Abstract
Background
Contrary to pancreatic adenocarcinoma, pancreatic neuroendocrine tumours (PNET) are commonly hyperenhancing on arterial phase computed tomography (APCT). However, a subset of these tumours can be hypoenhancing. The prognostic significance of the CT appearance of these tumors remains unclear.
Methods
From 2001 to 2012, 146 patients with well-differentiated PNET underwent surgical resection. The degree of tumour enhancement on APCT was recorded and correlated with clinicopathological variables and overall survival.
Results
APCT images were available for re-review in 118 patients (81%). The majority had hyperenhancing tumours (n = 80, 68%), 12 (10%) were isoenhancing (including cases where no mass was visualized) and 26 (22%) were hypoenhancing. Hypoenhancing PNET were larger, more commonly intermediate grade, and had higher rates of lymph node and synchronous liver metastases. Hypoenhancing PNET were also associated with significantly worse overall survival after a resection as opposed to isoenhancing and hyperenhancing tumours (5-year, 54% versus 89% versus 93%). On multivariate analysis of factors available pre-operatively, only hypoenhancement (HR 2.32, P = 0.02) was independently associated with survival.
Discussion
Hypoenhancement on APCT was noted in 22% of well-differentiated PNET and was an independent predictor of poor outcome. This information can inform pre-operative decisions in the multidisciplinary treatment of these neoplasms.
Introduction
Neuroendocrine tumours are a heterogeneous group of epithelial neoplasms that can originate from almost any organ derived from the primitive endoderm, including pancreatic islet cells.1 While pancreatic neuroendocrine tumours (PNET) account for only 2% of all pancreatic neoplasms, with an annual incidence between 2 to 5 cases per million individuals, their incidence appears to be rising.2–6 PNET are classically characterized as slow-growing and indolent tumors; however, aggressive tumours with early invasion and metastases have also been described. Definitive management of PNET includes complete removal of the tumour and any metastatic disease, as surgery remains the only treatment modality that can result in a cure.7–12 As many of these tumours display an indolent course, many surgeons opt for enucleation of small, superficial lesions in order to preserve pancreatic parenchyma and reduce the risk of pancreatic insufficiency.13–16 Larger tumours or those with pre-operative evidence of locoregional spread or distant metastases generally require formal pancreatic resection; indeed, when feasible, aggressive resection including vascular reconstruction and liver metastasectomy may allow for excellent long-term prognosis given the generally slow progression of these tumours.17–21
Numerous post-operative variables have been identified to predict prognosis after resection of PNET, including histological grade, Ki-67 proliferative index, mitotic count, evidence of necrosis, perineural or lymphovascular invasion and lymph node metastasis.22–29 Identification of pre-operative prognostic variables remains somewhat vague yet important, as these could potentially guide treatment strategy. Although discrepancies exist, older age, tumour size, the presence of distant metastases and a lack of hormone hypersecretion (likely owing to late presentation) have previously been shown to be independent pre-operative predictors of a worse prognosis.27–33
In an attempt to improve pre-operative prognostic stratification, recent studies have evaluated imaging characteristics of PNET that may suggest a more aggressive behaviour.34–37 Our group recently showed that the presence of calcifications on pre-operative computed tomography correlated with intermediate grade and metastases in well-differentiated PNET.34 Typically, PNET display a characteristic hyperenhancing pattern on the arterial phase of computed tomography (APCT), owing to the hypervascular nature of these tumours. A consistent number of PNET, however, appear hypoenhancing or heterogeneous on APCT.38–41 A previous pilot study by Rodallec et al37 has demonstrated a correlation between hypoenhancing tumours and poor differentiation as well as decreased survival; however, almost half (18 of 37) of the patients included in this study did not undergo surgery, making survival comparisons and definitive confirmation of the tumour's pathological characteristics problematic. In addition, this study only analysed the area of highest enhancement and did not account for tumour heterogeneity.
The goal of this study was to more accurately examine the enhancement patterns of well-differentiated PNET on pre-operative CT. Specifically we hoped to delineate the prevalence of hypoenhancement on APCT in these tumours and correlate this finding with histopathological features and survival after resection.
Patients and methods
A retrospective cohort study was conducted on all patients who underwent a surgical resection of a PNET at Stanford University Medical Center between 2001 and 2012, and for whom pre-operative APCT images were available for re-review. Patients with synchronous liver metastases who underwent a combined or staged liver resection or ablation were included in the study. Patients with high-grade PNET (G3), defined as having a mitotic rate of >20 per 10 High Power Fields (HPF), and/or a Ki-67of >20%, are typically managed with systemic chemotherapy at our institution and were excluded from the study.1 The study was approved by the Stanford University institutional review board.
The operative techniques have been described previously.19,20,42 Small, superficial tumours were enucleated provided the integrity of the pancreatic duct could be maintained. Additionally, unless the tumour enucleated was an insulinoma, a detailed removal of peripancreatic lymph nodes was performed.43,44 Specifically, for head/neck tumors, after a wide Kocher manoeuver, the lymphatic tissue posterior to the head of the pancreas and in the portocaval/hepatic artery space was removed. For body/tail tumours, peri-pancreatic lymph node sampling was accomplished with a deliberate dissection to remove nodes around the hepatic and splenic arteries. Larger tumours that could not be enucleated were resected with a pancreaticoduodenectomy, central pancreatectomy or distal pancreatectomy. The latter was done via open or laparoscopic approach, per the discretion of the surgeon. If liver metastases were present, they were addressed primarily with anatomic or non-anatomic liver resection, or alternatively with thermal ablation or a combination of resection and ablation.17
Demographic, clinical and pathological data were retrospectively reviewed. Age, gender, operative details and tumour characteristics (location, size, multifocality, differentiation, mitotic rate, Ki-67 immunostaining, necrosis, perineural or lymphovascular invasion and lymph node or liver metastases) were recorded. Intermediate grade tumours (G2) were defined by the presence of at least one of the following criteria: mitotic rate of 2–20 per 10 HPF, Ki-67 labelling index of 3–20%, the presence of necrosis and perineural or lymphovascular invasion.1,45 In the absence of any of the aforementioned criteria, the tumour was considered low grade (G1, mitotic rate <2/10 HPF, Ki-67 <3%). Functionality was based on the presence of the relevant clinical syndromes combined with biochemical evidence of hormonal excess. Vital status at last follow-up was obtained through a combined review of the medical record, Social Security Index and the California Cancer Registry.
All APCT images were re-reviewed using Centricity PACS (picture archiving and communication system; General Electric, Fairfield, CT, USA). Images were reviewed simultaneously by the first (D.J.W.) and last (G.A.P.) authors after a consensus was defined by these authors and the radiologist involved (P.D.P.) as to how the lesions should be categorized. The degree of tumour arterial enhancement was determined by comparison with the surrounding pancreatic parenchyma. Care was taken to avoid areas of calcifications, peritumoral areas of pancreatitis or adjacent normal vasculature when assessing tumour enhancement. When multiple tumours were present in one patient, the characteristics of the largest tumour were recorded. Non-contrast-phase CT images were also reviewed to record the presence of calcifications within the primary tumour. Finally, uptake on octreoscan was correlated with the enhancement pattern on APCT.
Statistical analysis
Categorical variables were presented as counts (percentages) and compared using Fisher's exact and chi-square tests. Continuous variables were presented as mean [standard deviation (SD)] and compared by one-way analysis of variance (anova) tests. Survival probabilities were calculated by the Kaplan–Meier method and compared using the log rank test. Multivariate analysis of pre-operative factors predictive of survival was performed by Cox regression analysis. The level of statistical significance was set to P < 0.05.
Results
From 2001 to 2012, 146 patients with well-differentiated PNET underwent surgical resection at our institution. Pre-operative APCT images were available for 118 patients (81%), and this group constituted our study cohort. The clinicopathological characteristics of the cohort are shown in Table 1. Synchronous liver metastases were present in 30 patients (25%) and were addressed with combined or staged liver resection or thermal ablation with potentially curative intent. Additional work-up to include biopsy or fine-needle aspiration was performed in 66 patients (56%) and rendered a diagnosis of neuroendocrine tumour in 58 patients (49%).
Table 1.
Clinicopathological characteristics of 118 patients with pancreatic neuroendocrine tumours
| Characteristic | |
|---|---|
| Age, mean (SD) | 55 (14) |
| Male gender (%) | 62 (53) |
| Pre-operative tissue diagnosis | 58 (49) |
| Mean size of tumour, cm (SD) | 3.68 (3.3) |
| Multifocal tumours (%) | 11 (9) |
| Enucleation (%) | 14 (12) |
| Vascular resection/reconstruction (%) | 11 (9) |
| Location | |
| Head/Uncinate (%) | 41 (34) |
| Body/Tail (%) | 74 (62) |
| Diffuse (%) | 4 (3) |
| Calcifications present (%) | 22 (19) |
| Functional (%) | 30 (25) |
| Insulinoma (%) | 21 (18) |
| Gastrinoma (%) | 4 (3) |
| Glucagonoma (%) | 3 (3) |
| VIPoma (%) | 1 (1) |
| MEN I (%) | 11 (9) |
| Positive Margin | 35 (30) |
| Grade | |
| Low grade (%) | 67 (57) |
| Intermediate grade (%) | 51 (43) |
| Lymph node metastasis (%)* | 33 (33) |
| Synchronous liver metastasis (%) | 30 (25) |
| * 99 patients had at least 1 lymph node evaluated pathologically | |
Review of pre-operative APCT images identified five dominant patterns for PNET enhancement (Fig. 1). Eighty (68%) tumours were hyperenhancing, of which 75 (64%) were solid appearing and 5 (4%) were cystic with rim enhancement. Two (2%) tumours were isoenhancing whereas 10 (8%) tumours were not visualized on pre-operative CT. A total of 26 (22%) tumours were hypoenhancing, of which 12 (10%) were homogeneously hypoenhancing and 14 (12%) were heterogeneous but mostly hypoenhancing, with some degree of peripheral enhancement (Fig. 2). Of the 38 patients in our cohort who had isoenhancing or hypoenhancing tumours, 28 patients underwent a pre-operative biopsy. Seventeen were endoscopic ultrasound-guided and 11 were percutaneous (mostly of liver metastases). Twenty-five of these yielded a diagnosis of neuroendocrine tumour. Of the 10 patients with no mass visualized, 3 had pre-operative tissue diagnosis via endoscopic ultrasound/fine-needle aspiration, 3 had tumours detectable by EUS without definitive pathological diagnosis, 1 had a pre-operative diagnosis based on percutaneous biopsy of a liver mass, 1 had clinical and biochemical evidence of Zollinger–Ellison syndrome and positive octreoscan, 1 had clinical and biochemical diagnosis of insulinoma and a calcium angiogram localizing the lesion and 1 had an ERCP showing a biliary stricture.
Figure 1.
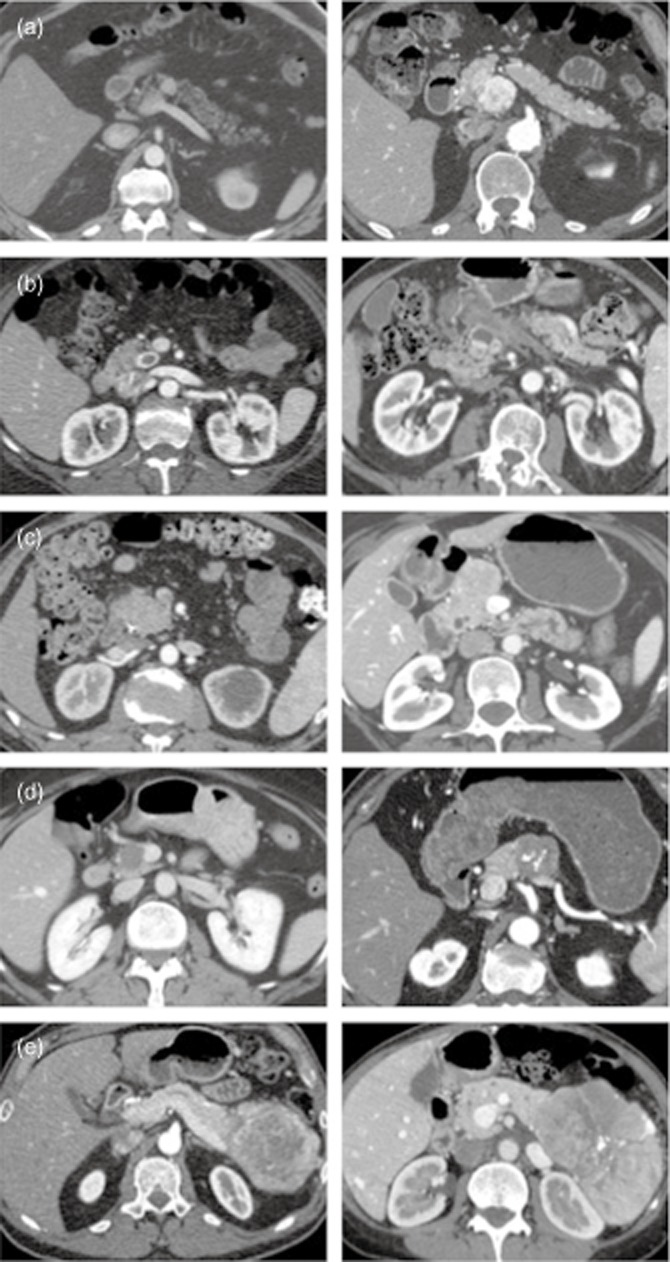
Representative images of the 5 types of enhancement pattern on arterial phase computed tomography. Two images are shown for each type. Hyperenhancing, solid (a); Cystic with hyperenhancing rim (b); Isoenhancing or no mass visualized (c); Homogeneously hypoenhancing (d); Heterogeneous but mostly hypoenhancing with some peripheral enhancement (e)
Figure 2.
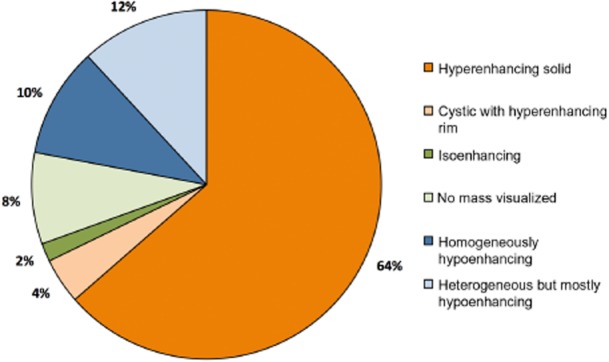
Distribution of enhancement pattern on arterial phase computed tomography for 118 patients with pancreatic neuroendocrine tumor
In our cohort, 11 patients were found to have multifocal tumours on final pathology. Five (45%) had multifocal tumours on pre-operative imaging. The enhancement pattern in these five patients was uniform across all tumours. Additionally, 30 patients (25%) were found to have synchronous liver metastases. Interestingly, the enhancement pattern of the liver metastases matched the pattern of the primary pancreatic tumour in only 60% of these patients. The consistency between pancreatic tumour and liver metastasis enhancement was highest for the hyperenhancing pancreatic tumours (86%). Twelve of the 14 patients with hyperenhancing pancreatic tumours had hyperenhancing liver metastases, whereas 1 patient had hypoenhancing liver metastases and the remaining patient did not have metastases noted on pre-operative imaging. The 2 patients with isoenhancing pancreatic tumours had hyperenhancing liver metastases. Of the 14 patients with hypoenhancing pancreatic tumours, only 6 had hypoenhancing liver metastases and the remaining 8 had hyperenhancing liver metastases. A pre-operative octreoscan was performed in 32 patients (27%), with 20 of these (63%) having a positive scan (identifying the pancreatic tumor). Uptake on octreoscan did not correlate with degree of enhancement on APCT. Octreoscan was positive in 62% of patients with hyperenhancing tumours (13 out of 21), 60% of patients with isoenhancing tumours (3 out of 5) and 67% of patients with hypoenhancing tumours (4 out of 6).
A comparison of clinicopathological characteristics among the five patterns of enhancement is shown in Table 2. Age, gender, multifocality, location in the pancreas, presence of calcifications, tumour functionality and margin status were similar among the groups. Additionally, the rates of enucleation (versus formal resection) and vascular resection or reconstruction were similar among the groups. However, both groups of hypoenhancing PNET were larger (P < 0.001), more likely to have necrosis on pathological evaluation, more likely to be intermediate grade (P < 0.001) and associated with lymph node (P < 0.001) or synchronous liver metastases (P = 0.01). Simplifying our enhancement classification scheme further into three categories (hyperenhancing, isoenhancing and hypoenhancing) produced the same result, with larger size, necrosis, intermediate grade, lymph node involvement and synchronous liver metastases all more prevalent in patients with a hypoenhancing tumour on preoperative imaging. In addition, there was a trend for hypoenhancing tumours to be more frequently calcified (P = 0.07).
Table 2.
Clinicopathological features of pancreatic neuroendocrine tumours based on their degree of enhancement on arterial phase computed tomography
| Hyperenhancing (n = 80) |
Isoenhancing (n = 12) | Hypoenhancing (n = 26) |
P* | ||||
|---|---|---|---|---|---|---|---|
| Hyper-enhancing Solid(n = 75) | Cystic with Hyper-enhancing Rim (n = 5) | Iso-enhancing (n = 2) or No Mass Visualized (n = 10) | Homogeneously Hypo-enhancing (n = 12) | Heterogeneous but Mostly Hypo-enhancing(n = 14) | P** | ||
| Mean Age, years (SD) | 56 (14) | 54 (10) | 52 (14) | 53 (15) | 54 (12) | 0.94 | 0.48 |
| Male gender | 40 (53%) | 3 (60%) | 6 (50%) | 6 (50%) | 7 (50%) | 1.00 | 0.95 |
| Pre-operative tissue diagnosis | 32 (43%) | 1 (20%) | 5 (42%) | 9 (75%) | 11 (79%) | 0.01 | 0.002 |
| Mean size, cm | 3.1 (2.6) | 2.1 (0.8) | 1.7 (1.3) | 4.4 (3.2) | 8.6 (4.3) | <0.001 | <0.001 |
| (SD) | |||||||
| Enucleation | 10 (13%) | 2 (40%) | 1 (8%) | 1 (8%) | 0 (0%) | 0.20 | 0.36 |
| Vascular resection | 6 (8%) | 0 (0%) | 0 (0%) | 3 (25%) | 2 (14%) | 0.21 | 0.17 |
| Multifocal tumours | 9 (12%) | 1 (20%) | 1 (8%) | 0 (0%) | 0 (0%) | 0.39 | 0.15 |
| Head/uncinate location | 24 (33%) | 3 (60%) | 7 (58%) | 3 (25%) | 4 (29%) | 0.30 | 0.17 |
| (versus body/tail) | |||||||
| Calcifications | 13 (17%) | 1 (20%) | 0 (0%) | 4 (33%) | 4 (29%) | 0.18 | 0.07 |
| Functional | 23 (31%) | 1 (20%) | 2 (17%) | 3 (25%) | 1 (7%) | 0.41 | 0.29 |
| Positive margin | 23 (31%) | 0 (0%) | 4 (33%) | 6 (50%) | 2 (14%) | 0.46 | 0.92 |
| Necrosisa | 5 (19%) | 0 (0%) | 0 (0%) | 4 (50%) | 5 (63%) | 0.02 | 0.02 |
| Intermediate gradeb | 27 (36%) | 0 (0%) | 4 (33%) | 8 (67%) | 12 (86%) | <0.001 | <0.001 |
| Lymph node metastasisc | 15 (24%) | 0 (0%) | 1 (10%) | 7 (70%) | 10 (71%) | <0.001 | <0.001 |
| Synchronous liver metastasis | 13 (17%) | 1 (20%) | 2 (17%) | 6 (50%) | 8 (57%) | 0.01 | <0.001 |
P-value across three patterns of enhancement (hyperenhancing, isoenhancing and hypoenhancing).
P-value across five patterns of enhancement.
Percentage reflects only patients for whom necrosis was commented upon in the pathology report (n = 48).
Intermediate grade was defined by the presence of at least one of the following: mitotic rate of 2–20/10 HPF, Ki-67 of 3–20%, necrosis, perineural or lymphovascular invasion on pathologic evaluation.
99 of 118 patients had at least one lymph node evaluated pathologically.
SD, standard deviation.
Because hypoenhancing PNET appeared to display a more aggressive phenotype, we explored whether survival differences existed among the three enhancement patterns. After a mean follow-up of 31 months for the entire cohort, hypoenhancing PNET were associated with a significantly worse overall survival after a resection, as opposed to isoenhancing and hyperenhancing tumours (5-year survival, 93% versus 89% versus 54% for hyperenhancing, isoenhancing and hypoenhancing tumours, respectively) (Fig. 3).
Figure 3.
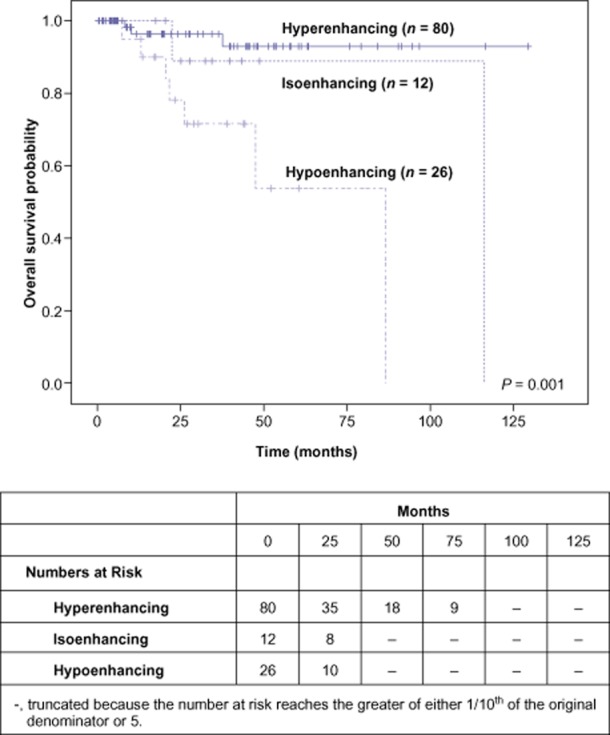
Overall survival after resection of pancreatic neuroendocrine tumors, stratified by their degree of enhancement on arterial phase computed tomography
As hypoenhancing tumours were more commonly larger and associated with synchronous liver metastases, we sought to examine whether the association between hypoenhancement and worse survival held true in multivariate analysis. After controlling for other variables that are available pre-operatively, such as tumour size, the presence of synchronous liver metastasis and functionality, only hypoenhancement on APCT (versus iso-and hyperenhancement) was independently associated with worse survival (P = 0.026, Table 3).
Table 3.
Multivariate analysis of pre-operative characteristics associated with mortality
| Characteristic | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Size | 0.99 (0.84–1.17) | 0.906 |
| Functional | 1.01 (0.47–2.39) | 0.879 |
| Synchronous liver metastasis | 0.64 (0.15–2.67) | 0.538 |
| Hypoenhancement on APCT | 2.32 (1.11–4.86) | 0.026 |
APCT, arterial phase computed tomography.
Discussion
This study examined the enhancement patterns of well-differentiated PNET on APCT and its prognostic significance. While the majority of these tumours are hyperenhancing on APCT, a subset of them is hypoenhancing (22% in our study). Hypoenhancing PNET are more likely to have an aggressive phenotype, more commonly associated with intermediate grade and lymph node or synchronous liver metastases. Additionally, hypoenhancement was associated with an approximate two-fold reduction in survival after a curative resection, a reduction that held true even when controlling for other preoperative variables, such as size, the presence of liver metastases and functionality.
Interestingly, the rates of necrosis were highest in the hypoenhancing tumours, although this analysis is somewhat limited as the minority of pathology reports commented on the presence or absence of necrosis (41%). Additionally, it is difficult to conclude that the pattern of enhancement is a function of necrosis (or percentage of necrosis), as one would expect a higher (or even 100%) rate of necrosis among the hypoenhancing tumours if this were the case. Alternatively, the presence of necrosis has been shown to be associated with more aggressive (and higher grade) tumours; therefore, hypoenhancing tumours may have higher rates of necrosis simply as a function of their being more aggressive tumours.
Our findings are in keeping with the findings of a previous smaller study of 37 patients reported by Rodallec et al.37 In this study (which included seven patients with high-grade PNET) hypoenhancing tumours were more likely to be associated with poor differentiation and worse overall survival. However, probably because of the small sample size, these findings did not hold true in multivariate analysis. In a subsequent prospective study from the same institution, d'Assignies et al. utilized perfusion CT on 28 patients with PNET and found lower intratumoural blood flow to be associated with lower microvascular density (the standard technique used to quantify angiogenesis in histologic studies by counting vessels on tissue specimens using CD34 immunostaining), as well as higher grade tumours, by WHO criteria and the Ki-67 proliferation index.36 Along the same lines, studies have suggested that as PNET progress, they lose their angiogenic potential and their microvascular density decreases.46 This phenomenon is in contrast to what is seen in pancreatic adenocarcinoma, where high microvascular density is associated with decreased survival.47 This differential relationship between microvascular density and outcomes underlines the biological differences between PNET and pancreatic adenocarcinoma and warrants further investigation.
Our study has several potential limitations. First, all patients included in the present study underwent surgery, so there is considerable selection bias towards operative treatment and potentially less advanced disease. However, we elected to include only patients who underwent surgery so that complete pathological information is available and survival analysis is meaningful. Second, evaluating the level of enhancement on APCT was not quantitative. This was done for several reasons. Many PNET are heterogeneous or cystic and therefore quantifying the degree of enhancement using Hounsfield units would be limited by differences within a single tumour. Also, it was our hope that we could provide a simplified system for categorizing tumours as enhancing, isoenhancing or hypoenhancing that relied on the overall appearance of the tumour. This eventually led to classifying cystic tumours with rim enhancement as ‘hyperenhancing’ and heterogeneous tumours with large areas of hypoenhancement as ‘hypoenhancing’ (even if portions of the latter were hyperenhancing) based on the biological behaviour of these groups. For the former, this is actually consistent with the reported literature on cystic PNET, as these tumours have been reported to have lower rates of tumour necrosis, perineural and vascular invasion, lymph node metastasis and synchronous distant metastasis; all findings consistent with our own study.48 Ultimately, we found that nearly all the tumours we examined were easily categorized using this system making it more readily applicable to everyday clinical use. Third, we concede that variations in the timing of contrast will play a major role in the enhancement pattern on CT. Although the vast majority of our study cohort (107 or 91%) had their pre-operative CT at our institution, where it is our practice to perform a dedicated pancreatic protocol CT, there can still be minor differences in contrast timing that can affect radiological interpretation of the images.
In conclusion, our study shows that hypoenhancement on arterial phase CT is present in 22% of well-differentiated PNET and is associated with a more aggressive tumour biology. Hypoenhancement was a more powerful predictor of decreased survival than established predictors for PNET such as size and presence of synchronous liver metastases. This information is available pre-operatively and may inform decision-making in the multidisciplinary treatment of these neoplasms. Patients with hypoenhancing tumours (even small in size) may benefit from a formal pancreatectomy with extended lymph node dissection rather than enucleation. Furthermore, as newer targeted agents inhibiting angiogenesis, such as everolimus and sunitinib,49,50 are increasingly being used for advanced PNET, the vascularity of these tumours based on imaging could be prospectively studied as a predictor of a response to these systemic agents.
Acknowledgments
The authors wish to thank Harriet and Alan Elisofon for their research support.
Conflicts of interest
None declared.
References
- 1.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas. 2008;37:134–138. doi: 10.1097/MPA.0b013e318163a329. [DOI] [PubMed] [Google Scholar]
- 3.Caldarella A, Crocetti E, Paci E. Distribution, incidence, and prognosis in neuroendocrine tumors: a population based study from a cancer registry. Pathol Oncol Res. 2011;17:759–763. doi: 10.1007/s12253-011-9382-y. [DOI] [PubMed] [Google Scholar]
- 4.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vagefi PA, Razo O, Deshpande V, McGrath DJ, Lauwers GY, Thayer SP, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg. 2007;142:347–354. doi: 10.1001/archsurg.142.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after ‘carcinoid’: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 7.Burns WR, Edil BH. Neuroendocrine pancreatic tumors: guidelines for management and update. Curr Treat Options Oncol. 2012;13:24–34. doi: 10.1007/s11864-011-0172-2. [DOI] [PubMed] [Google Scholar]
- 8.Norton JA. Neuroendocrine tumors of the pancreas and duodenum. Curr Probl Surg. 1994;31:77–156. doi: 10.1016/0011-3840(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 9.Norton JA. Surgery for primary pancreatic neuroendocrine tumors. J Gastrointest Surg. 2006;10:327–331. doi: 10.1016/j.gassur.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Nikfarjam M, Warshaw AL, Axelrod L, Deshpande V, Thayer SP, Ferrone CR, et al. Improved contemporary surgical management of insulinomas: a 25-year experience at the Massachusetts General Hospital. Ann Surg. 2008;247:165–172. doi: 10.1097/SLA.0b013e31815792ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen SQ, Angel LP, Divino CM, Schluender S, Warner RRP. Surgery in malignant pancreatic neuroendocrine tumors. J Surg Oncol. 2007;96:397–403. doi: 10.1002/jso.20824. [DOI] [PubMed] [Google Scholar]
- 12.Kazanjian KK, Reber HA, Hines OJ. Resection of pancreatic neuroendocrine tumors: results of 70 cases. Arch Surg. 2006;141:765–769. doi: 10.1001/archsurg.141.8.765. discussion 9–70. [DOI] [PubMed] [Google Scholar]
- 13.Casadei R, Ricci C, Rega D, D'Ambra M, Pezzilli R, Tomassetti P, et al. Pancreatic endocrine tumors less than 4 cm in diameter: resect or enucleate? a single-center experience. Pancreas. 2010;39:825–828. doi: 10.1097/MPA.0b013e3181cf155c. [DOI] [PubMed] [Google Scholar]
- 14.Pitt SC, Pitt HA, Baker MS, Christians K, Touzios JG, Kiely JM, et al. Small pancreatic and periampullary neuroendocrine tumors: resect or enucleate? J Gastrointest Surg. 2009;13:1692–1698. doi: 10.1007/s11605-009-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crippa S, Zerbi A, Boninsegna L, Capitanio V, Partelli S, Balzano G, et al. Surgical management of insulinomas: short-and long-term outcomes after enucleations and pancreatic resections. Arch Surg. 2012;147:261–266. doi: 10.1001/archsurg.2011.1843. [DOI] [PubMed] [Google Scholar]
- 16.Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 17.Norton JA, Warren RS, Kelly MG, Zuraek MB, Jensen RT. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057–1063. doi: 10.1016/j.surg.2003.07.025. discussion 63–65. [DOI] [PubMed] [Google Scholar]
- 18.Schurr PG, Strate T, Rese K, Kaifi JT, Reichelt U, Petri S, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg. 2007;245:273–281. doi: 10.1097/01.sla.0000232556.24258.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–866. doi: 10.1001/archsurg.138.8.859. [DOI] [PubMed] [Google Scholar]
- 20.Norton JA, Harris EJ, Chen Y, Visser BC, Poultsides GA, Kunz PC, et al. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg. 2011;146:724–732. doi: 10.1001/archsurg.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton JA. Endocrine tumours of the gastrointestinal tract. Surgical treatment of neuroendocrine metastases. Best Pract Res Clin Gastroenterol. 2005;19:577–583. doi: 10.1016/j.bpg.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Fischer L, Kleeff J, Esposito I, Hinz U, Zimmermann A, Friess H, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95:627–635. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 23.Tomassetti P, Campana D, Piscitelli L, Casadei R, Santini D, Nori F, et al. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol. 2005;16:1806–1810. doi: 10.1093/annonc/mdi358. [DOI] [PubMed] [Google Scholar]
- 24.Demir R, Pohl J, Agaimy A, Peros G, Perrakis A, Merkel S, et al. Necrosis and angioinvasion predict adverse outcome in pancreatic neuroendocrine tumors after curative surgical resection: results of a single-center series. World J Surg. 2011;35:2764–2772. doi: 10.1007/s00268-011-1262-9. [DOI] [PubMed] [Google Scholar]
- 25.Chu QD, Hill HC, Douglass HO, Driscoll D, Smith JL, Nava HR, et al. Predictive factors associated with long-term survival in patients with neuroendocrine tumors of the pancreas. Ann Surg Oncol. 2002;9:855–862. doi: 10.1007/BF02557521. [DOI] [PubMed] [Google Scholar]
- 26.Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798–7803. doi: 10.1158/1078-0432.CCR-08-0734. [DOI] [PubMed] [Google Scholar]
- 27.Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors. Ann Surg. 2008;247:490–500. doi: 10.1097/SLA.0b013e31815b9cae. [DOI] [PubMed] [Google Scholar]
- 28.Martin RCG, Kooby DA, Weber SM, Merchant NB, Parikh AA, Cho CS, et al. Analysis of 6,747 pancreatic neuroendocrine tumors for a proposed staging system. J Gastrointest Surg. 2011;15:175–183. doi: 10.1007/s11605-010-1380-y. [DOI] [PubMed] [Google Scholar]
- 29.Krampitz GW, Norton JA, Poultsides GA, Visser BC, Sun L, Jensen RT. Lymph nodes and survival in pancreatic neuroendocrine tumors. Arch Surg. 2012;147:820–827. doi: 10.1001/archsurg.2012.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 31.Franko J, Feng W, Yip L, Genovese E, Moser AJ. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14:541–548. doi: 10.1007/s11605-009-1115-0. [DOI] [PubMed] [Google Scholar]
- 32.Gullo L, Migliori M, Falconi M, Pederzoli P, Bettini R, Casadei R, et al. Nonfunctioning pancreatic endocrine tumors: a multicenter clinical study. Am J Gastroenterol. 2003;98:2435–2439. doi: 10.1111/j.1572-0241.2003.07704.x. [DOI] [PubMed] [Google Scholar]
- 33.Ballian N, Loeffler AG, Rajamanickam V, Norstedt PA, Weber SM, Cho CS. A simplified prognostic system for resected pancreatic neuroendocrine neoplasms. HPB. 2009;11:422–428. doi: 10.1111/j.1477-2574.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poultsides GA, Huang LC, Chen Y, Visser BC, Pai RK, Jeffrey RB, et al. Pancreatic neuroendocrine tumors: radiographic calcifications correlate with grade and metastasis. Ann Surg Oncol. 2012;19:2295–2303. doi: 10.1245/s10434-012-2305-7. [DOI] [PubMed] [Google Scholar]
- 35.Tatsumoto S, Kodama Y, Sakurai Y, Shinohara T, Katanuma A, Maguchi H. Pancreatic neuroendocrine neoplasm: correlation between computed tomography enhancement patterns and prognostic factors of surgical and endoscopic ultrasound-guided fine-needle aspiration biopsy specimens. Abdom Imaging. 2013;38:358–366. doi: 10.1007/s00261-012-9953-8. [DOI] [PubMed] [Google Scholar]
- 36.d'Assignies G, Couvelard A, Bahrami S, Vullierme MP, Hammel P, Hentic O, et al. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology. 2009;250:407–416. doi: 10.1148/radiol.2501080291. [DOI] [PubMed] [Google Scholar]
- 37.Rodallec M, Vilgrain V, Couvelard A, Rufat P, O'Toole D, Barrau V, et al. Endocrine pancreatic tumours and helical CT: contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology. 2006;6:77–85. doi: 10.1159/000090026. [DOI] [PubMed] [Google Scholar]
- 38.Lewis RB, Lattin GE, Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics. 2010;30:1445–1464. doi: 10.1148/rg.306105523. [DOI] [PubMed] [Google Scholar]
- 39.Rockall AG, Reznek RH. Imaging of neuroendocrine tumours (CT/MR/US) Best Pract Res Clin Endocrinol Metab. 2007;21:43–68. doi: 10.1016/j.beem.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Buetow PC, Miller DL, Parrino TV, Buck JL. Islet cell tumors of the pancreas: clinical, radiologic, and pathologic correlation in diagnosis and localization. Radiographics. 1997;17:453–472. doi: 10.1148/radiographics.17.2.9084084. quiz 72A–72B. [DOI] [PubMed] [Google Scholar]
- 41.Rodallec M, Vilgrain V, Zins M, Couvelard A, Ruszniewski P, Menu Y. Helical CT of pancreatic endocrine tumors. J Comput Assist Tomogr. 2002;26:728–733. doi: 10.1097/00004728-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 43.Arnold WS, Fraker DL, Alexander HR, Weber HC, Norton JA, Jensen RT. Apparent lymph node primary gastrinoma. Surgery. 1994;116:1123–1129. [PubMed] [Google Scholar]
- 44.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinoma: occurrence, natural history, and predictive factors: a prospective study. Ann Surg. 2003;237:650–657. doi: 10.1097/01.SLA.0000064375.51939.48. discussion 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couvelard A, O'Toole D, Turley H, Leek R, Sauvanet A, Degott C, et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92:94–101. doi: 10.1038/sj.bjc.6602245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239–2245. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 48.Singhi AD, Chu LC, Tatsas AD, Shi C, Ellison TA, Fishman EK, et al. Cystic pancreatic neuroendocrine tumors: a clinicopathologic study. Am J Surg Pathol. 2012;36:1666–1673. doi: 10.1097/PAS.0b013e31826a0048. [DOI] [PubMed] [Google Scholar]
- 49.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]


