Abstract
Cigarette smoking (CS) has been strongly linked to several health conditions including heart disease, lung cancer, and other respiratory and circulatory ailments. Deleterious effects of cigarette smoking on skin have also been well documented, but unlike effects on other organs, damage does not depend upon inhalation. The upper layer of the skin, the stratum corneum (SC) (rich in cholesterol fatty acids and ceramide) is very susceptible to damage induced by exposure to environmental stressors that can modify its lipid composition and thereby affect its function of protecting skin from dehydration. Scavenger Receptor B1 (SR-B1) is involved in uptake of cholesterol in several tissues including skin. We previously demonstrated that CS exposure induces formation of aldehydes (HNE) adducts that decrease SR-B1 expression. As topical resveratrol, a well-known polyphenolic stilbene, has been demonstrated to show benefits against skin disorders, we investigated its possible role as a protective agent against CS-induced reduction of SR-B1 expression in cutaneous tissue. In this study, we have demonstrated that resveratrol at the doses ranging from 0.5 to 10μM is not toxic, is able to increase SR-B1 protein levels in a dose-dependent manner in human keratinocytes. Moreover, when the cells that were pre-treated with different doses of resveratrol, were exposed to CS, the loss of SR-B1 was prevented in a dose dependent manner. In addition, in keratinocytes, resveratrol was also able to prevent an increase in HNE protein adducts induced by CS. In particular resveratrol was able to prevent HNE-SRB1 adducts formation. Thus, resveratrol appears to be a natural compound that could provide skin with a defense against exogenous stressors by protecting the essential cholesterol receptor, SR-B1.
Keywords: Resveratrol, 4-hydroxynonenal, scavenger receptor B1, protein adducts, oxidative stress
Introduction
Because of its critical location, cutaneous tissue is the first barrier against environmental insults such as UV radiation, CS, diesel fuel exhaust, halogenated hydrocarbons, heavy metals and O3 [1]. Within the skin, the stratum corneum (SC) has been identified as the main target of oxidative damage. As the outer skin barrier, the SC has important functions, limiting transepidermal water loss and posing a mechanical barrier to penetration by exogenous chemicals and pathogens. It comprises a unique two-compartment system of structural, non-nucleated cells (corneocytes) embedded in a lipid enriched intercellular matrix, forming stacks of bilayers that are rich in ceramides, cholesterol and free fatty acids [2].
Cholesterol represents about one-quarter of the lipid content of the SC. It is an essential component for all cells. In the skin, cholesterol is implicated in corneocyte desquamation and cohesion and keratinocyte differentiation. In keratinocytes, cholesterol is required to form lamellar bodies that play a key role in maintaining barrier function in optimal conditions [2]. One of the main pathways by which cholesterol can be taken up by the tissues is via scavenger receptor class B type I (SR-BI) that mediates the cholesterol flux in a bidirectional manner dependent upon the cholesterol gradient [3]. SR-BI, a transmembrane protein, mediates selective lipid uptake from hydrophobic lipoprotein cores [4,5] and facilitates the cellular uptake of cholesterol (mainly cholesteryl esters) by intervening in the binding of the lipoprotein with the outer surfaces of cells. This happens through a process in which the cholesterol esters are internalized without the net internalization and degradation of the lipoprotein itself although Silver et al. have shown that in polarized liver cells SR-B1 is able to facilitate uptake of the whole HDL particle via a transcytosis process [6]. Among environmental insults to which cutaneous tissue is exposed, CS, particularly the oxidative compounds that derive from the combustion of cigarettes, can affect skin physiology [7, 8].
In a previous study we showed that exposure to CS, through its stimulation of endogenous H2O2 production, induced post-translational modifications of SR-B1 with the consequent loss of the receptor. Furthermore, this loss may play a role in altered skin physiology induced by CS exposure [9].
Resveratrol (3,5,4’-trihydroxy-trans-stilbene) is a natural polyphenolic stilbene found in grape skin, nuts, pomegranate and herbal medicines, including Polygonum cuspitatum. Resveratrol has been shown to exhibit various biochemical activities including regulation of the cell cycle, stimulation of endothelial nitric oxide synthase, and inhibition of platelet aggregation. Furthermore, several studies have reported that resveratrol protects against oxidative damage by enhancing the expression of antioxidant genes, such as heme oxygenase-1 and glutamate cysteine ligase [10, 11, 12].
Natural compounds have been used to regulate serum lipid concentration to reduce the incidence of hyperlipidemia and atherosclerosis. As resveratrol is a natural polyphenol that can induce antioxidant enzymes, it has been suggested to be responsible for the protective effect in different pathologies characterized by hyperlipidemia [13] In our previous study [14] a decrease of SR-B1 protein levels induced by CS through post-transductional modification (HNE adduct formation) was shown. Thus, the present work is aimed at evaluating the possible protective effect of resveratrol in preventing the CS-induced decrease of keratinocytes SR-B1 levels.
Material and Methods
METHODS
Cell Culture and Treatments
HaCaT cells, (a cell line gift from Dr. F. Virgili), were grown in Dulbecco’s modified Eagle’s medium High Glucose (Lonza, Milan, Italy), supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/mL streptomycin and 2 mM L-glutamine as previously described [15]. Cell suspension containing 10 or 1 × 105 viable cells/ml were used. Cells were incubated at 37 °C for 24 h in 95% air/5% CO2 until 80% confluency.
HaCaT cells were pretreated (24h) with resveratrol [0.5, 1, 5 and 10 μM] (purity ≥ 99% [GC], Sigma Aldrich, Milan, Italy), then the media was removed before CS exposure, and then the cells were resuspended in DMEM medium supplemented with 10% FBS. After treatments with different doses and various times, cells were collected by centrifugation for the several assays described below.
CS Exposure
HaCaT cells were exposed to fresh CS in an exposure system that generated CS by burning one UK unfiltered research cigarette (12 mg tar, 1.1 mg nicotine) using a vacuum pump to draw air through the burning cigarette and leading the smoke stream over the cell cultures [16]. Briefly, overnight serum-starved HaCaT cells in culture were placed with lids removed in a 37 °C chamber having a volume of 0.0127 m3, smoke from one cigarette was introduced into the chamber by readmitting air (a decrease of ~12.7 cm Hg over ~30 s) in the chamber after achieving a partial vacuum (63.5 cm Hg) via a short piece of tubing holding a lit cigarette (UK research cigarette; 12 mg tar, 1.1 mg nicotine).
Cells were exposed for 50 min to CS. Control cells were exposed to filtered air for the same duration (50 min). After the exposure (air or CS) fresh media was added to the cells.
Cellular Viability
Viability studies were performed 24 h after treatments by LDH release assay. The LDH release was measured by enzymatic assay: in the first step NAD+ is reduced to NADH by the LDH-catalyzed conversion of lactate to pyruvate; in the second step the catalyst (diaphorase) transfers a H- from NADH to the tetrazolium salt, which is reduced to the formazan.
Before LDH release measurement, the cells were lysed with 0.1% (V/V) Triton X-100 in culture media for 30 min at 37°C to obtain a representative maximal LDH release as the positive control with 100% toxicity. The amounts of LDH in the supernatant were determined and calculated according to kit instructions (Roche, Milan, Italy). Color changes were measured at 490 nm.
Immunocytochemistry
HaCaT cells were grown on cover slips at a density of 1 x 105 cell/ml, and after CS exposure fixed in 4% paraformaldehyde in PBS for 30 min at room temperature (RT). Cells were permeabilized for 15 min at room temperature with PBS containing 1% BSA, 0.2% Triton X-100, and 0.02% sodium azide, then the cover slips were blocked in PBS containing 1% BSA, 0.2% Nonidet P-40 and 0.02% sodium azide at room temperature for 1 h at RT. Cells were incubated with a mixture of goat anti-HNE (1:250) and rabbit anti-SR-B1 (1:250) for 1 h and then were incubated with a mixture of anti-goat (1:100) and anti rabbit (1:100) in PBS containing 0.5% BSA for 1 h at RT. Nuclei were stained with 1 μg/ml DAPI (Molecular Probes) for 1 min after removal of secondary antibodies. Cover slips were mounted onto glass slides using anti-fade mounting medium 1,4 diazabicyclooctane in glycerin (DABCO) and examined by the Zeiss Axioplan2 light microscope equipped with epifluorescence at 40× magnification. Negative controls for the immunostaining experiments were performed by omitting primary antibodies. Images were acquired and analyzed with Axio Vision Release 4.6.3 software.
Western blot Analysis
Total cell lysates were extracted in solubilization buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Nonidet P-40, 1 mM EGTA, 0.1% SDS, 5 mM N-ethylmaleamide (Sigma-Aldrich Corp.), protease and phosphatase inhibitor cocktails (Sigma–Aldrich Corp.) as described before [16].
Cells were harvested by centrifugation and proteins concentration was determined by the method of Bradford (Biorad Protein assay, Milan, Italy). Samples of 60 μg protein in 3X loading buffer (65 mM Tris base, pH 7.4, 20% glycerol, 2% sodium dodecyl sulfate, 5% β-mercaptoethanol and 1% bromophenol blue) were boiled for 5 min, loaded onto 10% sodium dodecyl sulphate–polyacrylamide electrophoresis gels and separated by molecular size. The gels were then electro-blotted onto nitrocellulose membranes which were then blocked for 1 h in Tris-buffered saline, pH 7.5, containing 0.5% Tween 20 and 5% milk. Membranes were incubated overnight at 4 °C with the primary antibody, rabbit anti SR-B1 (Novus Biologicals, Inc.; Littleton, CO) or goat anti-HNE (Millipore, Milan, Italy). The membranes were then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h, and the bound antibodies were detected using chemiluminescence (BioRad, Milan, Italy). The blots were then stripped and reprobed with β-actin (Cell Signalling; Celbio, Milan, Italy) as the loading control. Images of the bands were digitized and the densitometry of the bands were performed using Image-J software.
Quantitative real-time PCR
Total RNA was extracted, using an AURUM total RNA Mini Kit with DNAse digestion (Bio-Rad, Laboratories Inc., Benicia, CA, USA), from 2x105 HaCaT cells for each experimental condition, according to the manufacturer’s recommended procedure. First-strand cDNA was generated from 1 μg of total RNA using the iScript cDNA Synthesis Kit (Bio-Rad). The primer pairs (Table 1) capable of hybridization with unique regions of the appropriate gene sequence were obtained from the Real-time PCR GenBank Primer and Probe Database Primer Bank, RT primerDB [17]. Quantitative real-time PCR (qPCR) was performed using SYBR Green on the iQ5 Multicolor Real-time PCR Detection System (Bio-Rad). The final reaction mixture contained 300 nM of each primer, 1μL of cDNA and 7ul of iQ SYBR Green Supermix (Bio-Rad), with RNAse-free water being used to bring the reaction mixture volume to 15 μl. All reactions were run in triplicate. Real-time PCR was initiated with a 3-min hot-start denaturation step at 95°C, and then performed for 40 cycles at 95°C for 10 s and at 60°C for 20 s. During the reaction, fluorescence, and therefore the quantity of PCR products, was continuously monitored by Opticon Monitor 3 software (Bio-Rad). Primers were initially used to generate a standard curve over a large dynamic range of starting cDNA quantities, permitting calculation of the amplification efficiency (a critical value for the correct quantification of expression data) for each of the primer pairs. Ribosomal protein L13a (RPL13a), L11a (RPL11a) and GAPDH, were employed as reference genes. As previously described, samples were compared using the relative cycle threshold (CT) method [18]. After normalization to more stable mRNA RPL13a, RPL11a and GAPDH, the fold increase or decrease was determined with respect to control, using the formula 2-ΔΔCT, where ΔCT is: (gene of interest CT)(reference gene CT), and ΔΔCT is (ΔCT experimental) (ΔCT control).
Table 1.
Primer sequences and PCR condition
| Gene | Primer sequence | Ta °C | Product length (bp) | QPCR Amplification Efficiency* (%) | n° of cycles | Ref Primer Bank |
|---|---|---|---|---|---|---|
| SRB1 | F: 5′-gaattcgcctttcgtccccg -3′ R: 5′-ttgaaggacaggctactggg3′ |
60.1 | 236 | 96.2 | 39 | GenBank Accession BC112037. 1 |
| RPL13A | F: 5′-cctaagatgagcgcaagttgaa- 3′ R: 5′-ccacaggactagaacacctgctaa-3′ |
60.2 | 203 | 97.3 | 39 | Pattyn et al. 2006 |
| RPL11A | F: 5′-tgcgggaacttcgcatccgc-3′ R: 5′-gggtctgccctgtgagctgc-3′ |
60.1 | 108 | 96.5 | 39 | GenBank Accession NM 000975.2 |
| GAPDH | F: 5′-tgacgctggggctggcattg -3′ R: 5′-ggctggtggtccaggggtct -3′ |
60 | 134 | 94.6 | 39 | GenBank Accession NM 002046.3 |
Data calculated by OpticonMonitor 3 Software (Bio-Rad).
Statistical Analysis
For each of the variables tested, two-way analysis of variance (ANOVA) was used. A significant effect was indicated by a P-value < 0.05. Data are expressed as mean ± S.D. of triplicate determinations obtained in 5 separate experiments.
RESULTS
Resveratrol cytotoxicity
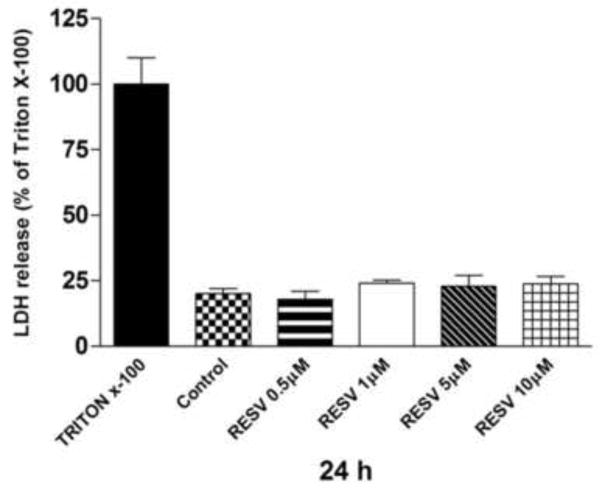
Cytotoxicity of resveratrol ranging from 0.5 to 10 μM was evaluated in the HaCaT cell line by LDH release. As shown in Fig.1, there were no significant differences between the resveratrol treated cells and the control (ethanol 0.1%) after 24 h. LDH release was around 20% in all sample, Triton X was used as positive control (100%).
Fig. 1.
Cytotoxicity of resveratrol ranging from 0.5 to 10 μM after 24 h was evaluated in HaCaT cell line by LDH release assay. Data are expressed as percentage of Triton X-100 [=positive control] (averages of five different experiments ± SEM).
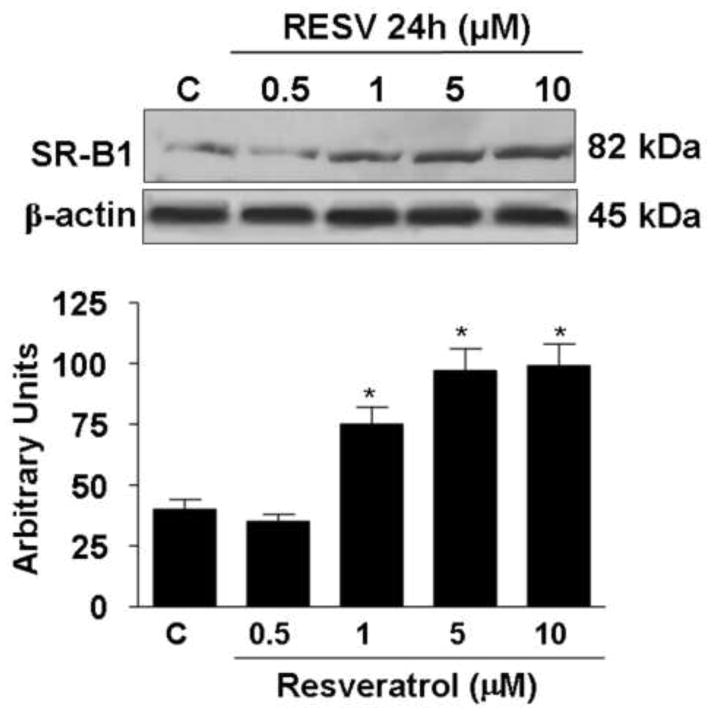
Resveratrol increased SR-B1 protein levels
To evaluate whether resveratrol treatment affect the SR-B1 protein level, we have performed Western blot analysis in HaCaT cells lysates. As shown in Fig.2, after 24 h of resveratrol treatment SR-B1 protein levels increased in a dose-dependent manner. The increase in the SR-B1 protein levels was already detectable after treatment with 1μM resveratrol (2 fold) and showed its highest increase at 5μM (2.3 fold); no further induction of the SR-B1 was observed with higher doses (10 μM).
Fig. 2.
Effect of resveratrol treatment on SR-B1 protein levels. Representative Western blot in the upper panel. Quantification of the SR-B1 bands is shown in the bottom panel. Data are expressed in arbitrary units (averages of five different experiments ± SEM, *p < 0.05). β-actin was used as loading control.
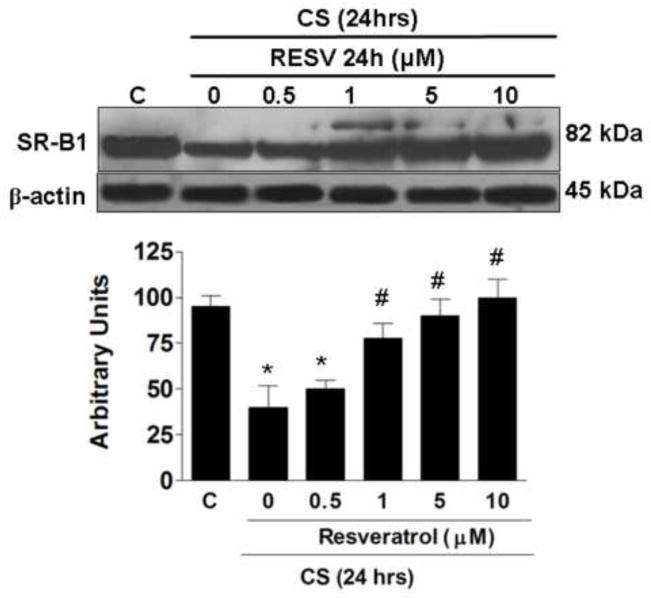
Resveratrol prevents SR-B1 decrease by CS exposure
In our previous work we have demonstrated that CS induces a decrease of the SR-B1 level in human keratinocytes as a consequence of increased oxidative stress [14], therefore we wanted to evaluate whether resveratrol treatment was able to protect against CS induced loss of SR-B1. As shown in Fig.3, 24 h after CS exposure, the protein levels of SR-B1 decreased significantly (circa 60%). On the other hand, pretreatment with resveratrol prevented the loss of the SR-B1 induced by CS in a dose dependent manner, starting from 1μM and reaching a maximum effect at 10μM resveratrol.
Fig. 3.
Effect of resveratrol pre-treatment on decreased SR-B1 levels induced by CS. Representative Western blot in the upper panel. Quantification of the SR-B1 bands is shown in the bottom panel. Data are expressed in arbitrary units (averages of five different experiments ± SEM, * vs. control; # vs. CS; p < 0.05). β-actin was used as loading control.
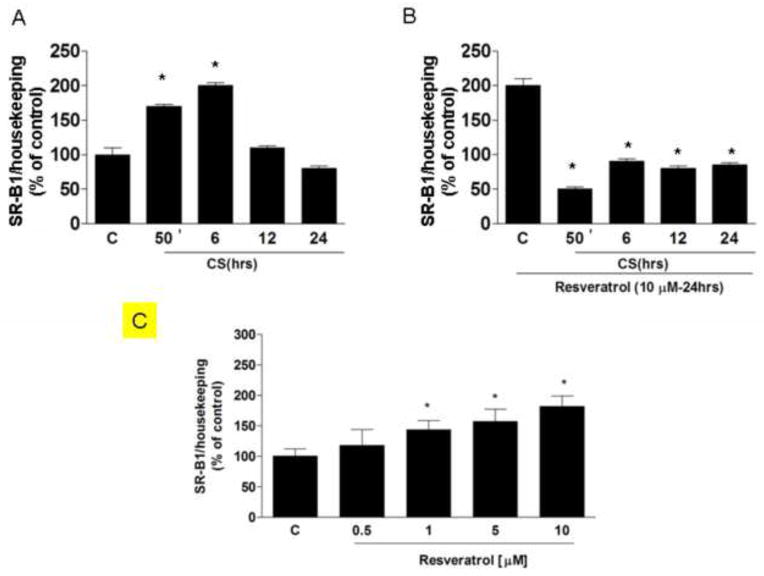
Effect of CS and Resveratrol on SR-B1 expression
To understand whether or not the effect of resveratrol on the SR-B1 protein levels was also a consequence of increased new transcription, we measured mRNA levels before and after resveratrol treatment. As shown in Fig.4A, SR-B1 mRNA levels increased significantly upon CS exposure (2 fold after 50 min). On the other hand, the opposite trend was observed in the resveratrol treated cells (Fig.4B), where the induction of mRNA was completely abolished, while, when the cells were treated with only resveratrol, SRB1 mRNA increased (panel C). Thus, it appears that resveratrol prevents the loss of the SR-B1 protein rather than induces replacement of the SR-B1 through transcription.
Fig. 4.
Effect of resveratrol with or without CS exposure on SR-B1 mRNA expression. Results are expressed in % 2–ΔΔCT with respect to the control value. Data are expressed as mean ± SEM of five independent experiments, * vs. control; p < 0.05.
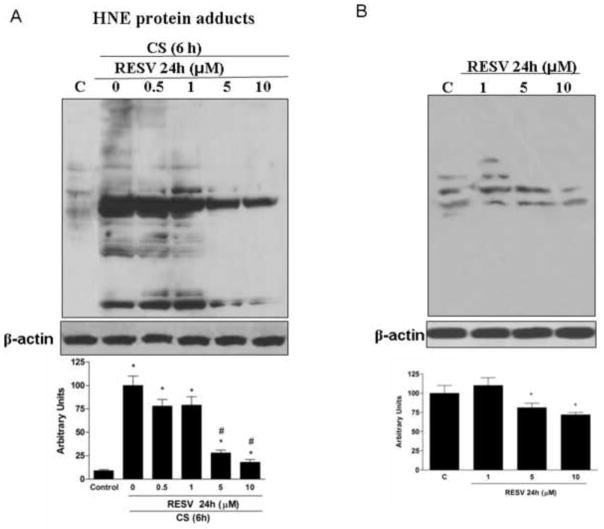
Resveratrol prevented the formation of HNE modified proteins induced by CS
Among the most reactive compounds present and generated by CS are the alpha-beta unsaturated aldehydes such as HNE. We therefore evaluated HNE adducts formation in keratinocytes exposed to CS with or without resveratrol pretreatment. As shown in Fig.5A, after CS exposure there was a significant increase of HNE protein adducts levels, and this effect was noticeable shortly after exposure (50’). Pretreatment with resveratrol produced dose-dependent prevention of CS-induced HNE protein adducts. This effect was observed with as little as 1μM resveratrol, but was more evident with higher resveratrol concentrations with a maximum effect at 10 μM. To evaluate the effect of resveratrol on HNE protein adducts formation, the cells were retreated with resveratrol at different doses and HNE adducts were determined. As shown in Fig.5B, resveratrol pretreatment was able to decrease the steady levels of cellular HNE proteins adducts, this effect was more evident at 5 and 10 μM concentration.
Fig. 5.
Effect of resveratrol with or without CS exposure on decreased HNE protein adducts. Representative Western blot in the upper panel. Quantification of the HNE bands is shown in the bottom panel. Data are expressed in arbitrary units (averages of five different experiments ± SEM, * vs. control; # vs. CS). β-actin was used as loading control.
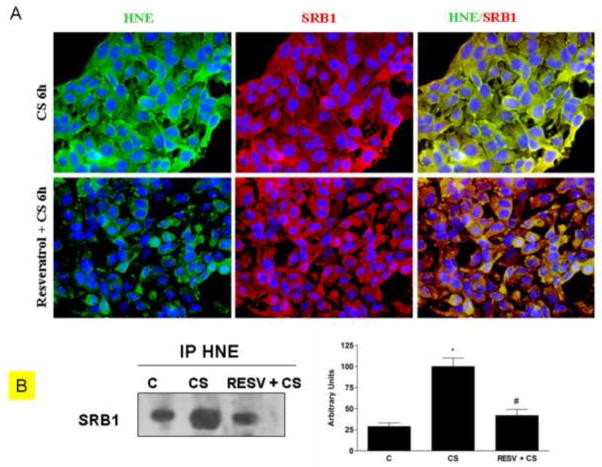
Resveratrol decreased the formation of HNE/SR-B1 adducts induced by CS
In our previous work we have reported that CS induces HNE/SR-B1 adducts [14]. Therefore in this study we evaluated by immunofluorescence and immunoprecipitation techniques whether resveratrol pretreatment was able to affect the formation of HNE/SR-B1 adducts in cells exposed to CS. As shown in Fig.6A, after CS exposure, there was a clear increase of HNE adducts (left column, green color) and a decrease in SR-B1 levels (central column, red color). The yellow color of merged images (right column) is indicative of the co-localization of HNE adduct and SR-B1, suggesting the formation of HNE-SR-B1 adducts. When the cells were pretreated with resveratrol and then exposed to CS, HNE adduct formation was less evident than that observed in the cells exposed to CS alone (bottom left panel). Moreover, the decrease in SR-B1 was prevented (bottom central panel) with very diminished co-localization (yellow) as can be observed in the bottom right panel. These data were also confirmed by immunoprecipitation assay as shown in Fig.6B, where immunoprecipitation of SR-B1 and immunoblotting for HNE shows a clear decrease of HNE-SR-B1 adducts after resveratrol pretreatment of circa 50–60%.
Fig. 6.
Effect of resveratrol on HNE/SRB1 adducts induced by CS. Immunocytochemistry of HaCaT cells showing localization of HNE-adducts (left column, green color), SR-B1 (central column, red color) and HNE/SR-B1 adducts (right column, yellow color) after Resveratrol pre-treatment and CS exposure(A). Images are merged in the right panel and the yellow color indicates overlap of the staining. These data were confirmed by immunoprecipitation for SR-B1 (B). HaCaT cells were pre-treated with resveratrol and then exposed to CS and cell lysates were immunoprecipitated using anti-HNE. Immunoprecipitated proteins were separated by SDS-PAGE, and then transferred to a nitrocellulose membrane and immunoblotted with anti-SRB1. Western blot shown is representative of five independent experiments. Quantification of the SR-B1 bands is shown on the right of the panel. Data are expressed in arbitrary units (averages of five different experiments ± SEM, * vs. control; # vs. CS; p < 0.05).
DISCUSSION
The purpose of this study was to evaluate the potential protective effect of resveratrol in CS induced loss of SR-B1 in keratinocytes. This work was a continuation of our previous work in which we demonstrated that CS was able to affect SR-B1 levels via post-translational modification as a consequence of increased H2O2 production.
Resveratrol, a polyphenolic stilbene ((E)-5-(4-h-hydroxystyryl)benzene-1,3-diol), was first isolated in the 1940s from white hellebores by Takaoka, and later from the Japanese knotweed Polygonum cuspidatum by Nonomura [19]. It is also found in some red wines and other components of the diet [20].
Due to several highly publicized claims and ‘low behaviors’ by a few investigators, resveratrol has been given a great amount of attention, some of which has been controversial. The French paradox is the apparently paradoxical association between lower cardiovascular disease despite a supposedly fat-laden French diet in which red wine consumption has been labeled as the ‘savior’ from fois gras [21, 22]. On closer investigation, it appeared that resveratrol was perhaps the active phytochemical in red wine. The concept of the French paradox has since been challenged, but the apparent cardiovascular protective properties of wine polyphenols, which have not been a principal subject of controversy, have been attributed to its inhibition of LDL cholesterol peroxidation, modulation of nitric oxide production, and ability when still in the gut with lipid-derived radicals [23–25].
Indeed, there has been an enormous amount of publications on the beneficial effects of resveratrol, showing its ability to prevent cancer formation [26] inhibit inflammation [27] and provide several other positive effects including cardiovascular protection [28] and neuroprotection [29]. Nonetheless, another controversy surrounds claims that resveratrol could extend lifespan by mimicking, without the need for self-denial, the benefits of a calorie-restricted diet [30–32]. The claims, particularly as the last failed to match its promise, has diminished enthusiasm for the compound despite those effects that appear to have stood up to further investigation.
In investigating the mechanism of how resveratrol acts, many have turned to studies of signaling in cellular models. The main concern about most in vitro cellular studies of the biological effect of resveratrol and other polyphenols has been the high concentration required to produce protection, as these would never be reached in vivo. It has been argued that following human oral absorption, resveratrol is very rapidly absorbed, mainly through transepithelial diffusion [33]. But, regardless of issues of absorption and fate of ingested resveratrol, in our studies that model topical application, the question of sufficient bioavailability was moot. Indeed, in this model, other factors affecting the delivery of resveratrol did not need to be considered. In addition the role of resveratrol in protecting skin inflammation in vivo has been elegantly demonstrated by the group of Surh, where animals feed with resveratrol showed a decreased activation of NF-κB and reduced COX2 expression [34]. Cutaneous tissue is continuously exposed to outdoor stressors such as UV, O3 and CS [35, 36]. The noxious effects of CS on skin have been now well recognized and its role in the pathogenesis of several pathologies is now well accepted [37, 38]. One of the mechanisms through which CS induces its noxious effect is through its ability to induce oxidative stress and generate bioactive products from lipid peroxidation including HNE. HNE derives from the oxidation of ω-6 polyunsaturated acids (PUFAs), essentially arachidonic and linoleic acid. It is a highly electrophilic molecule that primarily reacts with the amino acids cysteine, histidine and lysine, when free or as components of proteins. Cysteine, particularly as a thiolate (S−), and neutral imidazole and amino groups undergo Michael addition reactions to the C=C double bond of HNE.
In the present work, we have shown that, in keratinocytes, resveratrol was able to prevent CS induced SR-B1 protein modification by decreasing the formation of SR-B1 – HNE protein adducts. This protective effect can be achieved via multiple mechanisms. Although scavenging of reactive oxygen species has been claimed for resveratrol [39], within cells the induction of gene expression of phase II enzymes, such as heme oxygenase-1 or glutamate cysteine ligase, through activation of the transcription factor Nrf2 [40] or direct conjugation with HNE, as has been shown in epithelial lung cells [41], is more likely. In the present study we did not evaluate all the possible pathways mentioned above and we cannot exclude that more than one “defensive” mechanism is activated by resveratrol in keratinocytes exposed to CS.
We performed our study on HaCaT cells, a line of human non-tumorigenic skin epithelial cells, which represent a suitable, well-recognized and largely employed model of human skin keratinocytes [42] as they retain most of the functional differentiation properties of normal keratinocytes. In addition HaCaT cells in culture can revert back and forth between a differentiated and basal state upon changes in calcium concentration in the medium and express differentiation markers indicating their state [43, 44]. Our previous study [14] by demonstrating that CS adversely affects SR-B1 levels via posttranslational modification as demonstrated through immunoprecipitation and Western blotting. Here, we extend the investigation by showing that pre-treatment with resveratrol prevented the CS-induced loss of SR-B1. This is not the first finding showing that resveratrol can be effective in cutaneous topical usage. The root of Polygonum cuspidatum, which contains resveratrol, also called Ko-jo-kon in Japanese, was used in traditional Japanese and Chinese medicine to treat dermatitis [45]. In addition, resveratrol has also been shown to be chemopreventive against skin cancer by inhibiting carcinogenesis at all three stages, initiation, promotion, and progression [43, 46, 47].
Although most antioxidants cannot reach concentrations within cells that would allow them to function as chemical antioxidants, topical application may permit the required high concentration to compete with radicals at the cell surface. Certainly, outside the cell, resveratrol would have less competition and could act as a scavenger of reactive species. But, it is also known, that polyphenols, can be converted to electrophiles and thereby activate phase II enzyme as a protective mechanism [48]. So, there may be two contributing factors that allow exogenous resveratrol to protect keratinocytes, direct scavenging of radicals, which is usually only possible with vitamin E, and reaching high enough concentrations to induce antioxidant defenses.
The role of SR-B1 in protecting cutaneous tissue could be related to its ability to regulate cholesterol trafficking as suggested by the work Tsuruoka et al. [49] in which SR-B1 levels decreased as the keratinocytes differentiated but increased after insults, such as tape stripping, where the epidermis required more lipids to restore the permeability barrier in the SC. SR-B1 appears to possess additional functions in skin. Abnormal SC lipid profiles have been identified in several scaly skin diseases including ichtyosis [50] and atopic dermatitis. Atopic dermatitis is associated with abnormalities in the amounts of SC lipids and barrier function. Imokawa et al. [51] reported that in atopic dermatitis there was a marked reduction in the amount of CER in the forearm skin lesions compared with that of healthy individuals of the same age. These clinical studies indicated that alterations of SC lipid profile are responsible for the diminished permeability barrier function.
Although not directly connected to its ability to recognize HDL, SR-B1 has been shown to regulate calcium permeability in lymphocytes and be involved in bacteria recognition and vitamin E tissue uptake [3]; in addition it is now shown to be present in several tissues beside liver such as, lung, ovary, testis, brain, spleen, kidney [52] and, recently, even skin [50].
CS exposure was able to regulate SR-B1 also at the transcriptional level, and this effect was not observed when the cells were pretreated with resveratrol. This result may have been expected as SR-B1 is regulated by zinc-finger transcription factors Sp1, which can be activated by oxidative stress [53]. We therefore suggest that the role of resveratrol in preventing the loss of SR-B1 was due to either its direct or indirect increase in antioxidant protection that decreased the formation of HNE adducts. As noted above, topical application of resveratrol may allow it to reach high enough concentrations to act as a radical scavenger while its activation of phase II enzyme induction would increase the capacity to both prevent HNE formation and eliminate HNE when it is formed. Phase II induction increases the capacity to eliminate H2O2 and lipid hydroperoxides as well as accelerate HNE removal by conjugation and other metabolic routes [54]. In fact, in our recent work we have demonstrated that when HaCaT cells were exposed to CS give rise to a vicious cycle in which the aldehydes formed by CS led to the activation of NOX enzyme with increased H2O2 production. This leads to Fenton chemistry-dependent initiation of lipid peroxidation of cytosolic membrane lipids. A chain reaction in which increased formation of lipid peroxidation products including HNE and other aldehydes are produced then causes this cycle to spiral out of control [14].
Cigarette Smoke affect skin physiology by decreasing
SRB1 levels SRB1 forms adducts with 4HNE and then is ubiquitinated
Resveratrol prevents CS induced SRB1 loss by preventing the formation of SRB1-4HNE adducts
Acknowledgments
HJF is supported by NIH grant ES020942. The authors thank the association “Vino e Salute” for partial financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valacchi G, Weber SU, Luu C, Cross CE, Packer L. Ozone potentiates vitamin E depletion by ultraviolet radiation in the murine stratum corneum. FEBS Lett. 2000;466(1):165–8. doi: 10.1016/s0014-5793(99)01787-1. [DOI] [PubMed] [Google Scholar]
- 2.Schurer NY, Elias PM. The biochemistry and function of stratum corneum lipids. Adv Lipid Res. 1991;24:27–56. doi: 10.1016/b978-0-12-024924-4.50006-7. [DOI] [PubMed] [Google Scholar]
- 3.Valacchi G, Sticozzi C, Lim Y, Pecorelli A. Scavenger receptor class B type I: a multifunctional receptor. Ann N Y Acad Sci. 2011;1229:E1–7. doi: 10.1111/j.1749-6632.2011.06205.x. [DOI] [PubMed] [Google Scholar]
- 4.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 5.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 6.Silver DL, Wang N, Xiao X, Tall AR. High density lipoprotein (HDL) particle uptake mediated by scavenger receptor class B type 1 results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J Biol Chem. 2001;276(27):25287–93. doi: 10.1074/jbc.M101726200. [DOI] [PubMed] [Google Scholar]
- 7.Egawa M, Kohno Y, Kumano Y. Oxidative effects of cigarette smoke on the human skin. Int J Cosmet Sci. 1999;21:83–98. doi: 10.1046/j.1467-2494.1999.181656.x. [DOI] [PubMed] [Google Scholar]
- 8.Fortino V, Maioli E, Torricelli C, Davis P, Valacchi G. Cutaneous MMPs are differently modulated by environmental stressors in old and young mice. Toxicol Lett. 2007;173:73–79. doi: 10.1016/j.toxlet.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Model D. Smoker's face: an underrated clinical sign? Br Med J (Clin Res Ed) 1985;291(6511):1760–1762. doi: 10.1136/bmj.291.6511.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Shih A, Rinna A, Forman HJ. Resveratrol and 4-hydroxynonenal act in concert to increase glutamate cysteine ligase expression and glutathione in human bronchial epithelial cells. Arch Biochem Biophys. 2009;481:110–5. doi: 10.1016/j.abb.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Shih A, Rinna A, Forman HJ. Exacerbation of tobacco smoke mediated apoptosis by resveratrol: an unexpected consequence of its antioxidant action. Int J Biochem Cell Biol. 2011;43:1059–64. doi: 10.1016/j.biocel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331(4):993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 13.Penumathsa SV, Maulik N. Can J Physiol Pharmacol. Resveratrol: a promising agent in promoting cardioprotection against coronary heart disease. 2009;87(4):275–86. doi: 10.1139/Y09-013. [DOI] [PubMed] [Google Scholar]
- 14.Sticozzi C, Belmonte G, Pecorelli A, Arezzini B, Gardi C, Maioli E, Miracco C, Toscano M, Forman HJ, Valacchi G. Cigarette smoke affects keratinocytes SRB1 expression and localization via H2O2 production and HNE protein adducts formation. PLoS One. 2012;7(3):e33592. doi: 10.1371/journal.pone.0033592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valacchi G, Rimbach G, Saliou C, Weber SU, Packer L. Effect of benzoyl peroxide on antioxidant status, NF-kappaB activity and interleukin-1alpha gene expression in human keratinocytes. Toxicology. 2001;165:225–234. doi: 10.1016/s0300-483x(01)00430-9. [DOI] [PubMed] [Google Scholar]
- 16.Valacchi G, Davis PA, Khan EM, Lanir R, Maioli E, Pecorelli A, Cross CE, Goldkorn T. Cigarette smoke exposure causes changes in Scavenger Receptor B1 level and distribution in lung cells. Int J Biochem Cell Biol. 2011;43(7):1065–70. doi: 10.1016/j.biocel.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Pattyn F, Robbrecht P, De Paepe A, Speleman F, Vandesompele J. RTPrimerDB: the real-time PCR primer and probe database, major update 2006. Nucleic Acids Res. 2006;34:684–8. doi: 10.1093/nar/gkj155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orallo F. Review Comparative studies of the antioxidant effects of cis- and trans-resveratrol. Curr Med Chem. 2006;13(1):87–98. [PubMed] [Google Scholar]
- 20.Guerrero RF, García-Parrilla MC, Puertas B, Cantos-Villar E. Wine, resveratrol and health: a review. Nat Prod Commun. 2009;4(5):635–58. [PubMed] [Google Scholar]
- 21.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–6. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 22.Criqui MH, Ringel BL. Does diet or alcohol explain the French paradox? Lancet. 1994;344(8939–8940):1719–23. doi: 10.1016/s0140-6736(94)92883-5. [DOI] [PubMed] [Google Scholar]
- 23.Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341(8843):454–7. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- 24.Natella F, Ghiselli A, Guidi A, Ursini F, Scaccini C. Red wine mitigates the postprandial increase of LDL susceptibility to oxidation. Free Radic Biol Med. 2001;30(9):1036–44. doi: 10.1016/s0891-5849(01)00504-4. [DOI] [PubMed] [Google Scholar]
- 25.Ursini F, Sevanian A. Postprandial oxidative stress. Biol Chem. 2002;383:599–605. doi: 10.1515/BC.2002.062. [DOI] [PubMed] [Google Scholar]
- 26.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 27.Švajger U, Jeras M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int Rev Immunol. 2012;31(3):202–22. doi: 10.3109/08830185.2012.665108. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Xia N, Förstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide. 2012;26(2):102–10. doi: 10.1016/j.niox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Gong Q, Dong H, Shi J. Resveratrol, a neuroprotective supplement for Alzheimer's disease. Curr Pharm Des. 2012;18(1):27–33. doi: 10.2174/138161212798919075. [DOI] [PubMed] [Google Scholar]
- 30.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 31.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 34.Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27(7):1465–74. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 35.Valacchi G, Weber SU, Luu C, Cross CE, Packer L. Ozone potentiates vitamin E depletion by ultraviolet radiation in the murine stratum corneum. FEBS Lett. 2000;466(1):165–8. doi: 10.1016/s0014-5793(99)01787-1. [DOI] [PubMed] [Google Scholar]
- 36.Valacchi G, Pagnin E, Okamoto T, Corbacho AM, Olano E, Davis PA, van der Vliet A, Packer L, Cross CE. Induction of stress proteins and MMP-9 by 0.8 ppm of ozone in murine skin. Biochem Biophys Res Commun. 2003;305(3):741–6. doi: 10.1016/s0006-291x(03)00812-x. [DOI] [PubMed] [Google Scholar]
- 37.DeLancey JO, Hannan LM, Gapstur SM, Thun MJ. Cigarette smoking and the risk of incident and fatal melanoma in a large prospective cohort study. Cancer Causes Control. 2011;22(6):937–42. doi: 10.1007/s10552-011-9766-z. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles SV. Smoking and pathogenesis of psoriasis: a review of oxidative, inflammatory and genetic mechanisms. Br J Dermatol. 2011;165(6):1162–8. doi: 10.1111/j.1365-2133.2011.10526.x. [DOI] [PubMed] [Google Scholar]
- 39.Holthoff JH, Woodling KA, Doerge DR, Burns ST, Hinson JA, Mayeux PR. Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem Pharmacol. 2010;80(8):1260–5. doi: 10.1016/j.bcp.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He X, Wang L, Szklarz G, Bi Y, Ma QJ. Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2-related factor 2 pathway. Pharmacol Exp Ther. 2012;342(1):81–90. doi: 10.1124/jpet.112.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kode A, Rajendrasozhan S, Caito S, Yang S, Megson IL. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Irfan Rahman American Journal of Physiology - Lung Cellular and Molecular Physiology. 294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 42.Graf R, Kock M, Bock A, Schubert–Zsilavecz M, Steinhilber D, Kaufmann R, Gassenmeier T, Beschmann H, Bernd A, Kippenberger S. Lipophilic prodrugs of amino acids and vitamin E as osmolytes for the compensation of hyperosmotic stress in human keratinocytes. Exp Dermatol. 2009;18(4):370–7. doi: 10.1111/j.1600-0625.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- 43.Micallef L, Belaubre F, Pinon A, Jayat-Vignoles C, Delage C, Charveron M, Simon A. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp Dermatol. 2009;18(2):143–51. doi: 10.1111/j.1600-0625.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- 44.Deyrieux AF, Wilson VG. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology. 2007;54(2):77–83. doi: 10.1007/s10616-007-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clément MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92(3):996–1002. [PubMed] [Google Scholar]
- 46.Carbó N, Costelli P, Baccino FM, López-Soriano FJ, Argilés JM. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem Biophys Res Commun. 1999;254(3):739–43. doi: 10.1006/bbrc.1998.9916. [DOI] [PubMed] [Google Scholar]
- 47.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 48.Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. 2013 doi: 10.1016/j.freeradbiomed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuruoka H, Khovidhunkit W, Brown BE, Fluhr JW, Elias PM, Feingold KR. Scavenger receptor class B type I is expressed in cultured keratinocytes and epidermis. Regulation in response to changes in cholesterol homeostasis and barrier requirements. J Biol Chem. 2002;277(4):2916–22. doi: 10.1074/jbc.M106445200. [DOI] [PubMed] [Google Scholar]
- 50.Paige DG, Morse-Fisher N, Harper JI. Quantification of stratum corneum ceramides and lipid envelope ceramides in hereditary ichthyoses. Br J Dermatol. 1994;131:23–27. doi: 10.1111/j.1365-2133.1994.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 51.Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J Invest Dermatol. 1991;96:523–526. doi: 10.1111/1523-1747.ep12470233. [DOI] [PubMed] [Google Scholar]
- 52.Mardones P, Strobel P, Miranda S, Leighton F, Quiñones V, Amigo L, Rozowski J, Krieger M, Rigotti A. Alphatocopherol metabolism is abnormal in scavenger receptor class B type I (SR-BI)- deficient mice. J Nutr. 2002;132(3):443–9. doi: 10.1093/jn/132.3.443. [DOI] [PubMed] [Google Scholar]
- 53.Chu S, Ferro TJ. Identification of a hydrogen peroxide-induced PP1-JNK1-Sp1 signaling pathway for gene regulation. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L983–92. doi: 10.1152/ajplung.00454.2005. [DOI] [PubMed] [Google Scholar]
- 54.Forman HJ, Dickinson DA, Iles KE. HNE--signaling pathways leading to its elimination. Mol Aspects Med. 2003;24:189–94. doi: 10.1016/s0098-2997(03)00013-x. [DOI] [PubMed] [Google Scholar]