the ‘at risk’ genotypes) would be clinically important. Second, it is unclear whether this effect is specific to older adults. A similar study in young women with depression found no effects of citalopram on the digit span test, although some reduction in verbal fluency performance was noted (Wroolie et al. 2006). Finally, our finding should not be taken to suggest that SSRIs not be used in older adults. Instead, the findings suggest that there is room for drug development or personalization to provide a more beneficial neurocognitive profile.
For two serotonin receptors – 2A and 1B – we found evidence that high-expression genetic variants placed individuals at risk for significantly reduced attention with escitalopram, while the low-expression genetic variants did not. It is not surprising that we found that these receptor types are influential in the attentional effects of SSRIs. The serotonin 2A receptor (5-HTR2A) is an excitatory G protein coupled receptor (GPCR; Cook et al. 1994) and is expressed widely throughout the central nervous system, particularly in neocortical areas; it may also have an inhibitory effect in some of these brain regions (Martin et al. 1998). High concentrations of 5-HTR2A in cortical layer 5 pyramidal neurons demonstrate that this receptor is a key modulator of aspects of neurocognition (Aghajanian & Marek, 1999). The serotonin 1B receptor (5-HTR1B) is an inhibitory GPCR expressed widely across cortical and subcortical brain regions involved in neurocognition, including attention and motivation (Sari, 2004). Like 5-HTR2A, it is responsive to SSRI exposure and therefore is of interest as a moderator of antidepressant effects (Carr & Lucki, 2011; Murrough & Neumeister, 2011). Studies examining the neurocognitive effects of these antidepressants ought to model for functional genetic variability at these receptors.
Escitalopram drug level was not associated with change in attention performance in the overall sample or any genetic subgroup. Thus, although it has been posited that psychotropic adverse effects in older adults are related to higher and more variable drug concentrations (Pollock, 2005), we were unable to detect a pharmacokinetic effect in this study. We used a medication with simple pharmacokinetics and a fairly restricted dose range (10–20 mg daily); perhaps larger samples or antidepressants with more variable drug levels would show such an effect.
The main limitation of our study is a relatively small sample size, which may have led to some negative results due to lack of power. Additionally, our sample was not sufficiently powered to test group × genotype × time interactions (i.e. a statistical test of whether attentional performance is significantly worse in a high-transcription genotype group than a low transcription group). Sample size requirements for such a study would be daunting (Leon, 2008), but future confirmatory research could utilize larger open-label treatment or possibly pharmaco-epidemiological studies that include genetic and neuropsychological measures. Such research could identify genetic subgroups of individuals more likely to suffer neurocognitive side-effects of serotonergic antidepressants, leading to personalization of treatment for the growing number of older adults taking these medications (Olfson & Marcus, 2009; Pagura et al. 2011).
In summary, we found that older adults have reduced attentional performance with SSRI treatment. Individuals with high-transcription genotypes at serotonin receptors appear to be at particularly high risk for this effect. Our findings urge further investigation of genetic predictors of neurocognitive changes with these commonly prescribed psychotropics in older adults.
Supplementary Material
Acknowledgments
This study was supported by NIMH grants R01 MH070547, R01 MH072947, and K23 MH074012. Additionally, Forest Laboratories provided medication and placebo for this study.
Statement of Interest: Dr Lenze has received research funding from Forest Laboratories, Johnson & Johnson, Lundbeck and Roche, as well as research support in the form of medication from Pfizer and BMS. He has been a consultant for Fox Learning Systems. Dr Butters has been a consultant for Fox Learning Systems. She has also provided neuropsychological assessment services for clinical trials conducted by Medtronic. Dr Pollock discloses that within the past 3 years he served one time as a consultant for Wyeth (October 2008) and had been a member of the advisory board of Lundbeck Canada (final meeting, May 2009) as well as a faculty member of the Lundbeck International Neuroscience Foundation (final meeting, April 2010).
Footnotes
Supplementary material: For supplementary material accompanying this paper, please visit http://dx.doi.org/10.1017/S1461145712000351.
References
- Aghajanian GK, Marek GJ. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Research. 1999;825:161–171. doi: 10.1016/s0006-8993(99)01224-x. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Waldman S, Allgulander C. Evidence-based pharmacological treatment of generalized anxiety disorder. International Journal of Neuropsychopharmacology. 2011;14:697–710. doi: 10.1017/S1461145710001434. [DOI] [PubMed] [Google Scholar]
- Beal SL, Sheiner LB. NONMEM Users Guides. San Francisco, CA: University of California; 1992. [Google Scholar]
- Beevers CG, Marti CN, Lee HJ, Stote DL, et al. Associations between serotonin transporter gene promoter region (5-HTTLPR) polymorphism and gaze bias for emotional information. Journal of Abnormal Psychology. 2011;120:187–197. doi: 10.1037/a0022125. [DOI] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. American Journal of Geriatric Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, et al. Changes in cognitive functioning following treatment of late-life depression. American Journal of Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- Butters MA, Bhalla RK, Andreescu C, Wetherell JL, et al. Changes in neuropsychological functioning following treatment for late-life generalised anxiety disorder. British Journal of Psychiatry. 2011;199:211–218. doi: 10.1192/bjp.bp.110.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Lucki I. The role of serotonin receptor subtypes in treating depression: a review of animal studies. Psychopharmacology. 2011;213:265–287. doi: 10.1007/s00213-010-2097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. Does the use of SSRIs reduce medical care utilization and expenditures? Journal of Mental Health Policy and Economics. 2005;8:119–129. [PubMed] [Google Scholar]
- Conner TS, Jensen KP, Tennen H, Furneaux HM, et al. Functional polymorphisms in the serotonin 1B receptor gene (HTR1B) predict self-reported anger and hostility among young men. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2010;153B:67–78. doi: 10.1002/ajmg.b.30955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook EH, Jr, Fletcher KE, Wainwright M, Marks N, et al. Primary structure of the human platelet serotonin 5-HT2A receptor: identify with frontal cortex serotonin 5-HT2A receptor. Journal of Neurochemistry. 1994;63:465–469. doi: 10.1046/j.1471-4159.1994.63020465.x. [DOI] [PubMed] [Google Scholar]
- Diaconescu AO, Kramer E, Hermann C, Ma Y, et al. Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Human Brain Mapping. 2010;32:1677–1691. doi: 10.1002/hbm.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Mulsant BH, Ferrell RE, Lotrich FE, et al. Serotonin transporter triallelic genotype and response to citalopram and risperidone in dementia with behavioral symptoms. International Clinical Psychopharmacology. 2010;25:37–45. doi: 10.1097/YIC.0b013e328333ee10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drueke B, Baetz J, Boecker M, Moeller O, et al. Differential effects of escitalopram on attention: a placebo-controlled, double-blind cross-over study. Psychopharmacology. 2009;207:213–223. doi: 10.1007/s00213-009-1649-6. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, de Visser SJ, Cohen AF, van Gerven JM. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. British Journal of Clinical Pharmacology. 2005;59:495–510. doi: 10.1111/j.1365-2125.2005.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Graves LM, Benazzi F, Scalia MJ, et al. A cross-sectional study of the prevalence of cognitive and physical symptoms during long-term antidepressant treatment. Journal of Clinical Psychiatry. 2006;67:1754–1759. doi: 10.4088/jcp.v67n1113. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Clinician Version. Washington, DC: Administration Booklet; 1996. Structured Clinical Interview for DSM-IV Axis I Disorders(SCID) [Google Scholar]
- Foglia JP, Sorisio D, Kirshner M, Pollock BG. Quantitative determination of paroxetine in plasma by high-performance liquid chromatography and ultraviolet detection. Journal of Chromatography B: Biomedical Science Applications. 1997;693:147–151. doi: 10.1016/s0378-4347(97)00010-8. [DOI] [PubMed] [Google Scholar]
- Frodl T, Zill P, Baghai T, Schule C, et al. Reduced hippocampal volumes associated with the long variant of the tri- and diallelic serotonin transporter polymorphism in major depression. American Journal of Medical Genetics B: Neuropsychiatric Genetics. 2008;147B:1003–1007. doi: 10.1002/ajmg.b.30680. [DOI] [PubMed] [Google Scholar]
- Gagne JJ, Patrick AR, Mogun H, Solomon DH. Antidepressants and fracture risk in older adults: a comparative safety analysis. Clinical Pharmacology and Therapeutics. 2011;89:880–887. doi: 10.1038/clpt.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Pollock BG, Frank E, Florian J, et al. The effect of reporting methods for dosing times on the estimation of pharmacokinetic parameters of escitalopram. Journal of Clinical Pharmacology. 2009;49:176–184. doi: 10.1177/0091270008327538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Lenze EJ, Mulsant BH, Begley AE, et al. Risk factors for falls during treatment of late-life depression. Journal of Clinical Psychiatry. 2002;63:936–941. doi: 10.4088/jcp.v63n1012. [DOI] [PubMed] [Google Scholar]
- Laghrissi-Thode F, Pollock BG, Miller MC, Mulsant BH, et al. Double-blind comparison of paroxetine and nortriptyline on the postural stability of late-life depressed patients. Psychopharmacology Bulletin. 1995;31:659–663. [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, et al. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. International Journal of Neuropsychopharmacology. 2004;7:501–506. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Rollman BL, Shear MK, Dew MA, et al. Escitalopram for older adults with generalized anxiety disorder: a placebo-controlled trial. Journal of the American Medical Association. 2009;301:296–303. doi: 10.1001/jama.2008.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC. Implications of clinical trial design on sample size requirements. Schizophrenia Bulletin. 2008;34:664–669. doi: 10.1093/schbul/sbn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG. Candidate genes for antidepressant response to selective serotonin reuptake inhibitors. Neuropsychiatric Disease Treatment. 2005;1:17–35. doi: 10.2147/nedt.1.1.17.52301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli L, Serretti A, Colombo C, Florita M, et al. Improvement of cognitive functioning in mood disorder patients with depressive symptomatic recovery during treatment: an exploratory analysis. Psychiatry and Clinical Neurosciences. 2006;60:598–604. doi: 10.1111/j.1440-1819.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- Martin P, Waters N, Schmidt CJ, Carlsson A, et al. Rodent data and general hypothesis: antipsychotic action exerted through 5-Ht2A receptor antagonism is dependent on increased serotonergic tone. Journal of Neural Transmission. 1998;105:365–396. doi: 10.1007/s007020050064. [DOI] [PubMed] [Google Scholar]
- McMahon FJ, Buervenich S, Charney D, Lipsky R, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. American Journal of Human Genetics. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behavioral Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mogg K, Baldwin DS, Brodrick P, Bradley BP. Effect of short-term SSRI treatment on cognitive bias in generalised anxiety disorder. Psychopharmacology. 2004;176:466–470. doi: 10.1007/s00213-004-1902-y. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Neumeister A. The serotonin 1B receptor: a new target for depression therapeutics? Biological Psychiatry. 2011;69:714–715. doi: 10.1016/j.biopsych.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Houck PR, Butters MA, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. Journal of Psychiatric Research. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Archives of General Psychiatry. 2009;66:848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- Pae CU, Mandelli L, Han C, Ham BJ, et al. Do estradiol levels influence on the cognitive function during antidepressant treatments in post-menopausal women with major depressive disorder? A comparison with pre-menopausal women. Neuro Endocrinology Letters. 2008;29:500–506. [PubMed] [Google Scholar]
- Pagura J, Katz LY, Mojtabai R, Druss BG, et al. Antidepressant use in the absence of common mental disorders in the general population. Journal of Clinical Psychiatry. 2011;72:494–501. doi: 10.4088/JCP.09m05776blu. [DOI] [PubMed] [Google Scholar]
- Pollock BG. Adverse reactions of antidepressants in elderly patients. Journal of Clinical Psychiatry. 1999;60(Suppl. 20):4–8. [PubMed] [Google Scholar]
- Pollock BG. The pharmacokinetic imperative in late-life depression. Journal of Clinical Psychopharmacology. 2005;25(4 Suppl. 1):S19–S23. doi: 10.1097/01.jcp.0000162809.69323.66. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status Manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- Raskin J, Wiltse CG, Siegal A, Sheikh J, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. American Journal of Psychiatry. 2007;164:900–909. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neuroscience and Biobehavioral Reviews. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Saulin A, Savli M, Lanzenberger R. Serotonin and molecular neuroimaging in humans using PET. Amino Acids. 2011 doi: 10.1007/s00726-011-1078-9. Published online 24 September 2011. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Muller SE, Bohringer A, Schulz A, et al. Antidepressive therapy with escitalopram improves mood, cognitive symptoms, and identity memory for angry faces in elderly depressed patients. International Journal of Neuropsychopharmacology. 2008;11:381–388. doi: 10.1017/S1461145707007997. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kahn A, Sacher J, Rusjan P, et al. Serotonin transporter occupancy and the functional neuroanatomic effects of citalopram in geriatric depression. American Journal of Geriatric Psychiatry. 2011;19:1016–1025. doi: 10.1097/JGP.0b013e318227f83f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger H, Richardson J, Joober R, Gauvin L, et al. The 5HTTLPR polymorphism, prior maltreatment and dramatic-erratic personality manifestations in women with bulimic syndromes. Journal of Psychiatry and Neuroscience. 2007;32:354–362. [PMC free article] [PubMed] [Google Scholar]
- Thakur GA, Grizenko N, Sengupta SM, Schmitz N, et al. The 5-HTTLPR polymorphism of the serotonin transporter gene and short term behavioral response to methylphenidate in children with ADHD. BMC Psychiatry. 2010;10:50. doi: 10.1186/1471-244X-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte SM, Vallabhaneni K, Sliwerska E, McMahon FJ, et al. SSRI response in depression may be influenced by SNPs in HTR1B and HTR1A. Psychiatric Genetics. 2009;19:281–291. doi: 10.1097/YPG.0b013e32832a506e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wankerl M, Zyriax BC, Bondy B, Hinkelmann K, et al. Serotonin transporter gene-linked polymorphic region (5-HTTLPR) and diurnal cortisol: a sex by genotype interaction. Biological Psychology. 2010;85:344–346. doi: 10.1016/j.biopsycho.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, et al. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wilkie MJ, Smith G, Day RK, Matthews K, et al. Polymorphisms in the SLC6A4 and HTR2A genes influence treatment outcome following antidepressant therapy. Pharmacogenomics Journal. 2008;9:61–70. doi: 10.1038/sj.tpj.6500491. [DOI] [PubMed] [Google Scholar]
- Wroolie TE, Williams KE, Keller J, Zappert LN, et al. Mood and neuropsychological changes in women with midlife depression treated with escitalopram. Journal of Clinical Psychopharmacology. 2006;26:361–366. doi: 10.1097/01.jcp.0000227699.26375.f8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


