Abstract
Objectives
The role of serum α-fetoprotein (AFP) measurements in the diagnosis of hepatocellular carcinoma (HCC) remains controversial. Some guidelines have advised against the use of AFP in the diagnosis of HCC. This study was conducted to evaluate the performance of AFP in the diagnosis of HCC, and to identify the optimal cut-off value of serum AFP in the diagnosis of HCC in patients with a hepatic mass.
Methods
Patients who presented during the period from May 1997 to March 2003 with hepatic lesions, for whom paired data on serum AFP values at baseline and lesion histology were available, were reviewed. The performance of AFP in the diagnosis of HCC was determined using receiver operating characteristic curve analysis.
Results
Data for a total of 805 patients were evaluated. The mean AFP value was 26 900 ng/ml (range: 0–1 965 461 ng/ml). The histological diagnosis was HCC in 557 patients. The optimal AFP cut-off value was 10 ng/ml (for sensitivity of 82.6% and specificity of 70.4%). At a cut-off level of 200 ng/ml, sensitivity, specificity, and positive and negative predictive values were 47.7%, 97.1%, 97.5% and 44.4%, respectively. The diagnostic performance of AFP remains similar in patients with chronic hepatitis B virus infection, despite a lower negative predictive value. Common aetiologies of liver lesions associated with elevated AFP include cholangiocarcinoma and neuroendocrine tumours.
Conclusions
In Asian patients with suspicious liver lesions, the cut-off AFP level of 200 ng/ml is useful to achieve a diagnosis of HCC with high specificity and reasonable sensitivity. The measurement of serum AFP should not be excluded from guidelines for the diagnosis of HCC.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related death globally.1,2 A number of different methodologies exist for the diagnosis of HCC. Histological evidence used to be considered as the reference standard, but the biopsy procedure is hampered by concerns for bleeding complications in patients with co-morbid cirrhosis. Recent clinical practice has shifted its emphasis to the identification of typical patterns of contrast enhancement on dynamic imaging in the diagnosis of HCC.3–6 However, it is not uncommon to encounter patients whose hepatic lesions do not demonstrate typical patterns. It has been estimated that >30% of HCCs do not exhibit the radiological features regarded as ‘typical’ on dynamic contrast imaging.7,8 For hepatic tumours without typical radiological features, biopsy is still recommended in most international guidelines.3–5
In addition to the radiological methodology, clinicians frequently measure serum α-fetoprotein (AFP) during the workup of hepatic lesions. Serum AFP is elevated in 60–80% of patients with HCC, and is useful in screening and monitoring the treatment responses of HCC.9–12 However, serum AFP values are influenced by non-neoplastic factors, such as the presence of viral hepatitis or cirrhosis. Therefore, a cut-off value is required to improve specificity in the diagnosis of HCC. At present, the clinical use and optimal cut-off level of serum AFP in the diagnosis of HCC remain controversial. The American Association for the Study of Liver Disease (AASLD) guideline of 2005 recommends the use of an AFP value of 200 ng/ml as the cut-off level for the diagnosis of HCC.3 However, in a subsequent updated version of the AASLD guideline, this recommendation on the diagnostic use of AFP has been removed because of concern over false positivity.4 By contrast, European and Asian Pacific guidelines continue to recommend the use of an AFP level of 200 ng/ml as a reliable cut-off for the diagnosis of HCC.5,6
At the present centre, prior to the implementation of non-invasive diagnostic guidelines, histology was considered to represent the reference standard method of achieving a diagnosis of HCC. As a result, patients with inoperable HCC or suspicious hepatic lesions were subjected to liver biopsy in order to confirm the diagnosis. The current study was conducted to allow a retrospective review of baseline serum AFP values in patients who underwent hepatectomy or a percutaneous needle biopsy of a hepatic lesion. The aims of the study were: (i) to study the performance of AFP in the diagnosis of HCC, and (ii) to identify the optimal AFP cut-off value for the diagnosis of HCC in patients who present with a hepatic mass.
Materials and methods
Study design
This was a retrospective study conducted to review the level of serum AFP at baseline and correlate it with the final histological diagnosis. The study was reported according to the STARD (STAndards for the Reporting of Diagnostic accuracy) guidelines.13
Study population
The study population was composed of consecutive patients who presented at the study centre with liver lesions from May 1997 to March 2003. All patients were managed in either the hepatobiliary surgical unit or the joint hepatoma clinic in the hospital; the latter is a multidisciplinary clinic that includes surgeons, oncologists and radiologists. Inclusion criteria were: (i) presence of one or more focal liver lesions depicted on ultrasonography or computed tomography of the abdomen; (ii) availability of a histological diagnosis of the corresponding liver lesion(s) obtained by resection or percutaneous needle biopsy; (iii) availability of data on the serum AFP concentration within 1 month of the histological diagnosis and before the commencement of any treatment for cancer, and (iv) a patient age of ≥18 years. Patients with chronic hepatitis B virus (HBV) infection were identified according to positive results for hepatitis B surface antigen (HBsAg) for 6 months. Chronic hepatitis C virus (HCV) infection was indicated by the detection of HCV RNA persisting for >6 months. The study was approved by the institutional review board of the Chinese University of Hong Kong.
Histological diagnosis
Ultrasound-guided percutaneous biopsy was performed in single lesions or in the most suspicious of multiple lesions with one or two passes of an 18-gauge automated biopsy gun (Temno®; Cardinal Health, Inc., McGaw Park, IL, USA). The quality of biopsy was examined by a radiologist immediately after the procedure to ascertain that a good tissue core had been obtained. The biopsy was sent to the pathologist for histological examination. If a lesion was diagnosed histologically as non-tumorous or as representing a benign condition, an additional 2-year period of clinical and radiological follow-up was initiated. A diagnosis of a benign condition was considered definitive if there was no change in clinical and radiological outcomes in the 2-year follow-up. Any results of repeated biopsies during the 2-year period were reviewed to ensure the hepatic lesion was not malignant.
Quantification of level of AFP
Serum AFP concentration was measured by electrochemiluminescence immunoassay (E170 Analytics; Roche Diagnostics Corp., Indianapolis, IN, USA). The adult reference interval for serum AFP was <7 ng/ml and the coefficient of variation was ≤5% across the linear range (1–1210 ng/ml) of measurement. Laboratory staff involved in the measurement of AFP were unaware of final histological diagnoses. Serum AFP measured within 1 month prior to the histological diagnosis was reviewed. If more than one AFP measurement was recorded during this period, the level measured at the time-point closest to the histological diagnosis was chosen for analysis in the current study.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation. The sensitivity and specificity of serum AFP in the diagnosis of HCC in the whole study population and in the HBV-infected subgroup were analysed. Receiver operating characteristic (ROC) analysis using the area under the curve (AUC) was employed to identify the optimal threshold of any serum AFP level for the diagnosis of HCC by giving equal weighting to sensitivity and specificity. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of optimal thresholds were calculated. The value of the AUC and the corresponding 95% confidence interval (CI) were calculated and the significance of these thresholds re-evaluated using the chi-squared test or Fisher's exact test. Various cut-off values for the diagnosis of HCC were determined based on different criteria. Subgroup analyses were determined for the whole population, the HBV-infected population, and patients submitted to hepatectomy or percutaneous needle biopsy, respectively. Statistical analyses were performed using sas Version 8.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics
The study was commenced in August 2012 and completed in October 2012. A total of 805 patients were recruited in this study. Their baseline characteristics are summarized in Table 1. Their mean age was 55.9 years and 633 (78.6%) were male. A total of 563 (69.9%) patients were positive for HBsAg. Six patients had chronic HCV infection and five of these had concomitant HBV infection. The mean AFP value was 26 900 ng/ml (range: 0–1 965 461 ng/ml). Serum AFP was higher in HBV-infected patients than in patients without HBV infection (33 956 ng/ml versus 10 485 ng/ml; P = 0.013). Histological diagnoses indicated HCC in 557 patients. Histological indications of non-HCC disease are listed in Table 1. Common non-HCC histology included secondary liver disease, adenocarcinoma of unknown origin and cirrhosis or chronic hepatitis.
Table 1.
Baseline characteristics and histological diagnoses in the study cohort (n = 805)
| Variable | Value |
|---|---|
| Age, years, mean ± SD | 55.9 ± 12 |
| Sex, male : female | 632:173 |
| Specimen, n | |
| Biopsy of hepatic mass | 636 |
| Hepatectomy sample | 169 |
| HBsAg-positive, n | 563 |
| HCV-positive, n | 6 |
| AFP, ng/ml | |
| Mean ± SD | 26 900 ± 142 850 |
| Range | 0–1 965 461 |
| Log AFP, ng/ml | |
| Mean ± SD | 1.975 ± 1.486 |
| Range | −0.1 to 6.293 |
| Histological diagnosis, n | |
| Hepatocellular carcinoma | 557 |
| Cirrhosis/chronic hepatitis | 41 |
| Metastasis | 79 Colon (n = 48) Stomach (n = 15) Breast (n = 4) Cervix (n = 2) Nasopharynx (n = 3) Lung (n = 7) |
| Cholangiocarcinoma | 19 |
| Adenomatous hyperplasia | 7 |
| Haemangioma | 20 |
| Fatty change | 4 |
| Granulomatous reaction | 8 |
| Sarcoma | 2 |
| Abscess | 3 |
| Adenocarcinoma | 36 |
| Non-pathological diagnosis | 3 |
| Regenerative nodule | 7 |
| Amyloidosis | 1 |
| Cystadenoma | 2 |
| Adenoma | 3 |
| Focal nodular hyperplasia | 3 |
| Neuroendocrine tumour | 4 |
| Haemangiopericytoma | 1 |
| Atypia | 1 |
| Angiomyolipoma | 1 |
| Hepatoblastoma | 1 |
| Leiomyosarcoma | 2 |
SD, standard deviation; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; AFP, α-fetoprotein.
Performance of serum AFP in the diagnosis of HCC
The accuracy of serum AFP in predicting the diagnosis of HCC in patients with hepatic lesions was evaluated using ROC analyses (Table 2) and the AUC (Fig. 1). In the whole cohort (n = 805), the accuracy of AFP in the diagnosis of HCC produced an AUC of 0.83 (95% CI 0.81–0.86). The optimal cut-off AFP value was 10 ng/ml, at which sensitivity, specificity, PPV and NPV were 82.6%, 70.4%, 86.6% and 63.6%, respectively. At the conventional cut-off value of 200 ng/ml, sensitivity, specificity, PPV and NPV were 47.7%, 97.1%, 97.5% and 44.4%, respectively. For a definitive diagnosis of HCC (i.e. 100% specificity and PPV), the cut-off value was 500 ng/ml, with corresponding sensitivity and NPV of 38.1% and 41.1%, respectively.
Table 2.
Whole-cohort (n = 805) analysis: α-fetoprotein (AFP) thresholds obtained from receiver operating characteristic curves by various criteria to predict hepatocellular carcinoma
| Parameters | Optimal Se and Sp | Conventional cut-off | 100% Sp |
|---|---|---|---|
| AFP cut-off level | ≥10 ng/ml | ≥200 ng/ml | ≥500 ng/ml |
| True positives, n | 464 | 268 | 214 |
| False positives, n | 72 | 7 | 0 |
| True negatives, n | 171 | 236 | 243 |
| False negatives, n | 98 | 294 | 348 |
| Sensitivity | 82.6% | 47.7% | 38.1% |
| Specificity | 70.4% | 97.1% | 100% |
| PPV | 86.6% | 97.5% | 100% |
| NPV | 63.6% | 44.4% | 41.1% |
| Accuracy | 78.9% | 62.6% | 56.8% |
Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
Figure 1.
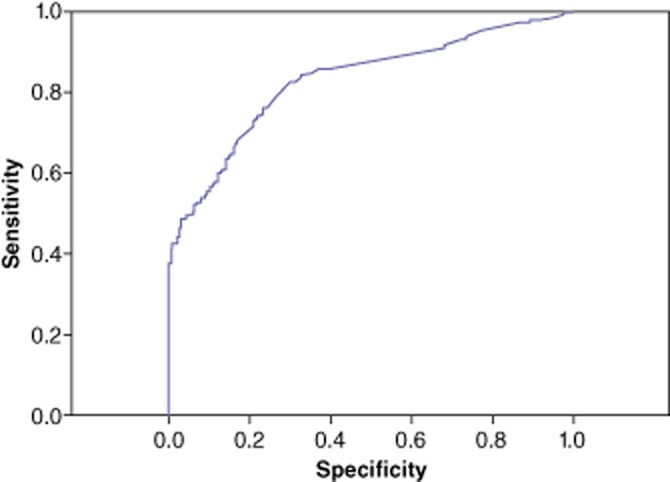
Receiver operating characteristic (ROC) curve showing the sensitivity and specificity of serum α-fetoprotein (AFP) values in the diagnosis of hepatocellular carcinoma in the entire cohort (n = 805). Area under the curve: 0.83 (95% confidence interval 0.81–0.86)
Performance of serum AFP in the diagnosis of HCC in HBV-infected patients
Subgroup analysis of the performance of AFP was conducted in patients with chronic HBV infection (Table 3). Five of the 563 patients with chronic HBV infection had concomitant HCV infection and were excluded from this analysis. In this subgroup (n = 558), the AUC for the accuracy of AFP in the diagnosis of HCC was 0.77 (95% CI 0.73–0.81) (Fig. 2). The optimal cut-off value of AFP was 10 ng/ml, at which sensitivity, specificity, PPV and NPV were 85.1%, 42.7%, 88.7% and 35.2%, respectively. At a cut-off level of 200 ng/ml, sensitivity and specificity were 49.5% and 95.5%, respectively, and the PPV and NPV were 98.4% and 27.3%, respectively. To achieve a definitive diagnosis of HCC (i.e. 100% specificity and PPV), the cut-off value was 400 ng/ml, at which sensitivity and NPV were 41.2% and 24.4%, respectively.
Table 3.
Subgroup analysis of patients with chronic hepatitis B virus (HBV) infection (n = 563): α-fetoprotein (AFP) thresholds obtained from receiver operating characteristic curves by various criteria to predict hepatocellular carcinoma
| Parameters | Optimal Se and Sp | Conventional cut-off | 100% Sp |
|---|---|---|---|
| AFP cut-off level | ≥10 ng/ml | ≥200 ng/ml | ≥400 ng/ml |
| True positives, n | 399 | 232 | 193 |
| False positives, n | 51 | 2 | 0 |
| True negatives, n | 38 | 87 | 89 |
| False negatives, n | 70 | 237 | 276 |
| Sensitivity | 85.1% | 49.5% | 41.2% |
| Specificity | 42.7% | 97.8% | 100% |
| PPV | 88.7% | 99.1% | 100% |
| NPV | 35.2% | 26.9% | 24.4% |
| Accuracy | 78.3% | 57.2% | 50.5% |
Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
Figure 2.
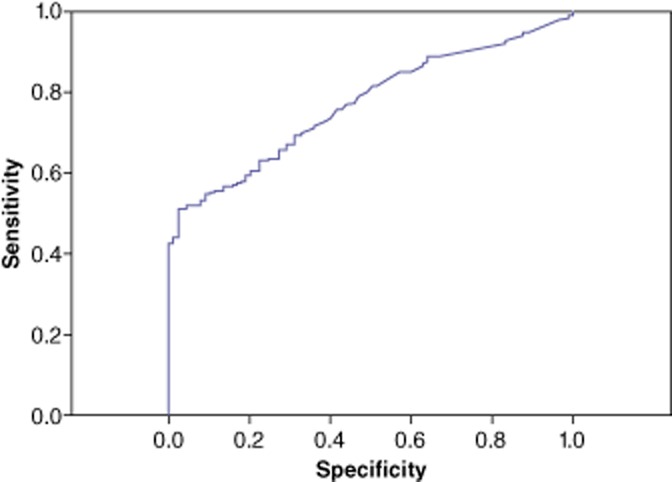
Receiver operating characteristic (ROC) curve showing the sensitivity and specificity of serum α-fetoprotein (AFP) values in the subgroup of patients with chronic hepatitis B infection (n = 563). Area under the curve: 0.77 (95% confidence interval 0.73–0.81)
Performance of serum AFP in the diagnosis of HCC in patients submitted to hepatectomy or biopsy
The accuracy of AFP was also analysed in the subgroups of patients submitted to hepatectomy and percutaneous needle biopsy, respectively (Table 4). The optimal cut-off values in the hepatectomy (n = 169) and biopsy (n = 636) subgroups were 5 ng/ml and 10 ng/ml, respectively. At the conventional cut-off of 200 ng/ml, specificity was similar between the two groups, whereas sensitivity was lower in the hepatectomy (35.2%) than the biopsy (51.3%) subgroup. Definitive diagnostic cut-off levels (i.e. to achieve 100% specificity) were 400 ng/ml and 500 ng/ml in the hepatectomy and biopsy subgroups, respectively.
Table 4.
Subgroup analysis of patients submitted to hepatectomy (n = 169) or biopsy (n = 636): α-fetoprotein (AFP) thresholds obtained from receiver operating characteristic curves by various criteria to predict hepatocellular carcinoma
| Parameters | Optimal Se and Sp | Conventional cut-off | 100% Sp |
|---|---|---|---|
| Hepatectomy group | |||
| AFP cut-off level | ≥5 ng/ml | ≥200 ng/ml | ≥400 ng/ml |
| True positives, n | 105 | 44 | 32 |
| False positives, n | 17 | 1 | 0 |
| True negatives, n | 27 | 43 | 44 |
| False negatives, n | 20 | 79 | 93 |
| Sensitivity | 84.0% | 35.2% | 25.6% |
| Specificity | 61.4% | 97.7% | 100% |
| PPV | 85.1% | 97.8% | 100% |
| NPV | 57.4% | 34.7% | 32.1% |
| Accuracy | 78.1% | 51.5% | 45.0% |
| Biopsy group | |||
| AFP cut-off level | ≥10 ng/ml | ≥200 ng/ml | ≥500 ng/ml |
| True positives, n | 378 | 224 | 186 |
| False positives, n | 64 | 6 | 0 |
| True negatives, n | 135 | 193 | 199 |
| False negatives, n | 59 | 213 | 251 |
| Sensitivity | 86.5% | 51.3% | 42.6% |
| Specificity | 67.8% | 97.0% | 100% |
| PPV | 85.5% | 97.4% | 100% |
| NPV | 69.6% | 47.5% | 44.2% |
| Accuracy | 80.7% | 65.6% | 60.5% |
Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value.
Histological diagnosis in patients with AFP of >200 ng/ml
Among the patients without chronic HBV infection were five patients in whom histological diagnosis did not indicate HCC but in whom AFP values higher than the conventional cut-off of 200 ng/ml were established. Three of these patients were found to have histologically confirmed cholangiocarcinoma; AFP values in these patients were 489 ng/ml, 307 ng/ml and 233 ng/ml, respectively. The fourth patient was found to have a neuroendocrine tumour (AFP = 307 ng/ml) and the fifth had a histological diagnosis of liver metastases of gastric cancer origin (AFP = 272 ng/ml). Among the patients with chronic HBV infection, two patients without HCC had AFP values of >200 ng/ml. Their respective diagnoses were adenocarcinoma of unknown origin (AFP = 298 ng/ml) and neuroendocrine tumour (AFP = 365 ng/ml).
Discussion
The patient cohort (n = 805) in the current study was characterized by the availability of histological evidence of hepatic lesions and data on serum AFP at diagnosis. These features enabled the study of the performance of AFP in the diagnosis of HCC in patients with hepatic lesions. The results have generated two important findings that pertain to the establishing of an optimum cut-off value of serum AFP for the diagnosis of HCC. Firstly, if both sensitivity and specificity are considered, the optimal cut-off is 10 ng/ml. Secondly, the conventional AFP cut-off value of 200 ng/ml is associated with satisfactory specificity and PPV (i.e. low false positivity) of >97%, but at a cost of lower sensitivity and NPV. The former cut-off is more important for the surveillance of HCC in patients without any hepatic lesions or symptoms. In the workup of a patient with a hepatic lesion(s), the cut-off of 200 ng/ml should be considered more relevant because a diagnosis of HCC can be regarded with substantial confidence when the AFP value is higher than the cut-off value. Although the cut-off value of 500 ng/ml proved able to contribute to a definitive diagnosis of HCC with 100% specificity and PPV in the current study, its lower range of sensitivity limits its widespread use in the clinical setting.
Most recent international guidelines recommend the use of dynamic imaging to characterize the vascular pattern of a liver lesion.4,6 However, in clinical practice, it is not uncommon to encounter patients in whom liver lesions do not demonstrate typical contrast enhancement in the arterial phase and washout in the portovenous phase. According to two Asian series, typical enhancement features may be absent in 30–60% of HCC cases.7,8 In addition, it has been suggested that poorly differentiated HCC is associated with an atypical pattern of enhancement, and infiltrative or diffuse HCC may have very subtle radiological features.14 Under such circumstances, it will be extremely difficult for clinicians to achieve a diagnosis of HCC based on radiological evidence alone.15,16 The current study provides evidence that the AFP value may assist clinicians in diagnosing HCC in a proportion of patients. This may help to avoid biopsy procedures in patients who are at risk for developing complications from the procedure.
In the current study, the sensitivity and specificity of AFP in the diagnosis of HCC was comparable with data reported in the literature.17–19 However, it should be noted that the PPV observed in the current study is higher than levels reported in other HCC screening studies.17–19 This is most probably because the patient cohort in the current study consisted of patients with suspected liver lesions on imaging which warranted biopsy or surgery. As a result, the prevalence of HCC in the current cohort can be expected to be higher than that in a patient population without suspicious liver lesions(s) and this will reduce the false positivity rate and lead to a higher PPV. This observation highlights the fact that an elevation in serum AFP is more informative in the setting of workup in patients with a high index of suspicion, such as in patients in whom the presence of symptoms or liver lesions is known, rather than in the setting of surveillance for HCC in asymptomatic patients.
Infection by HBV accounts for >80% of HCC in endemic regions, including Asia and sub-Saharan Africa.20 Not surprisingly, the present cohort was characterized by a high proportion of patients with chronic HBV infection. The influence of HBV infection on the performance of AFP in the diagnosis of HCC has been studied mainly in surveillance cohorts, from which variable conclusions have been obtained.17,21–24 In the current study, the performance of AFP in a subgroup of patients with chronic HBV infection was determined. The results showed that although the PPVs in the HBV-infected subgroup and the whole cohort were grossly similar, the NPVs were significantly lower in the HBV-infected subgroup. Even at the low cut-off value of 10 ng/ml, the NPV value in the HBV-infected subgroup was only 35.2%, whereas that in the entire cohort was 63.6%. The likely cause of this dissimilarity is that a large proportion of non-HCC cases in the cohort occur in patients without HBV infection and this contributes to the higher number of true negative cases in the whole-cohort analysis. By focusing analysis on HBV-infected populations, the proportion of true negative cases will be significantly diminished, resulting in a lower NPV. This finding has highlighted the limitation of AFP: although an elevation in AFP raises the suspicion of HCC in patients with chronic HBV infection, the clinician should not be falsely reassured by an AFP value lower than the cut-off level in these patients.
The current study also set out to determine the aetiologies of AFP elevation in non-HCC cases. Cholangiocarcinoma and neuroendocrine tumours emerged as the tumour types most commonly associated with an elevation in AFP to >200 ng/ml. To reduce the chance of a false positive finding, a number of developments have been made to improve the specificity of serum markers for the diagnosis of HCC. The most notable of these are the AFP Lens culinaris agglutinin reactive (AFP-L3) and the prothrombin induced by the absence of vitamin K or antagonist II (PIVKA-II). AFP-L3 is a fucosylated fraction of AFP that has been reported to be more specific for the diagnosis of HCC. At a cut-off value of 10%, AFP-L3 has specificity of >90% and sensitivity of about 40%.25–28 PIVKA-II is an abnormal prothrombin found in 50–60% of patients with HCC.29–32 At a cut-off value of 40 mAU/ml, the performance of PIVKA-II is similar to that of AFP and achieves sensitivity of around 50% and specificity of 80–90%.29–32 PIVKA-II may be particularly useful in low or negative AFP cases because its level is not correlated with AFP.33 The combination of these two additional markers may help to improve the accuracy of diagnosis of HCC.
The present study is subject to some limitations. Firstly, this was a retrospective study and its results are subject to selection bias. The authors aim to minimize this problem by recruiting a consecutive cohort of patients so that a more representative population can be analysed. However, the AFP values and histological diagnoses were objective findings, which were less subject to bias by investigators' interpretations. Secondly, dynamic contrast imaging was not available in most patients. Therefore, it was not feasible to correlate the radiological features of contrast imaging with serum AFP values in this study. Thirdly, some tumour-related information, such as details of tumour size or stage, was not available in the cohort, which rendered subgroup analyses of the performance of AFP according to tumour size or stage unfeasible. However, subgroup analyses were conducted in subgroups of patients submitted to hepatectomy and biopsy, respectively, and the results of these show that serum AFP measurements are consistently specific for the diagnosis of HCC in these groups of patients.
In conclusion, in Asian patients with suspicious liver lesions, serum levels of AFP are useful to achieve the diagnosis of HCC. The use of an AFP cut-off value of 200 ng/ml may help clinicians to achieve a diagnosis with high specificity and reasonable sensitivity. Such characteristics of AFP remain in patients with chronic HBV infection. Data from the current study suggest that the measurement of serum AFP should not be excluded from guidelines for the diagnosis of HCC.
Conflicts of interest
None declared.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verslype C, Van Cutsem E, Dicato M, Arber N, Berlin JD, Cunningham D, et al. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 10th World Congress on Gastrointestinal Cancer, Barcelona, 2008. Ann Oncol. 2009;20(Suppl. 7):1–6. doi: 10.1093/annonc/mdp281. [DOI] [PubMed] [Google Scholar]
- 7.Thian YL, Low AS, Chow PK, Ooi LL, Chung AY, Low SC, et al. Atypical enhancement pattern of hepatocellular carcinoma with portal vein thrombosis on multiphasic CT. Ann Acad Med Singapore. 2011;40:454–459. [PubMed] [Google Scholar]
- 8.Kim I, Kim MJ. Histologic characteristics of hepatocellular carcinomas showing atypical enhancement patterns on 4-phase MDCT examination. Korean J Radiol. 2012;13:586–593. doi: 10.3348/kjr.2012.13.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lok AS, Lai CL. Alpha-fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology. 1989;9:110–115. doi: 10.1002/hep.1840090119. [DOI] [PubMed] [Google Scholar]
- 10.Chan SL, Chan AT, Yeo W. Role of alpha-fetoprotein in hepatocellular carcinoma: prognostication, treatment monitoring or both? Future Oncol. 2009;5:889–899. doi: 10.2217/fon.09.64. [DOI] [PubMed] [Google Scholar]
- 11.Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64:1700–1707. doi: 10.1002/1097-0142(19891015)64:8<1700::aid-cncr2820640824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446–452. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 13.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898–906. doi: 10.1148/radiol.2443061520. [DOI] [PubMed] [Google Scholar]
- 15.Demirjian A, Peng P, Geschwind JF, Cosgrove D, Schutz J, Kamel IR, et al. Infiltrating hepatocellular carcinoma: seeing the tree through the forest. J Gastrointest Surg. 2011;15:2089–2097. doi: 10.1007/s11605-011-1614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myung SJ, Yoon JH, Kim KM, Gwak GY, Kim YJ, Yu JW, et al. Diffuse infiltrative hepatocellular carcinomas in a hepatitis B-endemic area: diagnostic and therapeutic impediments. Hepatogastroenterology. 2006;53:266–270. [PubMed] [Google Scholar]
- 17.Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–575. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 18.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–438. [PubMed] [Google Scholar]
- 19.Pateron D, Ganne N, Trinchet JC, Aurousseau MH, Mal F, Meicler C, et al. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20:65–71. doi: 10.1016/s0168-8278(05)80468-4. [DOI] [PubMed] [Google Scholar]
- 20.Chan HL, Sung JJ. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis. 2006;26:153–161. doi: 10.1055/s-2006-939753. [DOI] [PubMed] [Google Scholar]
- 21.Lee HS, Chung YH, Kim CY. Specificities of serum alpha-fetoprotein in HBsAg+ and HBsAg− patients in the diagnosis of hepatocellular carcinoma. Hepatology. 1991;14:68–72. doi: 10.1002/hep.1840140112. [DOI] [PubMed] [Google Scholar]
- 22.Tsai JF, Chang WY, Jeng JE, Ho MS, Lin ZY, Tsai JH. Frequency of raised alpha-fetoprotein level among Chinese patients with hepatocellular carcinoma related to hepatitis B and C. Br J Cancer. 1994;69:1157–1159. doi: 10.1038/bjc.1994.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang SJ, Tong MJ, Lai PP, Ko ES, Co RL, Chien D, et al. Evaluation of hepatitis B and C viral markers: clinical significance in Asian and Caucasian patients with hepatocellular carcinoma in the United States of America. J Gastroenterol Hepatol. 1996;11:949–954. [PubMed] [Google Scholar]
- 24.Fasani P, Sangiovanni A, De Fazio C, Borzio M, Bruno S, Ronchi G, et al. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology. 1999;29:1704–1707. doi: 10.1002/hep.510290604. [DOI] [PubMed] [Google Scholar]
- 25.Taketa K, Sekiya C, Namiki M, Akamatsu K, Ohta Y, Endo Y, et al. Lectin-reactive profiles of alpha-fetoprotein characterizing hepatocellular carcinoma and related conditions. Gastroenterology. 1990;99:508–518. doi: 10.1016/0016-5085(90)91034-4. [DOI] [PubMed] [Google Scholar]
- 26.Taketa K, Endo Y, Sekiya C, Tanikawa K, Koji T, Taga H, et al. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419–5423. [PubMed] [Google Scholar]
- 27.Johnson PJ, Poon TC, Hjelm NM, Ho CS, Blake C, Ho SK. Structures of disease-specific serum alpha-fetoprotein isoforms. Br J Cancer. 2000;83:1330–1337. doi: 10.1054/bjoc.2000.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oka H, Saito A, Ito K, Kumada T, Satomura S, Kasugai H, et al. Multicentre prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378–1383. doi: 10.1046/j.1440-1746.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- 29.Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427–1431. doi: 10.1056/NEJM198405313102204. [DOI] [PubMed] [Google Scholar]
- 30.Takikawa Y, Suzuki K, Yamazaki K, Goto T, Madarame T, Miura Y, et al. Plasma abnormal prothrombin (PIVKA-II): a new and reliable marker for the detection of hepatocellular carcinoma. J Gastroenterol Hepatol. 1992;7:1–6. doi: 10.1111/j.1440-1746.1992.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 31.Fujiyama S, Izuno K, Yamasaki K, Sato T, Taketa K. Determination of optimum cut-off levels of plasma des-gamma-carboxy prothrombin and serum alpha-fetoprotein for the diagnosis of hepatocellular carcinoma using receiver operating characteristic curves. Tumour Biol. 1992;13:316–323. doi: 10.1159/000217781. [DOI] [PubMed] [Google Scholar]
- 32.Tsai SL, Huang GT, Yang PM, Sheu JC, Sung JL, Chen DS. Plasma des-gamma-carboxyprothrombin in the early stage of hepatocellular carcinoma. Hepatology. 1990;11:481–488. doi: 10.1002/hep.1840110321. [DOI] [PubMed] [Google Scholar]
- 33.Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, et al. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036–1040. doi: 10.1111/j.1572-0241.2000.01978.x. [DOI] [PubMed] [Google Scholar]


