Abstract
Dispersal is a critical life history behavior for mosquitoes and is important for the spread of mosquito-borne disease. We implemented the first stable isotope mark-capture study to measure mosquito dispersal, focusing on Culex pipiens in southwest suburban Chicago, Illinois, a hotspot of West Nile virus (WNV) transmission. We enriched nine catch basins in 2010 and 2011 with 15N-potassium nitrate and detected dispersal of enriched adult females emerging from these catch basins using CDC light and gravid traps to distances as far as 3 km. We detected 12 isotopically enriched pools of mosquitoes out of 2,442 tested during the two years and calculated a mean dispersal distance of 1.15 km and maximum flight range of 2.48 km. According to a logistic distribution function, 90% of the female Culex mosquitoes stayed within 3 km of their larval habitat, which corresponds with the distance-limited genetic variation of WNV observed in this study region. This study provides new insights on the dispersal of the most important vector of WNV in the eastern United States and demonstrates the utility of stable isotope enrichment for studying the biology of mosquitoes in other disease systems.
Author Summary
The distance and direction of adult mosquitoes movement on the landscape are important processes in the spread of mosquito-borne diseases, and are critical to understand to the development of effective intervention programs. Here we present a novel approach to study adult mosquito dispersal by using stable isotope enrichment of natural larval habitats. We apply this technique in a focal hotspot of West Nile virus (WNV) transmission in suburban, Chicago, USA to measure dispersal of Culex spp. mosquitoes. We enriched larval mosquitoes in residential catch basins using 15N-potassium nitrate and captured adult mosquitoes in traps surrounding these catch basins. Of 10,817 adult female Culex mosquitoes trapped and tested for stable isotopes, 12 individuals were enriched with 15N, indicating they originated from the catch basins receiving stable isotope amendments. The mean dispersal distance was 1.15 km and maximum flight range was 2.48 km. Ninety percent of the female Culex mosquitoes stayed within 3 km of their larval habitat, which corresponds with the distance-limited genetic variation of WNV observed in this study region. This study provides new insights on the dispersal of the most important vector of WNV in the eastern United States and demonstrates the utility of stable isotope enrichment for studying the biology of mosquitoes in other disease systems.
Introduction
The distance and direction of mosquito movement on the landscape are critical factors in the development of effective strategies for control of both nuisance and vector mosquito species. At small spatial scales, effective mosquito abatement using adult insecticides or larvicides needs to incorporate information on flight range of the intended mosquito target [1]. For example, when controlling Aedes aegypti, the vectors of dengue virus, insecticides are sprayed at homes of infected patients and in a specified proximity to the homes based on studies quantifying adult female dispersal [2]–[4]. Short range dispersal of Ae. aegypti has been quantified in dengue-endemic areas using genetic markers in relation to habitat structure, in particular presence of road networks, which act as barriers to mosquito dispersal and further influence the local distribution and risk of dengue cases in humans [5], [6]. At large spatial scales, mosquito movement has been implicated in shaping the geographical spread of West Nile virus (WNV) across North America [7], underscoring the importance of vector dispersal for shaping spatial patterns of disease transmission.
Currently, many alternative strategies to insecticides for vector-borne disease control are being implemented, including sterile insect technique, biological control using Wolbachia, and genetically modified mosquitoes (reviewed by [8]). For these disease control strategies to succeed and reduce the global burden of vector-borne diseases, a critical parameter necessary for field implementation of these strategies is the distance mosquitoes travel across the landscape. For example, control programs that release sterile, Wolbachia-infected, or genetically modified mosquitoes need detailed understanding of flight ranges to determine the appropriate spatial resolution of the release points. Simulation models of these intervention programs often incorporate parameters to represent adult mosquito dispersal [9], [10], although limited data on actual dispersal presents challenges to these models [11]. Given the importance of adult mosquito behavior, mosquito biologists have utilized mark-release-recapture studies for several decades to estimate mosquito dispersal distance and patterns.
Diverse methods have been used to mark mosquitoes to study dispersal including dyes, paints, dusts, trace elements, and radioactive isotopes (reviewed by [1]). The ideal insect marker should persist without inhibiting normal biology, be environmentally safe, cost-effective, and easy to use [12]. However, existing techniques to mark mosquitoes tend to be labor intensive, as they require rearing mosquitoes, marking them in large quantities, and then inspecting large numbers of individuals to detect re-captures [13]. Additionally, the process of rearing adults, marking them, and releasing them may change behavior compared to natural populations [1], [14]. Further, the artificial release of mosquitoes inflates local populations that may contribute to pathogen transmission; this has led to studies where the proboscis has been glued or amputated to prevent feeding [15], a process with potential consequences for mosquito behavior.
In 2008, a meeting of international experts in vector biology discussed critical needs in vector-borne disease control [16]. Among the research priorities highlighted, the panel listed improved technologies for marking insects for studying basic biology. To meet this challenge and to overcome limitations of previous techniques, Hamer et al. [17] developed a stable isotope method to mark naturally-occurring Culex pipiens. The laboratory and field experiments from this study suggested life-long marker retention in adults with no apparent impact on survival or body size. Stable isotopes occur naturally in the environment, are non-toxic and non-radioactive, and incorporate into living tissue, which make them safe and useful tracers [18]. Several studies have utilized stable isotopes to study dispersal of adult insects; 15N was added to streams, immature aquatic insects incorporated the rare isotope into structural body tissues, and then the emergent adult insects were captured at different distances from the enriched stream [19]–[21]. Additionally, mosquitoes have been enriched with stable isotopes in the context of Sterile Insect Technique programs [22], [23].
Here we report the first application of stable isotopes to study mosquito dispersal in natural field conditions. We enriched naturally existing larval Culex mosquitoes with 15N in catch basins in Alsip, Illinois and used a large network of traps to capture marked females. This study demonstrates that female Culex mosquitoes were capable of flying up to 2.4 km with a logistic distribution function suggesting that 90% of the female Culex mosquitoes stayed within 3 km of their larval habitat. We discuss the advantages and disadvantages of the stable isotope enrichment of natural larval habitats and demonstrate how this approach could be a valuable new tool to study dispersal of medically important mosquitoes around the world.
Methods
Stable isotope enrichment of catch basins
From July to October 2010 and 2011, we treated nine catch basins with 15N-potassium nitrate in Alsip, Illinois (41°41′14.56″N; 87°44′32.84″W). The catch basins are stormwater drains designed with a sump to prevent organic debris from entering pipes that lead to an outlet at a creek. The catch basins we treated were the terminal catch basins before water drained through an outlet into a small stream (Stony Creek; Figure 1). The maximum distance between treated catch basins was 153 m. We added stable isotopes with the quantity based on the amount of water present in the catch basin sumps [17]. Briefly, the volume of water in each catch basin sump was estimated under the assumption that sediment comprised 50% of the sump volume. Initial treatment began with a targeted enrichment of 2.0 mg of stable isotope per liter of water in the catch basin. In subsequent treatments that followed rain events, we reduced this amount to a half dose or quarter dose, depending on the amount of flushing that had occurred. In 2010, we delivered 15N-potassium nitrate into the 9 catch basins on seven occasions for a total of 38.58 grams being delivered. In 2011, we treated these same catch basins on 14 occasions for a total of 51.36 grams. Fourth instar larvae, pupae, and adult male and female Culex mosquitoes were subsampled from these catch basins and submitted for stable isotope analysis to monitor the level of enrichment and adjust the frequency and quantity of isotopic amendment accordingly.
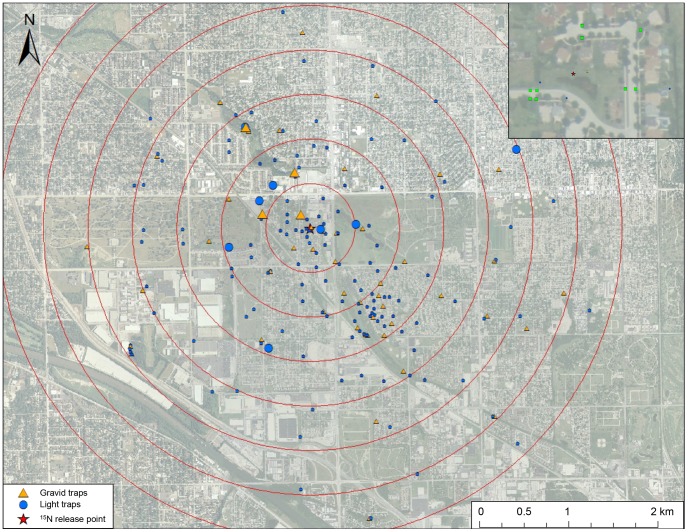
Figure 1. Map of 2010 and 2011 mark-capture study region in suburban Chicago, Illinois.
Gravid traps are orange triangles and light traps are blue circles and the larger trap symbols represent the 12 traps that captured marked Culex mosquitoes. Not all traps shown where trapped in both years. Red concentric rings represent the seven annuli used to calculate mean distance traveled. Inset shows nine catch basins enriched with 15N-potassium nitrate as green squares and the red star represents the mean center of these nine points.
We estimated the production of 15N-enriched mosquitoes from the amended catch basins using previously described emergence traps [24]. These traps were necessary because S-methoprene is used by mosquito control agencies in the study region and the presence of larvae or pupae does not necessarily reflect adult emergence. These standardized emergence traps covered a known surface area inside the catch basin and allowed an estimation of the total production of marked adults leaving the catch basin. In 2010 and 2011, we placed and continuously monitored three emergence traps in three of the treated catch basins from July to October. In addition, we estimated the number of Culex mosquitoes emerging from all nine catch basins per day from July to October [24].
To ensure that our enrichment activities were not affecting larval mosquitoes outside of our desired areas, we monitored down-stream enrichment by sampling immature mosquitoes and benthic invertebrates (Chironomidae, Amphipoda, Ephemeroptera, Coleoptera) in the Stony Creek; upstream, downstream, and at the outlet opening. We sampled these invertebrates on July 23 and August 31 in 2010 and on August 30 in 2011. Invertebrates from the creek were submitted for stable isotope testing and all δ15N values represented natural abundance levels (mean δ15N = 11.75) indicating no down-stream enrichment.
We obtained permission to add stable isotopes to the environment by the municipalities, Cook County Department of Public Health, Illinois Department of Public Health, and the Illinois Environmental Protection Agency.
Adult mosquito trapping
Mosquitoes were trapped from May to October, 2010 and 2011 in Alsip, Blue Island, Chicago, Chicago Ridge, and Oak Lawn, Illinois. We deployed CDC light traps at 100 different locations and gravid traps at 40 locations in 2010 and 83 light trap locations and 33 gravid trap locations in 2011 (Figure 1). The closest mosquito trap was 17.6 m from the centroid of the nine enriched catch basins and the farthest trap was 3.3 km. The mean trap distance from the centroid of the catch basins was 1.47 km and 0.89 km in 2010 and 2011, respectively. These trap locations were distributed in all directions from the catch basins and specific locations were dependent on obtaining permission from landowners. All locations were trapped once per week. Mosquitoes were identified by species and sex, and then pools of up to 50 female Culex spp. mosquitoes were tested for WNV using a quantitative RT-PCR [25]. Adult female Cx. pipiens and Cx. restuans collected in traps were pooled together as Culex spp. given the difficulty in distinguishing the two Culex species morphologically [26]. RNA was extracted using a MagMAX Viral Total RNA Isolation Kit (Applied Biosystems, Foster City, California). A subset of female Culex spp. mosquitoes were placed in pools of up to five individuals and prepared for stable isotope testing as previously described [17]. In 2010 and 2011, we deployed a Hobo weather station (Onset Computer Corporation, Pocasset, MA) placed 1.9 km from the amended catch basins. This station recorded hourly temperature, wind speed, wind direction, and precipitation. We calculated the average wind direction as a combined vector of the mean wind angle and speed [27]. The direction and speed were converted into N-S and E-W components and averaged over the July to September period. The average wind direction in 2010 was 216° (southwest) with a net speed of 1.9 kph and in 2011 was 170° (south) with a net speed of 0.66 kph.
Stable isotope analysis
All 4th instar larvae, pupae, adult mosquitoes, and other aquatic invertebrates were stored at −20°C and processed for stable isotope analysis as previously described [17]. Briefly, samples were dried at 50°C for 24 h, encapsulated into tin capsules to create a sphere, arranged into a 96-well plate, and submitted for stable isotope analysis at the University of California-Davis Stable Isotope Facility using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20Ð20 isotope ratio mass spectrometer (IRMS; Sercon Ltd., Cheshire, United Kingdom). Additional analyses, that required short turn-around of results in order to guide field enrichment activities, were performed by Isotech Laboratories Inc. by using a Carlo Erba CHNS-O EA1108 (CE Instruments, Milan, Italy) coupled to a ThermoFisher Delta V Plus IRMS (Thermo Fisher ScientiÞc, Bremen, Germany) via a ThermoFinnigan ConFlo III interface (Thermo Electron Corp., Waltham, MA).
Statistical analysis
Mosquito pools collected in the field and tested for stable isotopes were considered enriched if δ15N values were at least three standard deviations above the natural isotopic abundance of Culex mosquitoes in our study region [28]. We calculated the mean distance traveled (MDT) and incorporated a correction factor for each annulus given unequal trap density [29]–[32]. We calculated MDT for each year based on the equations in [33].
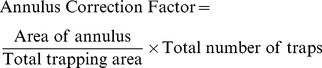 |
The estimated recaptures (ER) for each annulus were calculated as:
The mean distance traveled (MDT) was calculated as:
To estimate the probability of detecting a marked mosquito at different distances from the release point, we used a logistic regression in Program R [34]. Similar dispersion models have been used for relating the distance insects travel from a central point [35]. The data from two years were merged and each trap location (n = 167) was given a 1 for detecting a marked individual or a zero for no marked individuals. We used the centroid of the 9 catch basins receiving stable isotopes to calculate the distance from each light or gravid trap. The logistic distribution function of plogis (x) = (1+tanh (x/2))/2 was used for the predictions of detecting a marked mosquito at different distances from the release point.
Results
Stable isotope enrichment of catch basins
During the enrichment of nine catch basins receiving 15N-potassium nitrate in 2010 and 2011, a subsample of 4th instar larvae and pupae were collected and all were identified as Culex pipiens or Culex restuans. These immature specimens collected directly from the treated catch basins had a mean δ15N of 484.1±73.1 (n = 73) while immature Culex pipiens collected from nearby untreated catch basins had a mean δ15N of 4.7±0.74 (n = 15).
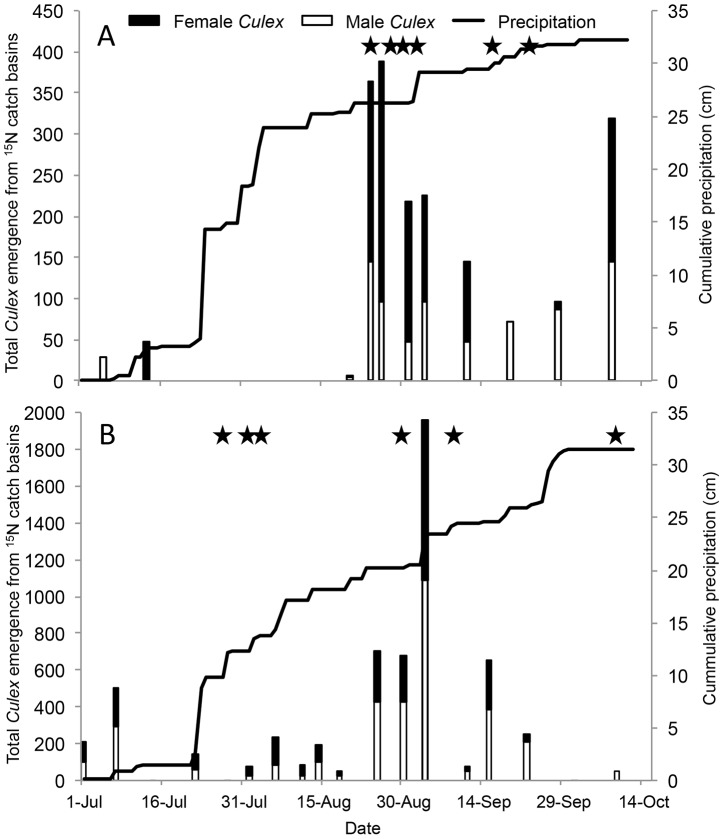
Using emergence traps, we estimated that the nine enriched catch basins produced 1,138 female and 769 male Culex spp. mosquitoes in 2010 and 2,624 female and 3,362 male Culex spp. mosquitoes in 2011 from July to October (Figure 2). The emergence of adults from the catch basins declined following large rain events (e.g. greater than 1 cm per day [24]) and the capture of marked pools from traps tended to occur following increased periods of emergence. A total of 343 larvae collected in catch basins or adults collected in emergence traps were identified to species during the two years and 333 (97%) were Cx. pipiens and 10 (3%) were Cx. restuans.
Figure 2. Emergence of Culex spp. mosquitoes from the nine catch basins receiving 15N enrichment in suburban Chicago in 2010 (A) and 2011 (B).
Stars represent the dates when 15N-enriched female Culex pools were captured in traps.
Adult mosquito trapping
In 2010, we collected 271,594 female mosquitoes of which 30,261 were Culex spp. mosquitoes (11.1%). Of the 2,255 Culex spp. mosquito pools (23,068 individuals) tested for WNV, 166 pools were positive with a peak infection rate of 42.6 per 1000 individuals (95% CI of 27.9–63.3) at the end of August. In 2011, we collected 227,036 individual mosquitoes of which 15,263 were Culex spp. mosquitoes (6.7%). Of the 1,954 Culex spp. mosquito pools (11,639 individuals) tested for WNV, 6 pools were positive with a peak infection rate of 2.31 per 1000 individuals (95% CI of 0.4–7.6) occurring in mid-August.
In 2010, 1,529 female Culex spp. mosquito pools (7,193 individuals) were collected and tested for stable isotopes and 6 pools were enriched (Figure 1). In 2011, 913 female Culex spp. mosquito pools (3,624 individuals) were collected and tested for stable isotopes and 6 pools were enriched. The 12 marked pools had a mean δ15N of 285.4±198.4. The mean δ15N of all un-enriched female Culex spp. mosquito pools was 6.6±0.04. Based on the estimated number of enriched female Culex mosquitoes emerging from the treated catch basins, we obtained a re-capture rate of 0.52% in 2010 and 0.23% in 2011, under the simplifying assumption that marked pools contained only one marked individual. The MDT for 2010 was 1.44 km and 2011 was 0.86 km. The closest trap containing a marked mosquito was 123.9 m from the release point and the farthest was 2.48 km (mean = 0.9 km, S.E. = 0.19). The marked female mosquito captured at 2.48 km occurred on September 22, 2010 in a trap with a 68 degree bearing from the release point. During the previous night before this female was captured (8pm to 8am) there was a mean wind speed of 3.3 km per hour (gusts up to 16.7 km per hour) with a mean bearing of 254 degrees (WSW at 74 degrees), which is in the direction of the trap that captured the marked female mosquito.
For the two years combined, the probability of detecting a marked mosquito at different distances from the release point was estimated using a logistic distribution function of y = (1+tanh((−0.76*x – 1.74)/2))/2 (Figure 3). Based on this model, 80% of the marked mosquitoes stayed within 2.1 km of the release point and 90% stayed within 3 km.
Figure 3. Probability of detecting a marked female Culex spp. mosquito at different distances from larval origin.
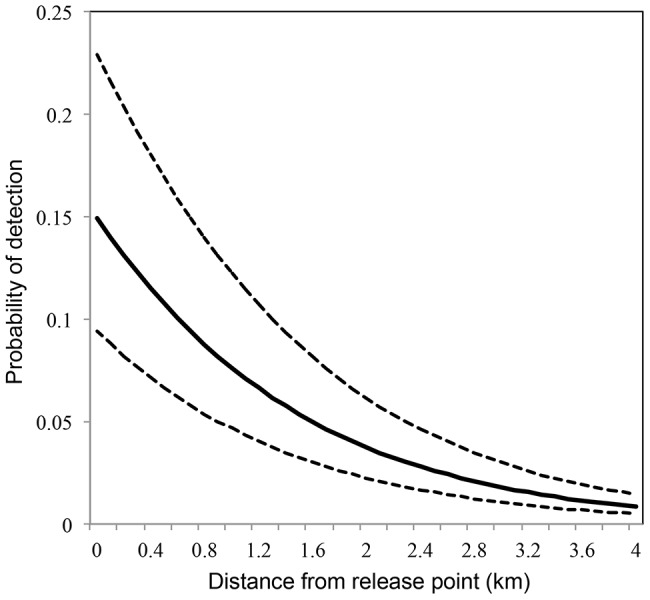
The prediction function is equal to (1+tanh((−0.76*x – 1.74)/2))/2. Dashed lines represent standard error.
Discussion
This study used stable isotope enrichment to measure the dispersal of Culex spp. mosquitoes in an urban hot spot of WNV transmission. The majority of the dispersal studies of Culex mosquitoes to date have focused on Cx. quinquefasciatus or Cx. tarsalis (reviewed by [1]) and there are very few dispersal studies for Cx. pipiens in the U.S. Given the importance of Cx. pipiens as a primary enzootic vector of WNV in the eastern half of the U.S. north of 36° latitude [36], [37], the paucity of data is unfortunate. One exception is a mark-release-recapture study by Jones et al. [38] conducted on Cx. pipiens, using fluorescent dust near Washington, DC. However, this study was primarily designed to study survival. A second study by Ciota et al. [39] analyzed dispersal of Cx. pipiens in New York using a novel labeling approach that allowed mosquitoes emerging from natural container habitats to self-mark with fluorescent dust. Ciota et al. [39] report a MDT of 1.37 km for Cx. pipiens (maximum flight range of 1.98 km) and the current study reports a MDT of 1.15 km (maximum flight range of 2.48 km), suggesting similar estimates between the two studies. Given the low detection probability of capturing a marked mosquito at large distances from the point of origin, these studies emphasize the potential for female Cx. pipiens to travel several kilometers from larval habitats of origin. However, the current dispersal estimates should be cautiously interpreted given the limited capture of marked female Cx. pipiens mosquitoes in the Ciota et al. [39] study (n = 10) and in the current study (n = 12). Future studies with designs that capture more marked mosquitoes will reduce the uncertainty of such estimates.
The mosquito dispersal documented in the current study is of direct relevance to the enzootic transmission of WNV and “spillover” to humans. From 2002 to 2012, the study region defined by the seven annuli contained the geocoded addresses of 57 human cases of WNV (Illinois Department of Public Health). Culex emerging from catch basins represent the same population that are part of the enzootic cycle feeding on birds [25] and are very likely also responsible for the bridge transmission to humans [40]. The study region has a radius of 3.5 km and includes 8 municipalities (Alsip, Oak Lawn, Chicago Ridge, Worth, Chicago, Palos Heights, Blue Island, and Midlothian), in which mosquito control efforts vary considerably. Our estimates that nearly 20% of Culex mosquitoes were able to travel over 2 km from their larval environment demonstrates that the mosquito control efficacy in one small municipality can affect the level of WNV transmission and of risk of human exposure in adjacent regions. Moreover, Bertolotti et al. [41] studied the fine-scale genetic variation of WNV in this suburban Chicago study region and found significant negative spatial autocorrelation at distances beyond 4 km. This evidence of distance-limited viral transmission corresponds well with the distance female Culex mosquitoes moved in the current study.
The stable isotope marking technique offers advantages and disadvantages over traditional mosquito dispersal studies. The ability to mark wild mosquitoes in natural containers with a non-invasive marker is an ideal approach to avoid artifacts of the marker or unnatural larval diet that may influence dispersal behavior [1], [12]. Additionally, our previous laboratory experiment revealed low decay rates of 15N in mosquitoes held for 55 days post-emergence [17]. Based on the enrichment achieved in the field during the current study, the stable isotope marker should offer sufficient retention for the life of the mosquito, which is fortunate given the significance of old females for disease transmission. The advantage of the larval site label also comes with the disadvantage of not being able to control how many marked mosquitoes emerge. In the current study, the relatively wet summers of 2010 and 2011 compromised this study given that the rain events washed the water and larvae out of the catch basins (Fig. 2; [24], [42]. Additionally, local mosquito control efforts used S-methoprene based products to treat mosquitoes in the same catch basins we were enriching with stable isotopes, although the immediate effect on the study was not quantified.
Another challenge of the larval site labeling approach is that the release of marked mosquitoes is over a prolonged time period and is thus not a defined release event. This confounds the effort to determine the age of the captured marked mosquitoes. Although this study estimated the number of marked mosquitoes emerging from the treated catch basins, the uncertainty associated with this estimate limits the ability to estimate the size of the adult mosquito population [1]. Another factor to consider with isotopic enrichment of mosquito larval environments in the field is the potential for downstream enrichment. In our case, we received many rain events during the study period, so dissolved potassium nitrate or microbes enriched with 15N would have washed into the Stony Creek at the catch basin outlets. However, we monitored aquatic invertebrates in these downstream areas and none were enriched, likely indicating that the large volume of water in the stream diluted the 15N to a concentration that was unable to bioaccumulate into the food chain and into invertebrates measurably.
The stable isotope mark-capture study offers a unique perspective on mosquito dispersal and should broadly be useful for other mosquito-borne disease systems. Aedes aegypti, responsible for an estimated 50–100 million annual human cases of dengue virus [43], is a container-breeding mosquito ideally suited for applying this stable isotope mark-capture study. Although Ae. aegypti is generally characterized as having limited dispersal [1], [44], the unique ability of life-long marker retention might yield a unique perspective on Ae. aegypti movement. This is especially important given current techniques of gene-driving and Wolbachia-induced population suppression or reduced vector competence aimed at the global elimination of dengue virus [8]. Understanding the distance between Anopheles spp. larval habitat and human exposure to malaria could improve control programs and mitigate disease transmission. Importantly, the enrichment of larval mosquito environments with stable isotopes is relatively inexpensive and easy to implement. Adult mosquitoes captured in traps need to be kept frozen or dried prior to the stable isotope analysis, either of which would be possible in remote field locations with limited facilities. Besides the labor and consumables to run mosquito traps, the most expensive aspect of this kind of project is the stable isotope analysis. Our project collected 2,442 pools between the two years that were tested at $8 per sample totaling $19,536. Different stable isotope facilities charge variable amounts, and the cost per sample is dropping [18]. Given the ability to measure mosquito dispersion, including epidemiologically important old females, this tool should be useful for studying the dispersal behavior of other medically important arthropods.
Acknowledgments
We thank the many municipalities, cemeteries, and private home owners for granting us permission to conduct this study. Timothy Thompson, Diane Gohde, Patrick Kelly, Carl Hutter, Marija Gorinshteyn, Zach Allison, and Mike Glester provided field assistance and Garrett Barry, Erica Brown, Geoffrey Grzesiak, and Monica MacDonald provided laboratory assistance. We appreciate the assistance from Rebecca Hood-Nowotny for helping to develop the stable-isotope mark-capture methodologies. We appreciate the constructive feedback from three anonymous reviewers.
Funding Statement
This project was supported by the National Science Foundation and National Institutes of Health Ecology of Infectious Disease program under Award No. 084040, NIAID grant R37AI21884, and the Michigan Agricultural Experiment Station and the Agricultural Research Service, multistate project NE-507. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Silver JB (2008) Mosquito Ecology: Field Sampling Methods. Springer. [Google Scholar]
- 2. Garcia-Rejon J, Lorono-Pino MA, Farfan-Ale JA, Flores-Flores L, Rosado-Paredes ED, et al. (2008) Dengue virus-infected Aedes aegypti in the home environment. Am J Trop Med Hyg 79: 940–950. [PubMed] [Google Scholar]
- 3. Eisen L, Beaty BJ, Morrison AC, Scott TW (2009) Proactive vector control strategies and improved monitoring and evaluation practices for dengue prevention. J Med Entomol 46: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 4. Chang AY, Parrales ME, Jimenez J, Sobieszczyk ME, Hammer SM, et al. (2009) Combining Google Earth and GIS mapping technologies in a dengue surveillance system for developing countries. Int J Health Geogr 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hemme RR, Thomas CL, Chadee DD, Severson DW (2010) Influence of urban landscapes on population dynamics in a short-distance migrant mosquito: evidence for the dengue vector Aedes aegypti . PLoS Negl Trop Dis 4: e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahabir RS, Severson DW, Chadee DD (2012) Impact of road networks on the distribution of dengue fever cases in Trinidad, West Indies. Acta Trop 123: 178–183. [DOI] [PubMed] [Google Scholar]
- 7. Venkatesan M, Rasgon JL (2010) Population genetic data suggest a role for mosquito-mediated dispersal of West Nile virus across the western United States. Mol Ecol 19: 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGraw EA, O'Neill SL (2013) Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol 11: 181–193. [DOI] [PubMed] [Google Scholar]
- 9. Okamoto KW, Robert MA, Lloyd AL, Gould F (2013) A reduce and replace strategy for suppressing vector-borne diseases: insights from a stochastic, spatial model. PLoS One 8: e81860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magori K, Legros M, Puente ME, Focks DA, Scott TW, et al. (2009) Skeeter Buster: a stochastic, spatially explicit modeling tool for studying Aedes aegypti population replacement and population suppression strategies. PLoS Negl Trop Dis 3: e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SS, Baker RE, Gaffney EA, White SM (2013) Optimal barrier zones for stopping the invasion of Aedes aegypti mosquitoes via transgenic or sterile insect techniques. Theor Ecol-Neth 6: 427–442. [Google Scholar]
- 12. Hagler JR, Jackson CG (2001) Methods for marking insects: Current techniques and future prospects. Annu Rev Entomol 46: 511–543. [DOI] [PubMed] [Google Scholar]
- 13. Walker ED, Copeland RS, Paulson SL, Munstermann LE (1987) Adult survivorship, population density, and body size in sympatric populations of Aedes triseriatus and Aedes hendersoni (Diptera, Culicidae). J Med Entomol 24: 485–493. [DOI] [PubMed] [Google Scholar]
- 14. Reisen WK, Lothrop HD, Lothrop B (2003) Factors influencing the outcome of mark-release-recapture studies with Culex tarsalis (Diptera : Culicidae). J Med Entomol 40: 820–829. [DOI] [PubMed] [Google Scholar]
- 15. Honorio NA, Silva WD, Leite PJ, Goncalves JM, Lounibos LP, et al. (2003) Dispersal of Aedes aegypti and Aedes albopictus (Diptera : Culicidae) in an urban endemic dengue area in the State of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 98: 191–198. [DOI] [PubMed] [Google Scholar]
- 16. Luckhart S, Lindsay SW, James AA, Scott TW (2010) Reframing critical needs in vector biology and management of vector-borne disease. PLoS Negl Trop Dis 4: e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamer GL, Donovan DJ, Hood-Nowotny R, Kaufman MG, Goldberg TL, et al. (2012) Evaluation of a stable isotope method to mark naturally-breeding larval mosquitoes for adult dispersal studies. J Med Entomol 49: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hood-Nowotny R, Knols BGJ (2007) Stable isotope methods in biological and ecological studies of arthropods. Entomol Exp Appl 124: 3–16. [Google Scholar]
- 19. Hershey AE, Pastor J, Peterson BJ, Kling GW (1993) Stable isotopes resolve the drift paradox for baetis mayflies in an arctic river. Ecology 74: 2315–2325. [Google Scholar]
- 20. Briers RA, Gee JHR, Cariss HM, Geoghegan R (2004) Inter-population dispersal by adult stoneflies detected by stable isotope enrichment. Freshw Biol 49: 425–431. [Google Scholar]
- 21. Macneale KH, Peckarsky BL, Likens GE (2005) Stable isotopes identify dispersal patterns of stonefly populations living along stream corridors. Freshw Biol 50: 1117–1130. [Google Scholar]
- 22. Hood-Nowotny R, Mayr L, Knols BGJ (2006) Use of carbon-13 as a population marker for Anopheles arabiensis in a sterile insect technique (SIT) context. Malar J 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Helinski MEH, Hood RC, Gludovacz D, Mayr L, Knols BGJ (2008) A N-15 stable isotope semen label to detect mating in the malaria mosquito Anopheles arabiensis Patton. Parasite Vector 1: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamer GL, Kelly PH, Focks DA, Goldberg TL, Walker ED (2011) Evaluation of a novel emergence trap to study Culex mosquitoes in urban catch basins. J Am Mosq Control Assoc 27: 142–147. [DOI] [PubMed] [Google Scholar]
- 25. Hamer GL, Walker ED, Brawn JD, Loss SR, Ruiz MO, et al. (2008) Rapid amplification of West Nile virus: The role of hatch-year birds. Vector-Borne Zoonotic Dis 8: 57–67. [DOI] [PubMed] [Google Scholar]
- 26. Harrington LC, Poulson RL (2008) Considerations for accurate identification of adult Culex restuans (Diptera : Culicidae) in field studies. J Med Entomol 45: 1–8. [DOI] [PubMed] [Google Scholar]
- 27.Burt S (2012) The Weather Observer's Handbook. Cambridge University Press. 456 p. [Google Scholar]
- 28.Internation Atomic Energy Agency (2009) Manual for the use of stable isotopes in entomology. Vienna, Austria. pp. 69. [Google Scholar]
- 29. Brenner RJ, Wargo MJ, Stains GS, Mulla MS (1984) The dispersal of Culicoides mohave (Diptera, Ceratopogonidae) in the desert of southern California. Mosq News 44: 343–350. [Google Scholar]
- 30. Lillie TH, Marquardt WC, Jones RH (1981) The flight range of Culicoides variipennis (Diptera, Ceratopogonidae). Can Entomol 113: 419–426. [Google Scholar]
- 31. Lillie TH, Kline DL, Hall DW (1985) The Dispersal of Culicoides-Mississippiensis (Diptera, Ceratopogonidae) in a Salt-Marsh near Yankeetown, Florida. J Am Mosq Control Assoc 1: 463–467. [PubMed] [Google Scholar]
- 32. White DJ, Morris CD (1985) Bionomics of anthropophilic Simuliidae (Diptera) from the Adirondack Mountains of New York State, USA. 1. Adult dispersal and longevity. J Med Entomol 22: 190–199. [DOI] [PubMed] [Google Scholar]
- 33. Morris CD, Larson VL, Lounibos LP (1991) Measuring mosquito dispersal for control programs. J Am Mosq Control Assoc 7: 608–615. [PubMed] [Google Scholar]
- 34.R Development Core Team (2012) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 35. Freeman GH (1977) Model relating numbers of dispersing insects to distance and time. J Appl Ecol 14: 477–487. [Google Scholar]
- 36. Barr AR (1957) The distribution of Culex p. pipiens and C. p. quinquefasciatus in North America. Am J Trop Med Hyg 6: 153–165. [DOI] [PubMed] [Google Scholar]
- 37. Petersen LR, Brault AC, Nasci RS (2013) West Nile virus: review of the literature. JAMA - J Am Med Assoc 310: 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones CE, Lounibos LP, Marra PP, Kilpatrick AM (2012) Rainfall influences survival of Culex pipiens (Diptera: Culicidae) in a residential neighborhood in the Mid-Atlantic United States. J Med Entomol 49: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ciota AT, Drummond CL, Ruby MA, Drobnack J, Ebel GD, et al. (2012) Dispersal of Culex Mosquitoes (Diptera: Culicidae) from a wastewater treatment facility. J Med Entomol 49: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, et al. (2008) Culex pipiens (Diptera : Culicidae): A bridge vector of West Nile virus to humans. J Med Entomol 45: 125–128. [DOI] [PubMed] [Google Scholar]
- 41. Bertolotti L, Kitron UD, Walker ED, Ruiz MO, Brawn JD, et al. (2008) Fine-scale genetic variation and evolution of West Nile Virus in a transmission “hot spot” in suburban Chicago, USA. Virology 374: 381–389. [DOI] [PubMed] [Google Scholar]
- 42. Gardner AM, Hamer GL, Hines AM, Newman CM, Walker ED, et al. (2012) Weather variability affects abundance of larval Culex (Diptera: Culicidae) in storm water catch basins in Suburban Chicago. J Med Entomol 49: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weaver SC, Reisen WK (2009) Present and future arboviral threats. Antiviral Res 85: 328–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, et al. (2005) Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg 72: 209–220. [PubMed] [Google Scholar]