Abstract
Objectives
Chronic infection with oncogenic HPV genotype is associated with the development of anal dysplasia. Antiretroviral therapy (ART) has been shown to decrease the incidence of cervical carcinoma in women with HIV. We sought to: 1) describe the prevalence and grade of anal dysplasia and HPV infection in our study subjects; 2) analyze the grade of correlation between anal cytology, PCR of high-risk HPV, and histology; 3) identify the factors associated with the appearance of ≥AIN2 lesions.
Design
Cross-sectional, prospective study.
Methods
A cohort of HIV-positive males (n = 140, mean age = 37 years) who have sex with males (MSM) had epidemiological, clinical and analytical data collected. Anal mucosa samples were taken for cytology, HPV PCR genotyping, and anoscopy for histological analysis.
Results
Within the cohort, 77.1% were being treated with ART, 8.5% anoscopy findings were AIN2, and 11.4% carcinoma in situ; 74.2% had high-risk (HR), 59.7% low-risk (LR) HPV genotypes and 46.8% had both. The combination of cytology with PCR identifying HR-HPV better predicts the histology findings than either of these factors alone. Logistic regression highlighted ART as a protective factor against ≥AIN2 lesions (OR: 0.214; 95%CI: 0.054–0.84). Anal/genital condylomas (OR: 4.26; 95%CI: 1.27–14.3), and HPV68 genotype (OR: 10.6; 95%CI: 1.23–91.47) were identified as risk factors.
Conclusions
In our cohort, ART has a protective effect against dysplastic anal lesions. Anal/genital warts and HPV68 genotype are predictors of ≥AIN2 lesions. Introducing PCR HPV genotype evaluation improves screening success over that of cytology alone.
Introduction
Over the last few decades there has been an increase in the incidence of anal cancer due, in large part, to the increase in risk groups such as males who have sex with males (MSM), the immunocompromised and, especially, patients with HIV infection [1].
The incidence of anal cancer in HIV-positive patients varies between 40 and 137 per 100,000 person/years, which is much higher than in the general seronegative population, with a predominance of males with AIDS staging [2], [3]. One of the principal risk factors associated with the appearance of this neoplasm is the chronic infection with high-risk genotypes of the human papilloma virus (HR-HPV), also termed oncogenic genotypes [4]. This infection in HIV patients is favored by specific factors such as low levels of CD4 [5], previous chlamydia infection, smoking habit [6] or being MSM [1]. Anal cancer has a considerable similarity with cancer of the cervix in terms of the different stages and natural history of the disease, such as the initial process of acquiring the HPV infection, followed by the persistence of the virus in the mucosa that favors the progression to high-grade anal intraepithelial neoplasia (HGAIN) and, subsequently, to invasive cancer [7]. Studies conducted in HIV-positive patients show a progression in the premalignant lesions of anal carcinoma from AIN1 to AIN2-3 in 12.8 cases/1000 patient-months [8]. In seronegative patients, the progression from AIN1 to AIN 2-3 is estimated at 62% within approximately 24 months, and that the progression from AIN3 to invasive carcinoma is around 9-13% within 5 years [9].
Anal cancer in HIV patients is currently considered one of the most-frequent non-AIDS-defining malignancies [2]. Several studies confirm its greater prevalence compared to the seronegative population and the appearance, according to the majority of authors, has not diminished despite anti-retroviral therapy (ART) [10], [11], [12], [13]; except in the case of the Swiss cohort study in which a reduction of this neoplasia was detected around the beginning of the late period of ART between the years 2002–2006 [14]. Along the same lines, there have been 2 studies on the benefits of ART in HIV-MSM patients not only in the progression of the pre-malignant anal lesions (derived from a Canadian cohort), but also in the prevalence of these lesions (derived from a Dutch transversal study) [15]. Finally, of note is that ART in HIV-positive women has demonstrated reduction in the incidence of cancer of the cervix [16], regression of dysplastic lesions, and elimination of the HPV genotypes [17].
Hence, we proposed as principal objective: 1) to describe the prevalence and grade of anal dysplasia, and of the infection by HPV, in our cohort of HIV-positive MSM patients; and as secondary objectives: 2) to analyze the degree of correlation between anal cytology and PCR of HR-HPV with the histology findings from biopsy and 3) to study the factors associated with the appearance of ≥AIN2 lesions in our cohort. The factors analyzed included that of ART.
Patients and Methods
Design
Cross-sectional study conducted between May 2010 and Sept. 2013 in a cohort of 140 patients with HIV-MSM recruited consecutively into a program of screening, diagnosis, treatment and follow-up of dysplastic lesions of the anal mucosa. The HIV-positive patients were from among those receiving attention in the Infectious Disease Unit of the Hospital Universitario Virgen de las Nieves (Granada, Spain) the ethics committee of which approved the study.
The inclusion criteria were: adult (≥18 years of age) MSM infected with HIV. The exclusion criteria were: females, heterosexual HIV-positive males, and history of anal canal neoplasia at the time of recruitment into the study.
The objectives and conditions of the study were explained to the patients at the first clinical visit, and written informed consent was obtained (as outlined in PLoS consent form). Clinical, epidemiological and analytical data were obtained and codified for anonymity according to the laws on protection of personal information currently in existence in Spain.
The variables collected were: age, history of perianal or genital condylomatosis, number of different sexual partners in the previous 12 months, condom use in sexual intercourse, tobacco use, alcohol use (standard units of consumption; SUC), intravenous drug abuse (IDA), HIV acquisition route, months since HIV diagnosis, HIV stage according to the CDC Atlanta; months on ART, virologic treatment failure (when the viral mRNA was >50 copies/mL in at least 2 measurements within the previous 6 months), use of concomitant treatments, other infections including chronic hepatitis B and C (HBV and HCV, respectively), luetic positive serum (syphilis), other sexually transmitted diseases (STD), latent tuberculosis or tuberculosis under treatment or active infection.
The laboratory analytes measured included: lymphocyte number, CD4 nadir, CD4, CD8, and viral load at the time of HIV diagnosis together with CD4, CD8 and viral load at the time of inclusion into the study.
At the clinical visit, 2 mucosa samples were taken from the anal canal with cotton swabs soaked in physiologic saline, and stored in liquid medium (thin layer liquid) for the detection and genotyping of the HPV using the polymerase chain reaction (PCR) technique (GeneAmp PCR System 9700, Applied Biosystems, Roche), and for cytology using the ThinPrep Pap Test (Thin Prep Processor 2000, Hologic Corp). Both samples were sent to the anatamo-pathology laboratory where the same senior pathologist of the research team (JE) carried out the cytology evaluation, validation of the PCR, and histology analyses. The genotypes 16, 18, 26, 31, 33, 35, 39, 45, 51–53, 56, 58, 59, 66, 68, 73 and 82 were considered high risk (HR-HPV). Genotypes 6, 11, 34, 40, 42–44, 54, 55, 57, 61, 70–72, 81, 83, 84 and 89 were considered low risk (LR-HPV) [18].
Subsequently, anoscopy by the digestive tract specialist of the research team (MLdeH) was performed within an interval of between 4 and 12 weeks from the cytology assessment. A standard endoscope of 9 mm with working channel of 2.8 mm was used, without any image enhancement. A short exploration of 15–20 cm was performed with retrovision maneuver to better visualize the pectinea line. Samples were taken for histology examination using an endoscopic retrograde cholangio-pancreatography (ERCP) catheter not only from the lesions (color change with irrigation with 5% acetic acid), but also from other parts of the quadrant with ostensibly-normal mucosa.
The cytology classification was that of Bethesda [19] which classifies the lesions into 3 types: atypical squamous cells (ASC), low-grade squamous intraepithelial lesions (LSIL) and high-grade squamous intraepithelial lesions (HSIL).
The histology classification employed divides the lesions into LSIL (AIN1/condyloma), HSIL (AIN2, AIN3/Carcinoma in situ), and invasive carcinoma [20]. We considered lesions ≥AIN1 those that spanned AIN1 to carcinoma in situ. We considered lesions ≥AIN2 those that proceeded beyond AIN2 to carcinoma in situ.
Statistical analyses
Sample size
To achieve a precision of 8% in the calculation of a rate using Normal asymptotic bilateral confidence interval of 95% assuming that the prevalence of infection and of any grade of anal dysplasia is 70%, it would be necessary to include 126 subjects in the study. With an expected loss of 10%, it would be necessary to recruit 140 patients.
Descriptive analyses
The general description of the principal variables included central tendency and dispersion (mean, standard deviation, median, percentiles) for the quantitative variables, and the absolute frequencies with 95% confidence intervals (95%CI) for the qualitative variables. The prevalence and 95%CI were calculated for HPV, dysplasias obtained from cytology, and dysplasias obtained from the histology evaluation. Diagnostic success, not only from cytology but also from PCR of HR-HPV of dysplasias that included lesions ≥AIN1 and ≥AIN2, was defined by the receiver operating characteristics (ROC) curves. The results were considered poor: 0.5 to 0.6, acceptable: 0.6 to 0.75, good: 0.75 to 0.9, very good: 0.9 to 0.97, and excellent 0.97 to 1. The degree of concordance between cytology, PCR of HR-HPV, and biopsy results was analyzed using the Kappa index. The results of the test were evaluated using the classification of Landis and Koch in which a value of k<0.20 would be considered poor; 0.21 to 0.40 weak; 0.41 to 0.60 moderate; 0.61 to 0.80 good; and 0.81 to 1.00 very good [21].
Bivariate analyses
We used bivariate analyses to assess the relationship between the possible risk factors and the presence of dysplastic lesions ≥AIN2. The Student t-test for independent samples was applied for quantitative variables that followed a normal distribution, while the Mann-Whitney test was employed for those variables that did not follow normal distributions. The Kolmogorov-Smirnov test was used to assess whether the different variables fulfilled the criteria of normal distribution. Comparison of differences between variables was with the Pearson χ2 test, or the Fisher exact test if the application criteria were not fulfilled.
Multivariate analyses
Logistic multivariate regression was applied based on the classic formula of Freeman [n = 10*(k+1)], [22]. Included in the model were the results that were statistically significant in the bivariate analyses, as well as those considered clinically relevant. Variables were introduced into the analyses manually one by one while leaving out those variables that did not modify the model so as, finally, to construct a model with those variables that did have a modifying effect (ART, current perianal condylomas, clinical history of syphilis, HPV 68, AIDS stage, duration of HIV, duration of ART, number of HR-HPV genotypes in anal mucosa, CD4 nadir, CD4 nadir <200 cells/uL). A method of selection using successive steps was employed considering, in each step, a probability of entry of 0.05 and that of 0.10 for exit. The Hosmer-Lemeshow test was employed to assess the goodness of adjustment for the logistic regression model.
A value of p<0.05 was considered statistically significant in all the analyses. The statistical software used was the SPSS (version 15.0).
Results
Outcomes data and details of methods are available from the corresponding author, on request.
1. Characteristics of the cohort of HIV-MSM patients
Included were 140 patients, mean age 37 years, CD4 nadir 356 cells/μL, median time of clinical evolution of the HIV infection around 33 months (IQR: 11–84), 78% on treatment with combination anti-retroviral therapy (ARTc) over a median period of 23.5 months at which time there were 652 cells/μL CD4, and only 6% in virologic treatment failure The characteristics are summarized in Table 1.
Table 1. General description of the patient cohort, and results of the PCR of HPV, cytology and anoscopy.
| Characteristics | Patients MSM-HIV; n = 140 |
| Mean age; years (± SD) | 37.27 (±8.9) |
| Nationality: Spanish; n (%) | 133 (95) |
| Retired; n (%) | 8 (5.8) |
| Education level; n (%) | |
| University | 73 (52.2) |
| Secondary school | 47 (33.6) |
| Primary school | 19 (13.6) |
| None | 1 (0.7) |
| Median number of partners over previous 12 months (IQR) | 1 (1–6.75) |
| Habitually using condoms; n (%), (95%CI) | 109 (77.9), (71–81) |
| Perianal/genital condylomatosis; n (%), (95%CI) | 42 (30), (20–36) |
| History of condylomas; n(%), (95%CI) | 50 (35.7), (28–44) |
| Median duration of HIV; months, (IQR) | 33 (11–84) |
| Mean VL of HIV (log), (±SD) | 3.83 (±4.33) |
| CD4 (cells/μL), (±SD) | 652.87 (±261.71) |
| CD8 (cells/μL), (±SD) | 1431.07 (±4366.35) |
| CD4 nadir (cells/μL), (±SD) | 356.29 (±246.92) |
| AIDS stage (A3, B3, C); n (%), (95%CI) | 46 (32.9), (27–43) |
| Receiving ART; n (%), (95%CI) | 108 (77), (70–84) |
| Number of months of ART; mean (IQR) | 23.5 (8–80.3) |
| Virological treatment failure; n (%), (95%CI) | 7 (6), (2–10) |
| History of syphilis treated; n (%), (95%CI) | 26 (18.6), (12–26) |
| Other STD; n (%), (95%CI) | 56 (40), (32–50) |
| Latent tuberculosis treated; n (%) | 17 (12.5) |
| Chronic HCV infection; n (%) | 6 (4.3) |
| Chronic HBV infection; n (%) | 3 (2.1) |
| Smoking habit; n (%), (95%CI) | 67 (47.9), (41–58) |
| Ex-IVDA, n (%) | 2 (1.5) |
| Median daily alcohol consumption; (SAU), (IQR) | 0 (0–1) |
MSM: males who have sex with males; VL: viral load; HCV: hepatitis C virus infection; HBV: hepatitis B virus infection; SAU: standard alcohol units; ex-IVDA: ex-intravenous drug abuser; SD: standard deviation; STD: sexually transmitted diseases; IQR (inter-quartile range).
2. Results of the cytology, PCR of the HPV and anal histology
We found that more than half the patients had dysplasia, 49.2% had LSIL, 2.5% and HSIL and 2.5% had ASC. Of the cohort, 88.6% were HPV-positive according to PCR, 74.2% (95%CI: 69–85) of whom had high-risk genotype, 59.7% (95%CI: 51–70) low-risk genotype and 46.8% (95%CI: 39–58) both genotypes. The HPV genotypes isolated most frequently in anal mucosa were: 16 (30.6%), 6 (16.1%), 51 (15.3%), 84 (13.7%), 11 (12.9%), 18 (12.1%), and 61 (12.1%) (Table 2). Of the 140 anoscopies performed, only 32.8% had normal histology, the rest having some grade of dysplasia, of which 20% had lesions ≥AIN2, and 11.4% (95%CI: 4–19) with carcinoma in situ (Table 2).
Table 2. Results of the PCR of HPV, cytology and anoscopy.
| Outcomes | MSM-HIV patients; n = 140 | |
| PCR of HPV-positive, n (%), 95%CI | 124 (88.6) | |
| High-risk HPV | 74 (59.7), (51–70) | |
| Low-risk HPV | 92 (74.2), (69–85) | |
| Low and High-risk HPV | 58 (46.8), (39–58) | |
| Genotypes, n (%) | HPV16 | 38 (30.6) |
| HPV6 | 20 (16.1) | |
| HPV51 | 19 (15.3) | |
| HPV 84 | 17 (13.7) | |
| HPV11 | 16 (12.9) | |
| HPV18 | 15 (12.1) | |
| HPV 61 | 16 (12.1) | |
| Anal cytology; n (%), 95%CI | 120 (85.7) | |
| Normal | 55 (45.8), (39–57) | |
| LSIL | 59 (49.2), (38–57) | |
| ASC | 3 (2.5), (0–6) | |
| HSIL | 3 (2.5), (0–6) | |
| Anoscopy; Histology, n (%), 95%CI | Normal | 46 (32.8), (28–44) |
| AIN1 | 66 (4.1), (38–56) | |
| AIN2 | 12 (8.5), (4–13) | |
| AIN3/Carcinoma in situ | 16 (11.4), (4–19) | |
HPV: human papilloma virus; HR-HPV: high-risk human papilloma virus, LR-HPV: low-risk human papilloma virus; LSIL: low-grade squamous intraepithelial lesion; HSIL: high-grade squamous intraepithelial lesion; ASC: atypical squamous cells; AIN: anal intraepithelial neoplasia. IQR (inter-quartile range).
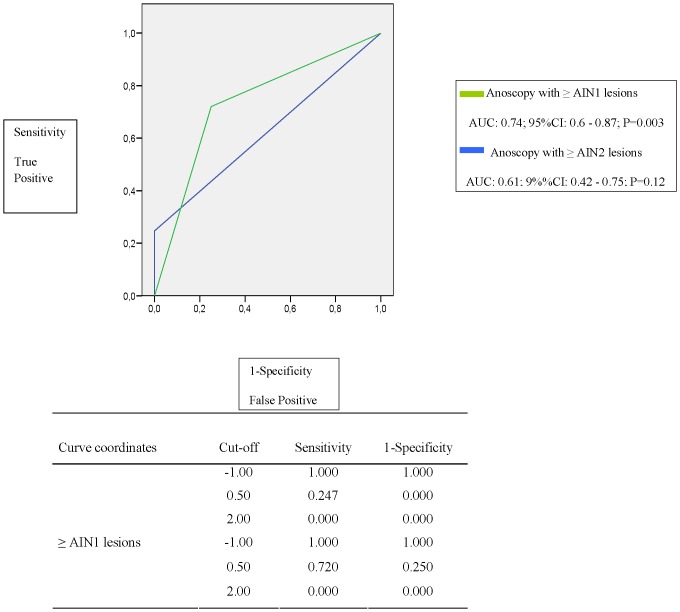
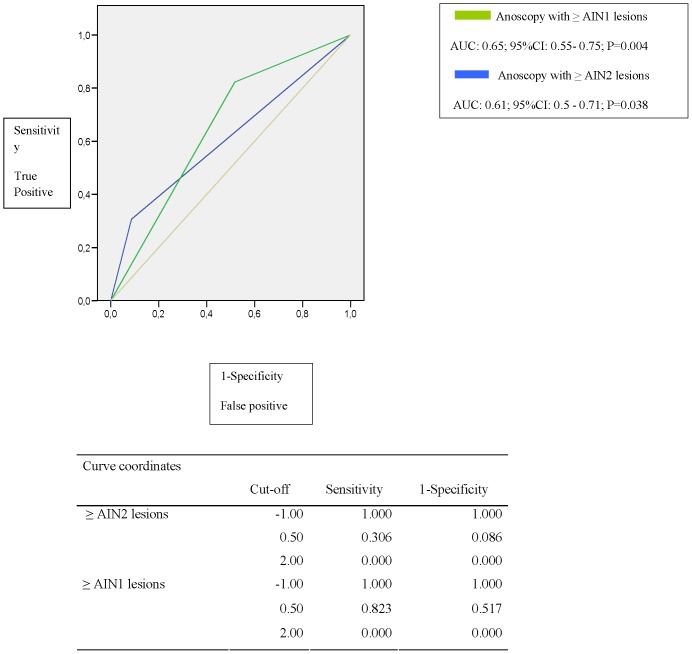
The diagnostic performance curves of the cytology, and those from PCR of HR-HPV genotypes used to predict the presence of dysplasia in the histology, revealed acceptable results. The area under the curve (AUC) values of the receiver operating characteristics (ROC) were not clearly discriminatory; the majority of values being around 6. The combination of HR-HPV PCR with cytology better predicts the grade of dysplasia than the cytology alone i.e. in those with normal cytology and HPV-negative PCR, the AUC of the ROC was 0.74 (p = 0.003) while, in the case of normal cytology alone, it was 0.65 (p = 0.04) (Figures 1 and 2).
Figure 1. ROC curve of HR-HPV-negative and normal cytology vs. ≥AIN 1 and ≥AIN 2 lesions.
Figure 2. ROC curve of normal cytology vs. ≥AIN 1 and ≥ AIN 2 lesions.
Correlations between the cytology results and PCR of HR-HPV were poor when each was considered independently, in relation to the histology of the anal mucosa. Of note is that only 48.3% of the patients with normal cytology had a normal biopsy; the rest of the patients presented anal dysplasia such as carcinoma in situ (3.4%) and AIN2 (5.2%). However, when we analyzed cytology and PCR of the HR-HPV in combination, the diagnostic efficiency was considerably improved. In case of normal cytology with PCR of HR-HPV-positive, 24.4% presented carcinoma in situ/AIN3 and 6.8% AIN2 i.e. 31.1% had lesions ≥AIN2. Of further note is that in case of normal cytology with PCR of HR-HPV-negative, 75% of the patients had normal histology, and none had lesions ≥AIN2 (Table 3)
Table 3. Degree of correlation between anal cytology and HR-HPV PCR, with histology.
| Variable | Normal histology | AIN1 | AIN2 | AIN3/Carcinoma in situ | ≥AIN2 lesions |
| n (%); Kappa; p* | n (%); Kappa; p* | n (%); Kappa; p* | n (%); Kappa; p* | n (%) ; Kappa; p* | |
| Normal cytology (n = 58) | 28 (48.3) | 25 (43.1) | 3(5.2) | 2 (3.4) | 5 (8.6) |
| 0.31; 0.0001 | -0.15; 0.1 | -0.46; 0.35 | -0.11;0.04 | -0.22; 0.003 | |
| ASC (n = 3) | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 0 (0) |
| 0.49; 0.22 | 0.048; 0.08 | 0.04; 0.6 | -0.41;0.57 | 0.47;0.38 | |
| LSIL (n = 59) | 11 (18.6) | 34 (57,6) | 5 (8.5) | 13 (2.03.3) | 18 (30.1) |
| 0.27; 0.001 | 013; 0.14 | 0.02; 0.6 | 0.19;0.02 | 0.21; 0.005 | |
| HSIL (n = 3) | 0 (0) | 2 (66.7) | 1 (33.3) | 0 (0) | 1 (33.3) |
| 0.02; 0.22 | 0.02; 0.5 | 0.12; 0.08 | -0.41;0.57 | 0.3; 0.56 | |
| HR-HPV positive (n = 92) | 29 (31.5) | 45 (48.9) | 7 (7.6) | 14(15.29) | 21 (22.8) |
| -0.17; 0.01 | 0.13; 0.83 | -0.03; 0.4 | 0.07;0.07 | 0.04; 0.39 | |
| Normal cytology | 9 (20) | 25 (55.5) | 3 (6.8) | 11 (24.4) | 14 (31.1) |
| + HR-HPV positive (n = 45) | -0.27; 0.005 | 0.14; 0.15 | -0.03; 0.6 | 0.22; 0.002 | 0.19; 0.029 |
| Normal cytology | 12 (75) | 4(25) | 0 | 0 | 0 |
| + HR-HPV-negative (n = 16) | 0.3; 0.0001 | -0.14; 05 | 0.12; 0.19 | -0.12;017 | -0.21;0.03 |
Kappa: Kappa index; p* significance.
3. Risk factors associated with the appearance of ≥AIN2 lesions
In the bivariate analysis of the different factors associated with the appearance of lesions ≥AIN2, we observed, as a risk variable, the number of HR-HPV genotypes in anal mucosa (p = 0.02) especially genotype 68 (p = 0.01). Longer-term ART was a protective factor (p = 0.02), as was a longer time since HIV diagnosis (p = 0.007) (Table 4).
Table 4. Risk factors associated with the appearance of lesions ≥AIN2. Univariate analysis.
| HIV-MSM with ≥AIN2 lesions; n = 28 | HIV-MSM without ≥AIN2 lesions; n = 112 | P* | |
| Age; mean years (±SD) | 35.1(±8.2) | 37.8(±8.9) | 0.149 |
| Nationality: Spanish, n (%) | 26 (92.9) | 107 (95.5) | 0.63 |
| Retired; n (%) | 1 (3.6) | 7 (6.3) | 1 |
| University education; n (%) | 17 (60.7) | 56 (50) | 0.31 |
| Partners previous year; median (IQR) | 1 (1–9.5) | 1 (1–6) | 0.4 |
| Condom use, n (%) | 21 (75) | 88 (78.6) | 0.7 |
| Perianal/genital condylomatosis, n (%) | 12 (42.9) | 30 (26.7) | 0.09 |
| History of condylomas, n (%) | 9 (32.1) | 41 (36.7) | 0.7 |
| Duration of HIV; mean months (IQR) | 18.5 (3–35.3) | 36 (14.25–84) | 0.007 |
| VL of HIV log10 (copies/mL) (± SD) | 4.03(±4.52) | 4.23(±3.2) | 0.3 |
| CD4 mean (cell/uL), (± SD) | 627.4(±289.4) | 659.8(±254.9) | 0.57 |
| CD8 mean (cell/uL), (± SD) | 967.8(±403.8) | 1558.7(±4924.6) | 0.54 |
| CD4 mean nadir (cell/uL), (± SD) | 354.5(±232) | 356.8(±251.9) | 0.96 |
| AIDS stage (A3, B3, C), n (%) | 6 (21.4) | 40 (34.5) | 0.15 |
| ART, n (%) | 17 (69.7) | 89 (79.5) | 0.02 |
| Median duration of ART; months (IQR) | 14.5 (0.25–27) | 28.5 (10–87.5) | 0.008 |
| Virological treatment failure, n (%) | 1 (4.5) | 6 (6.4) | 1 |
| Syphilis treated, n (%) | 4 (14.3) | 22 (19.6) | 0.5 |
| Latent tuberculosis treated, n (%) | 4 (14.3) | 13 (11.6) | 0.7 |
| HCV, n (%) | 2 (7.14) | 4 (3.6) | 0.5 |
| HBV, n (%) | 1 (3.6) | 2 (1.8) | 0.34 |
| Smoking habit, n (%) | 14 (50) | 53 (47.3) | 0.8 |
| PCR of HPV | |||
| LR-HPV, n (%) | 18 (69.2) | 56 (57.1) | 0.3 |
| HR-HPV, n (%) | 21 (80.8) | 71 (72.4) | 0.4 |
| HR+LR HPV, n (%) | 14 (53.8) | 44 (44.9) | 0.4 |
| Number of HR-HPV (IQR) | 2 (1–3.25) | 1 (0–2) | 0.2 |
| Number of LR-HPV (IQR) | 1 (0–2) | 1 (0–2) | 0.8 |
| Genotypes, n (%) | |||
| HPV6 | 7 (26.7) | 13 (13.2) | 0.1 |
| HPV11 | 6 (23.1) | 10 (10.2) | 0.1 |
| HPV16 | 11 (42.3) | 27 (27.5) | 0.2 |
| HPV18 | 6 (23.1) | 9 (9.2) | 0.08 |
| HPV51 | 6 (23.1) | 13 (12.2) | 0.2 |
| HPV 61 | 6 (23.1) | 10 (10.2) | 0.1 |
| HPV 68 | 5 (19.2) | 3 (13.2) | 0.01 |
| HPV 84 | 4 (4.1) | 13 (13.2) | 0.7 |
MSM-HIV-positive: males who have sex with males HIV-positive, VL: viral load; HCV: hepatitis C virus; HBV: hepatitis B virus; HPV: human papilloma virus; VL: HIV viral load; HR-HPV: high risk human papilloma virus; LR-HPV: low risk human papilloma virus; IQR: inter-quartile range.
Finally, in the logistic regression analysis assessing appearance of lesions ≥AIN2, we identified the risk factors as: the presence of perianal condylomas during the study (OR: 4.26; 95%CI: 1.27–14.3) and the HPV genotype 68 (OR: 10.6; 95%CI: 1.23–91.47) whose prevalence was around 6.5%. The factors protective against the appearance of these lesions were identified as: receiving ART (OR: 0.21; 95%CI: 0.054–0.84), and having had a previous diagnosis of syphilis (OR: 0.078; 95%CI: 0.008–0.72).
Discussion
Our cohort of HIV-positive MSM patients had a high prevalence not only of dysplastic lesions (50% LSIL in cytology and 20% biopsy lesion ≥AIN2) but also infection by high-risk HPV genotypes of around 74%. The results were very similar to those published by other authors who had observed the percentage of anal dysplasia with low grade cytology (LSIL) at around 40%, 19% ≥AIN2 lesions and the most-frequently identified HPV16 genotype [23].
The prevalence of anal carcinoma in situ/AIN3 in our group of patients, was 11.4% (95%CI: 4–19) which translates into 1/9 patients having anal cancer in situ. AIN2 was 8.5%, i.e. 1/5 of patients included had pre-malignant lesions (HSIL). These findings highlight the need to implement routine consultations in HIV clinics which, currently in Spain, are not performed systematically for screening, diagnosis, treatment and follow-up of dysplastic lesions of the anal mucosa. Anal cancer is one of the most frequent non-AIDS defining malignancies in HIV, and has become so probably because of the increase in survival of HIV patients [24]. Nevertheless, there has not been any consensus nor homogeneity of recommendations by the different scientific societies regarding the screening or treatment of anal dysplasia in HIV-positive patients [25], [26], [27].
The HPV genotype most frequently identified in the anal mucosa of our patient cohort was the oncogenic 16, present in about 30.6% of the patients included in this study. It is one of the most frequent, high-risk genotypes isolated in anal mucosa of HIV-positive MSM patients [28], as well as in HIV-negative males [29], HIV-positive women [30] and heterosexual HIV-positive men [31].
The correlation between the results of the cytology and histology that we observed are slightly more encouraging; only 48.3% of the patients with normal cytology had a normal biopsy (Kappa index: 0.31; p = 0.0001). Of the rest, 43.1% were AIN1, 5.2% AIN2 and 3.4% carcinoma in situ. Conversely, when the patient with normal cytology had high-risk HPV in anal mucosa, only 20% had a normal histology. Of the rest, 55.5% had AIN1, 6.8% AIN2 and 24.4% AIN3/carcinoma in situ. Of further note is that in patients with normal cytology with PCR of high-risk HPV-negative, 75% had normal histology, and none had ≥AIN2 lesions. Our results have some important consequences for recommendations for screening of premalignant lesions and carcinoma of the anal canal in HIV patients. To date, the different AIDS scientific societies recommend only anal cytology [25], [26], [27]. This would fail to diagnose, in our cohort for example, 1/3 patients with ≥AIN2 lesions. Hence, apart from the cytology in screening for dysplastic lesions of anal mucosa, we recommend the performance of HPV PCR. In case of normal cytology and HR-HPV-negative, anoscopy may be avoided and a new check-up scheduled for the next year, and to include cytology and PCR. In case the cytology was dysplastic or the HR-HPV PCR was positive, anoscopy would be performed. Also, the current recommendations of annual cytology assessments alone to discard diagnosis of AIN1 lesions would, as well, not be appropriate. The few studies that have addressed this issue in follow-up have detected progression to premalignant lesions in 62% within approximately 24 months, and progression from AIN3 to carcinoma of between 9% and 13% in 5 years [9].
We observed the presence of anal condylomatosis as well as HPV68 genotype as being risk factors associated with the appearance of ≥AIN2 lesions. Genital-anal condylomatosis is due to the infection by the HPV genotypes and, similar to those that present in the anal canal mucosa, are associated with the appearance of dysplasia not only in women [32] but also in men [33]. Hence, it can be considered a factor predictive of lesions ≥AIN2. As such, it would seem highly necessary to recommend, in HIV-positive MSM patients with genital and or perianal warts, that they are screened for anal dysplasia. Despite that genotype HPV16 was the most-frequently isolated in our cohort, it is the high-risk HPV68 that is associated with the appearance of ≥AIN2 lesions. This genotype has oncogenic capacity belonging to the C group of the phylogenetic tree of the papilloma virus, similar to genotypes 18, 39, 45 and 59 [34]. These genotypes, as opposed to the HPV16 in patients with similar immunological background, could more successfully avoid immune vigilance and give rise to dysplastic lesions in the anal mucosa [23].
We observed that receiving ART and previous diagnosis of luetic infection were factors predisposing against the appearance of ≥AIN2 lesions. With respect to ART, we support the findings of the Swiss study [14] and the probable underlying mechanism could be similar to that occurring in the cervix i.e. modulation of the oncogenic effect of the HR-HPV by maintaining suppression of the HIV replication. This reduction in HIV viral load in anal mucosa would reduce HPV replication and, subsequently, favor the clearance of the HPV. Also, our results support the findings of Pokomandy et al who observed, in a prospective cohort study of 3 years duration with 247 HIV-positive MSM. The patients received ART for >4 years and obtained a benefit against the appearance of AIN2-3 lesions [18] while, conversely, the low levels of CD4 as well as the presence of HPV genotypes 16/18 were the principal factors associated with progression. Finally, the data from a cross-sectional study in Holland composed of 250 HIV-positive MSM patients showed a lower prevalence of AIN2/3 lesions and HPV infection in those receiving ART compared to those who were not. As such there was a higher prevalence of these lesions in those with HPV infection; essentially genotypes 6 and 16 [15].
That previous infection with Treponema pallidum can be a protective factor against the appearance of ≥AIN2 lesions, could be interpreted as syphilis being a surrogate marker for early diagnosis of HIV and, thus, of the infection by HPV which is encountered in an earlier phase in which the appearance of the dysplastic lesions have not, as yet, occurred.
Finally, a weak point of our study needs to be addressed. The study was cross-sectional and, as such, no causal relationships between the measured variables could be demonstrated. The strength of the study is the consistency of the data. All the anoscopies, biopsy and cytology of anal mucosa, as well as the PCR of HPV, microbiology laboratory assessments, and clinical interviews were each performed by the relevant specialist, and this minimizes inter-observer bias.
In conclusion, in our cohort of HIV-positive MSM patients, 3/4 were infected with HPV genotypes of high-risk; 1/5 had high-grade lesions. There was a low correlation between the cytology findings and the anal histology. Adding PCR of the HPV genotypes to the screening for ≥AIN2 lesions would increase the diagnostic efficiency above that of cytology alone. Antiretroviral treatment could exercise a protective role against the presence of lesions ≥AIN2. The presence of genital or perianal warts and HPV68 genotype in anal mucosa are 2 factors predictive of ≥AIN2 lesions. These findings warrant further investigation.
Acknowledgments
We thank the nursing staff of the AIDS Unit for their invaluable help in managing the outpatient clinic. Editorial assistance was by Dr. Peter R. Turner of Tscimed.com.
Findings are to be presented at the CROI. March 3–6, 2014. Boston, Mass (Abstract P O6).
Funding Statement
These authors have no support or funding to report.
References
- 1. Kutlubay Z, Engin B, Zara T, Tuzun Y (2013) Anogenital malignancies and premalignancies: facts and controversies. Clin Dermatol 31: 362–373. [DOI] [PubMed] [Google Scholar]
- 2. Shiels MS, Cole SR, Kirk GD, Poole CA (2009) Meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 52: 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grulich AE, Van Leeuwen MT, Falster MO, Vajdic CM (2007) Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 370: 59–67. [DOI] [PubMed] [Google Scholar]
- 4. Salati SA, Al Kadi A (2012) Anal cancer - a review. Int J Health Sci 6: 206–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hidalgo-Tenorio C, Rivero Rodríguez M, Concha A, Gil Anguita C, López Castro R, et al. (2013) CD4 lymphocytes as a protective factor against infection by oncogenic genotypes of human papillomavirus in the anal mucosa of men who have sex with human immunodeficiency virus positive men. Med Clinica (Barna) 40: 193–199. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz LM, Castle PE, Follansbee S, Borgonovo S, Fetterman B, et al. (2013) Risk factors for anal HPV infection and anal precancer in HIV-infected men who have sex with men. J Infect Dis 208: 1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palefsky JM, Rubin M (2009) The epidemiology of anal human papillomavirus and related neoplasia. Obstet Gynecol Clin North Am 36: 187–200. [DOI] [PubMed] [Google Scholar]
- 8. de Pokomandy A, Rouleau D, Ghattas G, Trottier H, Vézina S, et al. (2011) HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis 52: 1174–1181. [DOI] [PubMed] [Google Scholar]
- 9. Scholefield JH, Harris D, Radcliffe A (2011) Guidelines for management of anal intraepithelial neoplasia. Colorectal Dis 201 13 Suppl 13–10 doi:10.1111/j.1463-1318.2010.02494.x [DOI] [PubMed] [Google Scholar]
- 10. Piketty C, Selinger-Leneman H, Grabar S, Duvivier C, Bonmarch M, et al. (2008) Marked increase in the incidence of invasive anal cancer among HIV-infected patients despite treatment with combination antiretroviral therapy. AIDS 22: 1203–1211. [DOI] [PubMed] [Google Scholar]
- 11. D'Souza G, Wiley DJ, Li X, Chmiel JS, Margolick JB, et al. (2008) Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. Acquir Immune Defic Syndr 48: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diamond C, Taylor TH, Aboumrad T, Bringman D, Anton-Culver H (2005) Increased incidence of squamous cell anal cancer among men with AIDS in the era of highly active antiretroviral therapy. Sex Transm Dis 32: 314–220. [DOI] [PubMed] [Google Scholar]
- 13. Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, et al. (2012) Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis 54: 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, et al. (2010) Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer 103: 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Der Snoek EM, Van Der Ende ME, Den Hollander JC, Schutten M, Neumann HA, et al. (2012) Use of highly active antiretroviral therapy is associated with lower prevalence of anal intraepithelial neoplastic lesions and lower prevalence of human papillomavirus in HIV-infected men who have sex with men. Sex Transm Dis 39: 495–500. [DOI] [PubMed] [Google Scholar]
- 16. Hleyhel M, Belot A, Bouvier AM, Tattevin P, Pacanowski J, et al. (2013) Risk of AIDS-defining cancers among HIV-1-infected patients in France between 1992 and 2009: Results from the FHDH-ANRS CO4 Cohort. Clin Infect Dis 57: 1638–1647. [DOI] [PubMed] [Google Scholar]
- 17. Blitz S, Baxter J, Raboud J, Walmsley S, Rachlis A, et al. (2013) on behalf of Canadian Women's HIV Study Group (2013) Evaluation of HIV and highly active antiretroviral therapy on the natural history of human papillomavirus infection and cervical cytopathologic findings in HIV-positive and high-risk HIV-negative women. J Infect Dis 208: 454–462. [DOI] [PubMed] [Google Scholar]
- 18. Muñoz N, Bosch FX, de Sanjose S, Herrero R, Castellsagué X, et al. (2003) on behalf of the International Agency for Research on Cancer Multicenter Cervical Cancer Study Group (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348: 518–527. [DOI] [PubMed] [Google Scholar]
- 19. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, et al. (2002) on behalf of the Forum Group Members, Bethesda 2001 Workshop (2002) The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA 287: 2114–2119. [DOI] [PubMed] [Google Scholar]
- 20. Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, et al. (2012) The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis 16: 205–42. [DOI] [PubMed] [Google Scholar]
- 21. Andrés AM, Marzo PF (2005) Chance-corrected measures of reliability and validity in K x K tables. Stat Methods Med Res 14: 473–492. [DOI] [PubMed] [Google Scholar]
- 22.Freeman DH (1987) Applied categorical data analysis. New York, USA, Marcel Dekker. [Google Scholar]
- 23. Darwich L, Videla S, Cañadas MP, Piñol M, García-Cuyàs F, et al. (2013) on behalf of the Can Ruti HIV-HPV Team (2013) Distribution of human papillomavirus genotypes in anal cytological and histological specimens from HIV-infected men who have sex with men and men who have sex with women. Dis Colon Rectum 56: 1043–1052. [DOI] [PubMed] [Google Scholar]
- 24. Pinzone MR, Fiorica F, Di Rosa M, Malaguarnera G, Malaguarnera L, et al. (2012) Non-AIDS-defining cancers among HIV-infected people. Eur Rev Med Pharmacol Sci 16: 1377–1388. [PubMed] [Google Scholar]
- 25.European AIDS Clinical Society (2012) 2012 Guidelines: 25. (version 6.1). Available: www.europeanaidsclinicalsociety.org.
- 26. Aberg JA, Kaplan JE, Libman H, Emmanuel P, Anderson JR, et al. (2009) on behalf of the HIV Medicine Association of the Infectious Diseases Society of America (2009) Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infec Dis 49: 651–581. [DOI] [PubMed] [Google Scholar]
- 27. Panel de expertos del Grupo de Estudio de Sida (GESIDA) y del Plan Nacional sobre el Sida (PNS) (2011) AIDS Study Group/Spanish AIDS Consensus Plan Document on sexually transmitted infections in HIV-infected patients. Enferm Infecc Microbiol Clin 29: 286. [DOI] [PubMed] [Google Scholar]
- 28. Yang Y, Li X, Zhang Z, Qian HZ, Ruan Y, et al. (2012) Association of human papillomavirus infection and abnormal anal cytology among HIV-infected MSM in Beijing, China. PLoS One. 7: e35983 doi:10.1371/journal.pone.0035983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mooij SH, Van der Klis FR, Van der Sande MA, Schepp RM, Speksnijder AG, et al. (2013) Seroepidemiology of high-risk HPV in HIV-negative and HIV-infected MSM: the H2M study. Cancer Epidemiol Biomarkers Prev 22: 1698–1708. [DOI] [PubMed] [Google Scholar]
- 30. Kojic EM, Cu-Uvin S, Conley L, Bush T, Onyekwuluje J, et al. (2011) Human papillomavirus infection and cytologic abnormalities of the anus and cervix among HIV-infected women in the study to understand the natural history of HIV/AIDS in the era of effective therapy (the SUN study). Sex Transm Dis 38: 253–259. [DOI] [PubMed] [Google Scholar]
- 31. Videla S, Darwich L, Cañadas MP, Coll J, Piñol M, et al. (2013) on behalf of the HIV-HPV Study Group (2013) Natural history of human papillomavirus infections involving anal, penile, and oral sites among HIV-positive men. Sex Transm Dis 40: 3–10. [DOI] [PubMed] [Google Scholar]
- 32. Heard I (2005) Ano-genital lesions due to human papillomavirus infection in women. Med Mal Infect 35: 302–305. [DOI] [PubMed] [Google Scholar]
- 33. Oon SF, Winter DC (2010) Perianal condylomas, anal squamous intraepithelial neoplasms and screening: a review of the literature. J Med Screen 17: 44–49. [DOI] [PubMed] [Google Scholar]
- 34. Chan SY, Bernard HU, Ong CK, Chan SP, Hofmann B, et al. (1992) Phylogenetic analysis of 48 papillomavirus types and 28 subtypes and variants: a showcase for the molecular evolution of DNA viruses. J Virol 66: 5714–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]