Abstract
Background
Approximately 1/3 of 1990-1 Gulf War veterans developed chronic multisymptom health problems. Implicated exposures bear mechanisms that adversely affect mitochondria. Symptoms emphasize fatigue, cognition and muscle (brain and muscle are aerobically demanding); with protean additional domains affected, compatible with mitochondrial impairment. Recent evidence supports treatments targeting cell bioenergetics (coenzyme10) to benefit Gulf War illness symptoms. However, no evidence has directly documented mitochondrial or bioenergetic impairment in Gulf War illness.
Objective
We sought to objectively assess for mitochondrial dysfunction, examining post-exercise phosphocreatine-recovery time constant (PCr-R) using 31Phosphorus Magnetic Resonance Spectroscopy (31P-MRS), in Gulf War veterans with Gulf War illness compared to matched healthy controls. PCr-R has been described as a “robust and practical” index of mitochondrial status.
Design and Participants
Case-control study from 2012–2013. Fourteen community-dwelling Gulf War veterans and matched controls from the San Diego area comprised 7 men meeting CDC and Kansas criteria for Gulf War illness, and 7 non-deployed healthy controls matched 1∶1 to cases on age, sex, and ethnicity.
Outcome Measure
Calf muscle phosphocreatine was evaluated by 31P-MRS at rest, through 5 minutes of foot pedal depression exercise, and in recovery, to assess PCr-R. Paired t-tests compared cases to matched controls.
Results
PCr-R was significantly prolonged in Gulf War illness cases vs their matched controls: control values, mean±SD, 29.0±8.7 seconds; case values 46.1±18.0 seconds; difference 17.1±14.9 seconds; p = 0.023. PCr-R was longer for cases relative to their matched controls for all but one pair; moreover while values clustered under 31 seconds for all but one control, they exceeded 35 seconds (with a spread up to 70 seconds) for all but one case.
Discussion
These data provide the first direct evidence supporting mitochondrial dysfunction in Gulf War illness. Findings merit replication in a larger study and/or corroboration with additional mitochondrial assessment tools.
Introduction
Of the ∼700,000 US troops deployed to the 1990-1 Persian Gulf theater, an estimated 175,000–250,000 (∼1/4–1/3 of those deployed), developed chronic multisymptom health problems often termed “Gulf War illness” (GWI) [1]. GWI is characterized by protean symptoms spanning multiple symptom “domains” (such as cognitive, fatigue, musculoskeletal). Gulf deployed veterans on average have more symptom domains affected, and greater severity and multiplicity of symptoms within domains, than Gulf era veterans who were not deployed [2]. Fatigue, exercise intolerance, cognitive difficulties, muscle pain and weakness, shortness of breath, gastrointestinal problems, sleep problems, behavior change, neurological findings, and skin problems are all elevated. GWI is defined by symptoms; a number of objective findings have been replicated, such as autonomic dysfunction, increased autoantibodies, reduced natural killer cell activity, and increased coagulation activation among others [3]. Studies generally show that affected veterans have not improved with time [4], [5], [6]; Gulf War veterans (GWV) continue to experience symptoms and impaired function 23 years later.
GWI is not equivalent to signature conditions of subsequent deployments to the region, such as posttraumatic stress disorder and traumatic brain injury. Indeed, stress and combat are demonstrably not the cause. While combat stress bears a dose-response relation to posttraumatic stress disorder (including in GWV); it bears no significant relationship to GWI in studies that adjust for other exposures [4]. Environmental factors are clearly inculpated in GWI. Many exposures were new, unique or excessive in the Gulf War. These include heat, sand, depleted uranium tanks/munitions, chemical agent resistant coating paint, and oil fires; as well as protections such as high numbers of multiple vaccines, anthrax vaccine, pyridostigmine bromide nerve agent pretreatment pills, pesticides and insect repellents, and permethrin-impregnated uniforms among others. Evidence most strongly implicates acetylcholinesterase inhibitor (AChEi) related exposures (which adhere to Hill's criteria for causality, include a dose-response relationship, and are buttressed by gene-exposure interaction data) [7]. Multiple vaccinations and anthrax vaccine also show relatively consistent epidemiological associations, but do not have the triangulating evidence for causality. AChEi related exposures include (carbamate) pyridostigmine bromide nerve agent pretreatment pills, given to an estimated 250,000 US troops [8]; carbamate and organophosphate pesticides [9], used aggressively and sometimes excessively to protect against insect vectors of disease [10], [11]; and organophosphate nerve gas, to which the Department of Defense estimates as many as ∼100,000 US troops were exposed in association with the demolition of the Khamisiyah munitions depot [12], with other possible nerve agent exposures [13]. Number of exposures experienced has also been linked to illness [14]; and exposure interactions, conceptually and empirically, may produce more problems [15].
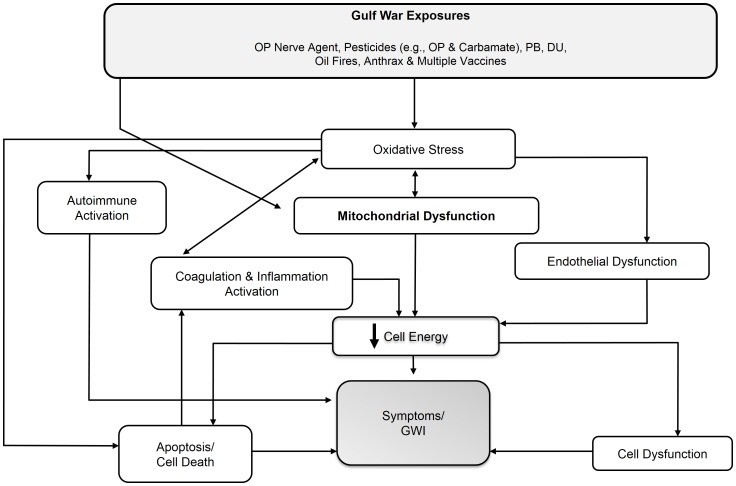
The involvement of AChEi provides important information regarding potential mechanisms. Whereas AChEi toxicity is often viewed in terms of acetylcholinesterase inhibition, evidence shows that toxicity and lethality in fact relate decisively to (intertwined) oxidative stress (OS) and mitochondrial dysfunction (MD) [16] (phenomena that are tightly intertwined because the mitochondria are a leading target and source of reactive oxygen species [17], [18], [19]): indeed, animal evidence shows that high quality antioxidants administered just before or just after organophosphate pesticide exposure protect against lethality and chronic sequelae [20], [21]. We have shown that a mechanism involving OS-MD would explain the symptom profile (including which symptoms dominate – fatigue as well as central nervous system and muscle symptoms dominate in MD), symptom multiplicity, protean symptom character, variable latency to onset of symptoms, and the objective markers linked to GWI [3]. This mechanism would also provide for a subsidiary role for multiple other exposures for which mechanisms of action are classically considered to be unrelated, but which share in common induction of OS – potentiating MD and further OS. Figure 1 depicts the mechanism we propose.
Figure 1. Hypothesized pathway (abridged version).
DU = depleted uranium, GWI = Gulf War illness, OP = organophosphate, PB = pyridostigmine bromide.
We sought to directly assess the hypothesis of MD in a pilot study of ill GWVs vs matched controls, using 31Phosphorus Magnetic Resonance Spectroscopy (31P-MRS) to measure post-exercise phosphocreatine-recovery time constant (PCr-R), a marker that serves as “an estimate of net oxidative ATP synthesis,” [22] and “a robust and practical way to study mitochondrial regulation and to quantify effective mitochondrial defects in vivo” [23]. We also assessed resting brain and muscle phosphocreatine (PCr).
Methods
Ethics statement
The study protocol was approved by the University of California, San Diego Human Research Protections Program. All subjects gave written informed consent.
Participants
GWI cases comprised 7 veterans who were deployed to the Persian Gulf theater between August 1990 and July 1991, and met Centers for Disease Control and Prevention (CDC) and Kansas criteria for GWI. CDC criteria require presence of one or more symptoms in each of at least 2 of the 3 domains of fatigue, musculoskeletal, and mood-cognitive. Kansas GWI criteria require that veterans have multiple symptoms within a qualifying domain, and/or symptom(s) of at least moderate severity, in at least 3 of the 6 domains of neurological-cognitive-mood, fatigue/sleep problems, respiratory, pain, gastrointestinal, and skin [2]. To qualify for either, symptoms must have been present for at least 6 months and not present prior to the Gulf conflict.
Controls were 7 non-deployed individuals matched 1∶1 to GWI cases on sex, age (within 38 months) and ethnicity. To qualify, controls must meet neither CDC nor Kansas symptom criteria for GWI, nor Kansas exclusionary criteria (concurrent significant conditions such as diabetes, heart disease, cancer, that could produce symptoms that might be confused for GWI), nor could any inquired-about symptom be greater than mild in severity.
All prospective participants were screened prior to enrollment and again on the day of their visit to ensure 31P-MRS eligibility. Requirements included no metal that might pose a problem with magnetic resonance assessment (e.g. no prosthetic devices, shrapnel, welding as a hobby); and weight ≤300 lbs.
31P-MRS (performed by Dr. Hamilton)
31P-MR spectra were acquired on a 3 Tesla GE Signa EXCITE HD scanner (GE Healthcare, Waukesha, WI). The 1H signal was acquired using the body coil for collection of multiplanar localization images and for shimming. Participants were scanned in the supine position. The 31P-MR spectra were collected with a 5-inch diameter surface coil, using a slice selective free induction decay sequence with a repetition time of 3 seconds. Spectra had a sampling interval of 0.2 ms; 2048 data points were collected. For muscle spectra, the coil was placed under the calf. The free induction decay sequence excited a thick slice (60 mm) parallel to the coil. The slice was positioned close to the coil to maximize signal-to-noise whilst attempting to minimize inclusion of surface features in the region close to the coil. The intrinsic weighting of the coil provided the remaining localization. For the exercise protocol, a spectrum was collected every 3 seconds during 2 minutes of rest (providing resting muscle spectra), then 5 minutes of exercise (repetitively depressing, as far as they were able, a metal-free pedal, similar to depressing a car pedal, with elastic bands providing resistance). This was followed by 6 minutes of recovery. A velcro strap across subjects' thighs limited extraneous movement during exercise.
Brain spectra were collected at rest with 128 signal averages, placing the coil at the front and back of the head, successively. A clear plexiglass head box with 3 sides reduced motion artifact; foam pads were placed for comfort and neck support on the MR table.
Headphones with a microphone were given to every participant to dampen noise generated from the MR scans, and to allow communication with study staff and MR technicians. Participants selected a radio station to listen to during the scans.
31P-MRS analysis
The raw spectra files were transferred and analyzed off-line. All spectroscopy analyses were carried out by a single observer (Dr. Hamilton, blinded to case-control status) who monitored spectral quality during the analysis process, to confirm quality of acquisition. The inherently low signal-to-noise of 31P-MR spectra and the complex overlapping peak structure of the spectra were addressed via AMARES algorithm [24] included in the MRUI software program [25], using prior knowledge adapted from an approach previously used by Dr. Hamilton at 1.5T [26]. For the resting spectra (only), signal to noise was adequate to permit truncation of the first 2 ms of the signal to remove the broad component to the residual, which is the difference between the full signal and the fit of the truncated signal. This broad component corresponds to signals from motion-restricted phospholipid in cell membrane and vesicle bilayers [27]. The post-exercise PCr-R time constant was procured, as relative change in the area. For the rest spectra, the areas of the PCr peak were expressed as a fraction of the total 31P-MRS signal (sum of the areas of all visible peaks); intracellular pH values were determined [28], [29].
PCr-R analysis
Paired t-tests compared values of PCr-R (and of secondary measures) in cases relative to their matched controls. Two-sided p-values <0.05 designated statistical significance. Analyses were conducted using Stata version 8.0.
Results
Subjects (cases and controls) were male, with mean age 53.9 (range 44 to 65 years). 85.7% were Caucasian, 14.3% African American.
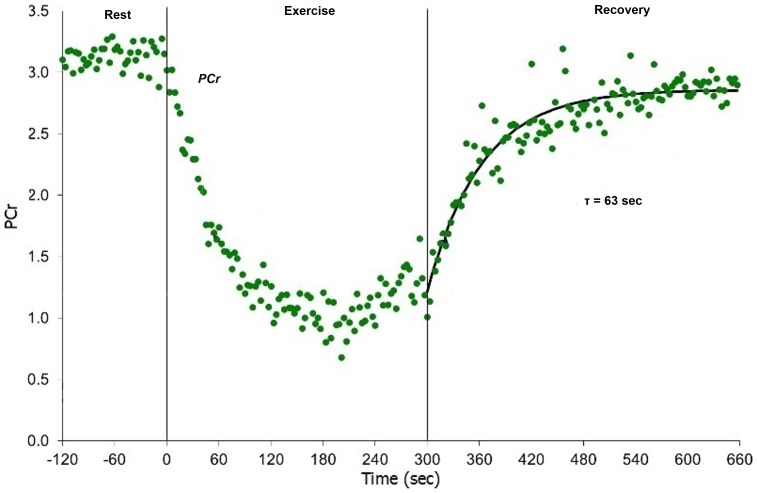
All participants performed the exercise rest/recovery task and provided PCr-R data. Figure 2 shows an example PCr rest-exercise-recovery curve. Resting PCr and pH values did not significantly differ in cases vs controls.
Figure 2. PCr-R rest-exercise-recovery profile for a GWI case.
GWI = Gulf War illness; PCr = phosphocreatine; PCr-R = phosphocreatine-recovery post-exercise. Rest-exercise-recovery phosphocreatine response curve for a Gulf War veteran (rest −120–0 sec, exercise 0–300 sec, recovery 300–660 sec) (Case 5).
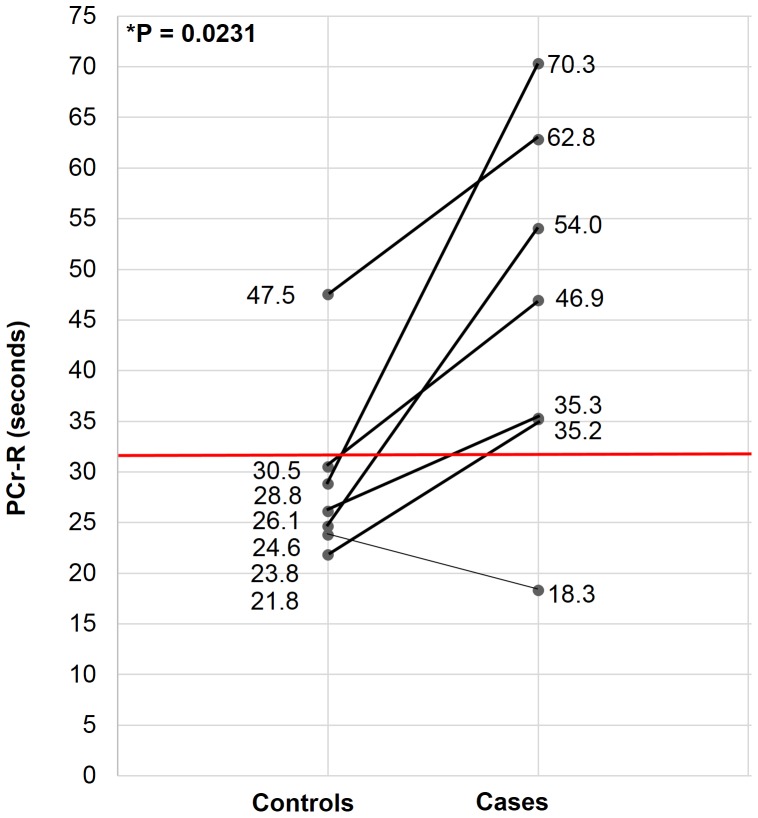
Figure 3 and Table 1 show PCr-R findings. Veterans with GWI had significantly prolonged PCr-R relative to their matched healthy controls (p = 0.023). For 6 of the 7 pairs, PCr-R was greater in the GWI case than in their matched healthy control. Moreover, with only one exception, control participants all had PCr-R values clustered under 31 seconds; while in contrast, all but one case had values distributed over 35 seconds, up to a maximum of ∼70 seconds.
Figure 3. PCr-R of GWV vs Matched Controls.
GWV = Gulf War veteran; PCr-R = phosphocreatine-recovery post-exercise. Line segments connect controls to their case matches. Note clustering of most control (but not case) PCr-R values under 31 seconds, with widely distributed case values primarily in excess of 35 seconds.
Table 1. PCr-R by 31P-MRS in GWI Cases vs Matched Controls.
| Case PCr-R (sec) | Difference | Control PCr-R (matched to that case) (sec) | Difference (sec) | |
| Pair 1 | 35.200 | > | 21.800 | +13.4 |
| Pair 2 | 70.300 | > | 28.800 | +41.2 |
| Pair 3 | 54.000 | > | 24.600 | +29.4 |
| Pair 4 | 18.300 | < | 23.800 | −5.5 |
| Pair 5 | 62.800 | > | 47.500 | +15.3 |
| Pair 6 | 35.300 | > | 26.100 | +9.2 |
| Pair 7 | 46.900 | > | 30.500 | +16.4 |
GWI = Gulf War illness; PCr-R = phosphocreatine-recovery post-exercise; 31P-MRS = 31Phosphorus Magnetic Resonance Spectroscopy; sec = seconds.
The case that provided the exception had the lowest PCr-R value of all subjects – cases or controls. Of note, whereas all other participants with GWI cited their pre-Gulf health as “excellent” or “very good”, this was the sole case to rate their health prior to their Gulf War participation as merely “good,” perhaps suggesting an unrelated prior health condition.
Discussion
To our knowledge, this is the first study to document prolongation of PCr-R in GWI, or indeed to assess PCr-R or MD in GWI. PCr-R is viewed as a “robust” index of MD [23]; thus, this study supports the hypothesis that mitochondrial pathology is present in GWI. Not only did all-but-one matched case-control pair show the hypothesized directionality, but values for all controls except one were clustered under 31 seconds, while values for all cases but one were distributed over 35 seconds, and up to 70 seconds, providing relatively striking separation. Lower pre-Gulf health was reported by the PCr-R-nonconforming veteran, relative to all other assessed veterans, affording the prospect that different health factors may have been at play in that individual.
These findings add empirical support to evidence previously assembled, making a case for a role for MD (and interrelated OS, which promotes and is caused by MD [17], [18]) in GWI [3].They also fit with evidence suggesting benefit of coenzyme Q10 for symptoms in GWI [30]. MD is expected based on known exposure relations to GWI [3], [16], [31]; and indeed, fully accounts for key “perplexing” clinical features of GWI not equally accounted for by any proposed alternative hypothesis, including which symptoms dominate (fatigue, muscle and brain, the latter two because they are energetically demanding postmitotic tissues), the spectrum of other symptoms less consistently present, the high symptom multiplicity, protean character, and variable latency to onset [3]. A role for MD also coheres with evidence of amyotrophic lateral sclerosis, observed in several studies to be present at elevated rates among GWV [32], [33], [34]: this condition involves MD, in a vicious cycle with OS [35].
Physiological dysfunction in GWI made evident following exercise has been reported for gene expression and imaging parameters [36], [37], [38]. Our finding of prolongation of post-exercise PCr-R represents a further instance in which exercise unmasks altered physiology in GWI. Of note, an oxidative-mitochondrial foundation could be postulated to underlie the other observed exercise-related findings.
This study has limitations. Most importantly, the sample size was small. However significance of the difference between cases and matched controls in the face of a small sample requires a correspondingly large effect size. Additionally, close matching of cases with their controls on key parameters advantages authority of the comparison. So too does the relatively striking separation between PCr-R values for the case and control groups. Exercise and diet patterns of controls were not elicited. Some otherwise eligible GWV with GWI were excluded from participation for MR safety reasons, due to historical or current hobbies involving welding, soldering, or grinding (associated with risk of unrecognized embedded metal around the eyes). However, this exclusion applied equally to cases and controls.
In summary, veterans of the 1990-1 Persian Gulf War who are affected by GWI show prolonged post-exercise recovery of PCr on 31P-MRS, consistent with a role for MD in GWI. 31P-MRS may provide an objective diagnostic tool for GWI that can be tracked with treatment. Findings of this study require replication and ideally assessment in a larger sample, and will benefit from triangulating validation with additional mitochondrial assessment tools.
Acknowledgments
We sincerely thank the GWV and control subjects, who generously gave their time to this effort, and without whom this would not have been possible. We thank Dr. Graeme M. Bydder, Lilly Pacheco, John Firebaugh, and Richard Znamirowski from the UC San Diego Bydder MR Center for their technical support and contributions. We thank Janis Ritchie and Kate Bloom for their assistance with this project.
Funding Statement
This study was funded by a University of California, San Diego, Academic Senate Award (MED126M) and the Department of Defense Congressionally Directed Medical Research Program (GW120071). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Binns JH, Cherry N, Golomb BA, Graves JC, Haley RW, et al. (2004) Research Advisory Committee on Gulf War Veterans' Illnesses: Scientific Progress in Understanding Gulf War Veterans' Illnesses: Report and Recommendations. Washington, D.C.
- 2. Steele L (2000) Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemiol 152: 992–1002. [DOI] [PubMed] [Google Scholar]
- 3.Golomb BA (2012) Oxidative Stress and Mitochondrial Injury in Chronic Multisymptom Conditions: From Gulf War Illness to Autism Spectrum Disorder. Available: http://precedings.nature.com/documents/6847/version/1. Accessed 2014 Mar 3.
- 4.Binns JH, Barlow C, Bloom FE, Clauw DJ, Golomb BA, et al. (2008) Gulf War Illness and the Health of Gulf War Veterans. Scientific Findings and Recommendations. Washington D.C.: U.S. Government Printing Office.
- 5. Ozakinci G, Hallman WK, Kipen HM (2006) Persistence of symptoms in veterans of the First Gulf War: 5-year follow-up. Environ Health Perspect 114: 1553–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hotopf M, David AS, Hull L, Nikalaou V, Unwin C, et al. (2003) Gulf war illness–better, worse, or just the same? A cohort study. BMJ 327: 1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golomb BA (2008) Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci U S A 105: 4295–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golomb BA (1999) A Review of the Scientific Literature as it Pertains to Gulf War Illnesses, Vol 2: Pyridostigmine Bromide. Washington, DC: RAND. 385 p. [Google Scholar]
- 9.Cecchine G, Golomb BA, Hilborne LH, Spektor DM, Anthony CR (2000) A Review of the Scientific Literature as it Pertains to Gulf War Illnesses, Vol 8: Pesticides. Santa Monica, CA: RAND. 182 p. [Google Scholar]
- 10.Department of Defense (2003) Environmental Exposure Report. Pesticides. Final Report. Available: http://www.gulflink.osd.mil/pest_final/index.html. Accessed 2014 Mar 3.
- 11.Fricker RD, Reardon E, Spektor DM, Cotton SK, Hawes-Dawson J, et al. (2000) A Review of the Scientific Literature as It Pertains to Gulf War Illnesses. Volume 12: Pesticide Use During the Gulf War: A Survey of Gulf War Veterans. Santa Monica, CA: RAND, MR-1018/12-OSD.
- 12.Gillert DJ (1997) DoD Says 98,910 Exposed to Low Levels of Nerve Agent. Available: http://www.defense.gov/News/NewsArticle.aspx?ID=41500. Accessed 2014 Mar 6.
- 13.General Accounting Office (2004) Gulf War Illnesses: DOD's Conclusions about U.S. Troops' Exposure Cannot Be Adequately Supported. GAO report number GAO-04-159. Available: http://www.gao.gov/new.items/d04159.pdf. Accessed 2014 Mar 3.
- 14. Kroenke K, Koslowe P, Roy M (1998) Symptoms in 18,495 Persian Gulf War veterans. Latency of onset and lack of association with self-reported exposures. J Occup Environ Med 40: 520–528. [DOI] [PubMed] [Google Scholar]
- 15. Abou-Donia MB, Wilmarth KR, Abdel-Rahman AA, Jensen KF, Oehme FW, et al. (1996) Increased neurotoxicity following concurrent exposure to pyridostigmine bromide, DEET, and chlorpyrifos. Fundam Appl Toxicol 34: 201–222. [DOI] [PubMed] [Google Scholar]
- 16. Milatovic D, Gupta RC, Aschner M (2006) Anticholinesterase toxicity and oxidative stress. ScientificWorldJournal 6: 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee HC, Wei YH (1997) Role of Mitochondria in Human Aging. J Biomed Sci 4: 319–326. [DOI] [PubMed] [Google Scholar]
- 18. Genova ML, Pich MM, Bernacchia A, Bianchi C, Biondi A, et al. (2004) The mitochondrial production of reactive oxygen species in relation to aging and pathology. Ann N Y Acad Sci 1011: 86–100. [DOI] [PubMed] [Google Scholar]
- 19. Wei YH (1998) Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med 217: 53–63. [DOI] [PubMed] [Google Scholar]
- 20. Pena-Llopis S, Ferrando MD, Pena JB (2003) Fish tolerance to organophosphate-induced oxidative stress is dependent on the glutathione metabolism and enhanced by N-acetylcysteine. Aquat Toxicol 65: 337–360. [DOI] [PubMed] [Google Scholar]
- 21. Pena-Llopis S, Ferrando MD, Pena JB (2003) Increased recovery of brain acetylcholinesterase activity in dichlorvos-intoxicated European eels Anguilla anguilla by bath treatment with N-acetylcysteine. Dis Aquat Organ 55: 237–245. [DOI] [PubMed] [Google Scholar]
- 22. Kemp GJ, Taylor DJ, Radda GK (1993) Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed 6: 66–72. [DOI] [PubMed] [Google Scholar]
- 23. Thompson CH, Kemp GJ, Sanderson AL, Radda GK (1995) Skeletal muscle mitochondrial function studied by kinetic analysis of postexercise phosphocreatine resynthesis. J Appl Physiol 78: 2131–2139. [DOI] [PubMed] [Google Scholar]
- 24. Vanhamme L, van den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35–43. [DOI] [PubMed] [Google Scholar]
- 25. Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D (2001) Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med 31: 269–286. [DOI] [PubMed] [Google Scholar]
- 26. Hamilton G, Patel N, Forton DM, Hajnal JV, Taylor-Robinson SD (2003) Prior knowledge for time domain quantification of in vivo brain or liver 31P MR spectra. NMR Biomed 16: 168–176. [DOI] [PubMed] [Google Scholar]
- 27. Murphy EJ, Rajagopalan B, Brindle KM, Radda GK (1989) Phospholipid bilayer contribution to 31P NMR spectra in vivo. Magn Reson Med 12: 282–289. [DOI] [PubMed] [Google Scholar]
- 28.Golomb BA, Erickson LC, Scott-Van Zeeland AA, Koperski S, Haas RH, et al. (2013) Assessing Bioenergetic Compromise in Autism Spectrum Disorder With 31P Magnetic Resonance Spectroscopy: Preliminary Report. J Child Neurol. [DOI] [PMC free article] [PubMed]
- 29. Hamilton G, Allsop JM, Patel N, Forton DM, Thomas HC, et al. (2006) Variations due to analysis technique in intracellular pH measurements in simulated and in vivo 31P MR spectra of the human brain. J Magn Reson Imaging 23: 459–464. [DOI] [PubMed] [Google Scholar]
- 30.Golomb BA, Koperski S, Rock CL, Devaraj S, Allison M, et al. (2014) Coenzyme Q10 Benefits Symptoms in Gulf War Veterans: Results of a Randomized Double-Blind Pilot Study. Neural Comput, in press. [DOI] [PubMed]
- 31. Chan JY, Chan SH, Dai KY, Cheng HL, Chou JL, et al. (2006) Cholinergic-receptor-independent dysfunction of mitochondrial respiratory chain enzymes, reduced mitochondrial transmembrane potential and ATP depletion underlie necrotic cell death induced by the organophosphate poison mevinphos. Neuropharmacology 51: 1109–1119. [DOI] [PubMed] [Google Scholar]
- 32. Coffman CJ, Horner RD, Grambow SC, Lindquist J (2005) Estimating the occurrence of amyotrophic lateral sclerosis among Gulf War (1990-1991) veterans using capture-recapture methods. Neuroepidemiology 24: 141–150. [DOI] [PubMed] [Google Scholar]
- 33. Haley RW (2003) Excess incidence of ALS in young Gulf War veterans. Neurology 61: 750–756. [DOI] [PubMed] [Google Scholar]
- 34. Horner RD, Kamins KG, Feussner JR, Grambow SC, Hoff-Lindquist J, et al. (2003) Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology 61: 742–749. [DOI] [PubMed] [Google Scholar]
- 35. Golomb BA, Kwon EK, Koperski S, Evans MA (2009) Amyotrophic lateral sclerosis-like conditions in possible association with cholesterol-lowering drugs: an analysis of patient reports to the University of California, San Diego (UCSD) Statin Effects Study. Drug Saf 32: 649–661. [DOI] [PubMed] [Google Scholar]
- 36. Whistler T, Fletcher M, Lonergan W, Zeng X-R, Lin J-M, et al. (2009) Impaired immune function in Gulf War Illness. BMC Medical Genomics 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Broderick G, Ben-Hamo R, Vashishtha S, Efroni S, Nathanson L, et al. (2013) Altered immune pathway activity under exercise challenge in Gulf War Illness: an exploratory analysis. Brain Behav Immun 28: 159–169. [DOI] [PubMed] [Google Scholar]
- 38. Rayhan RU, Stevens BW, Raksit MP, Ripple JA, Timbol CR, et al. (2013) Exercise Challenge in Gulf War Illness Reveals Two Subgroups with Altered Brain Structure and Function. PLoS ONE 8: e63903. [DOI] [PMC free article] [PubMed] [Google Scholar]