Abstract
Oxidative DNA damage is repaired primarily by the base excision repair (BER) pathway in a process initiated by removal of base lesions or mismatched bases by DNA glycosylases. MutY homolog (MYH, MUTYH, or Myh1) is a DNA glycosylase which excises adenine paired with the oxidative lesion 8-oxo-7,8-dihydroguanine (8-oxoG, or Go), thus reducing G:C to T:A mutations. The resulting apurinic/apyrimidinic (AP) site is processed by an AP-endonuclease or a bifunctional glycosylase/lyase. We show here that the major Schizosaccharomyces pombe AP endonuclease, Apn2, binds to the inter-domain connector located between the N- and C-terminal domains of Myh1. This Myh1 inter-domain connector also interacts with the Hus1 subunit of the Rad9-Rad1-Hus1 checkpoint clamp. Mutagenesis studies indicate that Apn2 and Hus1 bind overlapping but different sequence motifs on Myh1. Mutation on I261 of Myh1 reduces its interaction with Hus1, but only slightly attenuates its interaction with Apn2. However, E262 of Myh1 is a key determinant for both Apn2 and Hus1 interactions. Like human APE1, Apn2 has 3′-phosphodiesterase activity. However, unlike hAPE1, Apn2 has a weak AP endonuclease activity which cleaves the AP sites generated by Myh1 glycosylase. Functionally, Apn2 stimulates Myh1 glycosylase activity and Apn2 phosphodiesterase activity is stimulated by Myh1. The cross stimulation of Myh1 and Apn2 enzymatic activities is dependent on their physical interaction. Thus, Myh1 and Apn2 constitute an initial BER complex.
Keywords: DNA repair, DNA glycosylase, AP endonuclease, phosphodiesterase, yeast, Schizosaccharomyces pombe
1. Introduction
Reactive oxygen species and radiation can lead to DNA strand breaks and base lesions that must be repaired to maintain genomic stability, prevent carcinogenesis, and control aging [1]. Oxidative DNA lesions are repaired primarily by the base excision repair (BER) pathway [2]. A frequent and highly mutagenic oxidative lesion is 8-oxo-7,8-dihydroguanine (8-oxo-G or Go), which mispairs with adenine during DNA replication, leading to G:C to T:A mutations [3-5]. MutY homolog (MYH, MUTYH, or Myh1) is a DNA glycosylase which excises adenine from A/Go in the first step of BER pathway [3-5]. The resulting apurinic/apyrimidinic (AP) sites are generally processed by AP-endonucleases that catalyze a 5′ cleavage of the phosphodiester backbone, producing a 3′-OH [6,7]. Alternative pathways independent of AP-endonucleases have been identified [8,9]. The downstream BER enzymes then complete the repair process. These enzymes and basic steps of BER pathway are highly conserved among diverse organisms.
MYH/Myh1 repair is essential for genome stability because its deficiency leads to higher mutation rates in both mouse and fission yeast cells [10,11]. In addition, mutations in the human MYH (hMYH) gene are associated with colorectal cancer as in MYH-associated polyposis (MAP) [12-16]. Eukaryotic MYH enzymes contain unique motifs not found in prokaryotic MutY that mediate interactions with partner proteins involved in DNA replication, mismatch repair, and DNA damage response (reviewed in [3,17]). These interactions are critical to direct MYH repair to daughter DNA strands, to drive the repair pathway to completion, and to coordinate BER with DNA damage response. Cell cycle checkpoint provides surveillance mechanisms to activate the DNA damage response, thus preserving genomic integrity [18,19]. Checkpoint sensors Rad9, Rad1, and Hus1 form a heterotrimeric (9-1-1) complex [20,21] whose structure [22-24] is remarkably similar to that of proliferating cell nuclear antigen (PCNA) [25-27]. We recently showed that the interdomain connector (IDC) located between the N- and C-terminal domains of hMYH is uniquely oriented to interact with human AP endonuclease 1 (hAPE1) and the 9-1-1 complex [28]. We have shown that S. pombe Myh1 (SpMyh1) also interacts with the 9-1-1 complex and mutations in the IDC region of SpMyh1 cannot complement myh1Δ phenotypes [28-30].
AP-endonuclease is a multifunctional enzyme that participates in many aspects of DNA metabolism [31,32]. AP-endonuclease activity cleaves the phosphodiester bond 5′ to an AP site and its associated phosphodiesterase activity removes various forms of 3′-blocked lesions at DNA strand breaks to generate a 3′-OH DNA end [33,34]. Because AP sites are mutagenic and cytotoxic [17], they must be recognized by a downstream enzyme such as AP-endonuclease immediately after the action of a DNA glycosylase. A “passing-the-baton” model has been proposed for BER [35,36]. However, the underlying molecular mechanisms remain unclear. It has been proposed that the 9-1-1 complex may serve as a platform to coordinate BER at the sites of DNA damage because it interacts with nearly every enzyme in BER (reviewed in [37]). Human major repair AP-e 105 ndonuclease, APE1, has been shown to interact with several DNA glycosylases [38-43]. MYH is the only glycosylase that can form a stable complex with APE1 [39]. In S. pombe, there are three AP endonucleases (Apn1, Apn2, and Uve1) [33,44]. However, none of these AP endonucleases has been shown to interact with DNA glycosylases.
It has been suggested that S. pombe Apn2 is the major AP endonuclease [33,44]. Because the AP endonuclease activity of SpApn2 is very weak, it has been suggested that the AP lyase activity of the bifunctional glycosylase SpNth1 (endonuclease III) provides the major incision at AP sites [33,45]. The 3′-α,β-unsaturated aldehyde (3′-dRP) produced by SpNth1 is then further processed by the phosphodiesterase activity of SpApn2 [33,45]. Here, we provide the first biochemical characterization of Apn2. We show that recombinant Apn2 expressed in bacteria has 3′-phosphodiesterase activity but processes a weak AP endonuclease activity which cleaves the AP sites generated by Myh1 glycosylase. SpApn2 interacts with the IDC region (residues 245-293) of Myh1 which is also a Hus1 binding site, however, Apn2 and Hus1 use overlapping but different sequence motifs. Myh1 and Apn2 cross stimulate each other's enzymatic activity. Thus, Myh1 and Apn2 can act synergistically as a physical unit to maintain genomic stability.
2. Materials and methods
2.1. Cloning of glutathione S-transferase (GST) tagged SpApn2
The full-length cDNA of SpApn2 encoding 523 residues was amplified by PCR from the plasmid pNBR110 [44] (kindly provided by S. Mitra, University of Texas Medical Branch) using Pfu DNA polymerase (Stratagene) with the appropriate primers (listed in Table S1). All oligonucleotides were purchased and purified by HPLC from IDT. The PCR products were digested with BamHI and XhoI and then cloned into pGEX4T-2 to express GST fusion protein. The plasmids were transformed into E. coli DH5α cells (Invitrogen), and selected via ampicillin resistance. DNA sequencing revealed that the Apn2 clone in pGEX4T-2 vector contained Ser (AGT codon) at position 254. However, SpApn2 sequence in the gene bank (NCBI Reference Sequence: NP_595522.1) indicates Asn (AAT codon) at this position. Further analysis showed that the Apn2 gene in the original template plasmid (pNBR110) [44] already contained the same AAT to AGT change. The GST-tagged Apn2S254 was expressed in Rosetta cells (Invitrogen).
2.2. Cloning, expression, and purification of His-tagged full-length Apn2
The plasmid pGEX4T-Apn2 containing the full-length cDNA of SpApn2S254 was digested with BamHI and XhoI and isolated cDNA fragment was ligated into BamHI/XhoI digested pET21a vector to obtain pET21a-SpApn2S254. QuickChange site-directed mutagenesis (Strategene) using pET21a-SpApn2S254 plasmid as a template and primers listed in Table S1 was employed to obtain pET21a-SpApn2N254 plasmid. The pET21a-SpApn2N254 plasmid was then further used to construct pET21a-SpApn2N254/S295 plasmid by similar QuickChange site-directed mutagenesis. Both mutations were verified by DNA sequencing.
SpApn2S254, SpApn2N254, and SpApn2N254/S295 proteins were expressed in E. coli BW528 [nfo-1::kan Δ(xth-pncA)90] (kindly provided by Bernard Weiss) containing a lambda DE3 lysogen to avoid the contamination of E. coli AP endonucleases. The DE3 lysogenic stain was constructed according to the procedures described by Invitrogen. The cells were cultured in Luria-Bertani broth containing 100 μg/ml amplicilin at 37°C. Protein expression was induced at an A590of 0.6 by the addition of isopropyl 1-thio-β-D-galactopyranoside to a final concentration of 0.2 mM. After 16 hours at 20°C, the cells were harvested. The His-Apn2 proteins were first purified by Ni-NTA resin (Qiagen) under native conditions according to the manufacturer's protocol. The proteins from Ni column were diluted with buffer A (20 mM potassium phosphate, pH 7.4, 0.1 mM EDTA, 10% glycerol, 1 mM dithiothreitol and 0.1 mM PMSF) and further purified by 1 ml SP column (GE Health) equilibrated with buffer A containing 0.05 M KCl. Upon washing with 12 ml of equilibration buffer, the column was eluted with 20 ml of buffer A containing a linear gradient of KCl (0.05-1 M). The fractions that contain the His-Apn2 proteins (confirmed by SDS-polyacrylamide gel analysis) were pooled, further purified by 1 ml Heparin column (GE Health) equilibrated with buffer A containing 0.05 M KCl. Upon washing with 12 ml of equilibration buffer, the column was eluted with 20 ml of buffer A containing a linear gradient of KCl (0.05-1 M). Because heparin chromatography only increased Apn2 purify slightly, SpApn2N254/S295 protein was not further purified by Heparin column. The fractions that contain the His-Apn2 protein (confirmed by SDS-polyacrylamide gel analysis) were pooled, divided into small aliquots, and stored at −80°C. The concentrations of His- Apn2 proteins were determined by the Bradford method.
2.3. Cloning, expression, and purification of His- and maltose binding protein (MBP)-tagged Apn21-303
The cDNA (encoding residues 1-303) of SpApn2S254 was amplified by PCR from the plasmid pGEX4T-Apn2 using Pfu DNA polymerase (Stratagene) with the appropriate primers (listed in Table S1). The PCR products were digested with BamHI and NotI, ligated into BamHI/NotI digested pLM303 vector which can express dual N-terminal His- and MBP-tagged proteins. The plasmid was transformed into E. coli DH5α cells (Invitrogen), and selected via kanamycin resistance.
To express the His-MBP-tagged SpApn21-303 (His-MBP-Apn21-303) protein, the plasmid was transformed into the E. coli Rosetta (Invitrogen) strain. The cells were cultured in Luria-Bertani broth containing 25 μg/ml kanamycin at 37°C. Protein expression and purification procedures were similar to those described for His-Apn2. The fractions that contain the His-MBP-Apn21-303 protein (confirmed by SDS-polyacrylamide gel analysis) were pooled from SP column, divided into small aliquots, and stored at −80°C.
2.4. GST-Myh1(E262Q) and other GST fusion protein constructs
The Glu262 to Gln (E262Q) mutant of the Spmyh1 gene was constructed by QuickChange site-directed mutagenesis kit (Strategene) using the plasmid pGEX4T-SpMyh1 plasmid [29] and primers list in Table S1. The mutation was verified by DNA sequencing. The constructs of GST fusion of intact Myh1, Myh1(245-461), Myh1(294-461), Myh1(I261A), and Myh1(I261A/E262Q) have been described [28,29].
2.5. Other proteins used
The recombinant SpMyh1 [46] and SpMyh1(I261A/E262Q) [28] expressed in E. coli was purified as described. His-APE1 was purified from BL21(DE3) cells (Novagen) containing hAPE1-pET28 as published [47].
2.6. GST pull-down assay
Expression, immobilization of GST fusion proteins, and GST-pull-down assay were similar to the procedures described previously [29]. Briefly, cell extracts from a 0.5-liter culture were immobilized onto glutathione-Sepharose 4B (GE Health). Immobilized GST proteins were incubated with 0.1 mg of target proteins overnight at 4°C. After washing, the pellets were fractionated on a 10-12% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane. Western blot analyses were performed with respective antibody: polyclonal SpMyh1 antibodies [10] and His-probe antibody (sc-8036, Santa Cruz Biotechnology). Western blotting was detected by the Enhanced Chemiluminescence analysis system (USB Corporation) according to the manufacturer's protocol.
2.7. Myh1 glycosylase activity assay
The Myh1 substrate is a 20-mer duplex DNA containing an A/Go base mismatch (Table S1). The A-containing strand was labeled with fluorescein (FAM) at the 5′-end as described [28]. The Myh1 glycosylase assay with an A/Go-containing DNA was performed as described previously [28,46]. FAM-labeled A/Go-DNA substrate (5 nM) was incubated with 1 nM Myh1 and different amounts of His-Apn2 at 30°C for 30 min. The reaction mixture contained 10 mM Tris-HCl, pH 7.6, 0.5 mM dithiothreitol (DTT), 0.5 mM EDTA, 1.45% glycerol, 50 μg/ml bovine serum albumin (BSA), and 5 nM DNA in a total volume of 10 μl. The Myh1 glycosylase products were then treated with 0.1 M NaOH at 90°C for 30 min. For the Myh1-Apn2 coupled reactions, the mixture contained 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, , 1 mM DTT, 2.5% glycerol, and 50 μg/ml BSA. The coupled reaction products were not treated with 0.1 M NaOH. All reaction mixtures were supplemented with 5 μl of formamide gel loading dye (90% formamide, 10 mM EDTA, 0.1% xylene cyanol, and 0.1% bromphenol blue), heated at 90°C for 2 min, and 50% of reaction products loaded onto 14% 7 M urea sequencing gels. Fluorescence was detected by Typhoon FLA9500 and quantified by the ImageQuant Software (GE Healthcare).
2.8. Assay of Apn2 activity
Two types of DNA substrates were used to assay Apn2 activity. A 28-mer synthetic nucleotide (Table S1) containing a single tetrahydrofuran (THF, an AP analog) was synthesized with labeled FAM at the 3′ end and then annealed with the complementary oligonucleotide with G opposing THF. In some experiments, the strand containing THF before annealing was also labeled at the 5′-end with [32P]-phosphate as described [48]. The other Apn2 substrate is a DNA duplex (28-mer) containing a natural AP site pairing with G. DNA duplexes containing U/G (Table S1) (48 pmol) were fully converted to AP/G by treating with 30 units of E. coli uracil DNA glycosylase (UDG, Life Technologies) at 37°C for 1 h in a buffer consisting with 20 mM Tris-HCl, pH 7.6, 80 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, and 2.9% glycerol.
The AP endonuclease assay mixture (10 μl) contained 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 0.1 mg/ml BSA, 1 mM DTT, 10% glycerol and 20 nM 3′-FAM-labeled DNA or 0.18 nM 5′-[32P]-labeled DNA. The reaction was preceded by adding different amounts of His-Apn2 or hAPE1 at 30°C for different time intervals. After stopping the reaction by addition of formamide gel loading dyes, 50% of reaction products were separated by electrophoresis in a denaturing polyacrylamide gel (14%) containing 7 M urea. Fluorescence was detected by Typhoon FLA9500 and radioactive images were obtained by exposure to a storage phosphor screen and detected by Typhoon FLA9500. The bands were quantified by the ImageQuant Software (GE Healthcare).
The kinetics experiments used to determine rate constants (kobs) of His-Apn2 were performed using a saturating enzyme concentration (200 nM) and DNA substrate concentrations of 10 nM. The data were fitted by non-linear regression to eq. 1:
| (1) |
where A is the amplitude, kobs is the rate constant, and t is reaction time. Because the experiments were performed under saturating enzyme conditions, the kobsreflect the maximal rate of product formation (i.e., kobs≈ kmax) and are not influenced by product release or product inhibition.
3. Results
3.1. Expression of S. pombe Apn2 in E. coli
S. pombe Apn2 contains 523 residues in which the first 303 residues is highly homologous (with 57.4% conserved residues) to human APE1 [44]. The extra C-terminus of SpApn2 has unknown function. Ribar et al. [44] attempted to overexpress GST-Apn2 and His-Apn2 fusion proteins in the S. pombe apn1Δ apn2Δ mutant. Their recombinant His-Apn2S254 was found to be mostly insoluble and GST-Apn2S254 had a very weak AP endonuclease activity. Furthermore, the authors found that overexpression of the GST-Apn2S254 fusion protein appeared to be toxic because the transformed S. pombe stopped growing. To further study Apn2, we tried to express full-length Apn2 with His-tag or GST-tag and AP endonuclease domain (residues 1-303) with double His-MBP-tag in E. coli. DNA sequencing revealed that the Apn2 gene in the original template plasmid (pNBR110) [44] contained Ser (AGT codon) at position 254 that is different from the one in the gene bank (NCBI Reference Sequence: NP_595522.1) containing Asn (AAT codon) at this position. Thus, our three Apn2 clones in pET21a, pGEX4T-2, and pLM303 vectors derived from pNBR110 all contained Ser at position 254. We then sequenced several S. pombe genomic DNAs in our laboratory and showed that they all contained Apn2N254. Although the origin of SpApn2S254 variant is not clear at this point, we suggest that SpApn2S254 variant may be a rare form of SpApn2N254 for several reasons. First, Asn to Ser is a conservative change. Second, hAPE1 contains Thr268 and hAPE2 contains S262 at the corresponding position of Ser254 of SpApn2. Third, the Thr268 residue in hAPE1 is over 10 Å from the active site His309 [49,50]. Fourth, SpApn2S254 contains E42, D269, and His295 which are conserved in the catalytic sites of AP endonucleases. Thus, we proceeded to study Apn2S254 protein and to compare its activity with SpApn2N254.
To characterize the enzyme activity of Apn2, we purified His-Apn2S254, His-Apn2N254, and His-MBP-Apn21-303 proteins expressed in E. coli. His-Apn2S254 and His-Apn2N254 were expressed in E. coli BW528 which is deficient of both AP endonucleases (EndoIV and ExoIII). His-MBP-Apn21-303 protein could not be expressed in E. coli BW528 cells because the expression plasmid and bacteria are both kanamycin resistant. Thus, His-MBP-Apn21-303 protein was expressed in Rosetta strain and only used in physical interaction with Myh1. All the Apn2 proteins had low solubility and were not highly expressed in E. coli. We managed to purify them to greater than 90% purity; however, the preparations contained some degradation products (Fig. 1A). His-Apn2S254 and His-Apn2N254 preparations had several degraded products near 32 kDa (Fig. 1A, lanes 1-4) while His-MBP-Apn21-303 preparation had a major degraded product about 60 kDa (Fig. 1A, lanes 7 and 8).
Fig. 1.
SpApn2 interacts with SpMyh1. (A) SDS-polyacrylamide gel analysis of purified S. pombe His-Apn2S254, His-Apn2N254, His-Apn2S295, and His-MBP-Apn21-303. His-Apn2S254 (lanes 1 and 2), His-Apn2N254 (lanes 3 and 4), and His-Apn2S295 (lanes 5 and 6) were expressed in E. coli BW528 cells while His-MBP-Apn21-303 (lanes 7 and 8) was expressed in Rosetta cells. The proteins were separated on a 10% polyacrylamide gel in the presence of SDS. Odd lanes were stained with Coomassie Blue (S) and even lanes were from Western blots (WB) using an anti- His antibody. The positions of protein markers are indicated with arrows. The stars mark His-Apn2 and His-MBP-Apn21-303. Some degraded protein products were detected in all four preparations. (B)-(E) Immobilized GST, wild-type Myh1, Myh1(245-461), Myh1(294-461), Myh1(I261A), Myh1(E262Q), and Myh1(I261A/E262Q) were used to precipitate His-Apn2S254, His-Apn2N254, His-MBP-Apn21-303, and His-Apn2S295, respectively. Lane 1 contains 10% of input Apn2 proteins. Apn2 was detected by an anti-His antibody. (F) SpMyh1 can be pulled down by immobilized GST-Apn2S254. Lane 1 contains 10% of input Myh1. Myh1 was detected by an anti-Myh1 antibody. (G) Quantitative analyses of bound Apn2S254 on GST-tagged Myh1 constructs from three experiments.
3.2. S. pombe Apn2 interacts with Myh1
Mammalian APE1 has been shown to interact with many DNA glycosylases [38,39,41-43]. So far, only hMYH has been demonstrated to form a stable complex with hAPE1 [39]. Ribar et al. [44] have shown that inactivation of Apn2, but not Apn1 and Uve1, sensitizes S. pombe to alkylation and reactive oxygen species indicating Apn2 is the major AP endonuclease for BER. Therefore, we tested whether Apn2 interacts with Myh1. As shown in Figs. 1B and 1C, His-Apn2S254 and His-Apn2N254 could be pulled down by immobilized GST-Myh1 (lane 3). Thus, SpApn2 proteins containing Ser or Asn at position 254 interact similarly with Myh1. In addition, His-MBP-Apn21-303 and His-Apn2S295 (catalytically inactive mutant) also interacted with wild-type intact Myh1 at similar levels (Fig. 1D and 1E, lane 3). We also show that Myh1 could be pulled down by immobilized GST-Apn2S254 (Fig. 1F).
By using constructs containing different portions of Myh1 fused to GST, we determined the regions of Myh1 engaged in the physical interaction with Apn2S254 (Fig. 1B). These GST-tagged Myh1 deletion variants were constructed based on the domain structures of prokaryotic MutY [51] and human MYH [28]. Approximate equal molar amounts of GST or GST-tagged Myh1 constructs were immobilized on glutathione-sepharose (Fig. S1 in supplementary material) and incubated with His-Apn2S254. Myh1(245-461) (Δ245) had a two-fold weaker interaction with Apn2 than intact Myh1 (Fig. 1B, compare lane 4 with lane 3 and Fig. 1G). By contrast, Myh1(294-461) had no binding with Apn2 (Fig. 1B, lane 5). Thus, although the sequence upstream of the IDC contains an interaction element for Apn2 binding, the Apn2 interacting domain is mainly localized to residues 245-293 which constitute the IDC of SpMyh1. Interestingly, the IDC of Myh1 is also required for Hus1 interaction [30]. We have shown that both I261 and E262 are important for Hus1 interaction [28,29]. The interaction of Myh1(I261A) with Hus1 is reduced 5-fold as compared with that of wild-type Myh1 [30]. To test whether Apn2 and Hus1 bind to the same motif of Myh1, we analyzed the binding of Apn2 with GST-tagged Myh1(I261A), Myh1(E262Q), and Myh1(I261A/E262Q). The result (Fig. 1B, lanes 6-8) showed that I261A mutation of Myh1 slightly attenuated its interaction with Apn2 (Fig. 1B, lane 6 and Fig. 1G). However, Myh1(E262Q) single mutant and Myh1(I261A/E262Q) double mutant had no interaction with Apn2 (Fig. 1B, lanes 7 and 8). Thus, Apn2 and Hus1 bind to overlapping, but distinct, interaction sites of Myh1.
We observed that His-Apn2N254 exhibited identical binding patterns to various Myh1 constructs as His-Apn2S254 (compare Fig. 1C with Fig. 1B). However, His-MBP-Apn21-303 (Fig. 1D) and His-Apn2S295 (catalytically inactive mutant) (Fig. 1E) showed slightly different binding patterns with Myh1 mutant constructs. Particularly, their interactions with Myh1(245-461) were much weaker than the interactions of His-Apn2N254 and His-Apn2S254 with Myh1(245-461) (Figs. 1B-1E, lane 4). Thus, the sequence upstream of the IDC is more important for His-MBP-Apn21-303 and His-Apn2S295 binding.
3.3. Recombinant S. pombe Apn2 has 3′-phosphodiesterase activity and weak AP endonuclease activity
The total AP endonuclease activity was reported to be very weak in S. pombe extracts [33,45]. Ribar et al. [44] have shown that purified GST-Apn2S254 from the S. pombe apn1Δ apn2Δ mutant has very weak AP endonuclease activity with a THF-containing DNA. With 68-fold molar excess of GST-Apn2S254 over DNA substrate, only 25% of DNA were cleaved in 60 min incubation (Fig. 6B in [44]). Such poor AP endonuclease activity of Apn2 may be insignificant in the incision at AP sites in vivo [33,45]. We purified both His-Apn2S254 and His- Apn2N254 proteins expressed in E. coli and assayed their AP endonuclease activities with a DNA substrate containing a single THF and labeled with FAM at 3′ end (Fig. 2A). As shown in Fig. 2B (lanes 2-4), hAPE1 generated two cleavage products. Product 1 and product 2 are derived from cleavage at the 5′ of the THF and at the junction between DNA and FAM, respectively (Fig. 2A). Cleavage of FAM label linked to the 3′-terminal oxygen of DNA has been reported for hAPE1, presumably by hydrolyzing the phosphodiester bond [47]. Surprisingly, both His-Apn2S254 and His-Apn2N254 only generated product 2 (Fig. 2B, lanes 5-10). There is no difference in activity between His-Apn2S254 and His-Apn2N254. With equal molar of DNA substrate and His-Apn2, about 70% of 3′-FAM label was cleaved in 30 min incubation (Fig. 2B, lanes 7 and 10). The absence of product 1 in the reactions with His-Apn2 indicates that the enzyme does not incise at THF or the incision rate is much slower than the rate of 3′-FAM removal. To confirm this weak incision at THF, we labeled the same DNA with [32P]-phosphate at the 5′-end of the THF-containing strand (Fig. 2C). The autoradiograph detected the [32P]-labeled DNA (not the FAM-labeled product) and showed that hAPE1 predominately generated product 1 which is derived from cleavage at the 5′ of the THF (Fig. 2D, lanes 2-4). However, the major cleavage product of both His-Apn2S254 and His-Apn2N254 was product 2 derived from cleavage of 3′ FAM (Fig. 2D, lanes 5-10). There were very limited shorter products migrated faster than P2 indicating minimal 3’ to 5’ exonuclease activity after the removal of 3’ FAM. Thus, the AP endonuclease activity of Apn2 at the 5′ of the THF was almost undetectable.
Fig. 6.
Apn2 cleaves the products generated by Myh1 and stimulates Myh1 glycosylase activity. (A) Apn2 can cleave AP site generated by Myh1 in the coupled buffer. Lane 1 contains 5′-FAM-labeled A/Go-20 substrate (5 nM) alone. Lane 2, DNA (5 nM) was incubated 1 nM Myh1 in coupled buffer and the reaction mixture was supplemented with 1 μl of 1 M NaOH and heated at 90°C for 30 min to cleave the AP-DNA. Lane 3, similar to lane 2 but the reaction mixture was not treated with NaOH, thus the AP-DNA was not cleaved. Lanes 4-6, 5 nM DNA were incubated 1 nM Myh1 and 12.5, 25, or 50 nM His-Apn2S254, respectively; and the reaction mixtures were not treated with NaOH. Lane 7, DNA was incubated with His-Apn2S254 without Myh1 and the reaction mixture was not treated with NaOH. The reaction products were fractionated on a 14% sequencing gel. Fluorescence was detected by Typhoon FLA9500. Arrows mark the intact DNA substrate (I) and the cleavage product (N). The percentage (%) of the product generated is shown below each lane. (B) Reactions were similar to (A) except that Myh1(I261A/E262Q) mutant protein was used. (C) Reactions were similar to (A) except that Apn2S295 catalytically inactive mutant protein was used. (D) Quantitative analyses of percentage of nicked product of lanes 2-6 as in (A) (white bars), (B) (black bars), and (C) (grey bars) from three experiments. The error bars are the standard deviations of the averages. (E) Myh1 reactions with increasing amounts of His-Apn2S254 in the absence of MgCl2. Lane 1, 5′-FAM A/Go-DNA substrate (5 nM). Lane 2, 5 nM 5′-FAM A/Go-DNA substrate was incubated with 1 nM Myh1 in Myh1 buffer and treated with NaOH. Lanes 3-7 are similar to lane 2 but with added 1-16 nM of His-Apn2S254. Lane 8, DNA was incubated with 16 nM His-Apn2S254. (F) is similar to (E) except reactions were performed in the presence of 2 mM MgCl2. (G) Reactions were similar to (E) the reactions were performed in the presence of 2 mM MgCl2. (G) Reactions were similar to (E) except that Apn2S295 catalytically inactive mutant protein was used. (H) Quantitative analyses of the fold stimulation of Apn2 on the Myh1 glycosylase activity as in (E) (circles), (F) (diamonds), and (G) (triangles) from three experiments. The error bars reported are the standard deviations of the averages.
Fig. 2.
AP endonuclease and phosphodiesterase activities of SpApn2. (A) Double-stranded 28-mer DNA containing a single THF/G (as indicated) was used as a substrate of AP endonucleases. The THF-containing strand is labeled with FAM at the 3′ end. Product 1 and product 2 are derived from cleavage at the 5′ to the THF site and at the junction between DNA and FAM, respectively. (B) Comparison of the activities of SpApn2 and hAPE1 on 3′-FAM THF/G-DNA as in (A). Lane 1 contained 200 fmole of FAM-labeled THF/G-DNA (20 nM) (Fig. 2A). The assay mixture (10 μl) contained 200 fmole of FAM-labeled THF/G-DNA (20 nM) and indicated amounts of human APE1 (hAPE1) (lanes 2-4), His-Apn2S254 (lanes 5-7), or His-Apn2N254 (lanes 8-10). The samples were separated by electrophoresis in a denaturing polyacrylamide gel (14%) containing 7 M urea, and fluorescence was detected using a Typhoon FLA9500 (GE Healthcare). The positions of intact substrate (S), product 1 (P1), and product 2 (P2) are indicated. (C) The 3′- FAM labeled DNA containing a single THF/G (as in A) was labeled with [32P]-phosphate at 5′ end and used as a substrate of AP endonucleases. (D) Comparison of the activities of SpApn2 and hAPE1 on 5′-[32P]-phosphate, 3′-FAM THF/G-DNA (Fig. 2C). The assay is similar to that in (B) except the reactions contained 1.8 fmole of 32P-labeled THF/G-DNA (0.18 nM). Autoradiogram was detected by Typhoon FLA9500 (GE Healthcare) using a phosphor screen. (E) Double-stranded 28-mer DNA containing a single natural AP/G (as indicated) was used as a substrate of AP endonucleases. The DNA substrate is similar to (B) except the AP-site is generated from U after treatment with UDG. (F) Comparison of the activities of SpApn2 and hAPE1 on 3′-FAM AP/G-DNA (Fig. 2E). The assay is similar to that in (B). Lane 2, AP/G-DNA was treated with 0.1 M NaOH at 90°C for 30 min. Lanes 8-10 in (B), (D) and (F) were run on separate gels.
We also assayed the AP endonuclease activity of purified His-Apn2S254 and His-Apn2N254 with a 3’-FAM DNA substrate containing a natural AP site (with hydroxyl group at position 1’ of deoxyribose) (Fig. 2E). This AP/G-DNA substrate was derived from U/G-DNA after treatment with UDG. As shown in Fig. 2F (lane 2), the DNA was cleaved completely by 0.1 M NaOH at 90°C indicating that the U is completely removed by UDG. hAPE1 generated two cleavage products with AP/G-DNA (Fig. 2F, lanes 3 and 4). Both His-Apn2S254 and His- Apn2N254 had similar activity on AP/G-DNA. The major cleavage product of His-Apn2S254 and His-Apn2N254 was product 2 (Fig. 2F, lanes 7 and 10). The amount of product 1 is only slightly above background (Fig. 2F, lanes 5-10). Thus, Apn2 has very weak AP endonuclease activity. It is interesting to note that Apn2 activity on AP/G-DNA is weaker than on THF/G-DNA (compare Fig. 2B and 2F, lands 5-10).
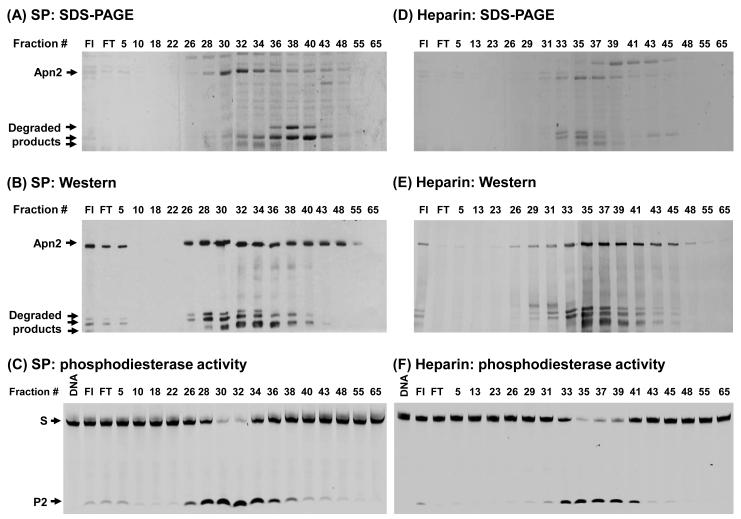
To investigate whether the phosphodiesterase activity that generates product 2 on 3’-FAM THF/G DNA results from a contaminant that co-purifies with His-Apn2, we assayed SP and Heparin chromatography fractions of His-Apn2N254 preparation with 3′-FAM labeled DNA. As shown in Figs. 3A-3C, both His-Apn2N254 protein and the activity of 3’-FAM excision peaked at fraction 30-32 of SP chromatography. Similarly, His-Apn2N254 protein co-purified with the activity of 3’-FAM excision during Heparin chromatography, both peaked at fractions 35-37 (Figs. 3D-3F). In addition, His-Apn2S254 co-purified with phosphodiesterase activity during SP and Heparin chromatography (Fig. S2 in supplementary material). We further purified a catalytically inactive His-Apn2H295S mutant protein. The SP fractions of this preparation did not have any phosphodiesterase activity (Fig. S3 in supplementary material). These results strongly suggested that the phosphodiesterase activity was intrinsic to the SpApn2 protein.
Fig. 3.
Co-purification of His-Apn2N254 with phosphodiesterase activity. (A) SDS-PAGE of fractions separated by SP chromatography. The proteins from each fraction (20 μl) were separated on a 10% polyacrylamide gel in the presence of SDS and stained with Coomassie Blue. The fraction numbers are indicated on the top of each lane. FI and FT represent the protein sample loaded onto the column and the flow-through fraction, respectively. The positions of His-Apn2 and its three major degraded products are indicated. (B) The same SP fractions on SDS-PAGE were detected by Western blotting using His-antibody. (C) Phosphodiesterase activity as measured by 3′-FAM cleavage of SP fractions. Protein fractions (1 μl of a 10-fold dilution of fractions) were incubated with 200 fmole (20 nM) of 3′-THF/G-28 DNA substrate (Fig. 2A) for 30 min at 30°C and the products were fractionated on a 14% sequencing gel. Lane 1 contains DNA alone. S and P2 label the intact DNA substrate and product 2, respectively. (D)- (F) are similar to (A) and (C), respectively, except using fractions from heparin chromatography and 1 μl of a 5-fold dilution of fractions for 3′-FAM cleavage assay. The gel images in (C) and (F) were obtained using a Typhoon FLA9500.
We further measured the rate of the 3′-phosphodiesterase activity of His-Apn2S254 protein. The kinetics experiments were performed under saturating enzyme conditions (Fig. 4A and 4B) to obtain the rate constant (kobs) that reflects the maximal rate of product formation and is not influenced by product release or product inhibition. With 20-fold molar excess of His-Apn2S254 relative to DNA substrate, the 3’-FAM label was completely cleaved in 64 min incubation (Fig. 4A, lane 12). Analysis of Fig. 4B showed that His-Apn2S254 cleaved the 3’-FAM label with a rate of kobs= 0.180 ± 0.005 min−1 at 30°C.
Fig. 4.
Kinetics of the phosphodiesterase activity of SpApn2 under substrate-limiting conditions. (A) Time course of His-Apn2S254 reaction with 3′-FAM THF/G-DNA under substrate-limiting conditions. Reactions are similar to Fig. 2B except with 10 nM 3′-FAM-labeled THF/G-DNA and 200 nM His-Apn2S254. The image was detected by Typhoon FLA9500 and quantified by PhosphorImager analysis using the ImageQuant Software (GE Healthcare). S and P2 label the intact DNA substrate and product 2, respectively. The percentage (%) of product 2 generated is shown below each lane. (B) Plot of data from three experiments as in (A). The error bars reported are the standard deviations of the averages.
3.4. Myh1 stimulates Apn2 activity
Since Apn2 physically interacted with Myh1, we tested whether they interacted functionally. First, we tested whether Myh1 has any effect on Apn2 3′-phosphodiesterase activity. We observed that Myh1 stimulated Apn2 hydrolysis of the phosphodiester bond between 3′-FAM and DNA (Fig. 5). To investigate the nature of Myh1 enhancement on Apn2 activity, we performed the time course experiments under DNA excess condition to measure the rate of turnover of His-Apn2S254 with 3′-FAM-labeled THF/G substrate in the presence and absence of 4-fold molar excess of Myh1. Compared the data in Fig. 5A and 5B, at 32 min incubation, the product amount generated by His-Apn2S254 was increased about 2-fold in the presence of Myh1 as compared to reactions without Myh1 (p-value=0.005) (Fig. 5D). From these data, we interpret that Myh1 stimulates the turnover of Apn2. We then tested the effect of Myh1(I261A/E262Q) mutant which is defective in Apn2 interaction (Fig. 1B, lane 8) on Apn2 activity. Myh1(I261A/E262Q) had no stimulation effect on the phosphodiesterase activity of His-Apn2S254 (Figs. 5C and 5D). Thus, the stimulation of Myh1 on Apn2 activity is dependent on their physical interaction.
Fig. 5.
The phosphodiesterase activity of Apn2 can be stimulated by Myh1. (A) Time course of the Apn2S254 reaction under enzyme limiting conditions. Reactions are similar to Fig. 2B except using 20 nM 3′-FAM THF/G-DNA and 2 nM His-Apn2S254. The percentage (%) of the product generated is shown below each lane. (B) and (C) Time course of the same Apn2 reactions in the presence of 8 nM of Myh1 and Myh1(I261A/E262Q), respectively. The percentage (%) of the product generated is shown below each lane. (D) Plot of data from three experiments as in (A) (closed circles), (B) (open squares), and (C) (open triangles).
3.5. Apn2 stimulates Myh1 glycosylase activity
Next, we examined Myh1 glycosylase activity on 5′-FAM-labeled A/Go-DNA in the presence of His-Apn2S254 in a coupled buffer containing MgCl2under which Apn2 is active (Fig. S4, lanes 4 and 5, supplementary material). As shown in Fig. 6A (lane 2), under a Myh1 limiting condition, Myh1 glycosylase removed the adenine base from A/Go-mismatch to generate AP site which is further cleaved by NaOH by β/δ elimination. Without NaOH treatment, no strand cleavage was observed at the AP site (Fig. 6A, lane 3). By adding His-Apn2S254 to the Myh1 reaction, the AP sites generated by Myh1 were incised by the AP endonuclease activity of Apn2 without NaOH treatment (Fig. 6, lanes 4-6). As expected, His-Apn2S254 did not cleave the 5′-FAM label and did not excise mispaired adenine (Fig. 6, lane 7). Thus, His-Apn2S254 can cleave the AP sites generated by Myh1 glycosylase. Moreover, with 50-fold molar excess of His-Apn2S254 over Myh1 in this coupled reaction, more than double amount of cleavage product was observed as compared to Myh1 reaction followed by NaOH treatment (p-value=0.01) (Fig. 6A, compare lanes 2 and 6; Fig. 6D). When Myh1(I261A/E262Q) mutant, which is defective in Apn2 interaction, was used in this coupled assay, the AP sites generated by Myh1 glycosylase can also be cleaved by His-Apn2S254. However, there is no enhancement in the production of cleavage product (p-value=0.4) (Fig. 6B, compare lanes 2 and 6; Fig. 6D). As a negative control, the AP site generated by wild-type Myh1 was not cleaved by the catalytically inactive His-Apn2S295 mutant protein (Fig. 6C, lanes 4-6 and Fig. 6D). Thus, Apn2 can cleave the AP sites generated by Myh1 glycosylase and stimulate Myh1 glycosylase activity.
We then analyzed the effect of Apn2 on the Myh1 glycosylase activity on A/Go-DNA in Myh1 buffer without MgCl2under which Apn2 activity is reduced by more than 4-fold (Fig. S4, lanes 6 and 7, supplementary material). In this assay, the resulting AP sites were cleaved by NaOH treatment. When increasing amounts of His-Apn2S254 were added to the Myh1 glycosylase reactions, the Myh1 activity was enhanced (Fig. 6E, compare lane 2 with lanes 3–7). The quantification results (Fig. 6H) showed that at an Apn2/Myh ratio of 16, Apn2 could enhance Myh1 activity by 2-fold (p-value=0.004). His-Apn2S254 alone at the highest concentration used (16 nM) did not have glycosylase or nicking activity on the A/Go-DNA substrate (Fig. 6E, lane 8). It has been shown that the stimulation of MYH activity by hAPE1 is enhanced about 2-fold by the presence of MgCl2[40]. We also observed a moderate effect of MgCl2on His-Apn2S254 stimulation on Myh1 (Fig. 6F and 6G). In the presence of MgCl2, at an Apn2/Myh ratio of 16, Apn2 could enhance Myh1 activity by about 3-fold which are 1.5-fold better than that without MgCl2(p-value=0.02) (Fig. 6H). When increasing amounts of catalytically inactive His-Apn2S295 were added to the Myh1 glycosylase reactions, the Myh1 activity was not enhanced (Fig. 6G, compare lane 2 with lanes 3–7). Thus, both Apn2 catalytic activity and a physical interaction are required to enhance Myh1 glycosylase activity.
4. Discussion
As suggested in the “passing the baton” model of the BER pathway [35,36], the product of each reaction must be sequestered and transferred to the next enzyme in order to mitigate the potential mutagenic and cytotoxic effects of the intermediates. Our results reveal that Myh1, primarily via its IDC, interacts with Apn2. Functionally, Apn2 and Myh1 stimulate each other's activities. The stimulation of Apn2 phosphodiesterase activity by Myh1 is dependent on their physical interaction. The stimulation of Myh1 glycosylase activity by Apn2 is dependent on their physical interaction and Apn2 catalytic activity. Importantly, Apn2 can incise at the AP site generated by Myh1, but has much weaker activity on incising the AP sites generated by UDG. Therefore, S. pombe Apn2 is likely the next enzyme to act after Myh1 glycosylase reaction, a relationship similar to the MYH/APE1 system in human cells.
It is interesting to note that the IDC region (residues 245-293) of Myh1 is also important for its interactions with Hus1, a subunit of the 9-1-1 complex. However, Apn2 and Hus1 bind Myh1 through overlapping but different sequence motifs. How these two partner proteins interact with Myh1 within this short 49-residue region is unclear. It has been shown that human Hus1 and APE1 bind to the IDC region of hMYH [30,39] and Hus1 stabilizes the MYH/APE1 complex [52]. These data suggest that Hus1 and APE1 may bind simultaneously to MYH forming a ternary complex. The ability of 9-1-1 to stabilize the MYH/APE1 complex might be part of an regulatory mechanism in BER.
We provide the first biochemical characterization of the activity of SpApn2. The AP endonuclease activity on THF of SpApn2 was first reported by Ribar et al. [44]. Due to low solubility, the activity detected was very low [44]. Our His-Apn2S254 preparation has the same sequence as the protein purified by Ribar et al. [44]. However, several S. pombe strains in our laboratory contain Apn2 with Asn254 which has been reported in the gene bank. Thus, we compared the activity of His-Apn2S254 and His-Apn2N254. We demonstrated that His-Apn2S254 and His-Apn2N254 interact with Myh1 constructs similarly and have identical activity on both THF/G- and AP/G-DNA (Fig. 2). Therefore, we suggest that SpApn2S254 variant may be a rare form of SpApn2N254. Both can remove FAM linked to the 3′-terminal oxygen of DNA by phosphodiesterase activity. However, their AP endonuclease activity cleaving at THF or natural AP sites generated by UDG are very low. Apn2 has a better activity on incising the AP sites generated by Myh1 (Fig. 6A) than the AP sites generated by UDG (Fig. 2F). In this regard, SpApn2 differs from hAPE1 protein, whose AP endonuclease activity is much stronger than the 3′-phosphodiesterase [53]. These properties of SpApn2 are similar to Apn2 of Saccharomyces cerevisiae whose 3′-phosphodiesterase activity are 30- to 40-fold more active than its AP endonuclease activity [54]. ScApn2 also has 3’ to 5’ exonuclease activity, however, we observed very weak exonuclease in our SpApn2 preparations (Fig. 2D, lanes 5-10). It has been shown that the ability of ScApn2 to remove 3′-end groups from DNA is critical in the repair of H2O2-induced DNA damage [54]. Thus, another major function of S. pombe Apn2 is to remove 3′-blocked lesions at DNA strand breaks by its phosphodiesterase activity.
The phosphodiesterase activity of Apn2 is comparable to that of hAPE1 (compare lane 4 with lanes 5 and 8, Fig. 2B). Time course studies indicated that the observed catalytic rate (kobs) is 0.180 ± 0.005 min−1. This Apn2 phosphodiesterase activity can be modestly stimulated by Myh1 and this stimulation relies on their physical interaction (Fig. 5). These similar properties have also been observed in the human MYH/APE1 system [52]. Our data suggest that Myh1 stimulates the turnover of Apn2. This leads us to propose that Myh1 remains in the complex composed of Myh1, Apn2, and AP-DNA. It has been shown that APE1 can enhance MYH glycosylase activity [40,43]. In those studies with mammalian enzymes, the turnover of MYH can be stimulated by large excess (i.e. 100 to 125-fold) of APE1 [40,43]. We observed a stronger stimulation of Myh1 by Apn2. With 50-fold molar excess of Apn2 relative to Myh1 in the coupled reaction, Myh1 glycosylase activity can be stimulated by two-fold (Fig. 6D). In the Myh1 buffer containing MgCl2, at an Apn2/Myh1 ratio of 16, Apn2 could enhance Myh1 activity by about 3-fold (Fig. 6H). Interestingly, both physical interaction and Apn2 catalytic activity are required to enhance Myh1 glycosylase activity. Taken together, these data suggest that Myh1 and Apn2 act as a synergistic catalytic unit.
SpApn2 contains 215 extra amino acids at its C-terminus that are not found in hAPE1. In S. cerevisiae, the C-terminal extension of ScApn2 makes no contribution to the AP endonuclease activity in vitro, but the truncated protein is defective in the removal of AP sites in vivo [55]. It is suggested that the C-terminal domain of ScApn2 is important to interact with other proteins. It has been established that PCNA associates with APE2 via a PIP box at the C-terminal domains in humans and S. cerevisiae [56-58]. We show here that the C-terminal domain of SpApn2 is not required for Myh1 interaction. The in vivo function of the extra C-terminal domain of SpApn2 is unknown.
Our results indicate that Apn2 acts immediately after Myh1 glycosylase reaction. However, the AP endonuclease activity of Apn2 is much weaker than its phosphodiesterase activity. It has been suggested that Apn2 is not the primary enzyme to incise DNA containing an AP site generated by DNA glycosylases in S. pombe [33,45]. Instead, it has been shown that SpNth1 bifunctional glycosylase provides the major incision at AP sites [33,45]. Thus, Nth1 may also be involved in Myh1-initiated BER. The 3′-phosphodiesterase activity of SpApn2 may remove the 3′-α,β-unsaturated aldehyde following SpNth1 action [33,45]. In this scenario, Apn2 is not the immediately downstream enzyme after Myh1 in BER. It will be interesting to see any physical or functional interactions of Nth1 with Myh1 or Apn2. The roles of the other AP endonucleases (Apn1 and Uve1) [33,44] in S. pombe Myh1-mediated repair pathway also need further investigation. Although SpApn1 has been shown to have no or only a limited role in AP site repair [44], it has been shown that laboratory strains derived from L972 h(-) contain an apn1 nonsense mutation [59]. The role of SpApn1 is unclear because expression of an active Apn1 in an apn1 defective S. pombe strain does not provide additional protection against methylation agents [59]. Further biochemical and genetics analyses are required to elucidate the second enzymes in BER.
Supplementary Material
Highlights.
The major S. pombe AP endonuclease, Apn2, binds to the inter-domain connector of Myh1.
Apn2 and Hus1 bind overlapping but different sequence motifs on Myh1.
Apn2 has 3′-phosphodiesterase activity and a weak AP endonuclease activity.
Apn2 stimulates Myh1 glycosylase activity and cleaves the AP sites generated by Myh1.
The phosphodiesterase activity Apn2 is stimulated by Myh1.
Acknowledgments
We thank Drs. Sankar Mitra (University of Texas Medical Branch) and Bernard Weiss (University of Rochester) for kindly providing Apn2 plasmid and E. coli strain, respectively. The authors would like to thank Dr. Laura Mizoue of the Center for Structural Biology at Vanderbilt University for providing the MBP expression plasmid used in these studies. This work was supported by grants (GM35132 and CA78391) from National Institute of Health to ALL and the American Cancer Society Research Scholar Grant RSG-09-058-01-GMC to EAT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflict of interest.
FOOTNOTES
The abbreviations used are: 3′-dRP, 3′-α,β-unsaturated aldehyde; 8-oxoG or Go, 7,8-dihydro-8-oxoguanine; 9-1-1, Rad9-Rad1-Hus1; AP, apurinic/apyrimidinic; APE1 or Apn1, AP-endonuclease 1; Apn2, AP-endonuclease 2; BER, base excision repair; BSA, bovine serum albumin; DTT, dithiothreitol; FAM, fluorescein; GST, glutathione S-transferase; h, human; IDC, interdomain connector; kobs,rate constants; MAP, MYH-associated polyposis; MBP, maltose binding protein; MYH, MUTYH, or Myh1, MutY homolog; Nth1, endonuclease III homolog; PCNA, proliferating cell nuclear antigen; S. cerevisiae or Sc, Saccharomyces cerevisiae; S. pombe or Sp, Schizosaccharomyces pombe; THF, tetrahydrofuran abasic site analog; UDG, uracil DNA glycosylase
References
- 1.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. ASM Press; Washington,D.C.: 2005. [Google Scholar]
- 2.Krokan HE, Nilsen H, Skorpen F, Otterlei M, Slupphaug G. Base excision repair of DNA in mammalian cells. FEBS Lett. 2000;476:73–77. doi: 10.1016/s0014-5793(00)01674-4. [DOI] [PubMed] [Google Scholar]
- 3.Lu A-L, Bai H, Shi G, Chang D-Y. MutY and MutY homologs (MYH) in genome maintenance. Front Biosci. 2006;11:3062–3080. doi: 10.2741/2033. [DOI] [PubMed] [Google Scholar]
- 4.Michaels ML, Miller JH. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxo-guanine) J. Bacteriol. 1992;174:6321–6325. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchou J, Grollman AP. Repair of DNA containing the oxidatively-damaged base 8-hydroxyguanine. Mutat. Res. 1993;299:277–287. doi: 10.1016/0165-1218(93)90104-l. [DOI] [PubMed] [Google Scholar]
- 6.Hitomi K, Iwai S, Tainer JA. The intricate structural chemistry of base excision repair machinery: implications for DNA damage recognition, removal, and repair. DNA Repair (Amst) 2007;6:410–428. doi: 10.1016/j.dnarep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Mol CD, Parikh SS, Putnam CD, Lo TP, Tainer JA. DNA repair mechanisms for the recognition and removal of damaged DNA bases. Annu. REv. Biophys. Biomol. Struct. 1999;28:101–128. doi: 10.1146/annurev.biophys.28.1.101. [DOI] [PubMed] [Google Scholar]
- 8.Nilsen L, Forstrom RJ, Bjoras M, Alseth I. AP endonuclease independent repair of abasic sites in Schizosaccharomyces pombe. Nucleic Acids Res. 2012;40:2000–2009. doi: 10.1093/nar/gkr933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiederhold L, Leppard JB, Kedar P, Karimi-Busheri F, Rasouli-Nia A, Weinfeld M, Tomkinson AE, Izumi T, Prasad R, Wilson SH, Mitra S, Hazra TK. AP endonuclease-independent DNA base excision repair in human cells. Mol. Cell. 2004;15:209–220. doi: 10.1016/j.molcel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Chang D-Y, Gu Y, Lu A-L. Fission yeast (Schizosaccharomyces pombe) cells defective in the MutY-homologous glycosylase activity have a mutator phenotype and are sensitive to hydrogen peroxide. Mol. Genet. Genomics. 2001;266:336–342. doi: 10.1007/s004380100567. [DOI] [PubMed] [Google Scholar]
- 11.Hirano S, Tominaga Y, Ichinoe A, Ushijima Y, Tsuchimoto D, Honda-Ohnishi Y, Ohtsubo T, Sakumi K, Nakabeppu Y. Mutator phenotype of MUTYH-null mouse embryonic stem cells. J. Biol. Chem. 2003;278:38121–38124. doi: 10.1074/jbc.C300316200. [DOI] [PubMed] [Google Scholar]
- 12.Al Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, Hodges AK, Davies DR, David SS, Sampson JR, Cheadle JP. Inherited variants of MYH associated with somatic G:C to T:A mutations in colorectal tumors. Nat. Genet. 2002;30:227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 13.Halford SE, Rowan AJ, Lipton L, Sieber OM, Pack K, Thomas HJ, Hodgson SV, Bodmer WF, Tomlinson IP, J. Pathol. Germline mutations but not somatic changes at the MYH locus contribute to the pathogenesis of unselected colorectal cancers, Am. 2003;162:1545–1548. doi: 10.1016/S0002-9440(10)64288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones S, Emmerson P, Maynard J, Best JM, Jordan S, Williams GT, Sampson JR, Cheadle JP. Biallelic germline mutations in MYH predispose to multiple colorectal adenoma and somatic G:C→T:A mutations. Hum. Mol. Genet. 2002;11:2961–2967. doi: 10.1093/hmg/11.23.2961. [DOI] [PubMed] [Google Scholar]
- 15.Sieber OM, Lipton L, Crabtree M, Heinimann K, Fidalgo P, Phillips RK, Bisgaard ML, Orntoft TF, Aaltonen LA, Hodgson SV, Thomas HJ, Tomlinson IP. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N. Engl. J. Med. 2003;348:791–799. doi: 10.1056/NEJMoa025283. [DOI] [PubMed] [Google Scholar]
- 16.Sampson JR, Dolwani S, Jones S, Eccles D, Ellis A, Evans DG, Frayling I, Jordan S, Maher ER, Mak T, Maynard J, Pigatto F, Shaw J, Cheadle JP. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39–41. doi: 10.1016/S0140-6736(03)13805-6. [DOI] [PubMed] [Google Scholar]
- 17.David SS, O'Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 19.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 20.Hang H, Lieberman HB. Physical interactions among human checkpoint control proteins HUS1p, RAD1p, and RAD9p, and implications for the regulation of cell cycle progression. Genomics. 2000;65:24–33. doi: 10.1006/geno.2000.6142. [DOI] [PubMed] [Google Scholar]
- 21.St Onge RP, Udell CM, Casselman R, Davey S. The human G2 checkpoint control protein hRAD9 is a nuclear phosphoprotein that forms complexes with hRAD1 and hHUS1. Mol. Biol. Cell. 1999;10:1985–1995. doi: 10.1091/mbc.10.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dore AS, Kilkenny ML, Rzechorzek NJ, Pearl LH. Crystal structure of the Rad9-Rad1-Hus1 DNA damage checkpoint complex--implications for clamp loading and regulation. Mol. Cell. 2009;34:735–745. doi: 10.1016/j.molcel.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Sohn SY, Cho Y. Crystal structure of the human Rad9-Hus1-Rad1 clamp. J. Mol. Biol. 2009;390:490–502. doi: 10.1016/j.jmb.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Xu M, Bai L, Gong Y, Xie W, Hang H, Jiang T. Structure and functional implications of the human Rad9-Hus1-Rad1 cell cycle checkpoint complex. J. Biol. Chem. 2009;284:20457–20461. doi: 10.1074/jbc.C109.022384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burtelow MA, Roos-Mattjus PM, Rauen M, Babendure JR, Karnitz LM. Reconstitution and molecular analysis of the hRad9-hHus1-hRad1 (9-1-1) DNA damage responsive checkpoint complex. J. Biol. Chem. 2001;276:25903–25909. doi: 10.1074/jbc.M102946200. [DOI] [PubMed] [Google Scholar]
- 26.Shiomi Y, Shinozaki A, Nakada D, Sugimoto K, Usukura J, Obuse C, Tsurimoto T. Clamp and clamp loader structures of the human checkpoint protein complexes, Rad9-Rad1-Hus1 and Rad17-RFC. Genes to Cells. 2002;7:861–868. doi: 10.1046/j.1365-2443.2002.00566.x. [DOI] [PubMed] [Google Scholar]
- 27.Venclovas C, Thelen MP. Structure-based predictions of Rad1, Rad9, Hus1 and Rad17 participation in sliding clamp and clamp-loading complexes. Nucleic Acids Res. 2000;28:2481–2493. doi: 10.1093/nar/28.13.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luncsford PJ, Chang DY, Shi G, Bernstein J, Madabushi A, Patterson DN, Lu A-L, Toth EA. A structural hinge in eukaryotic MutY homologues mediates catalytic activity and Rad9-Rad1-Hus1 checkpoint complex interactions. J. Mol. Biol. 2010;403:351–370. doi: 10.1016/j.jmb.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang DY, Lu A-L. Interaction of checkpoint proteins Hus1/Rad1/Rad9 with DNA base excision repair enzyme MutY homolog in fission yeast, Schizosaccharomyces pombe. J. Biol. Chem. 2005;280:408–417. doi: 10.1074/jbc.M406800200. [DOI] [PubMed] [Google Scholar]
- 30.Shi G, Chang D-Y, Cheng CC, Guan X, Venclovas C, Lu A-L. Physical and functional interactions between MutY homolog (MYH) and checkpoint proteins Rad9-Rad1-Hus1. Biochem. J. 2006;400:53–62. doi: 10.1042/BJ20060774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbotts R, Madhusudan S. Human AP endonuclease 1 (APE1): from mechanistic insights to druggable target in cancer. Cancer Treat. Rev. 2010;36:425–435. doi: 10.1016/j.ctrv.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Demple B, Sung JS. Molecular and biological roles of Ape1 protein in mammalian base excision repair. DNA Repair (Amst) 2005;4:1442–1449. doi: 10.1016/j.dnarep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Alseth I, Korvald H, Osman F, Seeberg E, Bjoras M. A general role of the DNA glycosylase Nth1 in the abasic sites cleavage step of base excision repair in Schizosaccharomyces pombe. Nucleic Acids Res. 2004;32:5119–5125. doi: 10.1093/nar/gkh851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 35.Prasad R, Shock DD, Beard WA, Wilson SH. Substrate channeling in mammalian base excision repair pathways: passing the baton. J. Biol. Chem. 2010;285:40479–40488. doi: 10.1074/jbc.M110.155267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson SH, Kunkel TA. Passing the baton in base excision repair. Nat. Struct. Biol. 2000;7:176–178. doi: 10.1038/73260. [DOI] [PubMed] [Google Scholar]
- 37.Madabushi A, Lu A-L. The novel role of cell cycle checkpoint clamp Rad9-Hus1-Rad1 (the 9-1-1 complex) in DNA repair. In: Berhardt LV, editor. Advances in Medicine and Biology. Nova Publishers; Hauppauge NY: 2011. pp. 41–74. [Google Scholar]
- 38.Baldwin MR, O'Brien PJ. Human AP endonuclease 1 stimulates multiple-turnover base excision by alkyladenine DNA glycosylase. Biochemistry. 2009;48:6022–6033. doi: 10.1021/bi900517y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker A, Gu Y, Mahoney W, Lee S-H, Singh KK, Lu A-L. Human homolog of the MutY protein (hMYH) physically interacts with protein involved in long-patch DNA base excision repair. J. Biol. Chem. 2001;276:5547–5555. doi: 10.1074/jbc.M008463200. [DOI] [PubMed] [Google Scholar]
- 40.Pope MA, Chmiel NH, David SS. Insight into the functional consequences of hMYH variants associated with colorectal cancer: distinct differences in the adenine glycosylase activity and the response to AP endonucleases of Y150C and G365D murine MYH. DNA Repair (Amst) 2005;4:315–325. doi: 10.1016/j.dnarep.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Sidorenko VS, Nevinsky GA, Zharkov DO. Mechanism of interaction between human 8-oxoguanine-DNA glycosylase and AP endonuclease. DNA Repair (Amst) 2007;6:317–328. doi: 10.1016/j.dnarep.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Xia L, Zheng L, Lee HW, Bates SE, Federico L, Shen B, O'Connor TR. Human 3-methyladenine-DNA glycosylase: effect of sequence context on excision, association with PCNA, and stimulation by AP endonuclease. J. Mol. Biol. 2005;346:1259–1274. doi: 10.1016/j.jmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Yang H, Clendenin WM, Wong D, Demple B, Slupska MM, Chiang JH, Miller JH. Enhanced activity of adenine-DNA glycosylase (Myh) by apurinic/apyrimidinic endonuclease (Ape1) in mammalian base excision repair of an A/GO mismatch. Nucleic Acids Res. 2001;29:743–752. doi: 10.1093/nar/29.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribar B, Izumi T, Mitra S. The major role of human AP-endonuclease homolog Apn2 in repair of abasic sites in Schizosaccharomyces pombe. Nucleic Acids Res. 2004;32:115–126. doi: 10.1093/nar/gkh151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugimoto T, Igawa E, Tanihigashi H, Matsubara M, Ide H, Ikeda S. Roles of base excision repair enzymes Nth1p and Apn2p from Schizosaccharomyces pombe in processing alkylation and oxidative DNA damage. DNA Repair (Amst) 2005;4:1270–1280. doi: 10.1016/j.dnarep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Lu A-L, Fawcett WP. Characterization of the recombinant MutY homolog, an adenine DNA glycosylase, from Schizosacchromyces pombe. J. Biol. Chem. 1998;273:25098–25105. doi: 10.1074/jbc.273.39.25098. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgerald ME, Drohat AC. Coordinating the initial steps of base excision repair. Apurinic/apyrimidinic endonuclease 1 actively stimulates thymine DNA glycosylase by disrupting the product complex. J. Biol. Chem. 2008;283:32680–32690. doi: 10.1074/jbc.M805504200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu A-L. Repair of A/G and A/8-oxoG mismatches by MutY adenine DNA glycosylase. In: Vaughan P, editor. DNA Repair Protocols, prokaryotic systems. Humana Press; Totowa, New Jersey: 2000. pp. 3–16. [Google Scholar]
- 49.Gorman MA, Morera S, Rothwell DG, de La FE, Mol CD, Tainer JA, Hickson ID, Freemont PS. The crystal structure of the human DNA repair endonuclease HAP1 suggests the recognition of extra-helical deoxyribose at DNA abasic sites. EMBO J. 1997;16:6548–6558. doi: 10.1093/emboj/16.21.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mol CD, Hosfield DJ, Tainer JA. Abasic site recognition by two apurinic/apyrimidinic endonuclease families in DNA base excision repair: the 3' ends justify the means. Mutat. Res. 2000;460:211–229. doi: 10.1016/s0921-8777(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 51.Fromme JC, Banerjee A, Huang SJ, Verdine GL. Structural basis for removal of adenine mispaired with 8-oxoguanine by MutY adenine DNA glycosylase. Nature. 2004;427:652–656. doi: 10.1038/nature02306. [DOI] [PubMed] [Google Scholar]
- 52.Luncsford PJ, Manvilla BA, Patterson DN, Malik SS, Jin J, Hwang BJ, Gunther R, Kalvakolanu S, Lipinski LJ, Yuan W, Lu W, Drohat AC, Lu A-L, Toth EA. Coordination of MYH DNA glycosylase and APE1 endonuclease activities via physical interactions. DNA Repair. 2013;12:1043–1052. doi: 10.1016/j.dnarep.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suh D, Wilson DM, III, Povirk LF. 3'-phosphodiesterase activity of human apurinic/apyrimidinic endonuclease at DNA double-strand break ends. Nucleic Acids Res. 1997;25:2495–2500. doi: 10.1093/nar/25.12.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unk I, Haracska L, Prakash S, Prakash L. 3'-phosphodiesterase and 3'-->5' exonuclease activities of yeast Apn2 protein and requirement of these activities for repair of oxidative DNA damage. Mol. Cell Biol. 2001;21:1656–1661. doi: 10.1128/MCB.21.5.1656-1661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Unk I, Haracska L, Johnson RE, Prakash S, Prakash L. Apurinic endonuclease activity of yeast Apn2 protein. J. Biol. Chem. 2000;275:22427–22434. doi: 10.1074/jbc.M002845200. [DOI] [PubMed] [Google Scholar]
- 56.Burkovics P, Hajdu I, Szukacsov V, Unk I, Haracska L. Role of PCNA-dependent stimulation of 3'-phosphodiesterase and 3'-5' exonuclease activities of human Ape2 in repair of oxidative DNA damage. Nucleic Acids Res. 2009;37:4247–4255. doi: 10.1093/nar/gkp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsuchimoto D, Sakai Y, Sakumi K, Nishioka K, Sasaki M, Fujiwara T, Nakabeppu Y. Human APE2 protein is mostly localized in the nuclei and to some extent in the mitochondria, while nuclear APE2 is partly associated with proliferating cell nuclear antigen. Nucleic Acids Res. 2001;29:2349–2360. doi: 10.1093/nar/29.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unk I, Haracska L, Gomes XV, Burgers PM, Prakash L, Prakash S. Stimulation of 3'→5' exonuclease and 3'-phosphodiesterase activities of yeast apn2 by proliferating cell nuclear antigen. Mol. Cell Biol. 2002;22:6480–6486. doi: 10.1128/MCB.22.18.6480-6486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laerdahl JK, Korvald H, Nilsen L, Dahl-Michelsen K, Rognes T, Bjoras M, Alseth I. Schizosaccharomyces pombe encodes a mutated AP endonuclease 1. DNA Repair (Amst) 2011;10:296–305. doi: 10.1016/j.dnarep.2010.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.