Abstract
Objectives
Current clinical studies report the results of laparoscopic resection of hepatocellular carcinoma (HCC) obtained in small cohorts of patients. Because France was involved in the very early development of laparoscopic surgery, the present study was conducted in order to report the results of a large, multicentre experience.
Methods
A total of 351 patients underwent laparoscopic liver resection for HCC during the period from 1998 to 2010 in nine French tertiary centres. Patient characteristics, postoperative mortality and morbidity, and longterm survival were retrospectively reviewed.
Results
Overall, 85% of the study patients had underlying liver disease. Types of resection included wedge resection (41%), left lateral sectionectomy (27%), segmentectomy (24%), and major hepatectomy (11%). Median operative time was 180 min. Conversion to laparotomy occurred in 13% of surgeries and intraoperative blood transfusion was necessary in 5% of patients. The overall morbidity rate was 22%. The 30-day postoperative mortality rate was 2%. Negative resection (R0) margins were achieved in 92% of patients. Rates of overall and progression-free survival at 1, 3 and 5 years were 90.3%, 70.1% and 65.9%, and 85.2%, 55.9% and 40.4%, respectively.
Conclusions
This multicentre, large-cohort study confirms that laparoscopic liver resection for HCC is a safe and efficient approach to treatment and can be proposed as a first-line treatment in patients with resectable HCC.
Introduction
Hepatocellular carcinoma (HCC), a cancer of frequent and worldwide occurrence, is most often associated with liver disease, mainly chronic hepatitis and cirrhosis.1 In recent years, liver transplantation has appeared to be successful in the treatment of both cancer and underlying liver disease.2 The limited number of liver grafts available does not permit transplantation in all patients with early HCC. In patients with normal liver function, partial hepatic resection is still indicated as the primary treatment and leads to survival rates within the same range as those of patients who undergo liver transplantation on an intention-to-treat basis.3 Moreover, partial resection allows complete information about the pathology and prognostic characteristics of the resected tumour to be obtained, and thus facilitates the selection of the best candidates for further liver transplantation.4 However, conventional partial resection still carries a high risk for complications.
Laparoscopy has rapidly come to be recognized as a surgical technique that can decrease morbidity and shorten the hospital length of stay, as well as allowing other benefits. The results of randomized studies have led both surgeons and patients to regard laparoscopy as appropriate in the surgical treatment of various digestive diseases, including colon cancer.5,6 However, liver surgeons have been reluctant to use laparoscopy for several reasons, including the technical difficulties involved in liver exploration and mobilization, as well as in vascular control and parenchyma transection.7 These technical challenges, which are even more hazardous in patients with cirrhosis, may lead to an increased risk for haemorrhage and compromise the improvements in oncological results that liver surgeons have achieved in open liver surgery in recent years. For these reasons, the diffusion of laparoscopic liver surgery remains limited and thus most articles report the results of fairly small cohorts of patients in non-controlled studies. In the absence of randomized controlled studies, robust clinical data are needed to define the role of laparoscopy in the resection of HCC. Thus, this study was designed to review the results of such laparoscopic liver resections in HCC at a nationwide level in France, a Western country in which laparoscopy gained early popularity among surgeons.
Materials and methods
Study population
Prospective data for patients who underwent laparoscopic resection of HCC during the period from 1998 to 2010 were retrospectively retrieved from the databases of nine French tertiary centres. These centres were experienced in both open and laparoscopic liver surgery. Preoperative general evaluation relied on the patient's American Society of Anesthesiologists (ASA) score, age, severity of underlying liver disease assessed by the Model for End-stage Liver Disease (MELD) score, Child–Pugh class and presence of significant portal hypertension with oesophageal varices (grade II or greater). The decision to undertake surgical resection was based on expert opinion delivered at a multidisciplinary meeting. Selection criteria for laparoscopic resection were determined by the surgeon or local surgical team on the basis of tumour size and location [assessed by computed tomography (CT) and/or magnetic resonance imaging (MRI)], according to which suitability for laparoscopic resection was evaluated, and on a preoperative general evaluation of the patient. Patients presenting with decompensated cirrhosis (Child–Pugh classes B and C) or an ASA score of >3 were deemed to be unsuitable for surgical resection, including by laparoscopy.
Surgical technique
The surgical techniques for laparoscopic liver resection have been described previously.7–9. All liver resections were intended to be totally laparoscopic and were performed according to the procedures described and the surgeon's usual practice. The patient was placed in a supine position with the legs apart, except in posterior liver (segments VI and VII) resections, in which left lateral decubitus positioning with an elevated right arm was chosen. Pneumoperitoneum was created by carbon dioxide insufflation at a pressure of 12–14 mmHg, and a 0-degree or 30-degree laparoscope was used. Parenchymal transection was performed with an ultrasonic dissector, or harmonic scalpel, in accordance with the surgeon's usual practice. Small vessels were controlled with bipolar coagulation or a harmonic scalpel. Larger vessels were clipped or electively stapled. The use of portal triad clamping was not systematic and depended upon the surgeon's habits and the amount of bleeding. The specimen was extracted through a suprapubic incision whenever possible in a dedicated laparoscopy bag. All resected specimens were sent to the pathology department for analysis. All surgeons were experienced in hepatic and laparoscopic surgery. Types of resection were defined according to the Brisbane 2000 classification.10 Minor resections involved two or fewer segments; major resections removed at least three segments. Resections of less than one segment (according to Couinaud's classification) were non-anatomical or wedge resections.
Postoperative management and follow-up
Postoperatively, patients were admitted to the intensive care unit only after major hepatic resection. After minor resections, patients were admitted to the surgical ward. Postoperative monitoring included liver biochemical tests on postoperative days 1, 3 and 5. Ultrasound and/or CT scans were indicated only in the event of complications. The occurrence of medical or surgery-related complications was closely monitored, as in open liver surgery. Morbidity was stratified by severity according to the Clavien–Dindo system of classification. Surgical complications included biliary leak, intra-abdominal collection, and parietal complications. Mid-and longterm follow-up included clinical, biological and radiological assessment at 1 month after surgery and subsequently every 6 months in all centres.
Evaluation criteria
Intraoperative evaluation criteria were operation duration, transfusion rate, need for and duration of a Pringle manoeuvre or portal triad clamping, and incidence of conversion to laparotomy. Postoperative parameters studied included: pathological margins; postoperative medical and surgical complications; 30-and 90-day mortality, and duration of hospital stay. Longterm survival and recurrence rates were estimated at 1, 3 and 5 years.
Data on clinicopathological factors, such as age, sex, ASA grade, preoperative hepatic function, underlying liver disease and its cause, number and size of tumours, fulfilling of the Milan Criteria, vascular invasion, presence of satellite nodules, and pathological margins, were collected.
An additional analysis referred to survival outcomes in a subgroup of patients with a good prognosis defined by Child–Pugh class A status, absence of portal hypertension, and the presence of a single well or moderately differentiated tumour without satellite nodule or vascular invasion and of ≤5 cm in diameter.
Margin status was assessed by the pathologist as follows: R0 resection was defined as a complete resection with no microscopic residual tumour; R1 resection was defined as a complete resection with no macroscopically visible tumour as defined by the surgeon, but with microscopically positive margins, and R2 resection was defined as a partial resection in which macroscopically visible tumour was retained.
Statistical analysis
Clinical data were expressed as the median (range) or frequency as appropriate.
Overall survival (OS) was computed from the date of operation. Progression-free survival (PFS) was the time to recurrence or death, whichever came first.
Survival curves were estimated using the Kaplan–Meier method and compared using log-rank tests. To detect factors associated with survival in patients who underwent laparoscopic resection of HCC, univariate analysis was used to verify the relationship between survival and the following variables: α-fetoprotein (AFP) level at the time of HCC resection (<200 ng/ml or >200 ng/ml); preoperative transarterial chemoembolization (performed or not performed); extent of hepatectomy (major or minor); pedicle clamping (required or not required); intraoperative blood transfusion (required or not required); postoperative complication (present or absent); cirrhosis (present or absent); fibrosis of F3 (present or absent); number of HCC nodules (single or multiple); tumour capsule (present or absent); satellite nodules (present or absent); differentiation of HCC (poor or good/moderate differentiation); vascular invasion (present or absent), and a histologically tumour-free margin (present or absent). All variables associated with survival that resulted in a P-value of <0.15 in univariate proportional hazards models were subsequently entered into a Cox multivariate regression model with backward elimination. Missing covariate values were considered to be missing at random and data for patients for whom such information was missing were not included in the regression. P-values of <0.05 were considered to indicate statistical significance.
Results
Patient characteristics
The clinicopathological characteristics of the 351 patients with HCC are given in Table 1. Their median age was 63 years; 260 (74%) patients were male. Preoperative underlying liver disease was known in 268 (76%) patients and was predominantly related to hepatitis C virus infection in 33% (113/345) and alcohol abuse in 30% (105/323) of patients (Fig. 1). In this series, 97% (275/284) of patients were of Child–Pugh class A. Preoperative portal hypertension was found in 70 (20%) patients, assessed by oesophageal varices [grade I (n = 45), grade II (n = 19), grade III (n = 6)]. Endoscopic ligation was performed before resection.
Table 1.
Clinicopathological characteristics of patients submitted to laparoscopic liver resection for hepatocellular carcinoma
| Characteristic | Patients assessed, n | Value |
|---|---|---|
| Age, years, median (range) | 351 | 63 (30–90) |
| Sex, male/female, n (%) | 351 | 260 (74%)/91 (26%) |
| Body mass index, kg/m2, median (range) | 194 | 26.4 (17.0–45.1) |
| α-fetoprotein, ng/ml, median (range) | 151 | 67 (0–65 000) |
| α-fetoprotein level ≥200 ng/ml, n (%) | 259 | 43 (17%) |
| American Society of Anesthesiologists (ASA) class, n (%) | 289 | |
| 1 | 47 (16%) | |
| 2 | 151 (52%) | |
| 3 | 90 (31%) | |
| 4 | 1 (0.003%) | |
| Child–Pugh class, n (%) | 284 | |
| A | 275 (97%) | |
| B | 8 (3%) | |
| C | 1 (0.003%) | |
| Known underlying liver disease, n (%) | 351 | 268 (76%) |
| Hepatitis B virus | 345 | 46 (13%) |
| Hepatitis C virus | 345 | 113 (33%) |
| Alcohol | 323 | 105 (33%) |
| Non-alcoholic steatohepatitis | 283 | 20 (7%) |
| Other | 283 | 60 (21%) |
| Preoperative transarterial chemoembolization, n (%) | 284 | 21 (7%) |
| Preoperative portal vein embolization, n (%) | 283 | 20 (7%) |
| Preoperative tumour biopsy, n (%) | 283 | 47 (17%) |
Figure 1.
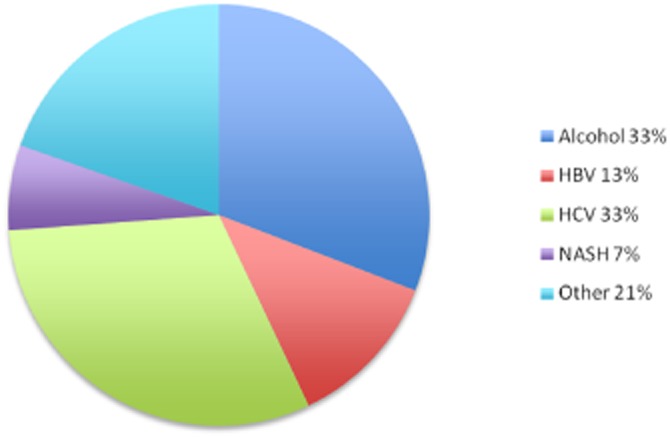
Aetiologies of underlying liver disease in patients submitted to laparoscopic liver resection for hepatocellular carcinoma. HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, non-alcoholic steatohepatitis
Figure 2 shows annual incidences of laparoscopic resection of HCC and indicates a steady increase over the study period to 30–40 procedures per year in the late 2000s.
Figure 2.
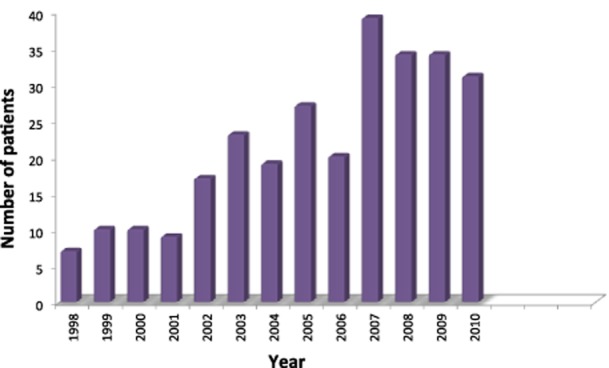
Annual incidences of laparoscopic liver resection for hepatocellular carcinoma in nine French tertiary centres during 1998–2010
Surgical procedures
Types of resection and intraoperative data are given in Table 2. Conversion to laparotomy was necessary in 13% of patients. The main cause for conversion was bleeding, but the reason for conversion was not recorded in 60% of conversions. The hand-assisted technique was not used for conversion.
Table 2.
Operative data in 351 patients submitted to laparoscopic liver resection for hepatocellular carcinoma
| Operative data | Value |
|---|---|
| Type of laparoscopic main liver resection, n (%) | |
| Major hepatectomy | 36 (10%) |
| Right hepatectomy | 20 (6%) |
| Left hepatectomy | 14 (4%) |
| Central hepatectomy | 2 (0.5%) |
| Left lateral sectionectomy, n (%) | 92 (26%) |
| Segmentectomy, n (%) | 83 (24%) |
| Wedge resection, n (%) | 140 (40%) |
| Per-procedure transfusion requirement, n (%) | 17 (5%) |
| Conversion to laparotomy, n | 45 (13%) |
| For dissection difficulties | 2 |
| For biliary leak | 2 |
| For haemorrhage | 14 |
| Reason not specified | 27 |
| Pedicle clamping, n (%) | 85 (24%) |
| Duration of clamping, min, median (range) | 36 (3–117) |
| Operating time, min, median (range) | 180 (15–655) |
| Tumour-free margin, mm, median (range) | 10 (0–78) |
Postoperative outcomes
Mortality rates at 30 days and 90 days were 2.0% and 2.8%, respectively (Table 3). Overall morbidity was 22.8% (80 patients) with an overall major morbidity (Clavien grades of 3 and 4) rate of 4.8% (17 patients). The most frequent complications were infections (18 patients) and ascites (16 patients). Bile leak occurred in seven patients. Postoperative red blood cell transfusions were given in 12 patients.
Table 3.
Outcomes in 351 patients submitted to laparoscopic liver resection for hepatocellular carcinoma
| Postoperative data | Value |
|---|---|
| Length of hospital stay, days, median (range) | 7 (1–90) |
| 30-day mortality, n (%) | 7 (2%) |
| 90-day mortality, n (%) | 10 (3%) |
| Overall morbidity, n (%) | 80 (23%) |
| Infections | 18 (5%) |
| Biliary leak | 7 (2%) |
| Ascites | 16 (5%) |
| Hepatic encephalopathy | 5 (1%) |
| Intra-abdominal fluid collection | 6 (2%) |
| Portal venous thrombosis | 3 (1%) |
| Postoperative blood transfusion requirement, n (%) | 12 (3%) |
| Clavien–Dindo classification, n (%) | |
| I | 35 (10%) |
| II | 21 (6%) |
| IIIa | 3 (1%) |
| IIIb | 8 (2%) |
| IVa | 5 (1%) |
| IVb | 1 (0.3%) |
| V | 6 (2%) |
Histological features are given in Table 4. Cirrhosis or severe fibrosis was present in 302 (86%) patients. Hepatocellular carcinoma presented as a single nodule in 86% of cases. Microvascular invasion was present in 34% of cases and tumour-free margins were obtained in 92% of patients.
Table 4.
Pathological features in 351 patients submitted to laparoscopic liver resection for hepatocellular carcinoma (HCC)
| Postoperative data | Value |
|---|---|
| Histological cirrhosis, n (%) | 247 (70%) |
| Histological fibrosis F2, F3, n (%) | 55 (16%) |
| Maximum tumour size, mm, median (range) | 35 (5–170) |
| Single HCC, n (%) | 302 (86%) |
| Multiple HCC, n (%) | 49 (14%) |
| Bilobar HCC, n (%) | 24 (7%) |
| Encapsulated HCC, n (%) | 162 (46%) |
| Satellite nodules, n (%) | 81 (23%) |
| Well or moderately differentiated HCC, n (%) | 319 (91%) |
| Poorly differentiated HCC, n (%) | 32 (9%) |
| Vascular invasion, n (%) | 119 (34%) |
| Tumour-free margin, n (%) | 323 (92%) |
| Margin, mm, median (range) | 10 (0–78) |
Survival
Median follow-up was 21 months (range: 1–134 months). Rates of OS and PFS at 5 years were 65.6% and 36.8%, respectively (Fig. 3). The subgroup of HCC patients with a better prognosis included 63 patients in whom median follow-up amounted to 32 months (range: 1–130 months). In this group, 5-year OS and PFS rates were 90.2% and 47.1%, respectively (Fig. 4).
Figure 3.
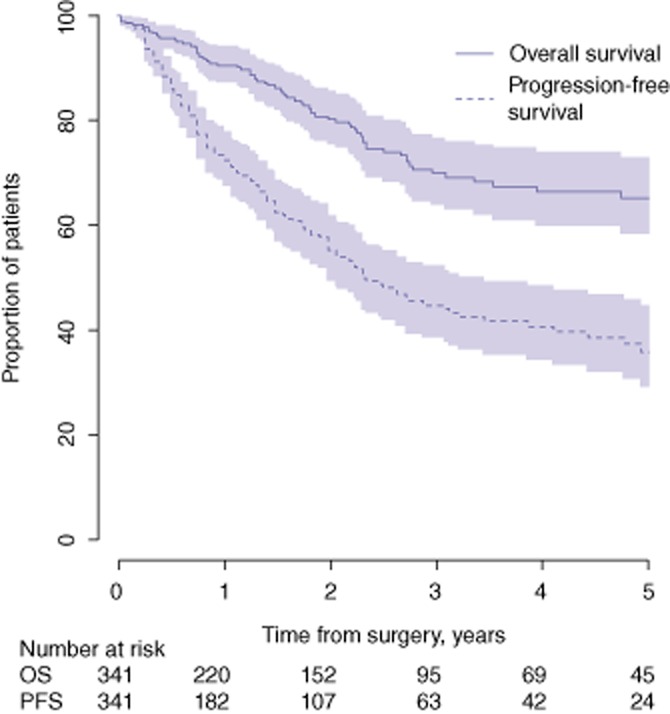
Overall survival (OS) and progression-free survival (PFS) in 351 patients submitted to laparoscopic liver resection for hepatocellular carcinoma during 1998–2010
Figure 4.
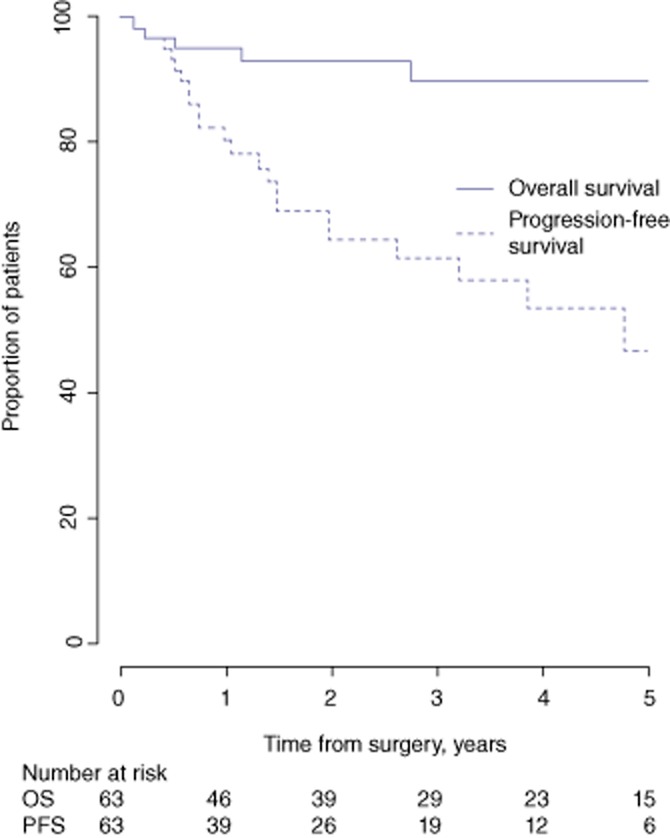
Overall survival (OS) and progression-free survival (PFS) in 63 patients with good prognoses submitted to laparoscopic liver resection for hepatocellular carcinoma during 1998–2010
Predictors of outcome
In univariate analysis, the presence of an AFP serum level of >200 ng/ml [hazard ratio (HR) = 2.6, P = 0.001], cirrhosis (HR = 3.2, P = 0.002), multiple tumour nodules (HR = 3.0, P < 0.0001), satellite nodules (HR = 2.2, P = 0.001), poorly differentiated tumour (HR = 3.0, P = 0.0003) and vascular invasion (P = 0.0001) were associated with worse OS after laparoscopic resection of HCC. In the multivariate analysis, AFP of >200 ng/ml, cirrhosis and vascular invasion remained independently associated with worse OS after laparoscopic resection of HCC (Table 5).
Table 5.
Univariate and multivariate analyses of factors associated with overall survival in 351 patients submitted to laparoscopic liver resection for hepatocellular carcinoma
| Predictor of overall survival | n (%) | Univariate analysis |
Multivariate analysisa |
||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| AFP ≥200 ng/ml versus AFP <200 ng/ml | 60 (17%) | 2.56 (1.46–4.48) | 0.001 | 2.4 (1.3–4.2) | 0.003 |
| Preoperative transarterial chemoembolization | 25 (7%) | 2.20 (0.94–5.19) | 0.07 | ||
| Major hepatectomy | 38 (11%) | 1.01 (0.46–2.23) | 0.98 | ||
| Pedicle clamping | 85 (24%) | 0.95 (0.58–1.56) | 0.84 | ||
| Blood transfusion requirement | 28 (8%) | 1.68 (0.72–3.88) | 0.23 | ||
| Cirrhosis | 247 (70%) | 3.2 (1.6–6.7) | 0.002 | 2.9 (1.2–6.7) | 0.02 |
| Single versus multiple HCC | 302 (86%) | 3.0 (1.74–5.12) | <0.0001 | ||
| Encapsulated HCC | 162 (46%) | 1.26 (0.79–2.01) | 0.33 | ||
| Satellite nodules | 81 (23%) | 2.21 (1.36–3.59) | 0.001 | ||
| Poor versus good/moderate differentiation | 32 (9%) | 3.0 (1.6–5.5) | 0.0003 | ||
| Vascular invasion | 99 (34%) | 2.5 (1.59–4.0) | <0.0001 | 2.65 (1.6–4.4) | 0.0002 |
| Tumour-free versus not tumour-free margin | 323 (92%) | 0.64 (0.27–1.49) | 0.3 | ||
Cox's regression model multivariate analysis included all variables with a P-value of <0.15 in univariate analysis.
HR, hazard ratio; 95% CI, 95% confidence interval; AFP, α-fetoprotein.
With respect to PFS, the presence of cirrhosis (P = 0.0008), multiple tumour nodules (P = 0.003), satellite nodules (P = 0.0003), poor tumour differentiation (P = 0.002) and vascular invasion (P = 0.001) were associated with worse tumour-free survival after laparoscopic resection of HCC. In the multivariate analysis, cirrhosis, poor differentiation and vascular invasion remained independently associated with an increased risk for recurrence (Table 6).
Table 6.
Univariate and multivariate analyses of factors associated with progression-free survival in 351 patients submitted to laparoscopic liver resection for hepatocellular carcinoma (HCC)
| Predictor of overall survival | n (%) | Univariate analysis |
Multivariate analysisa |
||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| AFP ≥200 ng/ml versus AFP <200 ng/ml | 60 (17%) | 1.6 (0.97–2.51) | 0.07 | ||
| Preoperative transarterial chemoembolization | 25 (7%) | 1.19 (0.58–2.45) | 0.64 | ||
| Major hepatectomy | 38 (11%) | 0.85 (0.49–1.49) | 0.57 | ||
| Pedicle clamping | 85 (24%) | 1.15 (0.8–1.66) | 0.45 | ||
| Blood transfusion requirement | 28 (8%) | 1.55 (0.86–2.8) | 0.15 | ||
| Cirrhosis | 247 (70%) | 2.03 (1.34–3.08) | 0.0008 | 2.3 (1.3–3.8) | 0.002 |
| Single versus multiple HCC | 302 (86%) | 2 (1.28–3.12) | 0.003 | ||
| Encapsulated HCC | 162 (46%) | 1.3 (0.91–1.85) | 0.15 | ||
| Satellite nodules | 81 (23%) | 1.98 (1.36–2.86) | 0.0003 | ||
| Poor versus good/moderate differentiation | 32 (9%) | 2.2 (1.33–3.6) | 0.0024 | 1.8 (1.1–2.9) | 0.004 |
| Vascular invasion | 119 (34%) | 1.75 (1.23–2.48) | 0.0017 | 2.1 (1.5–3.1) | <0.0001 |
| Tumour-free versus not tumour-free margin | 323 (92%) | 1.17 (0.54–2.51) | 0.69 | ||
Cox's regression model multivariate analysis included all variables with a P-value of <0.15 in univariate analysis.
HR, hazard ratio; 95% CI, 95% confidence interval; AFP, α-fetoprotein.
Discussion
This study provides evidence in support of the claim that laparoscopic resection of HCC is a safe and reliable procedure that is associated with prolonged survival in patients with HCC and in the majority of patients with chronic liver disease. The relevance of this study is derived from its large sample of patients, which reflects a nationwide collection of experience at nine tertiary hepatopancreatobiliary surgical centres in a single country. The present study found an OS rate of 65.6%. This result was achieved despite the fact that laparoscopic resection of HCC is a significantly challenging procedure in the presence of underlying liver disease. Indeed, severe fibrosis or cirrhosis, present in 85% of patients in the present series, increase the risk for haemorrhage and subsequent transfusion during liver resection.11 Moreover, the technical difficulties of the laparoscopic approach are increased by the characteristics of cirrhotic livers, such as dysmorphia, hypervascularized ligaments, and a hard parenchyma that is difficult to cut and to explore by ultrasound.
An 5-year OS rate of 65.6%, postoperative 30-and 90-day mortality rates of 2.0% and 2.8%, respectively, and an intraoperative transfusion rate of 5% fulfil modern standards for HCC resection in patients with cirrhosis as defined in the 2012 clinical practice guidelines recommended by the European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC), which include expected 5-year survival rates of 60%, perioperative mortality of 2–3% and blood transfusion requirements in <10% of patients.12 These results are within the range of those obtained by open resection and reported in recent series,3,13 in which 5-year survival rates range from 53% to 72%. Given that optimal partial resection can compete with liver transplantation, a pragmatic way to select patients and compare results would be to analyse results in patients with HCC that fall within the Milan Criteria.3
The overall morbidity rate of 22.8% identified in the present study was quite low and compares favourably with those observed after open surgery, especially in the setting of cirrhosis. Biliary fistula, a concern in laparoscopic liver resection, occurred infrequently at an incidence of 2%. With respect to specific morbidity, the incidence of postoperative ascites was as low as 5%. This finding has been underlined in previous studies and in meta-analyses of comparative studies, which indicate that laparoscopy is associated with a significantly lower incidence of postoperative cirrhosis decompensation than is open surgery.14–17
An interesting finding refers to that of the low transfusion rate of 5%. Allogeneic blood transfusion has been shown to be an independent risk factor for the recurrence of colorectal cancer,18,19 HCC20 and cholangiocarcinoma,21 amongst other diseases. Moreover, both intraoperative blood loss and allogeneic blood product transfusions have a negative impact on postoperative outcome, recurrence and survival in patients with HCC.11 The immunosuppressive effect of blood transfusion won early recognition in the setting of kidney transplantation, in which a donor-specific blood transfusion to the recipient was found to decrease the incidence of acute rejection. The non-specific immunosuppression induced by blood transfusion may explain the increased postoperative morbidity and recurrence of cancer observed after liver resection for HCC. Laparoscopy is usually associated with decreases in blood loss and transfusion requirements compared with open procedures in various types of surgery, such as colectomy,5 pancreaticoduodenectomy22 and liver resection for HCC.23 The usual explanation for the decrease in blood loss observed during laparoscopy is the presence of pneumoperitoneum,24 as well as the fact that the magnified vision afforded by laparoscopy allows for an optimal parenchymal transection. Most comparative studies and meta-analyses have found significant decreases in blood loss and requirements for blood transfusions in laparoscopy subgroups among patients undergoing liver resection for HCC.23,25,26 This key point may represent the major advantage of laparoscopy in liver surgery.
Surgical resection is the first-line treatment in patients with early HCC, preserved liver function, and without portal hypertension.12 This recommendation addresses the conventional approach through laparotomy; however, the indications for, associated morbidity and oncological results of laparoscopic resection of HCC have not been well defined, mainly because randomized controlled trials are lacking. Only rather small cohorts,27–29 with or without retrospective comparisons with data for open surgery patients,14–17 have been published, and have led to three meta-analyses.23,25,26 Large-scale retrospective research such as that conducted in the present study may prove more useful than small series in providing clinical data to help clarify potential indications for the laparoscopic approach.
Anatomical resections are recommended for HCC,9 but may be more difficult to perform through laparoscopy. In the present study, however, 60% of patients underwent anatomical resection, which, in the setting of cirrhosis, represents a high proportion compared with those in other series, even in open surgery, in which rates of anatomical resection range from 22% to 74%.13,30
The conversion rate of 13% was higher than those observed in other types of laparoscopic surgery, such as colectomy,5 but has significantly decreased since the first reports of HCC resection, in which it often reached ≥20%.28 An analysis of causes of conversion is not possible in the present series because the reasons for conversion were not specified in over half of the patients. However, difficult exposure and haemorrhage are the main causes of conversion reported in the literature.31
Oncological outcomes identified in the present study, in terms of survival and recurrence rates, were comparable with those reported after open surgery.23 However, it is difficult to analyse the R0 rate in the present study because definitions of R0 resection were not clearly stated and were subject to variation among centres. The multivariate analysis identified factors that are known to influence OS and PFS in patients with HCC, especially tumour-related factors such as poor differentiation, and the presence of satellite nodules and microvascular invasion, as well as the presence of underlying cirrhosis. These factors were significantly and independently associated with an increased risk for recurrence. The risk imposed by tumour-related factors such as vascular invasion underlines the impact of anatomic resection on prognosis.
The good results obtained in the subgroup of HCC patients in whom tumour characteristics were considered to indicate a better prognosis support the claim that partial liver resection can compete with liver transplantation in this group of patients and help to identify those patients who will eventually need a liver transplant.4,30 Here, laparoscopy may provide another advantage. Laurent et al. found that during liver transplantation, the duration of hepatectomy and volume of blood loss, as well as that of blood transfusion, were significantly decreased in patients who had undergone previous laparoscopic resection of HCC compared with those who had undergone open surgery.32 Laparoscopy is well known for making subsequent surgical procedures easier because the formation of intra-abdominal adhesions is reduced after laparoscopic surgery compared with after open surgery.
In view of the present results, which reflect findings collected over a long period in France, laparoscopic liver resection for HCC occurring on a background of chronic liver disease appears to represent a viable alternative to open surgery. Moreover, laparoscopic resection should be considered as a curative treatment of HCC in patients with preserved liver function, not simply as a bridge to liver transplantation, especially in patients with tumours characterized as affording a better prognosis. Laparoscopic liver resection for HCC may represent the missing link in the treatment of HCC because it provides both the oncological advantages of surgical resection and low morbidity rates that are closer to those of ablative techniques than of open surgery.
Conflicts of interest
None declared.
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ. The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1987 and 2001: 5-year survival has improved significantly with time. J Clin Oncol. 2003;21:4329–4335. doi: 10.1200/JCO.2003.11.137. [DOI] [PubMed] [Google Scholar]
- 3.Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Van Nhieu JT, et al. Liver resection for transplantable hepatocellular carcinoma. Longterm results and role of secondary liver transplantation. Ann Surg. 2009;250:738–746. doi: 10.1097/SLA.0b013e3181bd582b. [DOI] [PubMed] [Google Scholar]
- 4.Scatton O, Zalinski S, Terris B, Lefevre JH, Casali A, Massault PP, et al. Hepatocellular carcinoma developed on compensated cirrhosis: resection as a selection tool for liver transplantation. Liver Transpl. 2008;14:779–788. doi: 10.1002/lt.21431. [DOI] [PubMed] [Google Scholar]
- 5.Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomized trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Outcomes of Surgical Therapy (COST) Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 7.Buell JF, Cherqui D, Geller D, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery. The Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 8.Cherqui D, Husson E, Hammoud R, Malassagne B, Stephan F, Bensaid S, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–762. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen JC, Huang K, Wu J, Zhu H, Shi Y, Wang Y, et al. Survival after anatomic resection versus non-anatomic resection for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2010;56:1626–1633. doi: 10.1007/s10620-010-1482-0. [DOI] [PubMed] [Google Scholar]
- 10.Strasberg SM, Phillips BA. Use and dissemination of the Brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2012;257:377–382. doi: 10.1097/SLA.0b013e31825a01f6. [DOI] [PubMed] [Google Scholar]
- 11.Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–623. doi: 10.1097/SLA.0b013e31819ed22f. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver, European Organization for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Giuliante F, Ardito F, Pinna AD, Sarno G, Giulini SM, Ercolani G, et al. Liver resection for hepatocellular carcinoma <3 cm: results of an Italian multicentre study on 588 patients. J Am Coll Surg. 2012;215:244–254. doi: 10.1016/j.jamcollsurg.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Belli G, Fantini C, d'Agostino A, Cioffi L, Langella S, Russolillo N, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short-and middle-term results. Surg Endosc. 2007;21:2004–2011. doi: 10.1007/s00464-007-9503-6. [DOI] [PubMed] [Google Scholar]
- 15.Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg. 2003;138:763–769. doi: 10.1001/archsurg.138.7.763. [DOI] [PubMed] [Google Scholar]
- 16.Aldrighetti L, Guzzetti E, Pulitano C, Cipriani F, Catena M, Paganelli M, et al. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol. 2010;102:82–86. doi: 10.1002/jso.21541. [DOI] [PubMed] [Google Scholar]
- 17.Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, et al. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc. 2010;24:1170–1176. doi: 10.1007/s00464-009-0745-3. [DOI] [PubMed] [Google Scholar]
- 18.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery. A systematic review and meta-analysis. Ann Surg. 2012;256:235–244. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 19.Amato A, Pescatori M. Perioperative blood transfusions and recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;(25) doi: 10.1002/14651858.CD005033.pub2. CD005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001;91:771–778. [PubMed] [Google Scholar]
- 21.Young AL, Igami T, Senda Y, Adair R, Farid S, Toogood GJ, et al. Evolution of the surgical management of perihilar cholangiocarcinoma in a Western centre demonstrates improved survival with endoscopic biliary drainage and reduced use of blood transfusion. HPB. 2011;13:483–493. doi: 10.1111/j.1477-2574.2011.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810–819. doi: 10.1016/j.jamcollsurg.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Wu YR, Wu B, Lu MQ. Surgical and oncologic outcomes following laparoscopic versus open liver resection for hepatocellular carcinoma: a meta-analysis. Hepatol Res. 2012;42:51–59. doi: 10.1111/j.1872-034X.2011.00890.x. [DOI] [PubMed] [Google Scholar]
- 24.Papp A, Vereczkei M, Lantos J, Horvath OP. The effect of different levels of peritoneal CO2 pressure on bleeding time of spleen capsule injury. Surg Endosc. 2003;17:1125–1128. doi: 10.1007/s00464-002-9204-0. [DOI] [PubMed] [Google Scholar]
- 25.Fancellu A, Rosman AS, Sanna V, Nigri GR, Zorcolo L, Pisano M, et al. Meta-analysis of trials comparing minimally invasive and open liver resections for hepatocellular carcinoma. J Surg Res. 2011;171:e33–e45. doi: 10.1016/j.jss.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Zhou YM, Shao WY, Zhao YF, Xu DH, Li B. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci. 2003;56:1937–1943. doi: 10.1007/s10620-011-1572-7. [DOI] [PubMed] [Google Scholar]
- 27.Yoon YS, Han HS, Cho JY, Ahn KS. Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg Endosc. 2011;24:1630–1637. doi: 10.1007/s00464-009-0823-6. [DOI] [PubMed] [Google Scholar]
- 28.Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, et al. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease. Midterm results and perspectives. Ann Surg. 2006;243:499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dagher I, Belli G, Fantini C, Laurent A, Tayar C, Lainas P, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg. 2010;211:16–23. doi: 10.1016/j.jamcollsurg.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology. 2012;55:132–140. doi: 10.1002/hep.24680. [DOI] [PubMed] [Google Scholar]
- 31.Mirnezami R, Mirnezamin AH, Chandrakumaran K, Abu Hilal M, Pearce NW, Primrose JN, et al. Short-and longterm outcomes after laparoscopic and open hepatic resection: systematic review and meta-analysis. HPB. 2011;13:295–308. doi: 10.1111/j.1477-2574.2011.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurent A, Tayar C, Andréoletti M, Lauzet JY, Merle JC, Cherqui D. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:310–314. doi: 10.1007/s00534-009-0063-0. [DOI] [PubMed] [Google Scholar]


