Abstract
Background
Although an antecolic duodenojejunostomy was reported to reduce post-operative delayed gastric emptying (DGE) compared with a retrocolic duodenojejunostomy after a pylorus-preserving pancreaticoduodenectomy (PPPD), the long-term effects of these procedures have rarely been studied. The aim of this prospective, randomized, clinical trial was to investigate the influence of the reconstruction route on post-operative gastric emptying and nutrition.
Methods
Reconstruction was performed in 116 patients with an antecolic duodenojejunostomy (A group, n = 58) or a vertical retrocolic duodenojejunostomy (VR group, n = 58). Post-operative complications, including DGE, gastric emptying variables assessed by 13C-acetate breath test and nutrition, were compared between the two groups for 1 year post-operatively.
Results
The incidence of DGE was not significantly different between the procedures (A group: 12.1%; VR group: 20.7%, P = 0.316). At post-operative month 1, gastric emptying was prolonged in the VR versus the A group but not significantly so. At post-operative month 6, gastric emptying was accelerated significantly in the A versus the VR group. Post-operative weight recovery was significantly better in the VR versus the A group at post-operative month 12 (percentage of pre-operative weight, A group: 93.8 ± 1.2%; VR group: 98.5 ± 1.3%, P = 0.015).
Conclusions
A vertical retrocolic duodenojejunostomy was an acceptable procedure for the lower incidence of DGE and may contribute to better weight gain affected by moderate gastric emptying.
Introduction
A pylorus-preserving pancreaticoduodenectomy (PPPD) is the standard operation for peri-ampullary disease. Although operative mortality of PPPD has been reduced to less than 5%,1–4 post-operative morbidity remains high, at 30% to 60%.2–4 Delayed gastric emptying (DGE) is one of the most specific and frustrating complications after PPPD, with an incidence ranging from 5–60%.4,5 DGE is self-limiting and can be treated conservatively; however, this complication leads to a prolonged hospital stay and worsens the patient's quality of life.
Two reconstruction methods after PPPD are associated with the transverse colon: an antecolic duodenojejunostomy and a retrocolic duodenojejunostomy. An antecolic duodenojejunostomy is reported to offer equal or superior outcomes for the prevention of DGE compared with the retrocolic route.6–11 The reported incidence of DGE with the antecolic route is below 15%, whereas that with the retrocolic route is above 30%. The incidence of DGE reported by these studies for reconstruction by the retrocolic route was considered to be high compared with the authors' experience. The authors reported previously that a vertical retrocolic duodenojejunostomy, by which the stomach and duodenum are brought down the left side of the transverse mesocolon in a straight, vertical manner, reduces the incidence of DGE.12,13 However, the number of the patients was small and the period after PPPD was short in these two studies.
The aim of the present study was to perform a prospective, randomized, clinical trial to compare the incidence of DGE assessed according to the definition of the International Study Groups of Pancreatic Surgery (ISGPS)14 in 116 patients undergoing either an antecolic duodenojejunostomy or a vertical retrocolic duodenojejunostomy. Although some studies reported to notice an association between DGE and reconstruction route, the post-operative effects on gastric emptying function and nutritional status have rarely been compared between the two reconstruction methods. Therefore, in this study, nutritional status and gastric emptying variables assessed by the 13C-acetate breath test15–17 were compared before and at 1, 3, 6, 9 and 12 months during the first year after surgery between the two reconstruction methods.
Methods
The study protocol was approved by the ethics committee of Miyazaki University, Miyazaki, Japan, and was registered with the National Clinical Database (University Hospital Medical Information Network Clinical Trials Registry as UMIN000001712). From March 2005 until July 2011, 129 patients underwent a PPPD at the Department of Surgical Oncology and Regulation of Organ Function, Miyazaki University School of Medicine. The patients who underwent a pancreaticoduodenectomy with gastric resection, subtotal stomach-preserving PD (SSPPD), additional hepatic resection and a total pancreatectomy were excluded from the study. The patients underwent standard pretreatment evaluation, randomization to an antecolic or a vertical retrocolic duodenojejunostomy, and assessment of the results, DGE, gastric emptying and nutritional status for 1 year after surgery. Patients were recruited into the study before surgery, and informed consent was obtained from all participants. The study flow chart is shown in Fig. 1. Because of their pre-operative condition, four patients who did not give their informed consent and five patients with severe comorbidity were excluded. The remaining 120 patients underwent randomization; however, four patients with post-operative severe sepsis (two undergoing each reconstruction method) were excluded. A re-operation was performed in one patient (0.8%) in the antecolic group for ischaemic perforation of the duodenojejunostomy. Post-operative mortality occurred in two patients (1.7%), one patient in the antecolic group as a result of sepsis from an intravenous catheter infection, and one patient in the vertical retrocolic group owing to liver failure associated with a vascular problem. Thus, the remaining 116 patients were divided into two groups: the antecolic group (A group, n = 58) and the vertical retrocolic group (VR group, n = 58).
Figure 1.
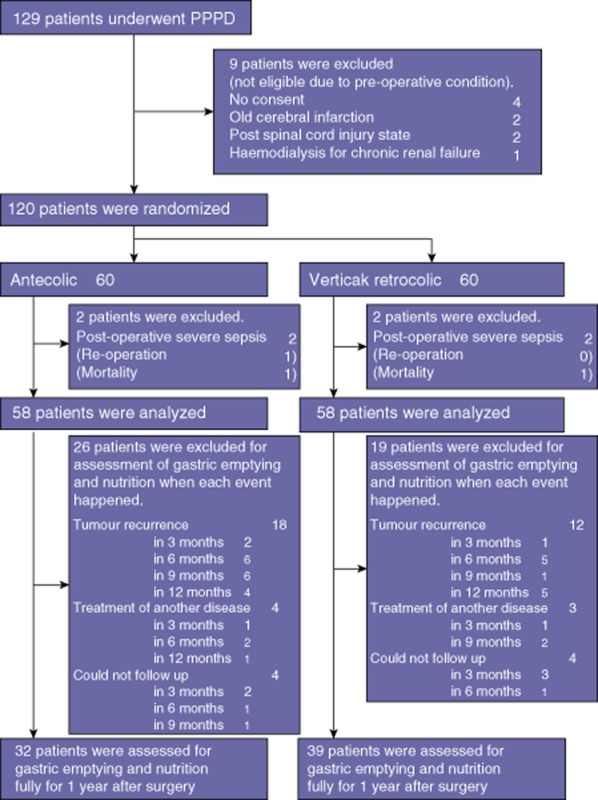
Participant flow chart
All patients underwent detailed pre-operative physical examination with haematological and biochemical assessment including measurement of tumour markers. The indication for surgery for all patients was suspected peri-ampullary lesion on the basis of computed tomography and additional imaging studies. Patients with distant metastases or locally far advanced tumours were judged to be inoperable. Patients with jaundice underwent pre-operative endoscopic or percutaneous transhepatic biliary drainage to decrease their serum bilirubin level. Pre-operative diabetes mellitus was assessed by the serum haemoglobin A1c level, fasting plasma glucose level, random glucose level and the oral glucose tolerance test. All patients except those with established diabetes mellitus were referred for oral glucose tolerance testing.
Prior to the surgeries, equal numbers of envelopes for an antecolic or a vertical retrocolic duodenojejunostomy were sequentially prepared in a blinded fashion to rule out any influence of bias in the choice of reconstruction technique during surgery.
The same team of surgeons performed all operations. The area resected during the PPPD included the gallbladder, common hepatic duct, pancreas head, duodenum (except the first portion) and 10 cm of the proximal jejunum. Lymph nodes in the hepatoduodenal ligament and those surrounding the common hepatic artery, peripancreatic tissue and the right side of the superior mesenteric artery were dissected. If necessary, combined portal vein resection or dissection of paraaortic lymph nodes was performed to accomplish a complete tumour resection. The duodenum was freed from the surrounding tissue and transected approximately 2–4 cm distal to the pyloric ring. The right gastric artery was divided at its origin in all patients. The lesser omentum close to the liver was dissected while preserving the vagus nerve to allow free movement of the stomach. These procedures allowed the stomach and the duodenum to be mobilized to the left in a straight, vertical manner. In reconstruction, the proximal jejunum was brought through the right side of the transverse mesocolon via the retrocolic route. An end-to-side pancreaticojejunostomy was performed with duct-to-mucosa anastomosis. A hepaticojejunostomy was performed 5–10 cm distal to the pancreaticojejunostomy. Then, an end-to-side duodenojejunostomy was performed about 50 cm distal to the hepaticojejunostomy based on randomization to either the antecolic or vertical retrocolic route. For vertical retrocolic duodenojejunostomy, the left side of the transverse mesocolon (to the left of the middle colic vessels) was opened, and the stomach and duodenum were brought down in a straight, vertical manner. The retrocolic duodenojejunostomy was performed at the caudal side of the transverse mesocolon, and the gastric antrum was fixed to the transverse mesocolon with several sutures. A Braun anastomosis was added in both reconstruction procedures. A schema of the reconstruction techniques used for both procedures is shown in Fig. 2. Two (or three) closed drains were placed around the pancreatic and biliary anastomoses. A pancreatic drainage tube and a biliary drainage tube were placed at the pancreatic duct and hepatic duct, respectively, and were exteriorized through the jejunal limb. A feeding tube was not placed in any of the patients.
Figure 2.
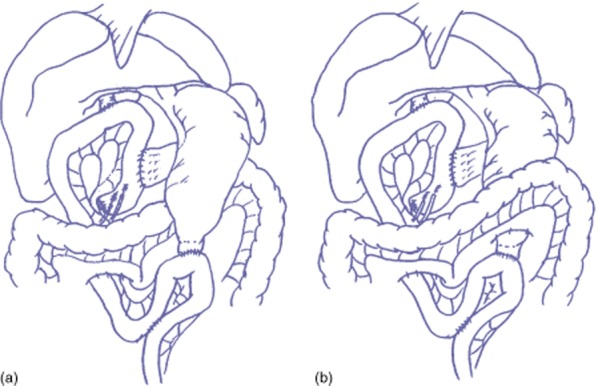
Schema showing reconstruction techniques after a pylorus-preserving pancreaticoduodenectomy. (a) Antecolic duodenojejunostomy. (b) Vertical retrocolic duodenojejunostomy
All patients received prophylactic antibiotics for 2 to 3 days post-operatively. The patients were given epidural anaesthesia for 4–5 days post-operatively and/or adequate analgesia, and early ambulation was encouraged. The general protocol for patient care was to remove the nasogastric tube (NGT) routinely on postoperative day (POD) 1 if the gastric amount was below 500 ml after the first post-operative night. If the patients vomited persistently after removal of the NGT, it was reinserted. The drinking of water was started from POD 3, and a liquid diet was commenced on POD 4, with progression to a soft diet as tolerated. The drains were checked for amylase every day from POD 1 to POD 5 and were removed if there was no evidence of any pancreatic or biliary leakage. A proton pump inhibitor was administered intravenously after surgery and converted to an oral dose once a diet was tolerated. Pancreatic enzyme supplements were prescribed once a soft diet was commenced. No patient was given prokinetic drugs such as erythromycin or octreotide. Parenteral nutrition was used in the patients with insufficient dietary intake owing to postoperative complications and was discontinued if the patients could tolerate more than half of their oral diet.
Post-operative complications
All resected specimens underwent definitive histological study after surgery. All patients with malignant disease underwent a gross complete (R0 or R1) resection. Post-operative complications were evaluated in all 116 patients (58 in each group). According to the ISGPS consensus criteria, DGE was defined by the need for maintenance or reinsertion of the NGT after POD 3 or inability to tolerate a solid diet after POD 7. The severity of DGE was classified into grades A, B, and C by the length of need for the NGT or inability to tolerate a solid diet, and clinical impact.14 A pancreatic fistula was defined and graded according to the International Study Groups on Pancreatic Fistula (ISGPF) definition, and clinically relevant a pancreatic fistula was defined as grade B or C.18 A post-pancreatic surgery haemorrhage was defined according to the ISGPS definition.19 An intra-abdominal abscess was defined as culture-positive purulent drainage or findings of intra-abdominal fluid collection by computed tomography accompanied with fever elevation or leukocytosis. Mortality was defined as the patient death occurring until POD 30.
Gastric emptying and nutritional status for 1 year after surgery
Gastric emptying function was evaluated pre-operatively and at months 1, 3, 6, 9, and 12 post-operatively using the 13C-acetate breath test.15–17 A proton pump inhibitor was not given for 3 days before the test. All patients ingested a liquid meal (200 Kcal/200 ml, RACOL; Ohtsuka Pharmaceutical Co., Tokyo, Japan) labelled with 100 mg sodium 13C-acetate (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) in the morning of the test day after an overnight fast. Breath samples were collected in the collection bag before and after ingestion of the test meal, i.e. before and at 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, 105, 120, 150 and 180 min after ingestion of the 13C-acetate. The recovery of 13C in the breath samples was analyzed by isotope-selective infrared spectrometry (UBiT-IR300; Otsuka Electronics Co., Ltd, Osaka, Japan). Gastric emptying was estimated by the values of the time when 13CO2 reaches maximum excretion (T max), half-emptying time (T 1/2) and total % excretion of 13CO2 in 2 h (%dose/2 h). These values were calculated with analysis software (Microsoft Office Excel; Microsoft Japan, Tokyo, Japan) from a calculated 13CO2 breath excretion curve.
Follow-up at intervals of least every 3 months comprised a physical examination, laboratory tests including tumour markers, computed tomography, estimation of tumour recurrence and survival. All 58 patients in each group received 13C-acetate breath test as the measure of gastric emptying function, but patients with tumour recurrence were excluded at that point as subjects for assessment of nutritional status and 13C-acetate breath test. In addition, any patients whose treatment required hospital admission or a laparotomy for another disease and those who could not be followed up at the study institution because they had moved to a different location were excluded at the follow-up evaluation. The number of the patients excluded and the reasons for exclusion are shown in Fig. 1.
Data collection and study end points
The patients' clinicopathological and follow-up data were collected prospectively. The primary end point was the incidence of DGE. Secondary end points were post-operative complications except DGE, evaluation of gastric emptying and nutritional status for 1 year after surgery.
Statistical analysis
For statistical analysis of post-operative complications, especially DGE, a power calculation indicated that 58 patients needed to be enrolled for each procedure to test the premise of improving the rate of DGE from 30% to 10% at the two-tailed significance level of 5% with a power of 80%. Results are reported as median (range) or mean ± standard error (SE). In comparisons between the A and VR groups, categorical variables were compared with chi-square test or Fisher's exact test, quantitative variables with the Student t-test, and non-parametric variables with the Mann–Whitney U-test. In addition, Dunnett's post hoc test was used to compare change from the baseline (preoperative) value for the post-operative parameters of gastric emptying and nutritional status for 1 year after surgery. The level of significance was set at P < 0.05.
Results
Clinical characteristics and operative findings of the enrolled patients are shown in Table 1. There were no statistically significant differences between the two groups with regards to age, gender ratio, body mass index, pre-operative body weight, the presence of diabetes mellitus, pre-operative nutritional biochemical parameters, pancreatic endocrine and exocrine function, pre-operative biliary drainage and type of disease (benign or malignant). The duration from presentation of disease to operation was also not significantly different between the two groups. Operative findings including operation time, operative blood loss, soft pancreas and portal vein resection were similar between the two groups (Table 1).
Table 1.
Characteristics of patients and operative findings
| Antecolic (A) group (n = 58) | Vertical retrocolic (VR) group (n = 58) | P-value | |
|---|---|---|---|
| Age (years)a | 70.0 (36–86) | 69.0 (46–86) | 0.540 |
| Gender (male/ female) | 36 (62.1%)/ 22 (37.9%) | 32 (55.2%)/ 26 (44.8%) | 0.451 |
| Body mass index (kg/m2)a | 21.8 (15.7–29.0) | 21.3 (14.7–29.3) | 0.463 |
| Pre-operative body weight (kg)a | 53.9 (35.6–78.8) | 51.9 (30.5–75.8) | 0.338 |
| Diabetes mellitus | 21 (36.2%) | 21 (36.2%) | 1.000 |
| Pre-existing on admission | 11 (19.0%) | 7 (12.1%) | 0.442 |
| Newly diagnosed | 10 (17.2%) | 14 (24.1%) | 0.359 |
| Insulin dependant | 4 (6.9%) | 5 (8.6%) | 0.729 |
| Oral administration | 9 (15.5%) | 5 (8.6%) | 0.393 |
| Pre-operative albumin (g/dl)a | 3.75 (2.94-4.78) | 3.66 (2.69-4.48) | 0.542 |
| Pre-operative total cholesterol (mg/dl)a | 177 (92–381) | 181 (115–372) | 0.204 |
| Pre-operative haemoglobin-A1c (%)a | 5.3 (3.6–11.0) | 5.2 (3.9–10.9) | 0.968 |
| Pre-operative BT-PABA test (%)a | 56.6 (10.6–82) | 53.0 (12.9–87.4) | 0.506 |
| Pre-operative biliary drainage | 38 (65.5%) | 43 (74.1%) | 0.312 |
| Length of time from presentation of disease to operation (weeks)a | 8 (3–36) | 8 (4–21) | 0.349 |
| Pathology | |||
| Benign/ malignancy | 12 (20.7%)/ 46 (79.3%) | 9 (15.5%)/ 49 (84.5%) | 0.425 |
| Pancreatic cancer | 17 (29.3%) | 16 (27.6%) | |
| Bile duct cancer | 17 (29.3%) | 20 (34.5%) | |
| Ampullary carcinoma | 4 (6.9%) | 9 (15.5%) | |
| Duodenal cancer | 2 (3.4%) | 0 | |
| Cystic tumour (IPMN, MCN) | 11 (19.0%) | 6 (10.3%) | |
| Chronic pancreatitis | 2 (3.4%) | 3 (5.2%) | |
| Benign bile duct disease | 2 (3.4%) | 2 (3.4%) | |
| Others | 3 (5.2%) | 2 (3.4%) | |
| Operative findings | |||
| Operating time (minutes)a | 558.0 (427–967) | 571.5 (422–769) | 0.712 |
| Operative blood loss (ml)a | 1380 (360–6870) | 1295 (230–3980) | 0.440 |
| Blood transfusion | 36 (62.1%) | 30 (51.7%) | 0.261 |
| Soft pancreas | 32 (55.2%) | 35 (60.3%) | 0.573 |
| Main pancreatic duct diameter (mm)a | 4 (2–10) | 3 (2–10) | 0.467 |
| Resected duodenum (cm)a | 4 (2–4) | 4 (3–4) | 0.064 |
| Portal vein resection | 8 (13.8%) | 4 (6.9%) | 0.223 |
Results are expressed as median (range).
BT-PABA, N-benzoyl-L-tyrosyl-para-aminobenzoic acid; IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm.
Post-operative complications
Post-operative complications before discharge from the hospital are shown in Table 2. Surgical morbidity between the two groups was not significantly different: 50.0% in the A group and 44.8% in the VR group. The overall incidence of clinically relevant pancreatic fistula (ISGPF grade B or C) occurred in 19 patients (16.4%). The incidence of all-grade pancreatic fistula was 37.9% in the A group and 29.3% in the VR group, and clinically relevant pancreatic fistula (ISGPF grade B or C) was 15.5% in the A group and 17.2% in the VR group, both without a statistically significant difference between the two groups. An intra-abdominal abscess occurred in 29 patients (25.0%), and the difference between the two groups was not statistically significant.
Table 2.
Post-operative complications, including clinical parameters related to delayed gastric emptying (DGE), and post-operative course
| Antecolic (A) group (n = 58) | Vertical retrocolic (VR) group (n = 58) | P-value | |
|---|---|---|---|
| Morbidity | 29 (50.0%) | 26 (44.8%) | 0.577 |
| Pancreatic fistula (PF) | 22 (37.9%) | 17 (29.3%) | 0.326 |
| ISGPF grading (A/B/C) | 13/6/3 | 7/8/2 | |
| ISGPF grade B/C | 9 (15.5%) | 10 (17.2%) | 0.802 |
| Intra-abdominal abscess | 16 (27.6%) | 13 (22.4%) | 0.520 |
| Post-operative haemorrhagea | 3 (5.2%) | 3 (5.2%) | 1.000 |
| Biliary leakage | 1 (1.7%) | 0 | 0.315 |
| Wound infection | 9 (15.5%) | 9 (15.5%) | 1.000 |
| Peptic ulcer | 2 (3.4%) | 1 (1.7%) | 0.559 |
| Cholangitis | 5 (8.6%) | 3 (5.2%) | 0.464 |
| DGE | 7 (12.1%) | 12 (20.7%) | 0.316 |
| ISGPS grading (A/B/C) | 4/1/2 | 6/0/6 | |
| ISGPS grade B/C | 3 (5.2%) | 6 (10.3%) | 0.298 |
| Clinical parameters related to DGE | |||
| Removal of NGT (day)b | 1 (0–2) | 1 (0–20) | 0.729 |
| Removal of NGT as per protocol | 55 (94.8%) | 55 (94.8%) | 1.000 |
| Reinsertion of NGT | 0 | 1 (1.7%) | 0.315 |
| Start of water drinking (day)b | 3 (3–17) | 3 (2–21) | 0.833 |
| Start of liquid diet (day)b | 4 (4–37) | 4 (4–28) | 0.633 |
| Start of solid diet (day)b | 5 (5–38) | 5.5 (5–29) | 0.258 |
| Start and progression of diet as per protocol | 44 (75.9%) | 42 (72.4%) | 0.672 |
| Duration of parenteral nutrition (days)b | 11 (5–43) | 14 (7–35) | 0.097 |
| Parenteral nutrition over 2 weeks | 19 (32.8%) | 21 (36.2%) | 0.696 |
| Post-operative course | |||
| Hospital stay (days)b | 36 (27–116) | 36 (23–75) | 0.910 |
| Adjuvant chemotherapy | 30 (51.7%) | 29 (50.0%) | 0.851 |
| Tumour recurrence in 1st postoperative year | 18 (31.0%) | 12 (20.7%) | 0.203 |
Post-pancreatic surgery haemorrhage was defined according to the ISGPS definition.
Results are expressed as median (range).
DGE, delayed gastric emptying; ISGPF, International Study Groups on Pancreatic Fistula; ISGPS, International Study Groups of Pancreatic Surgery; NGT, nasogastric tube.
Delayed gastric emptying
The overall incidence of DGE was 16.4% (19 of 116 patients) (Table 2). The DGE grades of these patients were A in 10, B in 1 and C in 8 patients. In A group, the incidence of DGE was 12.1% (7 of 58 patients), and the grades were A in 4, B in 1 and C in 2 patients. In the VR group, the incidence of DGE was 20.7% (12 of 58 patients), and the grades were A in 6, B in 0 and C in 6 patients. Although the incidence of DGE tended to be higher in the VR versus the A group, the difference was not statistically significant (P = 0.316). The incidence of clinically relevant DGE (grades B and C) was 5.2% (3 patients) in the A group and 10.3% (6 patients) in the VR group, and the difference was still not statistically significant (P = 0.298). Clinical parameters related to DGE are shown in Table 2. The day of NGT removal was similar, on median POD 1 in both groups. Only one patient in the VR group required reinsertion of the NGT, and the patient improved with conservative treatment and without additional interventions. The number of days to the start of liquid and solid diets, the duration of parenteral nutrition, and length of hospital stay were not significantly different between the two groups.
Changes in gastric emptying variables for 1 year after surgery
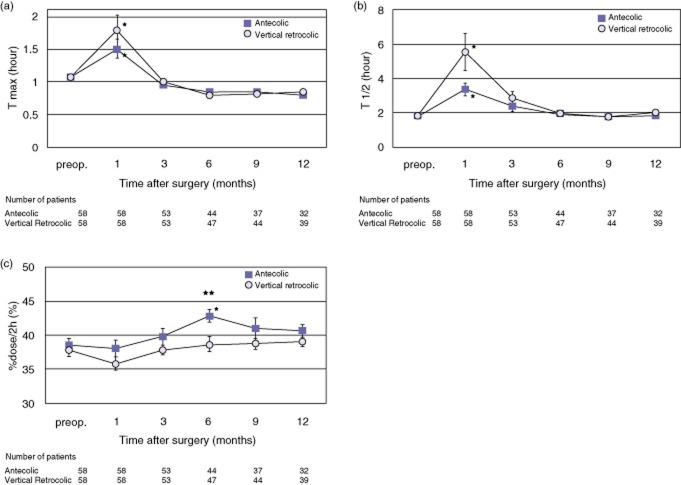
Results of the gastric emptying variables from the 13C-acetate breath test for 1 year after surgery are shown in Fig. 3. An increase in T max (the time when 13CO2 reaches maximum excretion) and in T 1/2 (half-emptying time) indicates prolonged gastric emptying, and an increase in %dose/2h (total % excretion of 13CO2 in 2 h) indicates accelerated gastric emptying.
Figure 3.
Post-operative change in parameters related to the 13C-acetate breath test: (a) the time when 13CO2 reaches maximum excretion (T max), (b) half-emptying time (T 1/2), and (c) total % excretion of 13CO2 in 2 h (%dose/2 h). Values are mean ± standard error. *P < 0.05 for comparison of each post-operative time point value with the pre-operative value in the same group. **P < 0.05 for comparison of the antecolic (A) group with the vertical retrocolic (VR) group. There were no significant differences at any time points compared between the two groups in (a) and (b), except for (c). Comparisons between the values of each time point and the pre-operative value showed the following. (a) The values at post-operative month 1 were significantly greater than the pre-operative values for each group. (b) The values at post-operative month 1 were significantly greater than the pre-operative values for each group. (c) The value at post-operative month 6 in the A group was significantly greater than the pre-operative value. The values were greater in the A group than in the VR group at every time point, and the difference was significant at month 6 after surgery (A group: 42.9 ± 1.0%, VR group: 38.7 ± 1.1%, P = 0.001)
The value of T max was significantly prolonged at post-operative month 1 in comparison with the pre-operative value in both groups. The value of T max in the VR group was greater than that in the A group at post-operative month 1, but it was not significantly different (A group: 1.50 ± 0.14 h, VR group: 1.79 ± 0.23 h, P = 0.593). The values of T max at post-operative months 3, 6, 9, and 12 were equal to or shorter than the pre-operative values in both groups, and there were no significant differences between the two groups. Similarly, the value of T 1/2 was significantly prolonged at post-operative month 1 in comparison with the pre-operative value in both groups. The value of T 1/2 in the VR group was greater than that in the A group at post-operative month 1, but the difference was not statistically significant (A group: 3.37 ± 0.37 h, VR group: 5.55 ± 1.10 hours, P = 0.164). Post-operative changes in the value of T 1/2 gradually decreased as time passed, but the difference was not significant between the two groups. The value of %dose/2h was higher in the A group than in the VR group at all post-operative time points and was significantly higher at post-operative month 6 (A group: 42.9 ± 1.0%, VR group: 38.7 ± 1.1%, P = 0.001). Collectively, the VR group showed prolonged gastric emptying without a significant difference in the short term post-operatively (post-operative month 1). Both groups showed prolonged gastric emptying at post-operative month 1, but gastric emptying was not prolonged after post-operative month 3 compared with the pre-operative value. The A group continued accelerated gastric emptying after post-operative month 3, whereas the VR group values were essentially close to their own pre-operative values.
Nutritional parameters for 1 year after surgery
Post-operative backgrounds of the study subjects, such as undergoing of adjuvant chemotherapy and tumour recurrence within the first postoperative year, were not significantly different between the A and VR groups (Table 2).
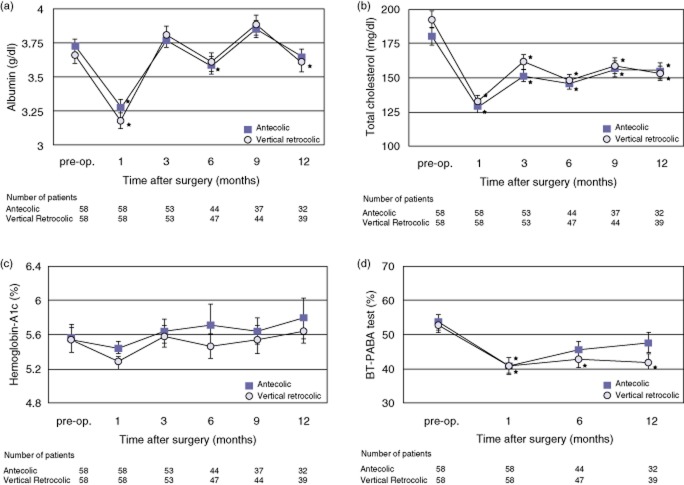
The results of changes in post-operative nutritional parameters are shown in Fig. 4. Comparisons of the nutritional biochemical parameters including serum albumin, total cholesterol, haemoglobin-A1c and the N-benzoyl-L-tyrosyl-para-aminobenzoic acid (BT-PABA) test were almost similar (Fig. 4a–d, respectively). The values of serum albumin returned to the pre-operative level at post-operative month 3, but the values of total cholesterol and pancreatic functions continued to remain below the pre-operative level during the first post-operative year in both groups. Weight change in all patients from admission to operation was −1.27 ± 0.2 kg and was without significant difference between the two groups (−1.18 ± 0.3 kg in the A group versus −1.36 ± 0.3 kg in the VR group; P = 0.969). The results of changes in post-operative patient weight are shown in Fig. 5. The post-operative weight in both groups decreased at post-operative month 1 compared with the pre-operative weight and in both groups gradually regained weight as time passed. Post-operative weight loss in the A group was prolonged compared with that in the VR group, and this tendency was observed at all post-operative time points. The post-operative body weight recovered to nearly the pre-operative weight in the VR group at 1 year after surgery. The percentage of pre-operative weight in the VR group was significantly greater than that in the A group at post-operative month 12 (A group: 93.8 ± 1.2%, VR group: 98.5 ± 1.3%, P = 0.015).
Figure 4.
Post-operative changes in nutritional parameters: serum albumin (a), total cholesterol (b), haemoglobin-A1c (c) and the BT-PABA (N-benzoyl-L-tyrosyl-para-aminobenzoic acid) test (d). Values are mean ± standard error. *P < 0.05 for comparison of the value of each post-operative time point with the pre-operative value in the same group. **P < 0.05 for comparison of the antecolic (A) versus vertical retrocolic (VR) group. There were no significant differences at any time points compared between the two groups in (a)–(d). Comparisons between the values of each time point and the pre-operative value showed the following. (a) The values at post-operative months 1 and 6 were significantly lower than the pre-operative value in the A group, and the values at post-operative months 1 and 12 were significantly lower than the pre-operative value in the VR group. (b) The values at every post-operative time point were significantly lower than the pre-operative values in both groups. (c) There were no significant differences at any time point for both groups. (d) The value at post-operative month 1 was significantly lower than the pre-operative value in the A group, whereas the values at post-operative months 1, 6, and 12 were significantly lower than the pre-operative value in the VR group
Figure 5.
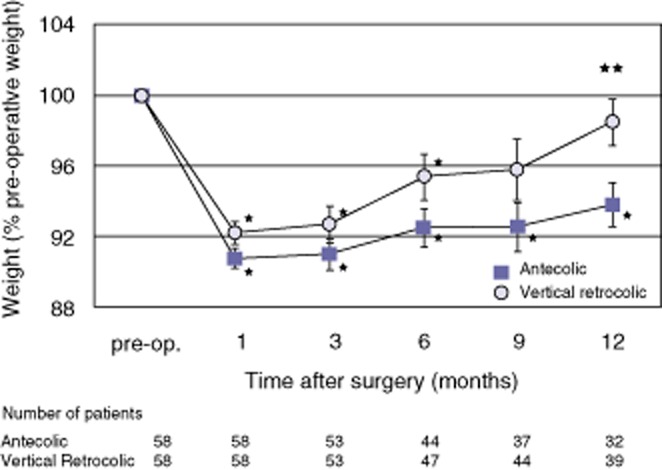
Post-operative change in patient weight. Post-operative weight was compared as a percentage of pre-operative weight. Values are mean ± standard error. *P < 0.05 for comparison of the value of each post-operative time point with the pre-operative value in the same group. **P < 0.05 for comparison of the antecolic (A) versus the vertical retrocolic (VR) group. The values at post-operative months 1, 3, 6, 9 and 12 were significantly lower than the pre-operative value in the A group, whereas the values at post-operative months 1, 3 and 6 were significantly lower than the pre-operative value in the VR group. In comparison between the two groups, the values were greater in the VR versus the A group at every time point, and the difference was significant at month 12 after surgery (percentage of preoperative weight, A group: 93.8 ± 1.2%; VR group: 98.5 ± 1.3%, P = 0.015)
Discussion
The results from the present prospective, randomized, clinical trial were that (i) the incidence of DGE was lower in an antecolic duodenojejunostomy versus a vertical retrocolic duodenojejunostomy, but the difference was not significant, (ii) gastric emptying was more accelerated in the patients reconstructed with the antecolic route for 1 year after surgery, and (iii) body weight gain during the first year after surgery was superior in patients reconstructed using the vertical retrocolic route.
The PPPD procedure was first described by Watson in 194420 and reintroduced by Traverso and Longmire in 197821 with the intent to improve post-operative nutritional status and avoid postgastrectomy syndromes, and DGE was considered as a specific complication after PPPD attributed to pylorus-sparing resection.4 The causative factors of DGE have been widely debated. These include anastomotic ischaemia, nerve damage, altered hormone levels, pylorospasm, gastric dysrhythmia, mechanical torsion and angularity, local inflammation and abdominal complications.4,7,10,22–26 To prevent or treat DGE after PPPD, the peri-operative use of prokinetic agents or different operative techniques has been tested.4,5,27–30 These peri-operative management or operative procedures were reported as possibly effective, but they have not gained wide acceptance. Several reports support an association between the reconstruction route used with PPPD and the incidence of DGE.4–13,26–29 Two reconstruction routes are used for a duodenojejunostomy during PPPD: the antecolic route and the retrocolic route. Many of the previous studies have suggested that the incidence of DGE is lower with an antecolic duodenojejunostomy because it may decrease the risk of mechanical problems as a result of angulation or torsion of the relatively fixed stomach.4–10 However, one randomized control trial11 and the authors' preliminary reports12,13 showed no significant difference in the incidence of DGE between antecolic and retrocolic reconstruction.
Although DGE occurrence in the VR group was higher than that in the A group in the present study, the difference was not statistically significant. Of note, the difference in DGE incidences in this study may be underestimated by a lack of power (type II error) because the number of patients was set on the basis of the hypothesis that antecolic reconstruction decreases the rate of DGE from 30% to 10%. However, the vertical retrocolic reconstruction showed a lower incidence of DGE than was expected. The 10% incidence of clinically relevant DGE with the authors' vertical retrocolic reconstruction method was much less than the 24%–72% incidence reported with retrocolic reconstruction in previous reports6,8,9,11,24,29 and was comparable to the rates of 3–34% reported with antecolic reconstruction.6–11,25,27,28,31,32 Two possible reasons for the decreased occurrence of DGE with the authors' vertical retrocolic reconstruction method include (i) the duodenojejunostomy and stomach were separate from the excisions and the anastomotic field in the right upper quadrant and thus were spared expected inflammation, and (ii) the vertical and straight reconstruction avoided flexion and angulation of the stomach and contributed to flow of gastric contents by gravity in the upright position in patients.
Gastric emptying as assessed by the results of T max and T 1/2 was more prolonged in the VR group than the A group at post-operative month 1, but the difference was not significant. Most patients in the VR group did not develop clinical problems including the need for interventional treatment. At post-operative months 3, 6, 9, and 12, the results of T max and T 1/2 were essentially similar between the two groups, recovering close to the pre-operative values. In contrast, gastric emptying as indicated by the %dose/2h results was more accelerated in the A group than in VR group patients, and the difference was significant at post-operative month 6. In addition, the %dose/2 h results in the VR group were essentially close to their own pre-operative values, whereas those in the A group were higher than the pre-operative values until post-operative month 12. These results imply that the vertical retrocolic reconstruction may maintain more physiological gastric emptying after surgery compared with the antecolic reconstruction.
With regard to nutritional status in the present study, the values of serum albumin returned to the pre-operative level at post-operative month 3, but the values of total cholesterol continued to remain below the pre-operative level during the first post-operative year in both groups. These results were similar to those in previous reports.31,32 The changes in biochemical parameters between the two groups were similar. In contrast, the patients in the A group had a prolonged body weight loss compared with those in the VR group. The factors relating to post-operative weight gain after PPPD have been reported to be pancreatic endocrine and exocrine function, disease, operative procedure, intra-or post-operative chemoradiation therapy and tumour recurrence.33–36 The nutritional parameters and tumour status were similar between the two groups in the present study. Thus, these results were difficult to explain in terms of the differences in body weight change between the two reconstruction methods. When the reason for the differences in body weight change between the two reconstructions is considered, gastric emptying may be highlighted. In a series of gastric surgeries, a pylorus-preserving gastrectomy was reported to lead to slower gastric emptying and better post-operative weight gain compared with a conventional distal gastrectomy with Billroth I anastomosis.37,38 In regard to bariatric surgeries, several reports suggested that operative procedures such as gastric Roux-en-Y bypass or sleeve gastrectomy may reduce weight owing to enhanced endogenous release of anorexigenic gut peptides (cholecystokinin, glucagon-like peptide-1, and polypeptide YY) by changing the acceleration of gastric emptying and increasing delivery of nutrients to the distal small intestine.39 In the present study, antecolic reconstruction is speculated to be potentially associated with prolonged post-operative weight loss affected by accelerated gastric emptying. However, the post-operative weight change is a result of multiple factors, and it may be difficult to explain weight change only on the basis of the influence of gastric emptying. Furthermore, post-operative patient eating habits and the status of health including quality of life were not evaluated. Further analysis focusing on post-operative nutritional status affected by subsequently changing gastrointestinal function such as gastric emptying and the profile and response of gut peptides may be required.
In conclusion, the results of this prospective, randomized, clinical trial showed that a vertical retrocolic duodenojejunostomy was an acceptable procedure for a lower incidence of DGE. In addition, a vertical retrocolic duodenojejunostomy may result in a better body weight recovery by maintaining moderate (not too accelerated) gastric emptying compared with that of an antecolic duodenojejunostomy. Although further studies are awaited, a vertical retrocolic duodenojejunostomy is proposed as a potential choice of reconstruction method in patients undergoing PPPD.
Acknowledgments
A part of this study was supported by grants-in-aid (Nos. 17591417 and 20591635) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Conflicts of interest
None declared.
References
- 1.McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schäfer M, Müllhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002;236:137–148. doi: 10.1097/00000658-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lytras D, Paraskevas KI, Avgerinos C, Manes C, Touloumis Z, Paraskeva KD, et al. Therapeutic strategies for the management of delayed gastric emptying after pancreatic resection. Langenbecks Arch Surg. 2007;392:1–12. doi: 10.1007/s00423-006-0096-7. [DOI] [PubMed] [Google Scholar]
- 5.Traverso LW, Hashimoto Y. Delayed gastric emptying: the state of the highest level of evidence. J Hepatobiliary Pancreat Surg. 2008;15:262–269. doi: 10.1007/s00534-007-1304-8. [DOI] [PubMed] [Google Scholar]
- 6.Tani M, Terasawa H, Kawai M, Ina S, Hirono S, Uchiyama K, et al. Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg. 2006;243:316–320. doi: 10.1097/01.sla.0000201479.84934.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horstmann O, Markus PM, Ghadimi MB, Becker H. Pylorus preservation has no impact on delayed gastric emptying after pancreatic head resection. Pancreas. 2004;28:69–74. doi: 10.1097/00006676-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Hartel M, Wente MN, Hinz U, Kleeff J, Wagner M, Müller MW, et al. Effect of antecolic reconstruction on delayed gastric emptying after the pylorus-preserving Whipple procedure. Arch Surg. 2005;140:1094–1099. doi: 10.1001/archsurg.140.11.1094. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama M, Abe N, Ueki H, Masaki T, Mori T, Atomi Y. A new reconstruction method for preventing delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. Am J Surg. 2004;187:743–746. doi: 10.1016/j.amjsurg.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Kurosaki I, Hatakeyama K. Preservation of the left gastric vein in delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy. J Gastrointest Surg. 2005;9:846–852. doi: 10.1016/j.gassur.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Gangavatiker R, Pal S, Javed A, Dash NR, Sahni P, Chattopadhyay TK. Effect of antecolic or retrocolic reconstruction of the gastro/duodenojejunostomy on delayed gastric emptying after pancreaticoduodenectomy: a randomized controlled trial. J Gastrointest Surg. 2011;15:843–852. doi: 10.1007/s11605-011-1480-3. [DOI] [PubMed] [Google Scholar]
- 12.Chijiiwa K, Imamura N, Ohuchida J, Hiyoshi M, Nagano M, Otani K, et al. Prospective randomized controlled study of gastric emptying assessed by (13)C-acetate breath test after pylorus preserving pancreaticoduodenectomy: comparison between antecolic and vertical retrocolic duodenojejunostomy. J Hepatobiliary Pancreat Surg. 2009;16:49–55. doi: 10.1007/s00534-008-0004-3. [DOI] [PubMed] [Google Scholar]
- 13.Chijiiwa K, Ohuchida J, Hiyoshi M, Nagano M, Kai M, Kondo K. Vertical retrocolic duodenojejunostomy decreases delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. Hepatogastroenterology. 2007;54:1874–1877. [PubMed] [Google Scholar]
- 14.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, et al. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108:1048–1055. doi: 10.1016/0016-5085(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 16.Nakata K, Aoyama N, Nakagawa M, Kawasaki S, Shirasaka D, Zai H, et al. The present and the future in gastric emptying study assessed by 13C-acetate breath test-with special reference to the standardization of the method-[in Japanese] J Smooth Muscle Res. 2002;6:J75–J91. [Google Scholar]
- 17.Takahashi Y, Amano Y, Yuki T, Ose T, Miyake T, Kushiyama Y, et al. Influence of acid suppressants on gastric emptying: cross-over analysis in healthy volunteers. J Gastroenterol Hepatol. 2006;21:1664–1668. doi: 10.1111/j.1440-1746.2006.04270.x. [DOI] [PubMed] [Google Scholar]
- 18.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Watson K. Carcinoma of the ampulla of Vater. Successful radical resection. Br J Surg. 1944;31:368–373. [Google Scholar]
- 21.Traverso LW, Longmire WP., Jr Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet. 1978;146:959–962. [PubMed] [Google Scholar]
- 22.Welsch T, Borm M, Degrate L, Hinz U, Büchler MW, Wente MN. Evaluation of the International Study Group of Pancreatic Surgery definition of delayed gastric emptying after pancreatoduodenectomy in a high-volume centre. Br J Surg. 2010;97:1043–1050. doi: 10.1002/bjs.7071. [DOI] [PubMed] [Google Scholar]
- 23.Malleo G, Crippa S, Butturini G, Salvia R, Partelli S, Rossini R, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors. HPB. 2010;12:610–618. doi: 10.1111/j.1477-2574.2010.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akizuki E, Kimura Y, Nobuoka T, Imamura M, Nagayama M, Sonoda T, et al. Reconsideration of postoperative oral intake tolerance after pancreaticoduodenectomy: prospective consecutive analysis of delayed gastric emptying according to the ISGPS definition and the amount of dietary intake. Ann Surg. 2009;249:986–994. doi: 10.1097/SLA.0b013e3181a63c4c. [DOI] [PubMed] [Google Scholar]
- 25.Park JS, Hwang HK, Kim JK, Cho SI, Yoon DS, Lee WJ, et al. Clinical validation and risk factors for delayed gastric emptying based on the International Study Group of Pancreatic Surgery (ISGPS) Classification. Surgery. 2009;146:882–887. doi: 10.1016/j.surg.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Qu H, Sun GR, Zhou SQ, He QS. Clinical risk factors of delayed gastric emptying in patients after pancreaticoduodenectomy: a systematic review and meta-analysis. Eur J Surg Oncol. 2013;39:213–223. doi: 10.1016/j.ejso.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Hochwald SN, Grobmyer SR, Hemming AW, Curran E, Bloom DA, Delano M, et al. Braun enteroenterostomy is associated with reduced delayed gastric emptying and early resumption of oral feeding following pancreaticoduodenectomy. J Surg Oncol. 2010;101:351–355. doi: 10.1002/jso.21490. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto Y, Yamamoto Y, Hata S, Nara S, Esaki M, Sano T, et al. Analysis of risk factors for delayed gastric emptying (DGE) after 387 pancreaticoduodenectomies with usage of 70 stapled reconstructions. J Gastrointest Surg. 2011;15:1789–1797. doi: 10.1007/s11605-011-1498-6. [DOI] [PubMed] [Google Scholar]
- 29.Kollmar O, Sperling J, Moussavian MR, Kubulus D, Richter S, Schilling MK. Delayed gastric emptying after pancreaticoduodenectomy: influence of the orthotopic technique of reconstruction and intestinal motilin receptor expression. J Gastrointest Surg. 2011;15:1158–1167. doi: 10.1007/s11605-011-1554-2. [DOI] [PubMed] [Google Scholar]
- 30.Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast-track recovery programme after pancreatico-duodenectomy reduces delayed gastric emptying. Br J Surg. 2008;95:1387–1393. doi: 10.1002/bjs.6324. [DOI] [PubMed] [Google Scholar]
- 31.Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, Uchiyama K, et al. Pylorus ring resection reduces delayed gastric emptying in patients undergoing pancreatoduodenectomy: a prospective, randomized, controlled trial of pylorus-resecting versus pylorus-preserving pancreatoduodenectomy. Ann Surg. 2011;253:495–501. doi: 10.1097/SLA.0b013e31820d98f1. [DOI] [PubMed] [Google Scholar]
- 32.Fujii T, Kanda M, Kodera Y, Nagai S, Sahin TT, Hayashi M, et al. Preservation of the pyloric ring has little value in surgery for pancreatic head cancer: a comparative study comparing three surgical procedures. Ann Surg Oncol. 2012;19:176–183. doi: 10.1245/s10434-011-1901-2. [DOI] [PubMed] [Google Scholar]
- 33.Niedergethmann M, Shang E, Farag Soliman M, Saar J, Berisha S, Willeke F, et al. Early and enduring nutritional and functional results of pylorus preservation vs classic Whipple procedure for pancreatic cancer. Langenbecks Arch Surg. 2006;391:195–202. doi: 10.1007/s00423-005-0015-3. [DOI] [PubMed] [Google Scholar]
- 34.van Berge Henegouwen MI, Moojen TM, van Gulik TM, Rauws EA, Obertop H, Gouma DJ. Postoperative weight gain after standard Whipple's procedure versus pylorus-preserving pancreatoduodenectomy: the influence of tumour status. Br J Surg. 1998;85:922–926. doi: 10.1046/j.1365-2168.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 35.Ohtsuka T, Yamaguchi K, Chijiiwa K, Tanaka M. Postoperative pancreatic exocrine function influences body weight maintenance after pylorus-preserving pancreatoduodenectomy. Am J Surg. 2001;182:524–529. doi: 10.1016/s0002-9610(01)00745-0. [DOI] [PubMed] [Google Scholar]
- 36.Ohuchida J, Chijiiwa K, Ohtsuka T, Konomi H, Tanaka M. Pylorus-preserving pancreatoduodenectomy: preoperative pancreatic function and outcome. Hepatogastroenterology. 2007;54:913–916. [PubMed] [Google Scholar]
- 37.Nunobe S, Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer. 2007;10:167–172. doi: 10.1007/s10120-007-0434-7. [DOI] [PubMed] [Google Scholar]
- 38.Park do J, Lee HJ, Jung HC, Kim WH, Lee KU, Yang HK. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg. 2008;32:1029–1036. doi: 10.1007/s00268-007-9441-4. [DOI] [PubMed] [Google Scholar]
- 39.Horner KM, Byrne NM, Cleghorn GJ, Näslund E, King NA. The effects of weight loss strategies on gastric emptying and appetite control. Obes Rev. 2011;12:935–951. doi: 10.1111/j.1467-789X.2011.00901.x. [DOI] [PubMed] [Google Scholar]