Abstract
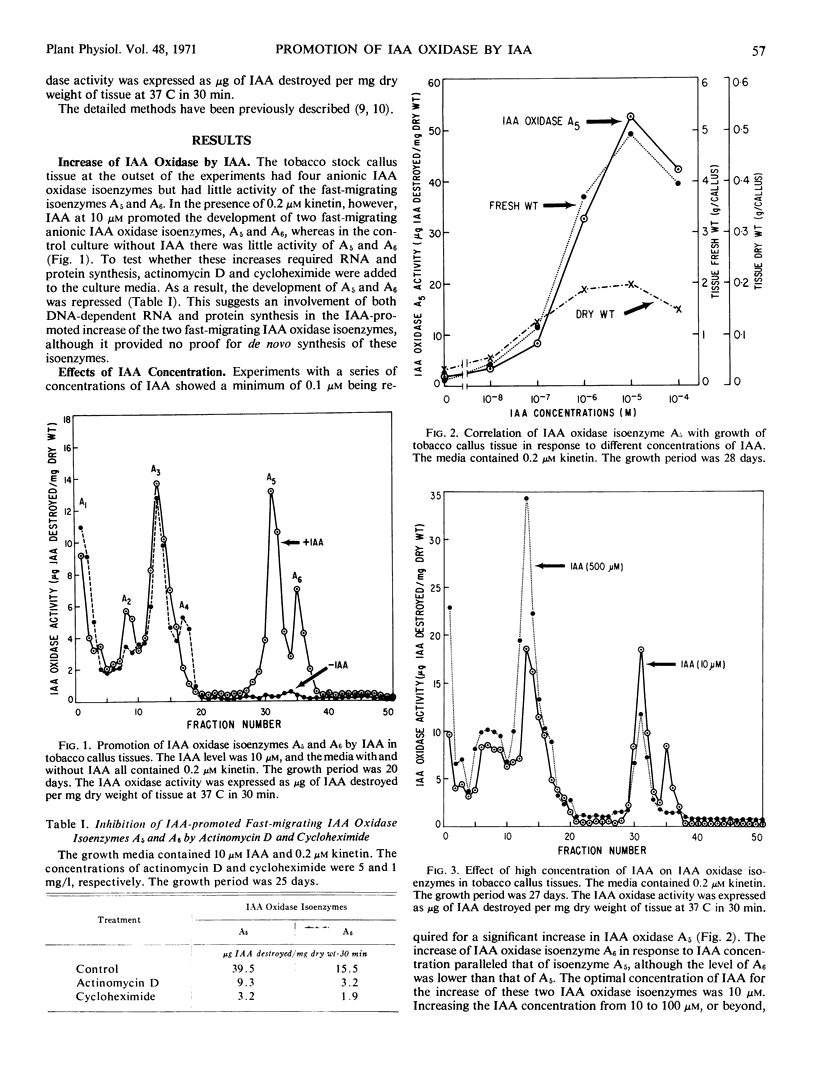
Indoleacetic acid oxidase in tobacco callus cultures (Nicotiana tabacum L., cv. White Gold) was composed of at least two groups of isoenzymes, which were distinctly different in electrophoretic mobilities and in responses to growth substances. Indoleacetic acid had dual effects; at low concentrations it promoted the development of two fast-migrating indoleacetic acid oxidase isoenzymes, but at high concentrations it increased the level of other indoleacetic acid oxidase isoenzymes with low and moderate electrophoretic mobilities. However, indoleacetic acid was not unique in such effects; 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid were effective at concentrations lower than that of indoleacetic acid.
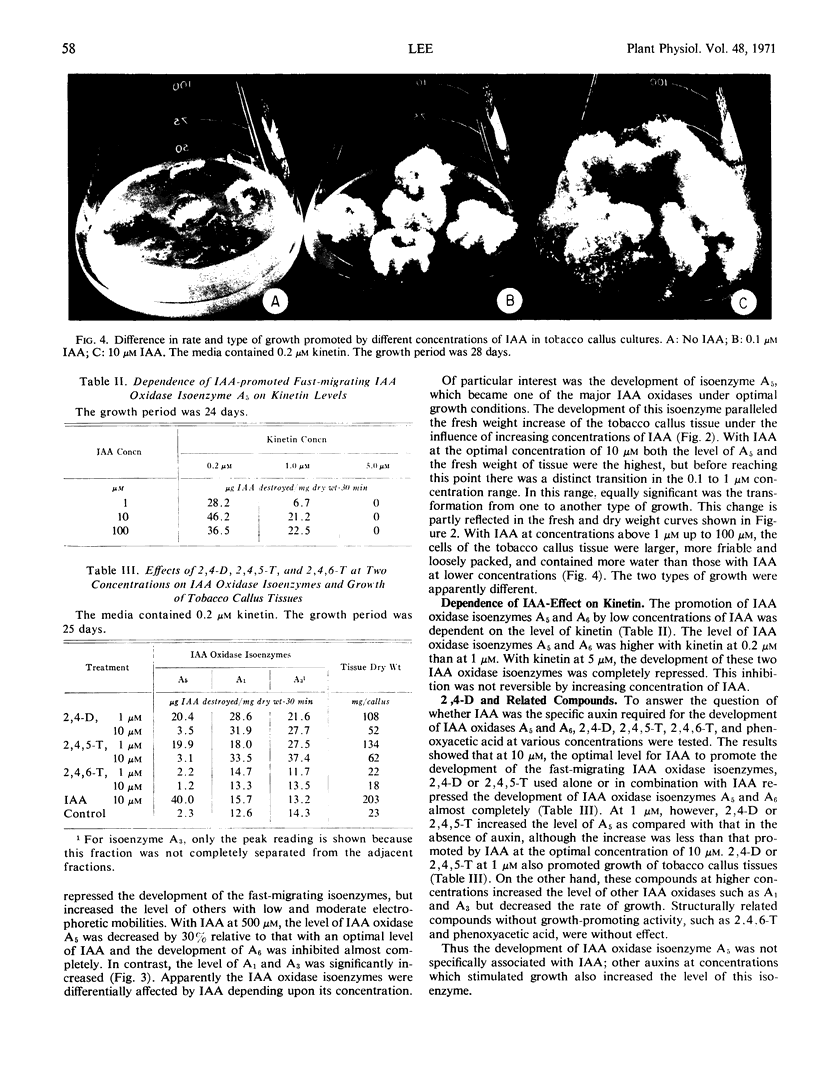
A correlation was apparent between the relative levels of the two groups of indoleacetic acid oxidase isoenzymes and the rate and type of growth, as affected by auxins. The development of the fast-migrating anionic indoleacetic acid oxidase isoenzymes was accompanied by a type of growth characterized by rapid growth rate, high water content, and friable tissues. On the other hand, a further increase in the isoenzymes of slower migrating rate was associated with growth retardation.
The indoleacetic acid-mediated increase of the fast-migrating indoleacetic acid oxidase isoenzymes was dependent on the level of kinetin, suggesting a multiple control by different types of growth substances. The inhibition of the formation of the fast-migrating isoenzymes by actinomycin D and cycloheximide suggests a requirement for both RNA and protein synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GALSTON A. W., BONNER J., BAKER R. S. Flavoprotein and peroxidase as components of the indoleacetic acid oxidase system of peas. Arch Biochem Biophys. 1953 Feb;42(2):456–470. doi: 10.1016/0003-9861(53)90373-7. [DOI] [PubMed] [Google Scholar]
- GOLDACRE P. L. Hydrogen peroxide in the enzymic oxidation of heteroauxin. Aust J Sci Res B. 1951 Aug;4(3):293–302. doi: 10.1071/bi9510293. [DOI] [PubMed] [Google Scholar]
- Gordon S. A., Weber R. P. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENTEN R. H. The oxidation of indolyl-3-acetic acid by waxpod bean root sap and peroxidase systems. Biochem J. 1955 Jan;59(1):110–121. doi: 10.1042/bj0590110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. T. Cytokinin-controlled Indoleacetic Acid Oxidase Isoenzymes in Tobacco Callus Cultures. Plant Physiol. 1971 Feb;47(2):181–185. doi: 10.1104/pp.47.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockerse R., Siegel B. Z., Galston A. W. Hormone-induced repression of a peroxidase isozyme in plant tissue. Science. 1966 Jan 28;151(3709):452–453. doi: 10.1126/science.151.3709.452. [DOI] [PubMed] [Google Scholar]



