Abstract
There is increasing interest in the optimization of polymyxin B dosing regimens to treat infections caused by multidrug-resistant Gram-negative bacteria. We aimed to develop and validate a liquid chromatography - single quadrupole mass spectrometry (LC-MS) method to quantify polymyxin B in two growth media commonly used in in vitro pharmacodynamic studies, cation-adjusted Mueller-Hinton and tryptone soya broth. Samples were pre-treated with sodium hydroxide (1.0 M) and formic acid in acetonitrile (1:100, v/v) before analysis. The summed peak areas of polymyxin B1 and B2 relative to the summed peak areas of colistin A and B (internal standard) were used to quantify polymyxin B. Quality control samples were prepared and analyzed to assess the intra- and inter-day accuracy and precision. The robustness of the assay in the presence of bacteria and commonly co-administered antibiotics (rifampicin, doripenem, imipenem, cefepime and tigecycline) was also examined. Chromatographic separation was achieved with retention times of approximately 9.7 min for polymyxin B2 and 10.4 min for polymyxin B1. Calibration curves were linear between 0.103 and 6.60 mg/L. Accuracy (% relative error) and precision (% coefficient of variation), pooled for all assay days and matrices (n=84), were −6.85% (8.17%) at 0.248 mg/L, 1.73% (6.15%) at 2.48 mg/L and 1.54% (5.49%) at 4.95 mg/L, and within acceptable ranges at all concentrations examined. Further, the presence of high bacterial concentrations or of commonly co-administered antibiotics in the samples did not affect the assay. The accuracy, precision and cost-efficiency of the assay make it ideally suited to quantifying polymyxin B in samples from in vitro pharmacodynamic models.
Keywords: Polymyxin B, Liquid chromatography - mass spectrometry, Mueller-Hinton broth, Tryptone soya broth
1. Introduction
Polymyxin B is a cationic lipopeptide antibiotic with activity against Gram-negative bacteria. It was first isolated from Paenibacillus polymyxa in 1947 and consists of a cyclic heptapeptide linked via a tripeptide chain to a fatty acyl tail.[1] Concerns about nephro- and neuro-toxicity led to waning clinical use of polymyxins in the 1960s, as newer and supposedly safer classes of antibiotics such as aminoglycosides became favored by clinicians.[2, 3] However, with increasing incidence of infections caused by multidrug-resistant (MDR) Gram-negative bacteria, there has been a recent resurgence in use of polymyxin B as a last-line therapy due to its activity against many of these MDR strains.[4]
Presently, there is a dearth of information to guide clinicians in the optimal use of polymyxin B [1, 4, 5] and further pharmacological investigations are urgently required to preserve its antibacterial activity by optimizing dosage regimens and minimizing the emergence of resistance. Such investigations are commonly conducted using in vitro experimental models.[6–9] Accurate and precise quantification of polymyxin B in microbiological media is therefore critical for determination of the pharmacokinetic/pharmacodynamic relationships that underpin both the antimicrobial activity of polymyxin B and emergence of bacterial resistance.[10–12]
Both polymyxin B and polymyxin E (colistin) (Figure 1), which differ by a single amino acid residue in the heptapeptide ring, are mixtures of several structurally related compounds. Polymyxin B1 and polymyxin B2 are the main constituents of polymyxin B while the corresponding major components of colistin are colistin A and colistin B; for both cases, these respective constituents generally account for over 85% of the total.[13–15]
Figure 1.
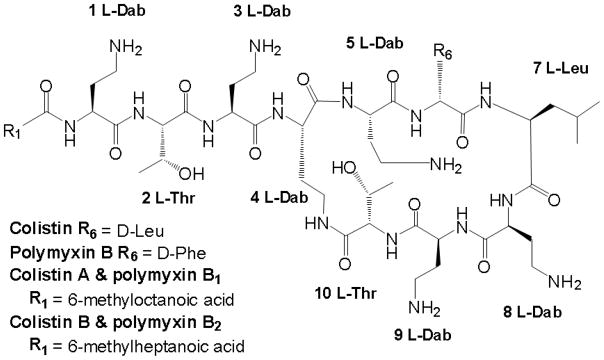
The chemical structure of polymyxin B and colistin
Quantification of polymyxins is complicated by their low UV absorption and lack of native fluorescence.[16] Several high-performance liquid chromatography (HPLC) and liquid chromatography - triple quadrupole mass spectrometry (LC-MS/MS) assays for polymyxins in plasma and other biological matrices have been reported.[17–24] However, to our knowledge, no liquid chromatography - mass spectrometry (LC-MS) assay for polymyxin B has been reported for cation-adjusted Mueller-Hinton broth (CAMHB) or tryptone soya broth (TSB), two microbiological growth media commonly used for antimicrobial susceptibility testing and in vitro infection models. In the present report we describe an accurate and reproducible LC-MS method for the quantification of polymyxin B in both of the above-mentioned growth media utilizing colistin as an internal standard.
2. Experimental
2.1. Apparatus
A Shimadzu (Kyoto, Japan) LC-MS system was used to obtain positive ion electro-spray mass spectra for the quantification of polymyxin B. This system consisted of a DGU-20A3 degasser, LC-20AD pump, SIL-20AC HT auto-sampler and CTO-20A column oven connected to an LCMS-2010EV single quadrupole mass spectrometer.
2.2. Materials and reagents
Mueller-Hinton broth powder was obtained from Oxoid (Basingstoke, Hampshire, England) and reconstituted with water in accordance with the manufacturer’s instructions. This broth was cation adjusted to 11.64 mg/L Mg2+ and 23.41 mg/L Ca2+ with magnesium chloride (Sigma Aldrich, St Louis, MO, USA) and calcium chloride (Univar, Redmond, WA, USA) before sterilization. Polymyxin B sulfate was obtained from BetaPharma (Branford, CT, USA) and colistin sulfate from Sigma Aldrich. Formic acid was purchased from Ajax Finechem (New South Wales, Australia); HPLC-grade acetonitrile was from Merck (New Jersey, USA) and analytical-grade sodium hydroxide from Sigma Aldrich. Rifampicin (Sigma Aldrich), imipenem/cilastatin (Merck Sharpe and Dohme, New Jersey, USA), doripenem (Janssen Pharmaceuticals, New Jersey, USA), cefepime (Omegapharm, Victoria, Australia) and tigecycline (Wyeth, New Jersey, USA) were obtained for assessing the specificity of the polymyxin B assay.
2.3. Sample pre-treatment
A 10 μL aliquot of the colistin internal standard solution (33.0 mg/L colistin base in acetonitrile/water [50:50, v/v]) was added to 100 μL of polymyxin B-containing growth medium (see Section 2.5 - Linearity, precision and accuracy) in a 1.7-mL polypropylene microcentrifuge tube (Quantum Scientific, Radnor, Pennsylvania, USA). A 20-μL aliquot of sodium hydroxide in water (1.0 M) was then added to each sample and the tube contents were vortex mixed for ~2 sec. Following the addition of 400 μL formic acid in acetonitrile (1:100, v/v), samples were vortex mixed for ~2 sec and allowed to stand at room temperature for 10 min prior to centrifugation at 20,800 g for 10 min. Subsequently, 150 μL of the supernatant was loaded into polypropylene auto-sampler vials for analysis by LC-MS.
2.4. LC-MS analysis
Samples were maintained at 4°C within the auto-sampler and an injection volume of 10 μL was used. Chromatographic separation was achieved using a Phenomenex Synergi™ Hydro-RP column (80 Å, 125 × 4.00 mm) maintained at 40°C. The mobile phase consisted of 0.5% aqueous formic acid (v/v, solvent A) and 0.5% formic acid in acetonitrile (v/v, solvent B). The flow rate was 0.4 mL/min with the following linear gradient elution program: 0% solvent B for 36 sec, increasing to 13.0% over 1.4 min and maintained for 30 sec before decreasing to 8% over 30 sec and subsequently increasing to 33.5% over 8 min. This was followed by a 1.0-min flush at 95% solvent B and re-equilibration at 0% solvent B for 2.8 min at an increased mobile phase flow rate of 0.5 mL/min. Following chromatographic separation, multiple ion monitoring was used to detect the [M+3H]3+ ions of polymyxin B (polymyxin B1 m/z 401.85, polymyxin B2 m/z 397.20) and colistin (colistin A m/z 390.55, colistin B m/z 385.95). The interface voltage was 4.5 kV, with a curved desolvation line temperature of 200 °C and voltage of 0 V.
2.5. Linearity, precision and accuracy
For preparation of calibration curve samples, stock solutions of polymyxin B (sulfate) were prepared in water and diluted to the required working solution concentrations in acetonitrile and water (50:50, v/v) before spiking samples. Aliquots (100 μL) of either sterile CAMHB or TSB were then spiked with 10 μL of polymyxin B working solution to give polymyxin B base concentrations of 0.103, 0.206, 0.413, 0.825, 1.65, 3.30 and 6.60 mg/L for the assessment of calibration curve linearity in each matrix. Quality control (QC) samples containing 0.103 (CAMHB only), 0.248, 2.48, 4.95 and 6.60 mg/L of polymyxin B base were prepared separately in CAMHB and TSB to assess the intra- and inter-day accuracy and precision of the assay. All QC samples were prepared with working solutions independent of those used for calibration curve samples. Further, a series of QC samples using growth media containing a high bacterial concentration of different species were prepared to evaluate the robustness of the assay to the presence of bacteria. Bacterial species causing infections in patients that are commonly treated with polymyxin B were specifically chosen for this aspect of the validation. These micro-organisms were: Acinetobacter baumannii ATCC (American Type Culture Collection) 19606, Pseudomonas aeruginosa ATCC 27853, and Klebsiella pneumoniae ATCC 13883. For each QC concentration, 6 replicates were prepared in either sterile media or an overnight bacterial culture (approximately 108 colony forming units [CFU] per mL). In total, 4 batches of sterile QC samples and 3 batches of overnight culture QC samples (1 for each strain) were prepared in each growth medium and analyzed as part of the assay validation.
2.6. Specificity
The specificity of the assay in the presence of the antibiotics from different classes that are commonly co-administered with polymyxin B was assessed by adding other antibiotics to blank samples and assessing chromatograms for interfering peaks. The antibiotics and their clinically relevant concentrations were rifampicin (2 mg/L), imipenem/cilastatin (12.5/12.5 mg/L), doripenem (25 mg/L), cefepime (100 mg/L) and tigecycline (0.5 mg/L).
3. Results and Discussion
3.1. Sample pre-treatment
During method development, various organic solvents and acids were trialed for protein precipitation, and 1% formic acid in acetonitrile effectively precipitated proteins from samples without introducing matrix effects that may adversely affect polymyxin B quantification. The addition of aqueous sodium hydroxide improved the accuracy of the assay at polymyxin B concentrations at or below 0.248 mg/L in samples containing high bacterial concentrations. This effect may have been due to the elevated pH favoring polymyxin B in its unionized state, leading to the dissociation of polymyxin B from negatively charged bacterial cells. [25] The pre-treatment of samples with aqueous sodium hydroxide and the protein precipitation method outlined above enabled the accurate and reproducible quantification of polymyxin B without the need for solid-phase extraction.
Sample pre-treatment methods detailed in previously reported polymyxin assays have included solid-phase extraction to minimize the effect of the sample matrix on quantification.[16, 17, 21, 23, 24] While effective, solid-phase extraction increases the cost, duration and complexity of analyzing samples. Our assay greatly simplifies sample preparation by eliminating the need for solid-phase extraction. Further, compared with HPLC methods which utilize ultraviolet fluorometric detection, [16, 18] the use of mass spectrometric detection in the present method eliminates the need for derivatization to improve fluorescence. These advantages significantly reduce the time and cost associated with sample preparation.
3.2. Optimization of liquid chromatography and mass spectroscopy
CAMHB and TSB are complex matrices which contain high concentrations (≥10 g/L) of peptides and proteins derived from casein and other sources.[26, 27] While sample pre-treatment precipitates a large number of these proteins, the remaining sample matrix contains residual peptides and proteins that generate m/z ratios (~300 – 400) similar to the [M+3H]3+ ions for polymyxins. When using isocratic elution with a mobile phase consisting of 0.5% formic acid in various mixtures of acetonitrile and water, these residual compounds produced chromatographic peaks which interfered with polymyxin B quantification. Detection of [M+2H]2+ ions with higher m/z ratios (~550 – 650) was explored as a strategy to ameliorate the interference caused by the growth media. Alteration of LC-MS conditions, including mobile phase composition and electrospray ionization parameters, was investigated to improve the abundance of [M+2H]2+ ions for both polymyxin B and colistin. However, [M+2H]2+ ions were consistently 5- to 10-fold less abundant compared with [M+3H]3+ ions in the single-quadrupole mass spectrometer. Further, the interfering peaks were also observed in the m/z range of the [M+2H]2+ ions. Ultimately, chromatographic separation was employed to overcome the interference caused by the components of the growth media. The final method used a polar end-capped C18 reversed-phase column and a gradient elution program. The gradient elution program was tuned to maximize chromatographic separation and minimize analysis time. This achieved good separation with a total run-time of 15 min per sample.
3.3. Assay performance
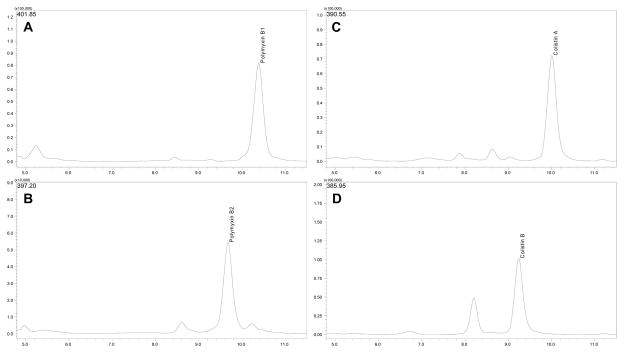
Typical chromatograms obtained from sterile growth media samples for the [M+3H]3+ ions of polymyxin B1 and B2 as well as colistin A and B are shown in Figures 2 and 3. Retention times were approximately 9.2 min for colistin B, 9.7 min for polymyxin B2, 10.0 min for colistin A and 10.4 min for polymyxin B1, in both media. Calibration curves were constructed using the ratio between the summed peaked areas of polymyxin B1 and polymyxin B2 and the summed peak areas of colistin A and B. In CAMHB, a linear regression weighted by the reciprocal of the squared concentration was used to describe the relationship between the peak area ratio and concentration of polymyxin B, while in TSB a linear regression weighted by the reciprocal of concentration was used to characterize this relationship. Coefficients of determination (R2) greater than 0.99 were achieved for all calibration curves (n = 14), with a slope of 0.199 ± 0.0121 (mean ± SD) and intercept of −0.0121 ± 0.0150 for CAMHB (n = 7). In TSB (n = 7), the slope was 0.245 ± 0.0162 and intercept 0.0170 ± 0.00408.
Figure 2.
Chromatograms obtained using multiple ion monitoring to detect [M+3H]3+ ions of polymyxin B1 (A - m/z 401.85), polymyxin B2 (B - m/z 397.20), colistin A (C - m/z 390.55) and colistin B (D - m/z 385.95), in CAMHB samples containing 3.30 mg/L of polymyxin B base and colistin base.
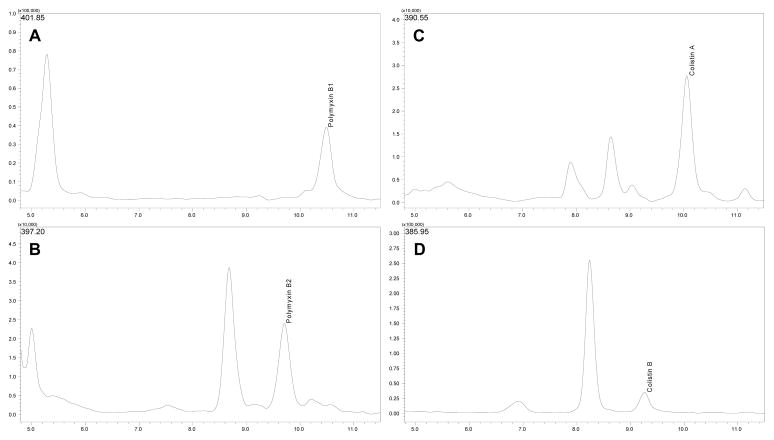
Figure 3.
Chromatograms obtained using multiple ion monitoring to detect [M+3H]3+ ions of polymyxin B1 (A - m/z 401.85), polymyxin B2 (B - m/z 397.20), colistin A (C - m/z 390.55) and colistin B (D - m/z 385.95), in TSB samples containing 3.30 mg/L of polymyxin B base and colistin base.
The ratio of summed peak areas of polymyxin B1 and polymyxin B2 relative to the summed peak areas of colistin A and B was utilized to determine polymyxin B concentration. This approach was favored in the development of this assay as polymyxin B1 and B2 combined account for over 85% of total polymyxin B [14, 15]. This choice also limits the potential effect of inter-batch variability in the relative proportions of these components when different batches of polymyxin B are used for in vitro experiments and quantification. An alternative approach [22], involving the quantification of polymyxin B1 only, was not applied as it does not account for the contribution of polymyxin B2 to overall antibacterial activity. This in turn can affect the characterization of pharmacokinetic/pharmacodynamic relationships that underpin polymyxin B activity.
The accuracy and precision of the assay for each matrix and bacterial strain, defined by the percentage relative error and percentage coefficient of variation (in parentheses), are summarized in Table 1. Accuracy and precision pooled for both sterile and bacteria-containing CAMHB across all assay days for each polymyxin B concentration (n = 42), was 3.01% (9.48%) at 0.103 mg/L, −6.18% (6.58%) at 0.248 mg/L, 0.250% (3.77%) at 2.48 mg/L, 3.18% (3.59%) at 4.95 mg/L and 5.39% (5.18%) at 6.60 mg/L. In TSB, the corresponding values for pooled data were −7.55% (9.64%) at 0.248 mg/L, 3.20% (7.54%) at 2.48 mg/L, −0.103% (6.61%) at 4.95 mg/L and 0.495% (6.15%) at 6.60 mg/L polymyxin B base. The lower limit of quantification was 0.103 mg/L in CAMHB and 0.248 mg/L in TSB. Rifampicin, doripenem, imipenem/cilastatin, tigecycline or cefepime did not affect the assay, with no significant changes observed in the chromatograms obtained from samples containing these antibiotics.
Table 1.
Accuracy (% relative error) and precision (% coefficient of variation) of the assay, grouped by sample matrix and polymyxin B base concentration. Overnight bacterial cultures contained a high bacterial concentration (~108 CFU/mL).
| Sample Type | Sterile |
A. baumannii ATCC 19606 |
P. aeruginosa ATCC 27853 |
K. pneumoniae ATCC 13883 |
|---|---|---|---|---|
| Number of samples at each concentration | 24a | 6b | 6b | 6b |
| % Relative error (% Coefficient of variation) | ||||
| Polymyxin B base concentration | Cation-adjusted Mueller-Hinton Broth | |||
| 0.103 mg/L | 1.89 (9.61) | 9.44 (8.96) | 4.99 (9.39) | −0.914 (8.41) |
| 0.248 mg/L | −7.86 (6.16) | −8.66 (3.17) | 1.45 (6.70) | −4.58 (4.11) |
| 2.48 mg/L | 0.186 (3.85) | −2.15 (2.14) | 1.55 (5.10) | 1.60 (2.46) |
| 4.95 mg/L | 3.06 (2.55) | 6.13 (1.02) | 0.639 (3.97) | 3.25 (6.53) |
| 6.60 mg/L | 5.67 (6.41) | 7.87 (1.92) | 1.67 (1.91) | 5.53 (1.04) |
| Tryptone Soya Broth | ||||
| 0.248 mg/L | −4.68 (10.5) | −17.50 (2.78) | −9.40 (5.56) | −6.24 (3.60) |
| 2.48 mg/L | 2.86 (7.57) | −0.95 (6.54) | 6.49 (8.98) | 5.41 (6.28) |
| 4.95 mg/L | −1.05 (6.72) | −5.29 (3.34) | 3.61 (6.56) | 5.16 (3.21) |
| 6.60 mg/L | −0.586 (6.31) | −6.51 (6.11) | 1.12 (3.58) | 4.27 (2.46) |
Pooled over 4 separate assay days, with 6 replicates assayed per day (Inter-day precision represented)
All 6 replicates completed on the same day (Intra-day precision represented)
Polymyxin B remains one of the very few antimicrobial agents with good activity against very problematic pathogens such as A. baumannii, P. aeruginosa and K. pneumoniae. Therefore, developing optimized polymyxin B dosage regimens via in vitro infection models is critical to preserving its activity, minimizing emergence of resistance, and combating MDR bacterial isolates.[1, 4, 28–30] This is particularly critical since the frequency of serious infections by the above-mentioned bacterial pathogens in hospitalized patients is increasing worldwide and presents a serious global threat to public health, as identified by the Infectious Diseases Society of America. [30, 31]
The assay described in this report demonstrated good accuracy and precision at clinically relevant polymyxin B concentrations and was not affected by non-specific binding of polymyxin B to laboratory ware [32] even at low concentrations (≤0.248 mg/L). Furthermore, the presence of commonly co-administered antibiotics or high concentrations of bacteria did not adversely affect the assay. Our assay is able to achieve satisfactory accuracy and precision utilizing LC-MS rather than LC-MS/MS [19, 20, 24], lowering the instrument costs associated with performing the assay and greatly increasing its accessibility to scientists. These attributes make our assay well suited to the quantification of polymyxin B in in vitro infection models, which are well accepted and widely used to guide optimization of antibiotic dosage regimens. [6–9]
4. Conclusion
In summary, we describe here the development and validation of a robust, simple, accurate and reproducible assay for polymyxin B in two commonly used bacterial growth media, CAMHB and TSB. The dynamic range of the assay and its robustness to the presence of high bacterial concentrations and other clinically important antibiotics within samples make it particularly well suited for application in in vitro pharmacokinetic/pharmacodynamic infection models.
Highlights.
A novel LC-MS assay for polymyxin B in biological growth media was developed.
Good accuracy and precision was achieved in two common in vitro growth media.
The assay is robust to the presence of bacteria and other antibiotics.
The assay requires neither triple quadrupole MS nor solid phase extraction.
Suitable for polymyxin B quantification to support in vitro experimental models.
Acknowledgments
The assistance provided by Jovan Jacob, Monash University, Melbourne, Australia is gratefully acknowledged.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (Award Numbers R01AI098771, R01AI079330 and R01AI070896 to RLN and JL). J.B.B. is an Australian Research Council DECRA Fellow (DE120103084). J.L. is an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellow.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60:1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- 2.Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22:535–543. doi: 10.1097/QCO.0b013e328332e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falagas ME, Kasiakou SK. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Critical care. 2006;10:R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouton JW, Ambrose PG, Canton R, Drusano GL, Harbarth S, MacGowan A, Theuretzbacher U, Turnidge J. Conserving antibiotics for the future: new ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective. Drug Resist Updates. 2011;14:107–117. doi: 10.1016/j.drup.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Tan CH, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2007;51:3413–3415. doi: 10.1128/AAC.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deris ZZ, Yu HH, Davis K, Soon RL, Jacob J, Ku CK, Poudyal A, Bergen PJ, Tsuji BT, Bulitta JB, Forrest A, Paterson DL, Velkov T, Li J, Nation RL. The combination of colistin and doripenem is synergistic against Klebsiella pneumoniae at multiple inocula and suppresses colistin resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2012;56:5103–5112. doi: 10.1128/AAC.01064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother. 2008;61:636–642. doi: 10.1093/jac/dkm511. [DOI] [PubMed] [Google Scholar]
- 9.Tam VH, Schilling AN, Neshat S, Poole K, Melnick DA, Coyle EA. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:4920–4927. doi: 10.1128/AAC.49.12.4920-4927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulitta JB, Landersdorfer CB, Forrest A, Brown SV, Neely MN, Tsuji BT, Louie A. Relevance of pharmacokinetic and pharmacodynamic modeling to clinical care of critically ill patients. Curr Pharm Biotechnol. 2011;12:2044–2061. doi: 10.2174/138920111798808428. [DOI] [PubMed] [Google Scholar]
- 11.Rybak MJ. Pharmacodynamics: relation to antimicrobial resistance. Am J Infect Control. 2006;34:S38–45. doi: 10.1016/j.ajic.2006.05.227. discussion S64–73. [DOI] [PubMed] [Google Scholar]
- 12.Singh R, Tam VH. Optimizing dosage to prevent emergence of resistance - lessons from in vitro models. Curr Opin Pharmacol. 2011;11:453–456. doi: 10.1016/j.coph.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Orwa JA, Govaerts C, Busson R, Roets E, Van Schepdael A, Hoogmartens J. Isolation and structural characterization of colistin components. J Antibiot (Tokyo) 2001;54:595–599. doi: 10.7164/antibiotics.54.595. [DOI] [PubMed] [Google Scholar]
- 14.Orwa JA, Govaerts C, Busson R, Roets E, Van Schepdael A, Hoogmartens J. Isolation and structural characterization of polymyxin B components. J Chromatogr A. 2001;912:369–373. doi: 10.1016/s0021-9673(01)00585-4. [DOI] [PubMed] [Google Scholar]
- 15.He J, Ledesma KR, Lam WY, Figueroa DA, Lim TP, Chow DS, Tam VH. Variability of polymyxin B major components in commercial formulations. Int J Antimicrob Agents. 2010;35:308–310. doi: 10.1016/j.ijantimicag.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J Chromatogr B. 2001;761:167–175. doi: 10.1016/s0378-4347(01)00326-7. [DOI] [PubMed] [Google Scholar]
- 17.Cao G, Ali FE, Chiu F, Zavascki AP, Nation RL, Li J. Development and validation of a reversed-phase high-performance liquid chromatography assay for polymyxin B in human plasma. J Antimicrob Chemother. 2008;62:1009–1014. doi: 10.1093/jac/dkn343. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Valentine J. Simple Method for Assaying Colistin Methanesulfonate in Plasma and Urine Using High-Performance Liquid Chromatography. Antimicrob Agents Chemother. 2002;46:3304–3307. doi: 10.1128/AAC.46.10.3304-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng C, Liu S, Xiao D, Hollembaek J, Yao L, Lin J, Hansel S. LC-MS/MS method development and validation for the determination of polymyxins and vancomycin in rat plasma. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2010;878:2831–2838. doi: 10.1016/j.jchromb.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 20.Jansson B, Karvanen M, Cars O, Plachouras D, Friberg LE. Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J Pharm Biomed Anal. 2009;49:760–767. doi: 10.1016/j.jpba.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Dotsikas Y, Markopoulou CK, Koundourellis JE, Loukas YL. Validation of a novel LC-MS/MS method for the quantitation of colistin A and B in human plasma. J Sep Sci. 2011;34:37–45. doi: 10.1002/jssc.201000680. [DOI] [PubMed] [Google Scholar]
- 22.Kwa AL, Lim TP, Low JG, Hou J, Kurup A, Prince RA, Tam VH. Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn Microbiol Infect Dis. 2008;60:163–167. doi: 10.1016/j.diagmicrobio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Gobin P, Lemaitre F, Marchand S, Couet W, Olivier JC. Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob Agents Chemother. 2010;54:1941–1948. doi: 10.1128/AAC.01367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gikas E, Bazoti FN, Katsimardou M, Anagnostopoulos D, Papanikolaou K, Inglezos I, Skoutelis A, Daikos GL, Tsarbopoulos A. Determination of colistin A and colistin B in human plasma by UPLC-ESI high resolution tandem MS: Application to a pharmacokinetic study. J Pharm Biomed Anal. 2013;83:228–236. doi: 10.1016/j.jpba.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Soon RL, Nation RL, Cockram S, Moffatt JH, Harper M, Adler B, Boyce JD, Larson I, Li J. Different surface charge of colistin-susceptible and -resistant Acinetobacter baumannii cells measured with zeta potential as a function of growth phase and colistin treatment. J Antimicrob Chemother. 2011;66:126–133. doi: 10.1093/jac/dkq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed: 19th August 2013];Product information: CM0129, Tryptone soya broth, Oxoid. http://www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0129.
- 27.Product information: CM0405, Mueller-Hinton Broth. Oxoid: [Accessed: 19th August 2013 ]. http://www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0405. [Google Scholar]
- 28.Nation RL, Li J. Optimizing use of colistin and polymyxin B in the critically ill. Semin Respir Crit Care Med. 2007;28:604–614. doi: 10.1055/s-2007-996407. [DOI] [PubMed] [Google Scholar]
- 29.Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis. 2008;47:1298–1304. doi: 10.1086/592577. [DOI] [PubMed] [Google Scholar]
- 30.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis. 2013;57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 31.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 32.Karvanen MC, Mohamed M, Lagerbäck A, Friberg P, Cars LEO. Program and abstract of the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 17–20; Chicago, IL: American Society for Microbiology; 2011. [Google Scholar]