Abstract
Human immunodeficiency virus (HIV) infection and methamphetamine (MA) dependence are associated with neural injury preferentially involving frontostriatal circuits. Little is known, however, about how these commonly comorbid conditions impact behavioral presentations typically associated with frontal systems dysfunction. Our sample comprised 47 HIV-uninfected/MA-nondependent; 25 HIV-uninfected/MA-dependent; 36 HIV-infected/MA-nondependent; and 28 HIV-infected/MA-dependent subjects. Participants completed self-report measures of “frontal systems” behaviors, including impulsivity/disinhibition, sensation-seeking, and apathy. They also underwent comprehensive neurocognitive and neuropsychiatric assessments that allowed for detailed characterization of neurocognitive deficits and comorbid/premorbid conditions, including lifetime Mood and Substance Use Disorders, Attention-Deficit/Hyperactivity Disorder, and Antisocial Personality Disorder. Multivariable regression models adjusting for potential confounds (i.e., demographics and comorbid/premorbid conditions) showed that MA dependence was independently associated with increased impulsivity/disinhibition, sensation-seeking and apathy, and HIV infection with greater apathy. However, we did not see synergistic/additive effects of HIV and MA on frontal systems behaviors. Global neurocognitive impairment was relatively independent of the frontal systems behaviors, which is consistent with the view that these constructs may have relatively separable biopsychosocial underpinnings. Future research should explore whether both neurocognitive impairment and frontal systems behaviors may independently contribute to everyday functioning outcomes relevant to HIV and MA.
Keywords: Substance abuse, HIV, Apathy, Impulsive behavior, Sensation
1. Introduction
Human immunodeficiency virus (HIV) infection and methamphetamine (MA) dependence are highly comorbid conditions, epidemiologically (Plankey et al., 2007; Purcell et al., 2005), and are thought to have synergistic neuropathologic effects (Cadet and Krasnova, 2007). Neuroimaging findings have shown both distinct and additive effects of HIV infection and MA dependence on brain structure and function, with preferential (although not exclusive) involvement of frontostriatal circuits (Chang et al., 2005; Jernigan et al., 2005). Independently, HIV and MA are associated with mild-to-moderate deficits in neurocognitive functions that also are typically mediated by frontostriatal brain systems, such as attention/working memory, psychomotor speed, executive functions, learning, and motor skills (Heaton et al., 2010; Scott et al., 2007). Although research on this topic is still sparse, there is some evidence to suggest that HIV infection and MA use might confer an additive vulnerability to neurocognitive impairment in these same areas (Carey et al., 2006; Cherner et al., 2005; Rippeth et al., 2004). For example Rippeth et al. (2004) demonstrated that neurocognitive impairment is increased in persons who have both conditions, with 58% of HIV-infected (HIV+) persons who also had a history of MA dependence showing neurocognitive impairment, as compared to 38-40% in those with only one risk factor. Neurocognitive deficits in HIV and MA are also each associated with notable functional outcomes, such as unemployment and problems in activities of daily living (Heaton et al., 1994, 2004a; Henry et al., 2010; Weber et al., 2012), and recent evidence suggests HIV infection might confer an increased concurrent risk of MA-associated disability (Blackstone et al., 2013). Identifying neurobehavioral factors contributing to functional decline in these risk groups is particularly important because such factors may be amenable to intervention.
Despite the high prevalence and adverse cognitive impact of the HIV/MA comorbidity, little is known about the combined effects of these risk factors on complex behavioral disturbances, other than cognition, that might also seriously affect functional outcomes. Research in other populations with frontostriatial injury, particularly involving medial prefrontal cortex, reveals behavioral disturbances in natural settings that may be relatively independent of abilities assessed by traditional neurocognitive tests, but can nevertheless impair everyday functioning (Anderson et al., 1999; Bechara et al., 1994). In fact, there is a large literature showing that frontally-mediated behavioral disturbances account for additional variance in real world outcomes, beyond that accounted for by cognition alone, in conditions such as Parkinson's disease (Leroi et al., 2011), Alzheimer's disease (Boyle, 2004; Boyle et al., 2003; Tekin et al., 2001), vascular dementia (Zawacki et al., 2002), schizophrenia (Velligan et al., 2002), stroke (Mikami et al., 2013), and traumatic brain injury (Reid-Arndt et al., 2007; Schwartz et al., 2003). The association between these complex behaviors and frontal-subcortical brain systems along with the potentially disabling effect of these behavioral disturbances, highlight the importance of further understanding their relationship to HIV infection and MA use (Bechara et al., 1994; Bonelli and Cummings, 2007). In the present study, we focus on impulsivity/disinhibition, sensation-seeking, and apathy because of existing evidence relating these behaviors to HIV infection and MA use separately (Castellon et al., 1998; Cattie et al., 2012; Gonzalez et al., 2005). Although the best way to conceptualize and measure these behaviors is a matter of debate, impulsivity/disinhibiton is generally understood to be a multidimensional concept that encompasses deficits in response inhibition, lack of premeditation or planning, and urgency (Grace and Malloy, 2001; Whiteside and Lynam, 2003). Sensation-seeking is defined as the tendency to prefer exciting, or novel stimulation or arousal, and readiness to take risks for the sake of such experiences (Zuckerman et al., 1964). Apathy has been referred to as a reduction in self-initiated, goal-directed behavior, and a lack of motivation (Marin, 1997). In the present study, we refer to these concepts as “frontal systems” behaviors, given the known importance of frontal brain areas and their underlying subcortical structures in their mediation (Bonelli and Cummings, 2007; Grace et al., 1999). However, we acknowledge that other brain regions, such as limbic systems (Horn et al., 2003; Martin et al., 2007) are also likely to be involved.
The aforementioned frontal systems behaviors have been related to HIV infection and MA use separately (Castellon et al., 1998; Cattie et al., 2012; Gonzalez et al., 2005). While research is still sparse, such neurobehavioral symptoms appear to be both prevalent and impactful in these risk groups. For example, studies have found clinically significant levels of self-reported disinhibiton in 36% of MA+ adults (Cattie et al., 2012) and apathy in 26-42% of HIV+ patients (Kamat et al., 2012; Tate et al., 2003). MA dependence has been associated with increased impulsivity/disinhibition as assessed by self-report instruments (Cattie et al., 2012; Lee et al., 2009; Semple et al., 2005; Winhusen et al., 2013). Further, the severity of these symptoms is related to declines in activities of daily living in MA use (Cattie et al., 2012). Higher impulsivity, as measured by the Iowa Gambling Task (Bechara, 2007), has been observed among HIV+ persons with a history of substance dependence (Martin et al., 2004). There is also evidence showing sensation-seeking to be associated with MA use (Brecht et al., 2004), and to risky sexual behavior in HIV+ mixed substance users (Gonzalez et al., 2005). Increased apathy has been related to MA use (Looby and Earleywine, 2007), and it is relatively common in HIV infection (Castellon et al., 1998; Rabkin et al., 2000), where it is independently associated with everyday functioning outcomes, including IADL declines (Kamat et al., 2012) and medication management (Barclay et al., 2007; Rabkin et al., 2000).
Although there is evidence linking these frontal systems behaviors to MA and HIV, behavioral disturbances in these conditions are not fully understood, especially regarding their comorbidity and possible influences of pre-existing/comorbid conditions (e.g., other substance use, mood, and relevant personality disorders). The main purpose of our study was to further our understanding of the patterns of behavioral dysfunctions related to MA dependence, HIV infection, and their combination. In order to do so, we evaluated frontal systems behaviors, as assessed by self-report measures of impulsivity, sensation-seeking and apathy, in a group of individuals with and without HIV-infection and histories of MA dependence.
As suggested above, an important challenge in studying these relationships is the need to account for comorbid/premorbid conditions, such as neurocognitive deficits, mood and personality disorders, and abuse of other substances, which may affect these frontal systems behaviors (Clarke, 2006). Thus, an important question is whether the potential relations between behavioral dysfunctions and HIV and/or MA might be better accounted for by these comorbidities. We measured these conditions, along with frontal systems behaviors, in order to look at the independent contribution of MA dependence and HIV infection. We hypothesized that MA dependence and HIV infection would be independently associated with increased behavioral disturbances, even after accounting for the effect of comorbid conditions. Moreover, we expected that the coexistence of HIV infection and MA dependence would be associated with an increased risk for behavioral dysfunction relative to groups with MA or HIV alone.
2. Methods
2.1. Participants
One-hundred and thirty-six individuals enrolled at the University of California San Diego Translational Methamphetamine AIDS Research Center (TMARC) were included in present analyses. Participants were recruited from the San Diego area through a variety of methods, such as flyers, and appearances at community events, HIV clinics, and residential drug treatment programs. Participants were recruited according to HIV serostatus and MA dependence into one of the following four groups: HIV-seronegative and MA-nondependent (HIV−/MA−; n=47); HIV-seronegative and MA-dependent (HIV−/MA+; n=25); HIV+ and MA-nondependent (HIV+/MA−; n=36); and HIV+ and MA-dependent (HIV+/MA+, n=28). The HIV+ groups included participants with self-reported HIV infection that was confirmed by laboratory testing. Participants in the MA+ groups met Diagnostic and Statistical Manual-Fourth Edition (DSM-IV); (American Psychiatric Association, 1994) criteria for MA dependence in their lifetime and MA abuse or dependence within the last 18 months. Participants in the MA-groups had never met criteria for MA abuse or dependence. An alcohol breath test and a urine drug screen (for amphetamine, methamphetamine, cocaine, opiates, phenylcyclidine, and cannabis) were performed on all participants prior to the other assessments. Assessments of participants who had a positive test for a non-prescribed drug (with the exception of cannabis) were rescheduled for a later date.
Exclusion criteria for all groups consisted of: (1) a history of comorbid neurological illness or injury that would be likely to affect cognitive functioning (e.g. seizure disorder, closed head injury with loss of consciousness for longer than 30 min, central nervous system neoplasms); (2) a history of psychotic disorder not related to substance use; (3) alcohol dependence within a year of participation in the study; (4) notable length of alcohol dependence during their lifetime; (5) dependence on another substance (other than marijuana) within 5 years of participation; (6) abuse of a substance other than MA, cannabis or alcohol within the past year; (7) history of Hepatitis C virus infection confirmed with an on-site diagnostic test; (8) participation in prior neuropsychological testing (which may bias results due to practice effects); and (9) Wide Range Achievement Test-4 (WRAT-4); (Wilkinson and Robertson, 2006) reading subtest standard scores ≤ 80.
2.2. Materials and procedure
Participants completed comprehensive standardized psychiatric and neuropsychological evaluations, as part of a longer evaluation that included a physical and neurological examination, as well as collection of a standardized medical history and typical clinical blood tests (e.g., HIV RNA level in plasma measured by reverse transcription-polymerase chain reaction, CD4+ T-cell count measured by flow cytometry, rapid plasma reagin).
2.2.1. Neurocognitive assessment
The neuropsychological test battery was constructed to optimize sensitivity to frontal-subcortical deficits associated with HIV infection and MA dependence. It included an estimate of premorbid cognitive ability (i.e. the reading subtest of the WRAT-4; Wilkinson and Robertson, 2006), and assessed seven cognitive domains, including speed of information processing, verbal fluency, learning, delayed recall, executive functions, attention/working memory and motor skills (see Weber et al., 2012 for a list of specific tests by domains). Raw test scores were transformed into demographically-adjusted T-scores, including adjustments for age, education, gender and ethnicity (Heaton et al., 2004b, 2002), which were then converted into deficit scores, ranging from 0 (T-score > 39, no impairment) to 5 (T-score < 20, severe impairment). Deficit scores for each test were then used to derive a global deficit score (GDS) that reflects the number and severity of impairments across the entire test battery (Carey et al., 2004; Heaton et al., 1995, 2004b).
2.2.2. Assessment of premorbid/comorbid conditions
2.2.2.1. Substance abuse or dependence and mood disorders
History of MA and other substance abuse and dependence was obtained by administration of the Composite International Diagnostic Interview (CIDI version 2.1); (Kessler and Ustun, 2004; Wittchen, 1994), which is a lay-administered structured diagnostic interview that follows DSM-IV criteria. Other Substance Use Disorder was defined as having a lifetime diagnosis of Alcohol Abuse, past diagnosis of Alcohol Dependence, or past diagnosis of Cocaine or Opioid Abuse or Dependence (see exclusions above concerning current substance use disorders). MA use characteristics (e.g., total quantity of use) were derived from a semi-structured timeline follow-back substance use interview (Rippeth et al., 2004). Lifetime DSM-IV diagnoses of mood disorder (i.e. Major Depressive Disorder, Bipolar I Disorder and Bipolar II Disorder) were also obtained via the CIDI.
2.2.2.2. Attention Deficit Hyperactivity Disorder (ADHD) and Antisocial Personality Disorder (ASPD)
DSM-IV diagnoses of childhood and current ASPD and ADHD were obtained using modules of the Diagnostic Interview Schedule (Robins et al., 1981).
2.2.3. Complex “frontal systems” behaviors
Participants completed the following self-report instruments to assess frontal systems dysfunction involving problems with impulsivity/inhibitory control, sensation-seeking, and apathy. Since there are no single, “gold standard” measures of these phenomena, we included multiple instruments that have been frequently used in prior studies.
The Frontal Systems Behavior Scale: self-report (Grace and Malloy, 2001) is a 46-item scale in which people use a 6-point Likert-type scale to rate the frequency of occurrence of a series of behaviors associated with damage to frontal brain systems. Participants rate their behavior “before illness or injury” and “at the present time”. The scale yields total scores and scores for three subscales (Apathy, Disinhibition and Executive Dysfunction). For the present study, only responses related to current behavior for the Apathy and Disinhibition subscales were used.
The UPPS Impulsive Behavior Scale (Whiteside and Lynam, 2001) is a 44-item scale designed to measure four impulsivity and sensation-seeking related traits (urgency, lack of premeditation, lack of perseverance, and sensation-seeking) for which corresponding subscale scores can be computed. Items are rated using a 4-point Likert-type scale. For the present study, we used responses to the urgency, lack of premeditation and sensation-seeking subscales.
The Barratt Impulsiveness Scale (Patton et al., 1995) is a 30-item 4-point Likert-type scale designed to assess impulsive behavior and preferences. Total scores on this measure were used for present analyses.
The Sexual and Non-Sexual Sensation-Seeking Scales (Kalichman et al., 1994; Kalichman and Rompa, 1995) are comprised of a total of 20 items in which individuals rate on a 4-point scale the degree to which a series of behaviors related to sensation seeking apply to them.
The Profile of Mood States (McNair et al., 1980) is a measure of psychological distress comprised by 65 items rated on a 5-point Likert scale. It contains six subscales and, for the present study, only responses to the Vigor-Activity subscale were used to help assess apathy.
The Beck Depression Inventory-II (Beck et al., 1996) is a 21-item instrument assessing mood symptoms. Each item has four graded statements ordered to show increasing symptomatology. In the present study we used responses to four items from this inventory that refer to apathy-related phenomena of loss of pleasure, loss of interest, difficulty making decisions, and feelings of tiredness and fatigue. Prior work in persons with HIV identified these symptoms as reflective of a motivational disturbance component of mood (Castellon et al., 2006).
Based on the constructs assessed by these measures we examined the relation among those that were concerned with impulsivity/disinhibition, sensation-seeking, and apathy to derive composite scores in these behavioral domains (see Table 1 for a list of measures by composite). Raw scores for these measures were converted to T-scores based on the mean and standard deviation of our HIV−/MA− group, and averaged to yield composite scores in these domains. For all domains, higher T scores represent higher levels of the corresponding behavior.
Table 1.
Frontal systems behaviors composites.
| Frontal system composite | Measures |
|---|---|
| Impulsivity/disinhibition | Frontal Systems Behavior Scale (Disinhibition subscale) |
| UPPS Impulsive Behavior Scale (Urgency and Lack of Premeditation subscales) | |
| Barratt Impulsiveness Scale total score | |
| Sensation-seeking | Sexual and Non-Sexual Sensation-Seeking Scales |
| UPPS Impulsive Behavior Scale (Sensation-Seeking subscale) | |
| Apathy | Frontal Systems Behavior Scale (Apathy subscale) |
| Profile of mood states (Vigor-Activity subscale) | |
| Motivation items from the Beck Depression Inventory-II |
Internal consistency was adequate for all composites (Cronbach's α: impulsivity/disinhibition=0.90, sensation-seeking=0.74, apathy=0.78). The mean Pearson product moment correlation coefficient among measures within the composites was strong (impulsivity/disinhibition: r=0.67; sensation-seeking: r=0.52; apathy: r=0.62), providing evidence of convergent validity. The mean correlations between the scales comprising the sensation-seeking and impulsivity/disinhibition (r=0.27), and the sensation-seeking and apathy composites (r=0.02) were small, which indicates divergent validity among these measures. However, the mean correlation between the scales comprising the Impulsivity/Disinhibition and Apathy composites was medium (r=0.45), indicating some degree of shared variance. This is consistent with prior findings showing increased impulsivity/ disinhibition and apathy within the same sample of HIV-infected (Kamat et al., 2012) and MA-dependent (Winhusen et al., 2013) participants, and might be explained by both behavioral presentations sharing similar neurocircuits (Bonelli and Cummings, 2007).
2.3. Statistical analyses
Demographic characteristics, GDS and comorbid/premorbid conditions (i.e. lifetime mood disorder and other substance use disorder, ADHD, and ASPD), and MA and HIV-disease characteristics were non-normally distributed as assessed with the Shapiro–Wilk W test or did not met homogeneity of variance assumptions across groups as assessed by Levene's test. Accordingly, we ran Kruskal–Wallis oneway analyses of variance and Wilcoxon rank-sum tests when comparing groups on continuous variables. We used Chi-Square (or Fisher's exact) tests for categorical ones.
To examine possible effects of MA dependence and HIV disease on frontal systems behaviors, we first conducted a series of linear regression models on impulsivity/disinhibiton, sensation-seeking, and apathy composite scores entering HIV, MA, and their interaction as predictors. Next, to investigate the independent contribution of the risk groups on frontal systems behaviors, we conducted similar analyses, but also including potential covariates in the models. We evaluated potential covariates for inclusion in these multivariable models by first examining the univariable relationships to frontal systems behaviors of variables that differed across groups, including demographic variables, GDS and comorbid conditions, using Pearson product moment correlation coefficients and independent sample t-tests. Those variables that reached a critical alpha p < 0.10 were entered into stepwise linear regression models that included terms for HIV, MA, and their interaction, using a forward selection method and minimum corrected Akaike Information Criterion (AICc) stopping rule. In order to adjust for multiple comparisons we used Tukey Honestly Significant Difference (HSD) tests to conservatively characterize significant interactions.
3. Results
3.1. Demographic characteristics
Table 2 shows demographic characteristics of the four study groups. The groups were comparable in age and ethnicity. The two MA+ groups (HIV−/MA+ and HIV+/MA+) had significantly more men than the HIV−/MA− group (p=0.01 and 0.02, respectively) and significantly lower levels of education than both MA− groups (ps ranged from < 0.001 to 0.01). However, there were no group differences in estimated premorbid cognitive ability, as assessed by the WRAT-4. The two HIV+ groups had higher percentages of people who reported their sexual orientation as gay or bisexual (HIV+/MA− : 83.33%; HIV+/MA+: 78.57%) than the HIV-groups (HIV−/MA−: 19.15%; HIV−/MA+: 25.00%; ps < 0.001), with no significant differences between the HIV−/MA− and HIV− MA+ groups (p=0.57), and HIV+/MA− and HIV+/MA+ (p = 0.63).
Table 2.
Demographics, neurocognitive deficits and comorbid psychiatric characteristics by group.
| HIV− | HIV+ | Group comparisons | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| MA− (n=47) a |
MA+ (n=25) b |
MA− (n=36) c |
MA+ (n=28) d |
p1; + | Pairwise2; ++ | |
| Demographics | ||||||
| Age (years) | 38.40 (13.83) | 38.00 (9.26) | 40.06 (11.38) | 41.04 (9.37) | 0.72 | |
| Sex (% male) | 72.34 | 96.00 | 86.11 | 92.86 | 0.02 | a <b, d |
| Ethnicity (%) | 0.22 | |||||
| Non-Hispanic White | 57.45 | 52.00 | 52.78 | 75.00 | ||
| Non-Hispanic Black | 10.64 | 28.00 | 22.22 | 7.14 | ||
| Hispanic | 25.53 | 16.00 | 19.44 | 14.29 | ||
| Education (years) | 14.04 (2.05) | 12.08 (1.66) | 14.06 (2.08) | 12.82 (2.44) | <0.001 | a, c> b, d |
| WRAT-4 | 103.38 (11.97) | 97.64 (11.22) | 101.28 (9.92) | 103.18 (12.34) | 0.41 | |
| Neurocognition (GDS) | 0.27 (0.27) | 0.40 (0.33) | 0.48 (0.59) | 0.46 (0.40) | 0.08 | a <b3;+++, c3;+++, d |
| Comorbid psychiatric characteristics | ||||||
| Lifetime Mood Disorder (%) | 14.89 | 68.00 | 63.89 | 75.00 | <0.001 | a <b, c, d |
| Other Substance Disorder (%) | 31.91 | 92.00 | 30.56 | 57.14 | <0.001 | a, c <b, d |
| ASPD (%) | 6.38 | 45.83 | 11.11 | 35.71 | <0.001 | a, c <b, d |
| ADHD (%) | 2.12 | 28.00 | 5.71 | 14.29 | <0.01 | a, c <b; a <d |
Values represent Mean (standard deviation) unless otherwise noted.
ADHD=Attention Deficit Hyperactivity Disorder; ASPD=Antisocial Personality Disorder; GDS=Global Deficit Score; and MA=methamphetamine.
Results from Kruskal-Wallis, Chi-Square or Fisher's Exact tests.
Indicates significant group differences from pairwise comparisons (p <0.05, unless otherwise noted).
p < 0.1.
3.2. Neurocognitive deficits and comorbid psychiatric conditions
As also shown in Table 2, and as expected, there were significant group differences in neurocognitive deficits and rates of premorbid/comorbid psychiatric conditions. Specifically, relative to the HIV−/MA− control group, the dually affected group (HIV+/MA+, p=0.03) showed significantly worse neurocognitive performance (i.e. higher GDS), and the solely affected groups showed marginally worse neurocognitive performance (HIV+/MA−, p=0.06; HIV−/MA+, p=0.09). A significantly higher number of participants in all risk groups (i.e. HIV−/MA+, HIV+, MA−, HIV+/MA+) met criteria for a lifetime diagnosis of Mood Disorder as compared to the HIV− MA− group (ps < 0.001). Participants in both of the MA+ groups had a higher prevalence of other Substance Use Disorder and ASPD, than those in the MA− groups (all ps < 0.05). A diagnosis of ADHD was more common in both of the MA+ groups (HIV−/MA+, p < 0.001; HIV+/MA+ p=0.04) as compared to the HIV −/MA− control group.
3.3. MA use and HIV disease characteristics
As shown in Table 3, the MA+ groups reported similar age at first MA use, current length of MA abstinence, total months of prior MA use, total grams of estimated MA use, and primary method of use. The HIV+ groups were comparable for estimated duration of infection, current and nadir CD4+ T-cell count, plasma HIV RNA level, and proportions of participants with an AIDS diagnosis, and reporting current prescribed antiretroviral therapy (Table 3).
Table 3.
Methamphetamine (MA) use and HIV disease characteristics by group.
| HIV−/MA+ (n=25) | HIV+/MA− (n=36) | HIV+/MA+ (n=28) | pa | |
|---|---|---|---|---|
| Methamphetamine use | ||||
| Age at first use (years) | 21.0 (16.0, 29.0) | 23.0 (18.0, 29.5) | 0.57 | |
| Last use (days) | 31.0 (15.5, 213.1) | 88.7 (30.4, 273.9) | 0.12 | |
| Total duration of use (months) | 52.6 (22.8, 112.0) | 65.8 (14.8, 135.9) | 1.00 | |
| Total quantity of use (grams) | 1060.3 (279.8, 6764.5) | 956.4 (370.6, 1847.3) | 0.49 | |
| Method of use (%) | 0.14 | |||
| Injection | 24 | 17.9 | ||
| Smoking | 60 | 53.6 | ||
| Intranasal | 16 | 14.3 | ||
| Mixed | 0 | 14.3 | ||
| HIV disease | ||||
| Duration of infection (months) | 63.5 (2.6, 154.3) | 90.2 (26.5, 178.9) | 0.22 | |
| Nadir CD4 | 250.0 (98.8, 459.5) | 188.0 (80.0, 326.3) | 0.17 | |
| Current CD4 | 467.5 (296.0, 606.8) | 538.0 (347.0, 603.0) | 0.63 | |
| Plasma viral load (% detectable) | 52.9 | 53.5 | 0.96 | |
| AIDS (% Yes) | 44.4 | 60.7 | 0.20 | |
| ART prescribed (% Yes) | 62.9 | 66.7 | 0.76 |
Values represent Median (interquartile range) unless otherwise noted.
AIDS=Acquired Immune Deficiency Syndrome; ART=Antiretroviral therapy.
Results from Wilcoxon Rank-Sum or chi-square (or Fisher's exact) tests.
3.4. The effect of HIV infection and MA dependence on frontal systems behaviors
Table 4 shows group comparisons on frontal systems behaviors. For impulsivity/disinhibition, there was a significant main effect of MA (β= 17.24, SE=2.35, p < 0.001) but not HIV (β=3.57, SE=2.10, p=0.09), and a significant interaction (β=−10.24, SE=3.35, p < 0.01). Both MA+ groups (HIV−/MA+ and HIV+/MA+) showed significantly higher impulsivity than controls (HIV−/MA−; ps< 0.001) and the HIV+/MA− group (ps < 0.03); the HIV by MA interaction reflected a (nonsignificant) tendency for the MA-only group (HIV−/MA+) to show higher impulsivity than the dual risk group (HIV+/MA+; p=0.06).
Table 4.
Frontal systems behaviors composite scores by group.
| HIV− | HIV+ | HIV and MA risk effects (p)a | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| MA− (n=47) | MA+ (n=25) | MA− (n = 36) | MA+ (n=28) | HIV | MA | HIV × MA | |
| Impulsivity/disinhibition | 50.04 (8.28) | 67.28 (12.57) | 53.61 (9.55) | 60.61 (7.99) | 0.09 | <0.001 | <0.01 |
| Sensation-seeking | 50.02 (8.43) | 55.85 (9.36) | 49.99 (8.97) | 54.43 (7.41) | 0.99 | <0.01 | 0.65 |
| Apathy | 50.01 (7.44) | 77.36 (18.62) | 68.12 (19.57) | 70.10 (22.56) | <0.001 | <0.001 | <0.001 |
Values represent Mean (standard deviation) unless otherwise noted.
MA=methamphetamine.
p Values of results from multiple linear regression models on frontal systems behaviors scores using HIV, MA, and their interaction as predictors. (For overall models: impulsivity/disinhibition, F [3, 132]=20.91, p<0.001; sensation-seeking, F [3, 132]=3.94, p<0.01; Apathy, F [3, 132]=17.72, p<0.001).
For sensation-seeking, there was also a significant main effect of MA (β=5.83, SE=2.12, p < 0.01) and no significant effect of HIV (β= −0.02, SE=1.90, p=0.99) or interaction (β= −1.39, SE=3.02, p=0.65), indicating that both MA+ groups showed higher sensation-seeking than the MA− groups.
For apathy, there were significant main effects of both HIV (β=18.09, SE=3.76, p < 0.001) and MA (β=27.34, SE=4.20, p < 0.001), plus a significant interaction (β= −25.36, SE=5.99, p < 0.001). All risk groups showed significantly more apathy than controls (ps < 0.001), with no significant differences among the risk groups (ps > 0.16).
3.5. Controlling for effects of potential confounding factors
We assessed several potentially confounding factors in order to determine if their effects might account for the observed risk group differences.
Univariable predictors of impulsivity/disinhibiton included male gender (p < 0.001), lower levels of education (p < 0.001), lifetime mood disorder (p < 0.001), other substance use disorder (p < 0.001), ASPD (p < 0.001), and ADHD (p < 0.01). When we included these variables in a multivariable analysis, we found a significant main effect of MA and a significant HIV by MA interaction. Both MA+ groups (HIV−/MA+ and HIV+/MA+) showed significantly higher impulsivity/disinhibition than controls (HIV−/MA−; ps < 0.01), and the MA-only group (HIV−/MA+) reported more of this frontal systems behavior than the HIV-only group (HIV+/MA−; p < 0.01). However, the dually affected group (HIV+/MA+) was not significantly different from the HIV only group (HIV+/MA−; p=0.25). In this multivariable analysis, a history of other substance use disorder and ASPD were also independently associated with impulsivity/disinhibition (Table 5).
Table 5.
Effects of HIV-infection, methamphetamine (MA) dependence and their interaction on frontal systems behaviors accounting for potential confounding factors.
| Impulsivity/disinhibition | Sensation-seeking | Apathy | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| β (SE) | p | β (SE) | p | β (SE) | p | |
| HIV | 2.93 (2.02) | 0.15 | – | – | 11.04 (3.76) | <0.01 |
| MA | 11.76 (2.60) | <0.001 | 3.59 (1.73) | 0.01 | 16.96 (4.41) | <0.001 |
| HIV × MA | −7.37 (3.27) | 0.03 | – | – | −18.60(5.70) | <0.01 |
| Cofactors | ||||||
| Sex (male) | 3.69 (2.23) | 0.10 | 9.36 (1.91) | <0.001 | – | – |
| Mood disorder | – | – | – | – | 13.58 (3.18) | <0.001 |
| Substance usea | 4.84 (1.79) | <0.01 | – | – | – | – |
| ASPD | 4.34 (2.11) | 0.04 | – | – | 8.61 (3.64) | 0.02 |
Results from stepwise regression models entering HIV, MA, HIV × MA as predictors along with demographic and comorbid conditions that differed across groups and were at least marginally (p <0.10) associated with each of the frontal systems behaviors in univariable analyses. Cells with no values represent variables not included in a given final model. (For overall models: impulsivity/disinhibition, F [6, 128]=14.38, Adj R2=0.37, p <0.001; sensation-seeking, F [2, 133]=18.81, Adj R2=0.21, p <0.001; Apathy, F [5, 129]=18.54, Adj R2=0.40, p <0.001).
β=regression coefficient; SE=standard error.
History of substance use disorder other than MA.
Univariable predictors of sensation-seeking included male gender (p < 0.001) and a history of other substance use disorder (p < 0.01). When these variables were entered into multivariable analyses, we found significant independent effects of MA and male gender (Table 5).
Univariable predictors of apathy included lower education (p=0.04), lifetime mood disorder (p < 0.001), other substance use disorder (p < 0.01), ASPD (p < 0.001) and ADHD (p=0.04). Multivariable modeling that included these variables showed significant independent effects of HIV and MA, in addition to a significant interaction, reflecting that while groups with either risk factor (HIV−/MA+, HIV+/MA−) reported significantly more apathy than controls (HIV−/MA−; ps < 0.03), the dually affected group (HIV+MA+) was not significantly different from controls (p=0.12). There were also an independent contributions of life-time mood disorder and ASPD on apathy scores (Table 5).
In follow-up analyses, we re-ran our models on frontal systems behaviors to adjust for covariates, but this time following a different methodology for the selection of potential covariates to be included in the models. Instead of first looking at the univariable relation with the outcomes, we selected covariates using stepwise regression models. Results from these final models were the same both for impulsivity/disinhibition and apathy. However, they differed somewhat for sensation-seeking, where MA dependence (β=4.01, SE= 1.41, p < 0.01), male sex (β=8.75, SE= 1.91, p < 0.01) and reduced neurocognitive deficits (β=−3.68, SE= 1.78, p=0.04), were independently associated with increased sensation-seeking, F(3, 132)= 14.26, Adj R2 = 0.23, p < 0.001.
3.6. Neurocognition and frontal systems behaviors
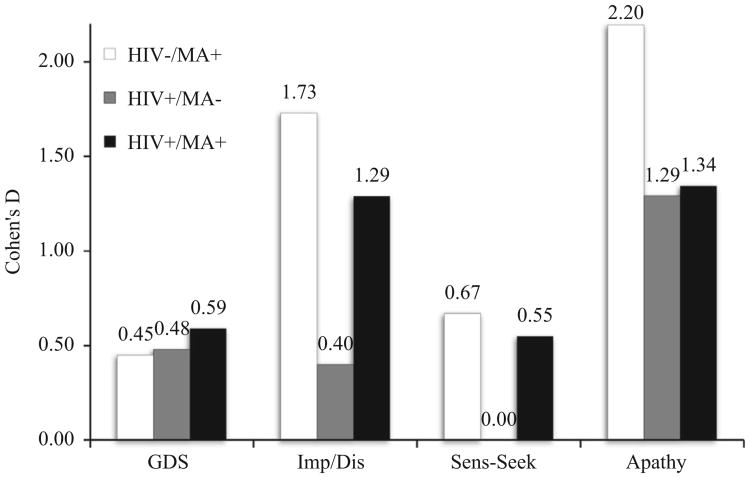
Given the established effect of MA and HIV on neurocognition (Rippeth et al., 2004) and current findings showing an effect of these conditions on frontal systems behaviors, we further investigated the relation between these variables in secondary analyses. Pearson product moment correlation coefficients between frontal systems composite scores and the GDS were small or non-existent (impulsivity/disinhibition: r=0.00, p=0.99; sensation-seeking: r= −0.18, p=0.04; apathy: r=0.18, p=0.04). In order to provide some perspective on the size of the effects of HIV and MA on frontal systems behaviors, we computed Cohen's d effect sizes (without adjusting for the effect of comorbidities) on neurocognition and frontal systems scores for the three risk groups (HIV−/MA+; HIV+/MA−; HIV+/MA+), with HIV−/MA− as the reference group (Fig. 1). Effect sizes on the GDS for the three risk groups were medium overall. Regarding impulsivity/disinhibition scores, there were large effect sizes for both MA+ groups, and small to medium effect sizes for the HIV-only group. Sensation-seeking scores showed generally medium effect sizes for both MA+ groups, and no effect for the HIV-only group. There were large effect sizes for all risk groups on apathy scores.
Fig. 1.
Cohen's d effect sizes on neurocognitive functioning (Global Deficit Scores, GDS) and Frontal System Behaviors (impulsivity/disinhibition, Imp/Dis; sensation-seeking, Sens-Seek; and apathy scores) by risk group (as compared to the HIV−/MA− groups).
4. Discussion
Results from the present study indicate that MA dependence, HIV infection, and the combination of these two risk factors are associated with behavioral presentations associated with frontal systems compromise. Further, these relationships cannot be explained by the higher rates of comorbid conditions in the HIV and MA groups. Specifically, MA history is associated with greater impulsivity/disinhibition, sensation-seeking, and apathy, and HIV with greater apathy. Surprisingly, the comorbidity of HIV and MA was not associated with increased frontal systems behaviors as compared to the groups with either risk factor alone. To the best of our knowledge this is the first study to explore the impact of HIV and MA on various frontal systems behaviors, while adjusting for the effect of potentially confounding demographic variables, neurocognitive deficits and pre-existing/comorbid psychiatric conditions, including history of lifetime mood and other substance use disorders, ADHD, and ASPD.
Our findings indicating a unique effect of MA dependence on impulsivity, sensation-seeking and apathy are consistent with studies showing higher self-reported impulsivity among MA+ than MA− persons (Cattie et al., 2012; Lee et al., 2009), and with the limited prior data available indicating that sensation-seeking is associated with MA use (Brecht et al., 2004). While there have been conflicting findings regarding the association of MA and apathy (Cattie et al., 2012), increased apathy has been found among stimulant users seeking treatment (Winhusen et al., 2013) and individuals who reported a history of MA use through an Internet survey (Looby and Earleywine, 2007). In order to examine the possible association between recency of use and frontal systems behaviors, in post-hoc analyses we ran a series of Spearman ρ correlations which showed that recency of use was not associated with impulsivity/disinhibition (ρ= −0.03, p=0.82), sensation-seeking (ρ= −0.05, p=0.71) or apathy, (ρ=0.02, p=0.89). We also divided our sample of MA users into those who had used MA in the prior two weeks (recent users, n = 10; 6 HIV−/MA+, 4 HIV+/MA+) and those who had not used MA within the past 6 months (remote users; n = 17, 7 HIV−/MA+, 10 HIV+/MA+), and compared these groups on our frontal systems behaviors. We found no significant group differences and small effect sizes for impulsivity/disinhibition (recent: M=67.34, SD=12.71; remote: M=64.17, SD=10.20 ; p=0.42, Cohen's d=0.28), sensation-seeking (recent: M=55.82, SD=7.27; remote: M=53.47, SD=8.13; p=0.32, Cohen's d=0.30) and apathy (recent: M=80.54, SD=18.39; remote: M=79.53, SD=22.40; p=0.88, Cohen's d=0.05). Taken together, these results suggest that recency of MA use did not significantly impact frontal systems behaviors in the current study.
We also observed an effect of HIV infection on apathy, which is in line with prior findings (Castellon et al., 1998; Kamat et al., 2012; Rabkin et al., 2000). Unexpectedly, we did not see a significant effect of HIV status on impulsivity/disinhibition. This is somewhat at odds with studies showing higher impulsivity in HIV+ persons who have a history of substance dependence (i.e. cocaine, heroin, alcohol, or polysubstance; Martin et al., 2004) relative to their HIV-counterparts. However, there are important differences across studies, including sample composition, and the assessment of impulsivity and potential confounds, which might account for the disparate findings. It is also worth noting that while the HIV-only group did not show increased impulsivity/disinhibition in our study, participants with both HIV and MA showed significantly higher impulsivity/disinhibition relative to controls. Based on sensation-seeking being associated with engagement in HIV-risk behaviors (Kalichman et al., 1996), we hypothesized that HIV status would be associated with sensation-seeking. However, we did not find such effect. Similarly, Gonzalez et al. (2005) found self-reported sensation-seeking to be comparable among polysubstance users with and without HIV infection. Interestingly, they still found that sensation-seeking was associated with risky sexual practices in HIV+ polysubstance users, which underscores the importance of understanding the impact of sensation-seeking on functional outcomes among HIV+ persons. In order to explore the possible effect on frontal systems behaviors of successful HIV viral suppression on antiretroviral therapy, in post-hoc analyses we ran a series of independent sample t-tests, which showed that frontal systems behaviors did not differ significantly among those HIV+ individuals with detectable (> 50 copies/ml; n=29) and undetectable (≤50 copies/ml; n=33) plasma viral loads (ps >0.14).
We did not find additive/synergistic effects of MA and HIV on any of the frontal systems behaviors. In fact, in analyses adjusting for potential confounds, the significant interaction we observed on apathy suggested that effects of HIV and MA were blunted in the dually affected participants. Further, although both MA+ groups showed higher impulsivity/disinhibition than controls, unlike the MA-only group, the dually affected group was not different from the HIV-only group. Sampling bias or naturalistic cohort effects (pre-existing differences among risk groups) may account for these unexpected findings. We controlled statistically for important comorbid/premorbid characteristics that differed across groups, but it cannot be assumed that the dual risk group shares all characteristics of both single risk groups. In many ways the dual risk group was more similar to the MA-only group than the HIV-only group; this includes several background characteristics that may well have pre-dated their HIV infection (e.g., lower education, higher rates of other substance use disorders, ASPD; See Table 2), as well as higher current levels of impulsivity/disinhibition and sensation-seeking (Fig. 1). Again, the MA+ effect on frontal systems behaviors remained significant after statistically controlling for potentially relevant pre-existing and concomitant disorders, which were quite common in both MA+ groups (Table 2). Further, compared to the HIV-only group, dually affected participants had somewhat (nonsignificant) longer disease duration, lower nadir CD4 cell counts and higher rates of AIDS. While individually these differences were not statistically significant, in combination they suggest a greater burden of HIV disease severity in the dually affected group. Thus, in post-hoc analyses we controlled statistically for these disease severity covariates in multiple regression models and MA continued to be independently associated with impulsivity/disinhibition (p=0.001) and sensation-seeking (p=0.01).
An alternative possibility for the reduced frontal systems effects in HIV+/MA+ participants is that, to some degree, the comorbidity of these conditions mitigates specific changes that may be caused by either risk alone, in certain neural circuits involved in frontal systems behaviors. While still very preliminary, this potential explanation is in line with functional neuroimaging findings showing blunted effects of HIV and MA in the striatum during motor programming (Archibald et al., 2012), as well as with results showing that neurocognitive impairment is associated with decreased cortical volumes in HIV but increased cortical volumes in MA dependence (Jernigan et al., 2005). Future studies incorporating neuroimaging data would be better suited to explore this hypothesis.
Interestingly, frontal systems behaviors were relatively independent of abnormalities in neurocognitive function in our study, as indexed by the GDS. This is consistent with the notion that they may have separable neurobiological underpinnings (Bonelli and Cummings, 2007; Freeman and Beer, 2010). Inspection of the effect sizes of frontal systems behaviors versus neurocognitive deficits in HIV and MA suggests that such frontal systems behavioral dysfunction might be at least as problematic (or more) as the well-established neurocognitive impairments in HIV and MA. Although we did not directly test this hypothesis, our observations highlight the importance of studying effects on functional outcomes of these complex behavioral symptoms in HIV and MA.
Of note, we observed a high prevalence of comorbid psychiatric conditions among our risk groups, particularly the MA+ groups. Specifically, all risk groups had much higher rates of lifetime Mood Disorder than controls, and both of the MA-dependent groups had higher rates of use of other substances, as well as ASPD and ADHD. We also observed significant associations between some comorbid conditions and frontal systems behaviors. Although the current study was not designed or powered to clarify effects of such comorbid conditions, they do merit additional study given their possible implications for treatment, particularly ASPD, which seemed to track with many of our effects.
Our study had several limitations. We relied on self-report measures to capture frontal systems behaviors rather than informant report, observation, or performance-based methods. Although self-report is limited by potential reporting bias or poor insight, it may be more sensitive to the participants' behavioral presentation in everyday life compared to other assessment approaches, and thus may yield clinically meaningful and incremental information to other methods. A large portion of our sample was male, and thus caution is warranted in generalizing our findings to females. Although there is evidence linking apathy, impulsivity and sensation-seeking to frontal-subcortical circuitry (Bonelli and Cummings, 2007; Freeman and Beer, 2010), our study did not include imaging, and thus cannot determine the degree to which these complex behaviors are associated with frontal brain dysfunction in our population of interest. However, consistent with this notion, a recent neuroimaging study in MA users indicated that ability to adapt a behavioral response is associated to prefrontal cortex brain activity and is compromised in this population (Salo et al., 2013). Further, our study is cross-sectional and correlational in nature and thus we cannot determine cause-effect of the relationships observed. It may be that persons with a predisposition toward frontal systems behaviors are more likely to engage in MA use and/or become HIV infected. Alternatively, MA dependence and HIV infection may result in behavioral dysfunction. Longitudinal studies would be best suited to address causality.
Overall, our findings show that there are important behavioral dysfunctions present in HIV and MA that are separate from comorbid psychiatric conditions, and relatively independent of neurocognitive impairments. Future studies should extend current findings by investigating the independent (or synergistic) contribution of frontal systems behaviors and neurocognitive deficits to everyday functioning outcomes relevant to HIV and MA, such as HIV transmission risk and medication adherence. Further, incorporating neuroimaging data might provide insights into the biological underpinnings of neurobehavioral symptoms in HIV and MA, and help to elucidate our unexpected results regarding the effect of comorbid HIV and MA on frontal systems behaviors.
Acknowledgments
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute (SBMRI).
Additional support for this research was provided by the NIDA-funded Institutional Training Grant award T32DA031098, Training in Research on Addictions in Interdisciplinary NeuroAIDS (TRAIN).
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: 1994. [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Jacobson MW, Fennema-Notestine C, Ogasawara M, Woods SP, Letendre S, Grant I, Jernigan TL. Functional interactions of HIV-infection and methamphetamine dependence during motor programming. Psychiatry Research-Neuroimaging. 2012;202:46–52. doi: 10.1016/j.pscychresns.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, Levine AJ, Durvasula RS. Age-associated predictors of medication adherence in HIV-positive adults: health beliefs, self-efficacy, and neurocognitive status. Health Psychology. 2007;26:40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Iowa Gambling Task Professional Manual. Psychological Assessment Resources; Boca Raton: 2007. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory – Manual. Second. The Psychological Corporation; San Antonio: 1996. [Google Scholar]
- Blackstone K, Iudicello JE, Morgan EE, Weber E, Moore DJ, Franklin D, Ellis R, Grant I, Wood S. HIV infection heightens concurrent risk of functional dependence in persons with chronic methamphetamine use. Journal of Addiction Medicine. 2013;7:255–263. doi: 10.1097/ADM.0b013e318293653d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues in Clinical Neuroscience. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA. Assessing and predicting functional impairment in Alzheimer's disease: the emerging role of frontal system dysfunction. Current Psychiatry Reports. 2004;6:20–24. doi: 10.1007/s11920-004-0033-9. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. American Journal of Geriatric Psychiatry. 2003;11:214–221. [PubMed] [Google Scholar]
- Brecht ML, O'Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addictive Behaviors. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Interactions of HIV and methamphetamine: cellular and molecular mechanisms of toxicity potentiation. Neurotoxicity Research. 2007;12:181–204. doi: 10.1007/BF03033915. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. the HNRC Group. 2004. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS and Behavior. 2006;10:185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Hardy DJ, Hinkin CH, Satz P, Stenquist PK, Van Gorp WG, Myers HF, Moore L. Components of depression in HIV-1 infection: their differential relationship to neurocognitive performance. Journal of Clinical and Experimental Neuropsychology. 2006;28:420–437. doi: 10.1080/13803390590935444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon SA, Hinkin CH, Wood S, Yarema KT. Apathy, depression, and cognitive performance in HIV-1 infection. Journal of Neuropsychiatry and Clinical Neuroscience. 1998;10:320–329. doi: 10.1176/jnp.10.3.320. [DOI] [PubMed] [Google Scholar]
- Cattie JE, Woods SP, Iudicello JE, Posada C, Grant I. Elevated neurobehavioral symptoms are associated with everyday functioning problems in chronic methamphetamine users. Journal of Neuropsychiatry and Clinical Neuroscience. 2012;24:331–339. doi: 10.1176/appi.neuropsych.11080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. American Journal of Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Letendre S, Heaton RK, Durelle J, Marquie-Beck J, Gragg B, Grant I. the HNRC Group. 2005. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64:1343–1347. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- Clarke D. Impulsivity as a mediator in the relationship between depression and problem gambling. Personality and Individual Differences. 2006;40:5–15. [Google Scholar]
- Freeman HD, Beer JS. Frontal lobe activation mediates the relation between sensation seeking and cortisol increases. Journal of Personality. 2010;78:1497–1528. doi: 10.1111/j.1467-6494.2010.00659.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Bechara A, Grbesic S, Sworowski L, Novak RM, Nunnally G, Martin EM. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. Journal of the International Neuropsychological Society. 2005;11:121–131. doi: 10.1017/s1355617705050186. [DOI] [PubMed] [Google Scholar]
- Grace J, Malloy PF. Professional Manual. Psychological Assessment Resources, Inc; Lutz: 2001. Frontal Systems Behavior Scale. [Google Scholar]
- Grace J, Stout JC, Malloy PF. Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment. 1999;6:269–284. doi: 10.1177/107319119900600307. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. the CHARTER Group. 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, Wolfson T, Velin R, Marcotte TD, Hesselink JR, Jernigan TL, Chandler J, Wallace M, Abramson I. the HNRC Group. 1995. The HNRC 500 – neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I. the HNRC Group. 2004. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society. 2004a;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller S, Taylor M, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African Americans and Caucasian Adults. Psychological Assessment Resources; Lutz: 2004b. [Google Scholar]
- Heaton RK, Taylor MJ, Manly JJ. Demographic Effects and Use of Demographically Corrected Norms with the WAIS-III and WMS-III. Academic Press; San Diego: 2002. [Google Scholar]
- Heaton RK, Velin RA, Mccutchan JA, Gulevich SJ, Atkinson JH, Wallace MR, Godfrey HPD, Kirson DA, Grant I. the HNRC Group. 1994. Neuropsychological impairment in human-immunodeficiency-virus infection – implications for employment. Psychosomatic Medicine. 1994;56:8–17. doi: 10.1097/00006842-199401000-00001. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addictive Behaviors. 2010;35:593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TL, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Heckman T, Kelly JA. Sensation seeking as an explanation for the association between substance use and HIV-related risky sexual behavior. Archives of Sexual Behavior. 1996;25:141–154. doi: 10.1007/BF02437933. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Johnson JR, Adair V, Rompa D, Multhauf K, Kelly JA. Sexual sensation seeking - scale development and predicting aids-risk behavior among homosexually active men. Journal of Personality Assessment. 1994;62:385–397. doi: 10.1207/s15327752jpa6203_1. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Rompa D. Sensation Seeking and Sexual Compulsivity Scales – reliability, validity, and predicting hiv risk behavior. Journal of Personality Assessment. 1995;65:586–601. doi: 10.1207/s15327752jpa6503_16. [DOI] [PubMed] [Google Scholar]
- Kamat R, Woods SP, Marcotte TD, Ellis RJ, Grant I. the HNRC Group. 2012. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Archives of Clinical Neuropsychology. 2012;27:520–531. doi: 10.1093/arclin/acs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The world mental health (WMH) survey initiative version of the World Health Organization (WHO) composite international diagnostic interview (CIDI) International Journal of Methods in Psychiatric Research. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal Dopamine D-2/D-3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. Journal of Neuroscience. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroi I, Ahearn DJ, Andrews M, McDonald KR, Byrne EJ, Burns A. Behavioural disorders, disability and quality of life in Parkinson's disease. Age and Ageing. 2011;40:614–621. doi: 10.1093/ageing/afr078. [DOI] [PubMed] [Google Scholar]
- Looby A, Earleywine M. The impact of methamphetamine use on subjective well-being in an Internet survey: preliminary findings. Human Psychopharmacology Clin Exp. 2007;22:167–172. doi: 10.1002/hup.831. [DOI] [PubMed] [Google Scholar]
- Marin RS. Differential diagnosis of apathy and related disorders of diminished motivation. Psychiatric Annals. 1997;27:30–33. [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Grbesic S, Vassileva J, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Martin SB, Covell DJ, Joseph JE, Chebrolu H, Smith CD, Kelly TH, Jiang Y, Gold BT. Human experience seeking correlates with hippocampus volume: convergent evidence from manual tracing and voxel-based morphometry. Neuropsychologia. 2007;45:2874–2881. doi: 10.1016/j.neuropsychologia.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1980. [Google Scholar]
- Mikami K, Jorge RE, Moser DJ, Jang M, Robinson RG. Incident apathy during the first year after stroke and its effect on physical and cognitive recovery. American Journal of Geriatric Psychiatry. 2013;21:848–854. doi: 10.1016/j.jagp.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Plankey MW, Ostrow DG, Stall R, Cox C, Li XH, Peck JA, Jacobson LP. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the Multicenter AIDS Cohort Study. Journal of Acquired Immunodeficiency Syndromes. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell DW, Moss S, Remien RH, Woods WJ, Parsons JT. Illicit substance use, sexual risk, and HIV-positive gay and bisexual men: differences by serostatus of casual partners. AIDS. 2005;19:S37–S47. doi: 10.1097/01.aids.0000167350.00503.db. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Ferrando SJ, van Gorp W, Rieppi R, McElhiney M, Sewell M. Relationships among apathy, depression, and cognitive impairment in HIV/ AIDS. Journal of Neuropsychiatry and Clinical Neuroscience. 2000;12:451–457. doi: 10.1176/jnp.12.4.451. [DOI] [PubMed] [Google Scholar]
- Reid-Arndt SA, Nehl C, Hinkebein J. The Frontal Systems Behaviour Scale (FrSBe) as a predictor of community integration following a traumatic brain injury. Brain Injury. 2007;21:1361–1369. doi: 10.1080/02699050701785062. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. the HNRC Group. 2004. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National-Institute-of-Mental-Health Diagnostic Interview Schedule – its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Salo R, Fassbender C, Buonocore MH, Ursu S. Behavioral regulation in methamphetamine abusers: An fMRI study. Psychiatry Research: Neuroimaging. 2013;211:234–238. doi: 10.1016/j.pscychresns.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L, Taylor HG, Drotar D, Yeates KO, Wade SL, Stancin T. Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. Journal of Pediatric Psychology. 2003;28:251–263. doi: 10.1093/jpepsy/jsg013. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and metaanalysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Zians J, Grant I, Patterson TL. Impulsivity and methamphetamine use. Journal of Substance Abuse Treatment. 2005;29:85–93. doi: 10.1016/j.jsat.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Tate D, Paul RH, Flanigan TP, Tashima K, Nash J, Adair C, Boland R, Cohen RA. The impact of apathy and depression on quality of life in patients infected with HIV. AIDS Patient Care and STDs. 2003;17:115–120. doi: 10.1089/108729103763807936. [DOI] [PubMed] [Google Scholar]
- Tekin S, Fairbanks LA, O'Connor S, Rosenberg S, Cummings JL. Activities of daily living in Alzheimer's disease: neuropsychiatric, cognitive, and medical illness influences. American Journal of Geriatric Psychiatry. 2001;9:81–86. [PubMed] [Google Scholar]
- Velligan DI, Ritch JL, Sui D, DiCocco M, Huntzinger CD. Frontal Systems Behavior Scale in schizophrenia: relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Research. 2002;113:227–236. doi: 10.1016/s0165-1781(02)00264-0. [DOI] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Iudicello JE, Morgan EE, Grant I, Moore DJ, Woods SP. the TMARC Group. 2012. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug and Alcohol Dependence. 2012;125:146–153. doi: 10.1016/j.drugalcdep.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Exp Clin Psychopharmacol. 2003;11:210–217. doi: 10.1037/1064-1297.11.3.210. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test-4: Professional Manual. Psychological Assessment Resources, Inc; Lutz. FL: 2006. [Google Scholar]
- Winhusen TM, Somoza EC, Lewis DF, Kropp FB, Horigian VE, Adinoff B. Frontal systems deficits in stimulant-dependent patients: evidence of pre-illness dysfunction and relationship to treatment response. Drug and Alcohol Dependence. 2013;127:94–100. doi: 10.1016/j.drugalcdep.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU. Reliability and validity studies of the WHO–composite international diagnostic interview (CIDI): a critical review. Journal of Psychiatric Research. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Zawacki TM, Grace J, Paul R, Moser DJ, Ott BR, Gordon N, Cohen RA. Behavioral problems as predictors of functional abilities of vascular dementia patients. Journal of Neuropsychiatry and Clinical Neuroscience. 2002;14:296–302. doi: 10.1176/jnp.14.3.296. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kolin EA, Price L, Zoob I. Development of a Sensation-Seeking Scale. Journal of Consulting Psychology. 1964;28:477–482. doi: 10.1037/h0040995. [DOI] [PubMed] [Google Scholar]