Abstract
The function of some hypothetical proteins, possibly regulated by key hrp regulators, in the pathogenicity of phytopathogenic bacteria remains largely unknown. In the present study, in silicon microarray data demonstrated that the expression of 17 HrpX-regulated protein (Xrp) genes of X. oryzae pv. oryzicola (Xoc), which causes bacterial leaf streak in rice, were either positively or negatively regulated by HrpX or/and HrpG. Bioinformatics analysis demonstrated that five Xrps possess a putative type III secretion (T3S) signal in the first 50 N-terminal amino acids, six xrp genes contain a PIP-box-like sequence (TTCGB-NX-TTCGB, 9≤X≤25) in the promoter regions, and two Xrps have both motifs. Twelve Xrps are widely conserved in Xanthomonas spp., whereas four are specific for X. oryzae (Xrp6) or Xoc (Xrp8, Xrp14 and Xrp17). In addition to the regulation by HrpG/HrpX, some of the 17 genes were also modulated by another hrp regulator HrpD6. Mutagenesis of these 17 genes indicated that five Xrps (Xrp1, Xrp2, Xrp5, Xrp8 and Xrp14) were required for full virulence and bacterial growth in planta. Immunoblotting assays and fusion with N-terminally truncated AvrXa10 indicated that Xrp3 and Xrp5 were secreted and translocated into rice cells through the type-III secretion system (T3S), suggesting they are novel T3S effectors. Our results suggest that Xoc exploits an orchestra of proteins that are regulated by HrpG, HrpX and HrpD6, and these proteins facilitate both infection and metabolism.
Introduction
Bacterial leaf streak (BLS) of rice, which is caused by Xanthomonas oryzae pv. oryzicola (Xoc), is a destructive plant disease in Asia. The pathogen infects rice through leaf stomata or wounds and colonizes intercellular spaces in the mesophyll, resulting in water-soaked interveinal lesions that develop into translucent streaks [1]. The infection routes and the symptoms caused by Xoc differ from those incited by the closely-related pathogen, X. oryzae pv. oryzae (Xoo). Xoo enters rice leaves through hydathodes or wounds, propagates in the intercellular spaces of the underlying epidermis, and then spreads throughout the plant in the xylem, where it presumably interacts with xylem parenchyma cells [2], [3], [4]. The Xoc-rice pathosystem is an important working model to elucidate how pathogens evade the plant host immune system [2], [5]. The complete genome sequence and comparative and functional genomic studies of three Xoo strains like KACC10331 [6], PXO99A [7], MAFF311018 [8] and Xoc strain BLS256 [9] have furthered our understanding of Xanthomonas-rice interactions. However, it remains unclear whether the numerous hypothetical proteins, maybe possibly regulated by HrpX (Xrps) annotated in X. oryzae, are involved in virulence.
The type III secretion system (T3S) is a pathogenicity determinant machine for Gram-negative pathogenic bacteria in host plants [10], [11]. The Xanthomonad T3S is encoded by the hrp-hrc-hpa genes [5], [12] and secretes a repertoire of effector proteins (T3SEs) into plant cells to trigger plant disease development [13]–[16]. These T3SEs may function to overcome PAMP- (pathogen-associated molecular pattern) triggered immunity (PTI) and Effector-triggered immunity (ETI), or promote effector-triggered susceptibility (ETS) [17]–[19]. In X. oryzae, T3SEs are classified into two types: transcriptional activator-like effectors (TALEs) [6], [20], [21] and NTALEs (non-TAL effectors); the latter group is also known as the Xop (Xanthomonas out protein) effectors [16], [22]. Some of the NTALEs are Xrps originally annotated in the genomes of Xanthomonas spp. [16], implying that some Xrps may be uncharacterized T3SEs.
The expression of genes coding for the T3S and effectors is generally plant-inducible and regulated by a key hrp regulatory factor, HrpX [12], [14], [23], [24]. HrpX is an AraC-type transcriptional regulator that controls the expression of genes in the HrpX regulon by binding the PIP (plant-inducible promoter)-box; this is a conserved cis-element with the consensus TTCGB-N15-TTCGB (‘B’ refers to any base except adenine) [25]–[28]. The PIP-box is normally followed by a −10 box that is located at 30–32 bp further downstream [29]. T3SEs also contain secretion signals in the first 50 N-terminal amino acids, which are characterized by one or more of the following: ≥20% Ser and Pro [22], [26], [30]; more than five Ser residues [13], [31], [32]; an aliphatic amino acid (Ile, Leu, or Val) or Pro at the third or fourth position; and a lack of negatively charged amino acids within the first 12 residues [33]. However, it is important to note that genes in the HrpX regulon may not be T3SEs; for example, HrpX-regulated proteins from Xoo recently identified by 2D-difference gel electrophoresis (2-DIGE) did not function as T3SEs [34]. The transcription and translocation of HrpX regulon candidates have been examined using several reporter systems, such as calmodulin-dependent adenylate cyclase (Cya) of Bordetella pertussis [22], [30], [31], [35], gusA [36]–[40] and avirulence proteins (e.g., AvrBs1 and AvrXa10) lacking the T3S signal sequence [24], [38], [41].
The expression of HrpX is regulated by HrpG, which belongs to the OmpR family of two-component signal transduction response regulators [42], [43]. HrpG regulates the expression of hrpX and hrpA operon and also controls the expression of several proteins that function as cell wall degrading enzymes (CWDEs), which are secreted by the type II secretion system (T2SS) [14], [44], [45]. Recently, a novel hrp regulator, HrpD6, was identified and shown to be regulated by HrpG and HrpX; HrpD6 regulates the expression of hpa2, hpa1, hpaB, hrcC, and hrcT [12]. However, it remains unclear whether Xrps in Xanthomonas are regulated by HrpG or HrpD6.
In this study, bioinformatic and genetic approaches were used to characterize 17 Xrp-coding genes from in silicon data. Different transcriptional profiles of these genes in the wild-type strain Xoc RS105, hrpG (RΔhrpG), and hrpX (RΔhrpX) mutants [12] were compared. Two Xrp proteins, XOC_3956 and XOC_1550, were identified as new T3SEs in Xoc.
Materials and Methods
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Table S1 in File S1. Escherichia coli was grown at 37°C in Luria-Bertani medium [46]. Xanthomonas strains and other derivatives were grown in NB, NA, NAN, NAS [47], XOM3 [48] or with rice suspension cells [48]. Antibiotics were added at the following concentrations (µg/ml) when required: kanamycin (Kan), 25; rifampicin (Rif), 50; ampicillin (Ap), 100; and spectinomycin (Sp), 50.
Microarray design
An oligonucleotide microarray was designed at the Shanghai Biotechnology Corporation (Shanghai, China). Each slide contained six arrays, and each array contained approximately 15,000 spots (our probes were represented in triplicate). For Xoc BLS256, the genome sequence was also available from the NCBI database as accession AAQN01000001(GI:94721269). Up to five candidate probes per target (sense orientation) were designed with the Agilent eArray web tool, using temperature-matching methodology, a preferred probe melting temperature of 80°C, no 3′bias, and a target length of 60 bp. Shorter probes were extended to 60 bp using the Agilent linker.
RNA isolation and microarray execution
Xoc strain RS105 and the hrpG and hrpX mutants (RΔhrpG and RΔhrpX, respectively) [12] were cultured overnight in NB broth at 28°C in a shaking incubator and collected the following day via centrifugation at 5,000 rpm. Each strain was washed once in rice suspension cells [49] before resuspension in rice cells at OD600 = 0.6. Strains were then incubated for 16 h at 60 rpm at 25°C. RNA was extracted from 1 ml of co-cultivated cells using TRIzol® Reagent (Invitrogen, Shanghai, China) as described by the manufacturer. All RNA was quantified using an Eppendorf BioSpectrometer kinetic (Eppendorf, Shanghai, China) and checked for quality using an RNA 6000 Nano Kit and a 2100 Bioanalyzer (Agilent Technologies). Fluorescent labeling of total RNA was performed as described previously [49] using the following array design on a single 6×15 k format slide: 1, WT rep 1 (Cy3) and RΔhrpG rep 1 (Cy5); 2, RΔhrpG rep 2 (Cy3) and WT rep 2 (Cy5); 3, WT rep 3 (Cy3) and RΔhrpG rep 3 (Cy5); 4, WT rep 1 (Cy3) and RΔhrpX rep 1 (Cy5); 5, RΔhrpX rep 2 (Cy3) and WT rep 2 (Cy5); 6, WT rep 3 (Cy3) and RΔhrpX rep 3 (Cy5). This design incorporated a dye-swap and balanced labeling of all samples. Levels and efficiencies of labeling were estimated using a spectrophotometer. Microarray hybridization, washing and scanning were performed in the JHI Sequencing and Microarray Facility as described previously [50]. Microarray images were imported into Agilent Feature Extraction (FE) (v.9.5.3) software and aligned with the appropriate array grid template file (021826_D_F_20081029). Intensity data and quality control (QC) metrics were extracted using the recommended FE protocol (GE2-v5_95_Feb07). Entire FE datasets for each array were loaded into GeneSpring (v.7.3) software for further analysis.
Microarray analysis
Data were normalized using default settings for two-channel arrays and transformed to account for dye-swaps. Data from each array were normalized using the Lowess algorithm to minimize differences in dye incorporation efficiency. Unreliable data flagged as absent in all replicate samples by the FE software were discarded. Gene lists with significant change were generated from combined replicate datasets for each pares, RΔhrpG/RS105 and RΔhrpX/RS105, using volcano plot filtering if the ratio is lower than 0.55 or higher than 1.75 with the P value less than 0.05 (Student's t test).
DNA manipulation and plasmid construction
DNA manipulation was performed following standard procedures [46]. Biparental conjugal transfer of plasmids from E. coli to Xoc was performed as described previously [51]. PCR amplification was performed with primers (Table S2 in File S1) and genomic DNA of Xoc RS105; the genome sequence of Xoc BLS256 was used as a reference (http://cmr.jcvi.org/cgi-bin/CMR/GenomePage.cgi?org=Xoc). All plasmids constructs were confirmed by restriction enzyme digestion and sequencing.
To construct transcriptional fusions of xrp genes with the promoterless gusA (β-glucuronidase) gene, a 500-bp region upstream of each xrp ORF was PCR-amplified with primer sets pxrpX-F/pxrpX-R (X refers to 17 xrp genes; 1 to 17) (Table S2 in File S1). The PCR products were fused in-frame with gusA in pUFR034GUS (Table S1 in File S1), resulting in pxrpXGUS (X refers to 17 xrp genes; 1 to 17). Recombinant plasmids were introduced into the wild-type strain RS105, and the hrpG, hrpX and hrpD6 mutants (see reference 12 for RΔhrpG, RΔhrpX, and RΔhrpD6) (Table S1 in File S1) using biparental conjugation as described above.
To generate transcriptional fusions of xrp genes with c-Myc tags, fragments containing native promoters and corresponding xrp ORFs were PCR-amplified with the xrpX-F/xrpX-R primer pairs (X refers to 17 xrp genes, Table S2 in File S1) using RS105 genomic DNA as the template. After sequencing for confirmation, PCR products were cloned in pUFR034Myc in-frame at suitable enzyme sites, generating pXrpXMyc (X refers 1 to 17 xrp genes) (Table S1 in File S1). The recombinant plasmids were introduced into wild-type RS105 and the hrcV mutant (RΔhrcV) by the biparental conjugation method.
Chimeric fusions were also generated between a variant of AvrXa10 lacking 28 amino acids at the N-terminus and xrp3 and xrp5. The truncated form of AvrXa10 was designated AvrXa10Δ28 and has been described previously [26]. The sequences obtained from xrp3 and xrp5 included approximately 500 bp upstream of the translational start site and the first 150 nucleotides. These regions of xrp3 and xrp5 were obtained using primer pairs xrp3-N-F/xrp3-N-R and xrp5-N-F/xrp5-N-R, respectively (Table S2 in File S1). The PCR products were ligated into pBlueAvrXa10Δ28 at SacΙ and PstΙ sites. The constructs were then cloned into pHMI at the SacΙ site, resulting in pXrp3AvrXa10Δ28 and pXrp5AvrXa10Δ28 (Table S1 in File S1). Recombinant plasmids were introduced into Xoo PXO99A and PΔhrcU by electroporation.
YFP was used as a reporter to investigate the subcellular localization of selected T3SEs. The complete ORFs encoding xrp3 and xrp5 without stop codons were PCR-amplified from genomic DNA of Xoc RS105 with xrp3-Y-F/xrp3-Y-R and xrp5-Y-F/xrp5-Y-R, respectively (Table S2 in File S1). The products were cloned in-frame with yfp in pA7-YFP [52], resulting in pXrp3YFP and pXrp5YFP (Table S1 in File S1), respectively. These two recombinants were then transferred into Arabidopsis (Ecotype Col-0) mesophyll protoplasts by PEG-calcium fusion as described previously [53].
Quantitative real-time PCR (qRT-PCR)
The expression of selected xrp genes was assayed by qRT-PCR with corresponding primer pairs (Table S2 in File S1). All primers were designed with Beacon Designer 7 software. RNA was extracted from Xanthomonas strains as described previously [47] using TRIzol® Reagent (Takara, Dalian, China) and the manufacturer's recommendations. cDNA synthesis was conducted with AMV random primers purchased from Takara. Prior to synthesis of the first-strand cDNA, total RNAs were digested with RNase-free DNase I (TaKaRa) to remove potential traces of genomic DNAs. qRT-PCR was performed on the Applied Biosystems 7500 qRT-PCR System using SYBR Premix ExTaq™ (Takara). PCR conditions included the following parameters: denaturation at 95°C for 30 s; 40 cycles at 95°C, 5 s; and 60°C, 34 s. Experiments were performed at least three times in triplicate. Internal controls included gyrB (XOC_0006, DNA gyrase B subunit) and rpoD (XOC_2329, RNA polymerase sigma factor 70) [28], [54].
β-glucuronidase (GUS) activity assays
For GUS activity assays, Xoc strains were preincubated in 5 ml NB broth at 28°C for 16–20 h until the OD600 = 0.6. An aliquot (100 µl) of this culture was transferred into 5 ml fresh NB broth. Bacterial cells were collected, washed twice, and resuspended in XOM3 to an OD600 = 2.0. After incubation at 28°C for 6 h, 1 ml of sonic buffer (40 mM Tris-HCl, pH 7.0, 20 mM β-mercaptoethanol, 10 mM EDTA, and 2% Triton X-100) was added into 1 ml of the bacterial culture. This mixture was frozen in liquid nitrogen and then thawed in at 37°C for 5 min. This procedure was repeated five times, and the mixture was centrifuged (12,000 rpm) at 4°C for 15 min. Then, 90 µl 4-methylumbelliferone-β-glucuronide (4-MUG) (Sigma, Shanghai, China) [55] was added into 10 µl supernatant and incubated at 37°C for 1 h. The reaction was terminated by adding 1 ml of 2 M Na2CO3. GUS activity was measured at 415 nm with the Modulus™ Single Tube Multifunction Tester (YuanPingHao, Beijing, China) [56]. One unit was defined as 1 nM of 4-methyl-umbelliferone (4-MU) produced per min per OD600 of bacterial cells as described [56]. Three independent experiments were performed, and similar results were obtained.
Mutant construction and complementation studies
To generate non-polar mutations in the 17 xrp genes, two fragments flanking each gene were PCR-amplified from Xoc RS105 genomic DNA with primer pairs xrpXI-F/xrpXI-R and xrpXII-F/xrpXII-R (X refers to the 17 xrp genes; 1 to 17, Table S2 in File S1). After verification by sequence analysis, the two fragments flanking each gene were fused and ligated to the suicide vector pKMS1 [57] at selected restriction sites (Table S2 in File S1), producing plasmids pKMSΔxrpX (X refers 1 to 17, Table S1 in File S1). Mutations in the 17 xrp genes were generated by two-step homologous recombination as described previously [57]. Deletions in the xrp genes were confirmed by PCR-amplification with the primer pairs, xrpXI-F/xrpXII-R (Table S2 in File S1); the results confirmed that the PCR products were smaller than those obtained in RS105 (data not shown). For complementation studies, constructs designated pXrpXMyc (X refers 1 to 17, Table S1 in File S1) were introduced into the corresponding xrp mutants by biparental conjugation. Mutants containing xrp genes in trans were verified by colony-PCR and named CRΔxrpX (X refers 1 to 17, Table S1 in File S1).
Bacterial virulence and growth in planta
Rice cultivar Shanyou 63 (two weeks old) was used to evaluate the virulence of Xoc RS105 and derivatives. Bacterial cells were adjusted to 1×108 cfu/ml and infiltrated into newly-expanded leaves with a needleless syringe at three locations per leaf. Three leaf discs (0.5 cm in diameter) were excised with a cork borer from each infiltrated area. After sterilization in 70% ethanol and 30% hypochlorite, the discs were macerated using a sterile mortar and pestle in 1 ml of distilled water, diluted and plated to determine cfu/cm2. Serial dilutions were spotted in triplicate on NA plates with appropriate antibiotics. The plates were incubated at 28°C for 3–4 days until single colonies could be counted. The bacterial population (cfu/cm2 of leaf area) was then estimated, and the standard deviation was calculated using colony counts from three triplicate spots of three samples obtained at each time point. All rice cultivars were grown in a greenhouse maintained at 25°C with a 12-h photoperiod. Experiments were repeated at least three times.
Near-isogenic lines of rice cultivar IRBB10 were used to assay the pathogenicity of Xoo PXO99A and derivatives as described previously [47]. Plant responses were scored 14 dpi for lesion lengths. Experiments were repeated at least three times.
Type III secretion assays
The pXrpXMyc plasmids were transformed into the wild-type RS105 and RΔhrcV for detection of secreted proteins. Xoc strains were preincubated in NB medium, washed twice, and suspended at OD600 = 2.0 with sterilized water. An aliquot (40 µl) of the bacterial suspension was inoculated into 1 ml of XOM3 medium (pH 6.0), adjusted to OD600 = 1.0, and incubated at 28°C for 6 h with the appropriate antibiotics. Supernatant fractions were separated using a 0.22 µm filter, and the supernatant fraction (50 ml) was reduced to 5 ml by vacuum evaporation. Proteins were precipitated with 12.5% trichloroacetic acid at 4°C for 16 h, centrifuged at 3000× g for 15 min, and then washed briefly with acetone and air-dried. The protein pellet was resuspended in SDS buffer containing dithiothreitol (DTT) [58]. Proteins were separated on 10% SDS-PAGE gels and transferred to membranes for immunoblotting using primary antibody anti-c-Myc (Huaan, Hangzhou, China). Primary antibodies were recognized by anti-rabbit secondary antibodies (Huaan) and visualized by autoradiography with the Western-Light chemiluminescence system (Transgene, Beijing, China). Experiments were repeated at least twice and Hpa1 was used as a positive control [59].
Results
Screening of T3SE candidates from Xoc
Genome-wide identification of bacterial virulence genes has been greatly facilitated by the availability of microarrays [60], [61]. Considering the critical fact that HrpG and HrpX are two key regulators for pathogenicity determinants in Xanthomonads [14], [26], [28], [29], [43], [44], the expression profiles of genome widely annotated hypothetical proteins in Xoc RS105 and hrpG and hrpX mutants were only evaluated based on the annotated sequence of Xoc BLS256 (AAQN01000001.1 (GI:94721269)). The three Xoc strains (RS105, RΔhrpG, RΔhrpX) were co-cultivated with rice cells for 16 h at 25°C, and bacterial RNAs were extracted and hybridized with the BLS256 genechip. The expression levels of hypothetical protein genes in the hrpG and hrpX mutants were compared with the wild-type RS105 and taken as the candidates if the ratio was lower than 0.55 or higher than 1.75 with the P value less than 0.05 (Table S3 in File S1). For a comparison, we checked the expression of hrp genes and known T3SE genes (Table S3 in File S1) and found that the expression patterns of these genes in silicon were consistent with those that were experimentally explored previously [12], [23], [44], [56], [61] Totally, there were 247 hypothetical protein genes significantly (P≤0.05, t test) altered their expression levels in the hrpG or/and hrpX mutants, comparing to the wild-type RS105 (Table S3 in File S1), indicating that these in silicon data are helpful to find HrpG/HrpX-regulated proteins (Xrp).
To reveal whether there is(are) any T3SE protein(s) in these Xrp candidates, three criteria are considered: (i) candidate genes are homologous to known T3SEs in other phytopathogenic bacteria [16], [22]; (ii) expression of candidate genes is HrpX-dependent with the presence of a PIP-box in the promoter [29], [38], and (iii) the candidate protein product contains targeting signals at the N-termini that are indicative of T3S secretion [22], [47]. For these, sequences in the xrp promoter regions and in the N-termini of Xrp translational products (identical correspondingly to these in Xoc BLS256 after sequencing confirmation) were analyzed and the protein IDs were changed into Table 1 and Table 2 according to the new version of the genome sequence of Xoc BLS256 (NZ_AAQN01000001.1, GI:353459993). In Xrp1, Xrp3, Xrp4,,Xrp5, Xrp7, Xrp8, Xrp9, Xrp11 and Xrp14, the total number of Ser and Pro residues in the first 50 N-terminal amino acids constitutes more than or equal to eight (Table 1). Xrp1, Xrp3, Xrp4, Xrp5, Xrp6, Xrp8 and Xrp11 contain at least five Ser residues at the N-terminus (Table 1). Promoter analysis showed that there are PIP-box-like sequences upstream of xrp1, xrp2, xrp8, xrp9, xrp13, xrp14, xrp15 and xrp16 ORFs (Table 1). Interestingly, these PIP-box-like sequences, which contained 9–25 nucleotides between TTCGB motifs, differ from the typical consensus PIP-box (TTCGB-N15-TTCGB). To have a better understanding, three candidates (Xrp10, Xrp12 and Xrp17) without either of two properties abovementioned were selected from 247 hypothetical protein genes (Table S3 in File S1) as the comparison.
Table 1. Protein and nucleotide sequence analysis of 17 xrp genes.
| RS105 Xrp | Protein ID in BLS256 | Gene ID in BLS256 | Ser and Proa | Ser | Pro | Leub | Asp and Gluc | 3rd AA | 4th AA | PIP box-liked | −10 Box-like | |
| position | 5′-3′ sequence | |||||||||||
| Xrp1 | YP_005627937 | XOC_1601 | 8 | 6 | 2 | 8 | 0 | T | H | −96/−75 | TTCGC-N12-TTCGC | Ne |
| Xrp2 | YP_005630809 | XOC_4584 | 6 | 4 | 2 | 2 | 0 | K | F | −106/−85 | TTCGT-N12-TTCGT | N |
| Xrp3 | YP_005630213 | XOC_3956 | 10 | 6 | 4 | 3 | 1 | T | R | N | N | |
| Xrp4 | YP_005630212 | XOC_3955 | 8 | 6 | 2 | 7 | 2 | R | H | N | N | |
| Xrp5 | YP_005627898 | XOC_1550 | 8 | 7 | 1 | 7 | 2 | G | E | N | N | |
| Xrp6 | YP_005629702 | XOC_3440 | 6 | 5 | 1 | 4 | 1 | V | E | N | N | |
| Xrp7 | YP_005630808 | XOC_4583 | 9 | 2 | 7 | 6 | 1 | S | L | N | N | |
| Xrp8 | YP_005628761 | XOC_2462 | 9 | 6 | 3 | 6 | 1 | G | L | −81/−56 | TTCGA-N16-TTCGA | N |
| Xrp9 | YP_005628272 | XOC_1951 | 11 | 3 | 8 | 8 | 0 | L | R | −114/−69 | TTCGT-N6-TTCGA-N25-TTCGC | -N33-TATGAT |
| Xrp10 | YP_005626937 | XOC_0560 | 6 | 2 | 4 | 1 | 0 | V | P | N | N | |
| Xrp11 | YP_005629396 | XOC_3130 | 8 | 8 | 0 | 4 | 1 | I | Q | N | N | |
| Xrp12 | YP_005630266 | XOC_4010 | 5 | 2 | 3 | 8 | 1 | A | L | N | N | |
| Xrp13 | YP_005627233 | XOC_0860 | 5 | 1 | 4 | 5 | 1 | L | A | −240/−211 | TTCGC-N20-TTCGG | N |
| Xrp14 | YP_005626509 | XOC_0084 | 10 | 3 | 7 | 4 | 2 | D | D | −61/−26 | TTCGC-N25-TTCGC | N |
| Xrp15 | YP_005629104 | XOC_2829 | 2 | 1 | 1 | 5 | 1 | I | E | −121/−90 | TTCGA-N21-TTCGC | N |
| Xrp16 | YP_005629103 | XOC_2828 | 5 | 3 | 2 | 5 | 0 | A | P | −362/−331 | TTCGA-N22-TTCGG | N |
| Xrp17 | hrpFB | 2 | 2 | 0 | 6 | 1 | Y | F | N | N | ||
Number of Ser and Pro residues in the N-terminal 50 amino acids.
Number of Leu residues in the N-terminal 50 amino acids.
Number of Asp and Glu residues in the N-terminal 12 amino acids.
Includes an imperfect PIP-box like sequence (TTCGB-Nx-TTCGB,B,A/T/C/G) in the respective promoter.
No corresponding characteristics.
Table 2. Conservation of 17 Xrps of X. oryzae pv. oryzicola RS105 in other Xanthomonas species.
| Xoc RS105 | Description | Xoc BLS256 | Xoo | Xac 306 | Xcv 85-10 | Xcc ATCC33913 | ||
| PXO99A | MAFF311018 | KACC10331 | ||||||
| Xrp1 | cysteine protease | XOC_1601 (100%)a | PXO_04730 (83%) | XOO1385 (84%) | XOO1487 (83%) | XAC2853 (88%) | XCV3013 (87%) | XCC2693 (83%) |
| Xrp2 | hypothetical protein | XOC_4584 (100%) | PXO_03859 (82%) | XOO4169 (82%) | XOO4426 (82%) | N | XCV0093 (54%) | N |
| Xrp3 | hypothetical protein | XOC_3956 (100%) | PXO_03076 (88%) | XOO0633 (89%) | XOO0696 (89%) | XAC3685 (88%) | XCV3806 (86%) | XCC3645 (79%) |
| Xrp4 | hypothetical protein | XOC_3955 (100%) | PXO_03077 (85%) | XOO0634 (85%) | XOO0697 (85%) | XAC3684 (78%) | XCV3805 (80%) | XCC3644 (60%) |
| Xrp5 | hypothetical protein | XOC_1550 (100%) | PXO_04764 (89%) | XOO1359(89%) | XOO1457 (89%) | XAC2878 (81%) | XCV3033 (82%) | XCC2715 (80.8%) |
| Xrp6 | hypothetical protein | XOC_3440 (100%)b | PXO_01952 (93%) | N | N | N | N | N |
| Xrp7 | glucuronate isomerase | XOC_4583 (100%) | PXO_03860 (96%) | XOO4170 (96%) | XOO4427 (95%) | XAC4251 (92%) | XCV4357 (92%) | XCC4117 (86%) |
| Xrp8 | hypothetical protein | XOC_2462 (100%) | Na | N | N | XAC2155 (91%) | XCV2099 (91%) | XCC2020 (86%) |
| Xrp9 | hypothetical protein | XOC_1951 (100%) | PXO_00529 (98%) | XOO2357 (99%) | XOO2488 (96%) | XAC2517 (96%) | XCV2699 (92%) | XCC2382 (84%) |
| Xrp10 | hypothetical protein | XOC_0560 100%) | PXO_04113 (90%) | XOO3892 (91%) | XOO4113 (90%) | XAC3866 (94%) | XCV3985 (94%) | XCC3811 (81%) |
| Xrp11 | hypothetical protein | XOC_3130 (100%) | PXO_01766 (85%) | XOO1798 (85%) | XOO1902 (85%) | XAC1364 (85%) | XCV1420 (85%) | XCC1318 (83%) |
| Xrp12 | S-(hydroxymethyl) glutathione dehydrogenase | XOC_4010 (100%) | N | XOO0585 (96%) | XOO0635 (95%) | XAC3747 (92%) | XCV3866 (94%) | XCC3703 (90%) |
| Xrp13 | Protease | XOC_0860 (100%) | PXO_04349 (96%) | XOO3588 (97%) | XOO3806 (96%) | XAC0795 (79%) | XCV0845 (80%) | N |
| Xrp14 | cytochrome P450 BJ-1 | XOC_0084 (100%) | N | N | N | XAC3170 (29%) | N | N |
| Xrp15 | NDP-hexose isomerase | XOC_2829 (100%) | PXO_00207 (98%) | XOO2849 (96%) | XOO2998 (98%) | XAC1687 (92%) | XCV1723 (93%) | XCC1670 (86%) |
| Xrp16 | transferase hexapeptide repeat | XOC_2828 (100%) | PXO_00206 (94%) | XOO2848 (94%) | XOO2997 (94%) | XAC1688 (86%) | XCV2006 (33%) | N |
| Xrp17 | hypothetical protein | HrpFBc (100%) | N | N | N | N | N | N |
Parentheses indicate percent amino acid identity. N, no homologous gene was identified.
XOC_3440,an ORF located between hrpG and hrpX.
HrpFB, an ORF located upstream of hrpF.
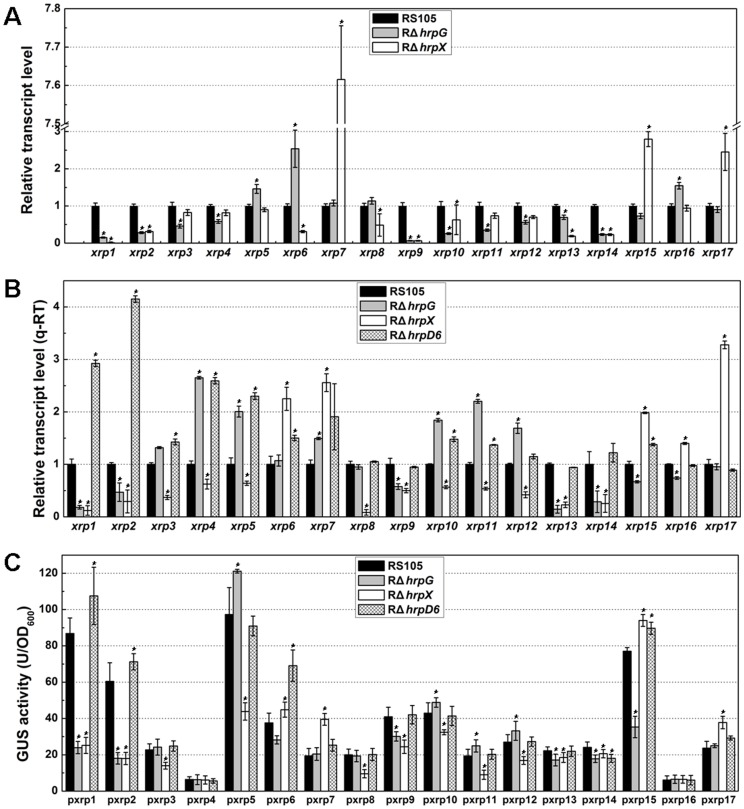
The in silicon data showed that the expression of xrp1, xrp2, xrp9, xrp13, xrp14 was significantly (t test, P≤0.05) positively regulated by both HrpG and HrpX. the expression of xrp8 was positively regulated only by HrpX, the expression of xrp3, xrp4, xrp11 and xrp12 was positively regulated only by HrpG, the expression of xrp7 xrp15, and xrp17 was negatively regulated by HrpX, and the expression of xrp5, xrp6 and xrp16 was significantly negative regulated only by HrpG with respect to expression levels in the wild-type RS105 (Fig. 1A, Table S3 in File S1). These results suggest that mutations in hrpG or hrpX may alter the expression of these 17 xrp genes during bacterial infection in rice.
Figure 1. Transcriptional expression of 17 X. oryzae pv. oryzicola xrp genes exposed to three hrp-inducing conditions.
(A) The transcriptional profiles in silicon of 17 xrp genes in Xoc RS105, RΔhrpG and RΔhrpX. The three strains were incubated with rice cells at 28°C for 16 h, and RNA was extracted and hybridized to the genechip arrays described in Materials and Methods. (B) Expression of 17 xrp genes using qRT-PCR. RNAs were extracted from Xoc RS105, RΔhrpG, RΔhrpX and the hrpD6 mutant (RΔhrpD6) and used to synthesize cDNA for qRT-PCR. gyrB and rpoD were used as internal controls. (C) GUS activities of 17 xrp promoter-GUS fusions in strains RS105, RΔhrpG, RΔhrpX and RΔhrpD6. Strains were cultured in XOM3 at 28°C for 6 h, and GUS activities were determined by measuring using 4-MUG as a substrate. Data are the mean ± SD of triplicate measurements from a representative experiment; and similar results were obtained in two other independent experiments. Asterisks in horizontal columns indicate significant differences using the Student's t test (*P<0.05).
Expression patterns of xrp genes controlled by HrpG, HrpX and HrpD6
To confirm the expression profiles of these 17 xrp genes in RS105, RΔhrpG, and RΔhrpX (Fig. 1A), we used quantitative real-time PCR (q-RT PCR) to determine whether the expression was HrpG or HrpX-dependent. Primers (Table S2 in File S1) selected for this experiment were based on the genome of BLS256 (NZ_AAQN01000001.1, GI:353459993)(10). Since HrpD6 is a newly identified hrp regulatory factor, we also investigated whether the expression of these 17 xrp genes was altered in the mutant RΔhrpD6. Controls included Hpa1, which contains a T3S signal at the N-terminus and the PIP-box promoter [24], [59] and XOC_0618, which is homologous to XopX [22]. Hpa1 and XOC_0618 were positively regulated by HrpG and HrpX, but negatively regulated by HrpD6 (Fig. S1 in File S1); this observation is consistent with previous results [12]. As shown in Fig. 1B, the expression of xrp1, xrp2, xrp9, xrp13 and xrp14 was significantly positively regulated by both HrpG and HrpX, which is consistent with our in silicon data (Fig. 1A, Table S3 in File S1); the expression of xrp3 and xrp8 was decreased in the hrpX mutant, relative to the wild-type and hrpG mutant; the expression of xrp4, xrp5, xrp10, xrp11 and xrp12 was decreased in RΔhrpX, but increased not only in RΔhrpG but also in RΔhrpD6; and the expression of xrp6, xrp7, xrp15 xrp16 and xrp17 was significantly increased in the hrpX mutant. The qRT-PCR results (Fig. 1A) were generally consistent with the expression in silicon (Fig. 1). In contrast, the expression of xrp1, xrp2, xrp3, xrp4, xrp5, xrp6, xrp7, xrp10, xrp11, and xrp15 was significantly increased in the hrpD6 mutant (Fig. 1B).
To further investigate the expression of these 17 xrp genes, we fused the putative promoter regions (500 bp upstream of the translational start site) to a promoterless gusA gene. The promoters were PCR-amplified from genomic DNA of strain RS105 using the primers listed in Table S2 in File S1. After sequencing these PCR products from RS105, no obvious difference was found in corresponding regions in BLS256 genome (data not shown). The pxrp-gusA fusions were then transferred into the wild-type RS105, the hrpG, hrpX and hrpD6 mutants, respectively, then incubated in XOM3 (a hrp-inducing medium) [49] at 28°C for 6 h. The GUS expression patterns (Fig. 1C) detected by these 17 xrp promoters was similar to the regulation observed by genechip and qRT-PCR analysis (Figs. 1A, B). However, there were no obvious differences in GUS activities expressed from the xrp4, xrp13, xrp14 and xrp16 putative promoters in RS105, and the hrpG, hrpX and hrpD6 mutants (Fig. 1C), possibly because that these four genes localize within adjacent operons where the tested promoters are not real for GUS activity detection (data not shown).
xrp1, xrp2, xrp5, xrp8 and xrp14 genes are required for Xoc virulence to rice
The expression patterns of these 17 xrp genes in the hrpG, hrpX and hrpD6 mutant backgrounds prompted us to determine whether they are involved in Xoc virulence. Each xrp gene was PCR-amplified using primers derived from the BLS256 genome (Table S2 in File S1). Sequence and BLAST analysis of xrp PCR products from RS105 showed no differences in these xrp genes with the corresponding ORFs in BLS256 (Table 2). All these Xrp proteins of RS105 showed 100% identity to the homologs of BLS256 (Table 2). Homologs of Xrp2 were not identified in X. axonopodis pv. citri strain 306 (Xac 306) and X. campestris pv. campestris (Xcc) ATCC33913. Xrp6 was only identified in Xoc BLS256 and Xoo PXO99A; Xrp8 had no orthologs in Xoo strains but in other three Xanthomonas species, and Xrp12 was not present in Xoo PXO99A. Xrp14 was identified in Xoc BLS256, but only had lower similarity (29%) in Xac 306; Xrp13 and Xrp15 had no homologous counterparts in Xcc; and Xrp17 was a putative ORF upstream of HrpF only in Xoc BLS256 genome (designed HrpFB herein) (Table 2). It should be mentioned here that the ORFs and putative products for xrp1, xrp7, xrp12, xrp13, xrp14, xrp15 and xrp16 were re-annotated in the new version of Xoc BLS256 genome sequence (NZ_AAQN01000001.1, GI:353459993) (Table 2).
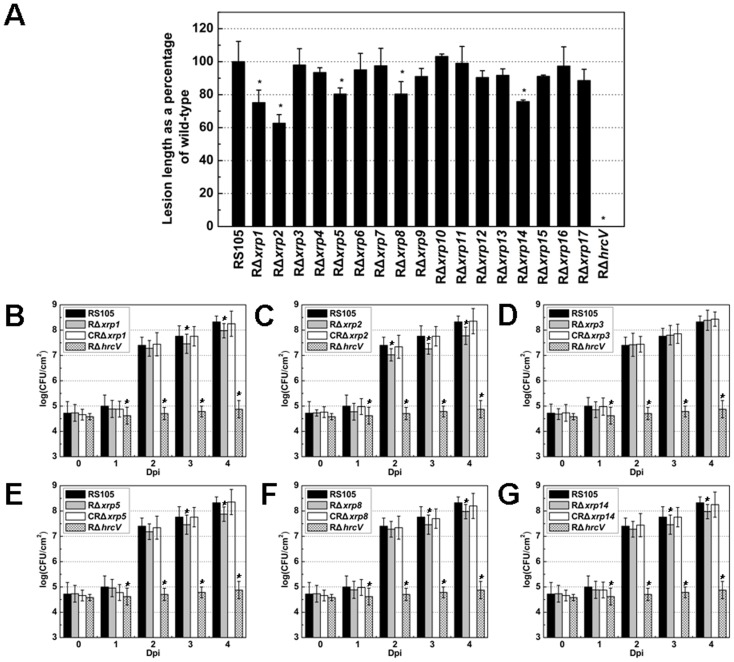
In order to explore the putative role of the xrp genes in virulence, we generated in-frame deletion mutants of the 17 xrp genes (Table S1 in File S1) in Xoc RS105 as described previously [57]. The left and right fragments flanking the xrp genes were PCR-amplified with primers listed in Table S2 in File S1 using RS105 genomic DNA as the template, fused, transferred into the suicide vector pKMS1 and generated deletion mutants correspondingly (Table S1 in File S1). These 17 nonpolar deletion mutants were inoculated into leaves of rice cv. Shanyou 63, which is susceptible to Xoc. The lesion lengths generated by xrp1, xrp2, xrp5, xrp8 and xrp14 mutants were significantly shorter than those produced by Xoc RS105 (Fig. 2A), and these five mutants were impaired in their ability to grow in rice leaves compared to the wild-type (Fig. 2B–G). Virulence and bacterial growth in planta were restored to wild-type levels by the introduction of corresponding xrp genes in trans (Fig. 2B–G). The above data indicate that xrp1, xrp2, xrp5, xrp8 and xrp14 genes are required by Xoc for bacterial virulence and growth in rice.
Figure 2. Virulence evaluation of 17 xrp mutants of X. oryzae pv. oryzicola in rice.
(A) Lesion lengths of 17 xrp mutants derived from the wild-type strain RS105. Lesion lengths were measured as the ratio of lesion length caused by an xrp mutant compared to the wild-type RS105. The hrcV mutant was used as a negative control. Xoc strains (∼1×108 cfu/mL) were inoculated to rice cv. Shanyou 63 (two-months old) by leaf-needling [58]. Lesion lengths were scored 14 days post inoculation. Data are the mean ± standard deviation (SD) of three replicates, and the data shown are representative of three independent experiments. Asterisks at the top of columns indicate significant differences by the Student's t test (*P<0.05). Panels B through G show the population dynamics of Xoc RS105 and derivatives in rice leaves. Leaf discs (0.5 cm in diameter) were excised from the inoculated areas, homogenized in sterile water, diluted and plated on nutrient agar (NA) plates. Panels: (B), xrp1 mutant, RΔxrp1; (C), xrp2 mutant, RΔxrp2; (D), xrp3 mutant, RΔxrp3; (E), xrp5 mutant, RΔxrp5; (F), xrp8 mutant, RΔxrp8; and (G) xrp14 mutant, RΔxrp14. Similar results were obtained in two other independent experiments.
Xrp3 and Xrp5 are novel T3SEs
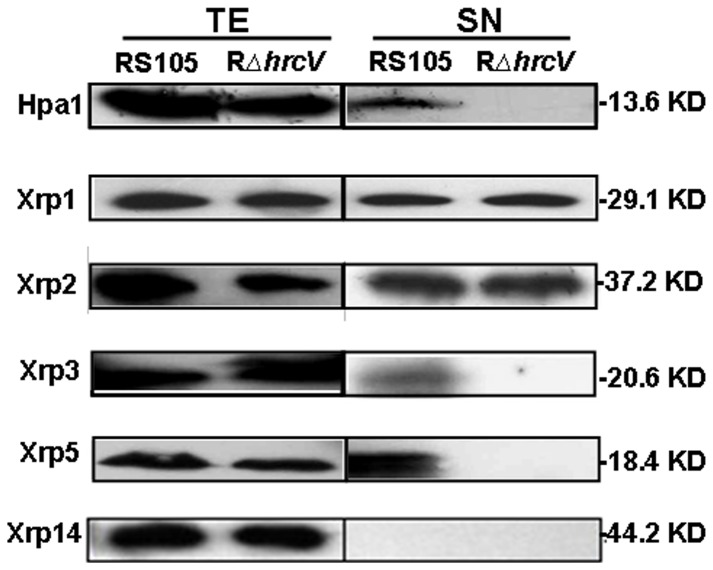
In the complementation studies mentioned above, a c-Myc tag was fused in frame at the C-termini of the 17 Xrp proteins (see Table S1 in File S1), which facilitated subsequent expression studies. The c-Myc-tagged constructs were introduced into Xoc RS105 and RΔhrcV [62] and incubated in hrp-inducing medium XOM3 [48] for 6 h. The supernatants (SN) and total extracts (TE) of bacterial cells were used to investigate whether the Xrps were expressed by Western blotting using a c-Myc specific polyclonal antibody (Huaan, Hangzhou, China). With the exception of Xrp4, Xrp6, Xrp9, Xrp10 and Xrp13, the other 12 Xrp proteins were detectable in TEs of both RS105 and RΔhrcV (representative data are shown in Fig. 3). However, Xrp3 and Xrp5, like Hpa1, were detected in SNs of the wild-type, but not in the T3S mutant (Fig. 3). Xrp1 and Xrp2 were detected in the SN fractions of both the wild-type and the T3S mutant, whereas Xrp14 were not (Fig. 3). These data indicate that Xrp3 and Xrp5 are secreted via the T3S, but Xrp1 and Xrp2 may be secreted via other systems.
Figure 3. Secretion detection of c-Myc epitope-tagged derivatives of Xrp proteins through the T3S.
Xoc wild-type strain RS105 and the T3S mutant RΔhrcV, harboring c-Myc eptope-tagged Xrp protein constructs were incubated in a hrp-inducing medium XOM3. Total protein extracts (TE) and culture supernatants (SN) of the tested strains were analyzed by SDS-PAGE and immunoblotted using anti-c-Myc antibodies. Immunoblots were performed described in Materials and Methods. Hpa1 was used as a positive control. Similar results were obtained in two other independent experiments.
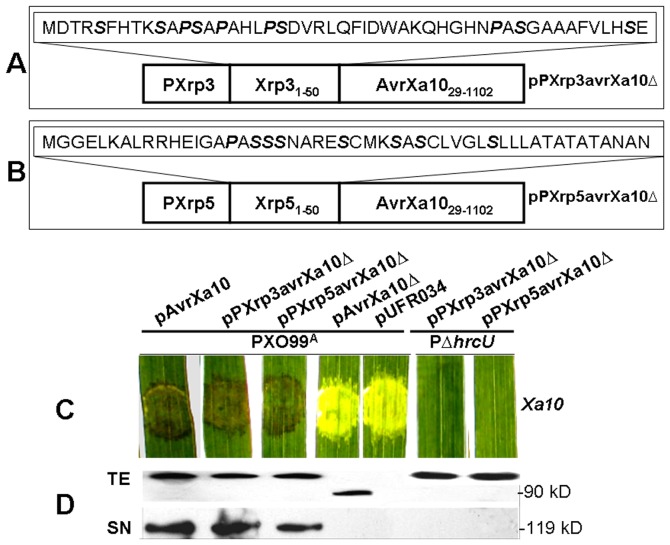
Xanthomonas T3SEs containing up to 20% Ser/Pro residues or at least five Ser residues in their N-terminal sequences are deemed to contain a T3S signal [22], [33]. One strategy for determination of T3SE secretion via the T3S is to fuse the N-terminus of a candidate effector with an Avr protein that lacks the secretion signal, and investigate whether the fused effector can be translocated into plant cells [24], [38]. The first 50 N-terminal amino acid residues of Xrp3 and Xrp5 contain a total of 10 and 8 Ser/Pro residues, respectively (Fig. 4A, B), indicating that Xrp3 and Xrp5 may be translocated into host cells. To further investigate this possibility, the N-terminal 50 amino acids of Xrp3 and Xrp5 were fused with a truncated AvrXa10 that lacked 28 amino acid residues at the N-terminus (avrXa10Δ). The chimeric proteins Xrp3avrXa10Δ and Xrp5avrXa10Δ were constructed using native xrp promoters (see Materials and Methods), resulting in pPXrp3avrXa10Δ and pPXrp5avrXa10Δ, respectively (Table S1 in File S1, Fig. 4A, B). The wild-type RS105 harbouring avrXa10 does not trigger an HR in rice line IRBB10, which carries the cognate R gene Xa10 [24]; thus, we utilized Xoo strain PXO99A to investigate whether the fused proteins can trigger an HR in IRBB10. The T3S mutant PΔhrcU was used as a negative control. PXO99A containing pPXrp3avrXa10Δ or pPXrp5avrXa10Δ produced an HR in IRBB10, as did PXO99A (pavrXa10) (Fig. 4C). However, HR was not elicited by PXO99A containing pavrXa10Δ or the empty vector pUFR034 (Fig. 4C). As predicted, the T3S mutant PΔhrcU did not elicit symptoms in cultivar IRBB10 (Fig. 4C). We then used immunoblotting to determine whether the translational fusions present in pPXrp3avrXa10Δ and pPXrp5avrXa10Δ were expressed in the tested strains. As expected, the chimeric proteins PXrp3avrXa10Δ and PXrp5avrXa10, like the wild-type AvrXa10 and the N-terminal truncated AvrXa10Δ, were detectable in the TEs of both wild-type PXO99A and PΔhrcU, but not in SN fraction of PΔhrcU (Fig. 4D). The above data indicate that the N-terminal portions of Xrp3 and Xrp5 enable the N-terminal truncated AvrXa10 to be secreted through the T3S and translocated into rice cells for HR induction.
Figure 4. Translocation of Xrp3 and Xrp5 into plant cells via the T3S.
Panels (A) and (B) show schematic maps of fusion proteins Xrp3 and Xrp5, respectively. The putative promoter and 5′ coding regions of xrp3 and xrp5 were fused to AvrXa10Δ28; this resulted in constructs containing in-frame fusions between with the N-terminus of Xrp3/Xrp5 and truncated AvrXa10Δ28 (AvrXa1029-1211). The 50 N-terminal amino acids in Xrp3 and Xrp5 are indicated in dashed boxes. (C) Avirulence activities of pXrp3avrXa10Δ28 and pXrp5avrXa10Δ28 in leaves of rice IRBB10 carrying the resistance gene Xa10. The secondary leaves of 14-day-old seedlings of IRBB10 were inoculated by needleless syringe with the respective bacterial strains as illustrated. Photos were taken 3 days after inoculation. The procedure was performed as in Materials and Methods, and similar results were obtained in two other independent experiments. (D) Detection of Xrp3 and Xrp5 secretion via the T3S in Xoc strains by immunoblotting. PXO99A and RΔhrcU containing c-Myc tagged derivatives of pXrp3avrXa10Δ28 and pXrp5avrXa10Δ28 were incubated in secretion medium XOM3. Total protein extracts (TE) and culture supernatants (SN) were analyzed by SDS-PAGE and immunoblotted using anti-c-Myc antibodies. The experiment was performed as described in Materials and Methods, and similar results were obtained in two other independent experiments.
Subcellular localization of Xrp3 and Xrp5
The localization of Xrp3 and Xrp5 was examined by utilizing YFP-tagged Xrp3 and Xrp5 proteins. The protocol (see Materials and Methods) utilizes mesophyll protoplasts of Arabidopsis and PEG-calcium-mediated transfection to deliver pXrp3-YFP and pXrp5-YFP (Table S1 in File S1) into plant cells; GUS-NLS-YFP and YFP were used as a reference. Fluorescence confocal microscopy indicated that YFP-tagged Xrp3 proteins are localized throughout the cell, but Xrp5-YFP is targeted to the plasma membrane and cytoplasm. The latter result was clearly different from the YFP control, which was partially retained in the nucleus (Fig. 5). In general, our results suggest that Xrp3-YFP is localized throughout the cell (cytoplasm, nucleus, plasma membrane) and Xrp5-YFP preferentially targets the cytoplasm.
Figure 5. Subcellular localization of Xrp3 and Xrp5 in plant cells.
The subcellular localization of Xrp3 and Xrp5 was examined by fusing these proteins with yellow fluorescence protein (YFP) and evaluating their expression in Arabidopsis mesophyll cells. Images were generated by fluorescence at 525–550 nm (YFP, yellow) and 610–700 nm (chloroplasts, red) using excitation at 514 nm. Expression was driven by the CaMV 35S promoter. PEG-calcium-mediated transfection was used to deliver DNA into protoplasts, and photos were taken 12 h after transformation. Controls included YFP, which localizes in both cytoplasm and nucleus, and GUS-NLS-YFP, which localizes in the nucleus. Bars correspond to 10 µm. The experiment procedure was performed as described previously [54] and repeated three times with similar results.
Discussion
In this report, two new T3SEs, Xrp3 (XOC_3956) and Xrp5 (XOC_1550), were identified and shown to be HrpX-dependent based on transcriptional data in silicon (Fig. 1A). These two newly-identified T3SEs can be added to the extensive list of Xanthomonas T3SEs at http://www.Xanthomonas.org/t3e.html [16]. Interestingly, xrp3 and xrp5 are highly conserved in Xanthomonads rather than other plant pathogenic bacteria whose genomes have been completely sequenced (Table 2). Xrp5, but not Xrp3, was important for a full level of virulence in rice (Fig. 2). This is consistent with a previous study that many NTALEs but XopZ in Xoo do not impact bacterial virulence in rice [63].
The in silicon and qRT-PCR data (Figs. 1A, B) consistently showed that the expression of 17 xrp genes was regulated by HrpX and/or HrpG at the transcriptional level. However, GUS activity detection showed that the expression of xrp4 (XOC_3955) and xrp16 (XOC_2828) did not display any obvious differences in regulation in WT and mutant strains (Fig. 1C), being due to that they are members of their adjacent operons (data not shown). Giving that the expression of xrp1 (XOC_1601), xrp2 (XOC_4584), xrp3 (XOC_3956), xrp4 (XOC_3955), xrp5 (XOC_1550), xrp6 (XOC_3440), xrp7 (XOC_4583), xrp10 (XOC_0560), xrp11 (XOC_3130) and xrp15 (XOC_2829) was negatively regulated by HrpD6 but either positively regulated by HrpX and/or HrpG or negatively regulated by HrpX (Fig. 1B), HrpG-, HrpX- and HrpD6-mediated regulation in xanthomonads is very complicated to follow the concept that hrpX expression is controlled by HrpG [14], [44] and HrpX regulates the expression of hrpD6 [12]. In general, one criterion for identification of HrpX regulon genes is the PIP-box [26], [27], [43], [64]. However, PIP-box-like sequences are absent in the xrp3 and xrp5 upstream regions (Table 1). This is consistent with reports that the expression of some HrpX-regulated genes that lack PIP-box promoters is modulated directly by HrpX and indirectly by HrpG [22]. For example, there is no PIP-box in the promoter regions of hpaJ, XCV_0869 or XCV_3406 genes, but their expression is HrpX-dependent [26]. Thus, there may be an alternative regulatory system in Xanthomonas where HrpX activates genes independently via an unknown regulator(s), like HrpD6.
It should be emphasized that the location of xrp6 (XOC_3440) and xrp17 (hrpFB) is within the hrp-hrc-hpa cluster. xrp6 is a putative ORF between hrpG and hrpX, and xrp17 is hrpFB upstream of hrpF. These two ORFs have not been annotated in other Xanthomonas genomes (Table 1). Our data suggest that xrp6 and xrp17 are not involved in Xoc virulence (Fig. 2A); however, the expression of these two ORFs was negatively regulated by HrpX, but not affected by HrpG (Fig. 1B), and the gene product of xrp17 was not detectable in the SN of the WT and mutant strains (data not shown). It remains unclear whether Xrp6 or Xrp17 function in the regulation of the hrp-hrc-hpa cluster.
New Xoc virulence factors identified in this study included Xrp1 (XOC_1601), Xrp2 (XOC_4584), Xrp8 (XOC_2462), and Xrp14 (XOC_0084) (Fig. 2). Xrp1 (XOC_1601) encodes a putative cysteine protease, which is highly conserved in Xoo, Xac, Xcc, and Xcv (Table 2); this protein shows 84% identity with the T2SS protein CysP2 in Xoo [14], XOC_1601 is controlled by DSF-mediating QS (quorum-sensing) in Xoc RS105, involved in extracellular protease activity, cell motility, antioxidative ability and EPS biosynthesis [65]. However, Xrp1 is not related to XopD, AvrXv4, AvrPphB or AvrRpt2, which have cysteine protease functions that alter plant immunity [66]–[71]. Given that Xrp1 is involved in Xoc virulence in rice (Fig. 2) and T3S-independent secretion (Fig. 3), we assume that this cysteine protein may be secreted via the T2SS, like the homolog of CysP2 in Xoo [14]. Xrp1 could potentially degrade a component of the plant cell walls, and the proteolysis of host substrates may be employed by the pathogen to alter plant physiological processes.
Xrp2 (XOC_4584) is conserved in Xoc, Xoo and Xcv, but not in Xcc or Xac (Table 2); database searches provided no functional clues regarding Xrp2 function. The expression of Xrp2 is HrpG- and HrpX-dependent, but upregulated by HrpD6, like the expression of Xrp1 (Fig. 1). Thus, in addition to regulating hrp gene expression [12], HrpD6 may also regulate other virulence factors that are not secreted via the T3S. We found that Xrp8 (XOC_2462) is highly conserved in Xoc, Xcv, Xac and Xcc, but has no homolog in Xoo strains PXO99A, MAFF311018 or KACC10331 (Table 2); thus, this virulence factor (Fig. 2F) may potentially be required for full virulence of Xcv, Xac and Xcc in plants.
Xrp14 (XOC_0084) is present in Xoc BLS256 and Xac 306 (Table 2) and encodes a putative gene belonging to the cytochrome P450 family [9]. The P450 family proteins use heme iron to oxidize molecules, often making them more water-soluble [72]. It is interesting to note that there are three Ser and seven Pro residues at the N-terminus of Xrp14 (Table 1), indicating that Xrp14 is a potential T3SE. However, the protein is undetectable in the SN of the wild-type strain and T3S mutant (Fig. 3), suggesting that the involvement of Xrp14 in Xoc virulence is worthy of further investigation.
In Xanthomonas spp., T3SEs have been identified based on sequence similarities identified from genome sequence data [16], [22], avirulence reporter fusion assays [38], Cya-fusion approaches [22] and functional assays of T3S-dependent expression and secretion [73]. It has been demonstrated that the fusion expression of an N-terminally truncated avr gene (avrXa10) with the first 50 N-terminal amino acids of a T3SE is an important tool for identifying Xoc T3SEs by utilizing the Xoo-rice pathosystem [24], [47], [74]. However, it may be important to utilize the highly sensitive Cya assay to identify Xoc T3SEs [22], [33], [75]. In R. solanacearum, 72 T3SEs have been characterized [76]. To date, approximately 26 NTALEs and 29 TALEs have been confirmed or predicted in Xoc BLS256 (http://www.xanthomonas.org/t3e.html), excluding HrpE3 [74], and Xrp3 (XOC_3956) and Xrp5 (XOC_1550). Considering the long period of co-evolution of Xoc and rice, we speculate that the number of T3SEs in Xoc or Xoo may possibly be more than the currently identified. Hence, identifying T3SEs is still an important endeavor in Xoc where HrpX-regulated proteins could potentially function as T3SEs.
Supporting Information
File containing supporting Figure S1 and Tables S1–S3.
(DOC)
Funding Statement
This work was supported by the State Key Basic Research and Development Project of China (20112CB114003), the Natural Science Foundation of China (31230059), and the Special Fund for Agro-Scientific Research in the Public Interest of China (201303015). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chen G, Zou L, Wu X-M, Li Y, Wang J (2005) avr/pth13 gene of Xanthomonas oryzae pv. oryzicola, a novel virulence member of avrBs3/PthA family, strengthening virulence of Xanthomonas oryzae pv. oryzae on rice. Chin J Rice Sci 19: 291–296. [Google Scholar]
- 2. Hilaire E, Young SA, Willard LH, McGee JD, Sweat T, et al. (2001) Vascular defense responses in rice: peroxidase accumulation in xylem parenchyma cells and xylem wall thickening. Molecular Plant-Microbe Interactions 14: 1411–1419. [DOI] [PubMed] [Google Scholar]
- 3. Niño-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Molecular Plant Pathology 7: 303–324. [DOI] [PubMed] [Google Scholar]
- 4. Ou SH (1985) Rice diseases: IRRI. [Google Scholar]
- 5. Zou L, Wang X, Xiang Y, Zhang B, Li Y, et al. (2006) Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl Environ Microbiol 72: 6212–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee BM, Park YJ, Park DS, Kang HW, Kim JG, et al. (2005) The genome sequence of Xanthomonas oryzae pathovar oryzae KACC10331, the bacterial blight pathogen of rice. Nucl Acids Res 33: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salzberg SL, Sommer DD, Schatz MC, Phillippy AM, Rabinowicz PD, et al. (2008) Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A . Bmc Genomics 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ochiai H, Inoue Y, Takeya M, Sasaki A, Kaku H (2005) Genome sequence of Xanthomonas oryzae pv. oryzae suggests contribution of large numbers of effector genes and insertion sequences to its race diversity. Japan Agricultural Research Quarterly 39: 275. [Google Scholar]
- 9. Bogdanove AJ, Koebnik R, Lu H, Furutani A, Angiuoli SV, et al. (2011) Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J Bacteriol 193: 5450–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alfano JR, Collmer A (1997) The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J Bacteriol 179: 5655–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Büttner D, Bonas U (2002) Getting across - bacterial type III effector proteins on their way to the plant cell. EMBO J 21: 5313–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li YR, Zou HS, Che YZ, Cui YP, Guo W, et al. (2011) A novel regulatory role of HrpD6 in regulating hrp-hrc-hpa genes in Xanthomonas oryzae pv. oryzicola . Mol Plant-Microbe Interact 24: 1086–1101. [DOI] [PubMed] [Google Scholar]
- 13. Alfano JR, Collmer A (2004) Type III secretion system effector proteins: Double agents in bacterial disease and plant defense. Annu Rev Phytopathol 42: 385–414. [DOI] [PubMed] [Google Scholar]
- 14. Furutani A, Tsuge S, Ohnishi K, Hikichi Y, Oku T, et al. (2004) Evidence for HrpXo-dependent expression of type II secretory proteins in Xanthomonas oryzae pv. oryzae . J Bacteriol 186: 1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kay S, Bonas U (2009) How Xanthomonas type III effectors manipulate the host plant. Curr Opin Microbiol 12: 37–43. [DOI] [PubMed] [Google Scholar]
- 16. White FF, Potnis N, Jones JB, Koebnik R (2009) The type III effectors of Xanthomonas . Mol Plant Pathol 10: 749–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 124: 803–814. [DOI] [PubMed] [Google Scholar]
- 18. Jones JDG, Dangl JL (2006) The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- 19. Kim J-G, Li X, Roden JA, Taylor KW, Aakre CD, et al. (2009) Xanthomonas T3S effector XopN suppresses PAMP-triggered immunity and interacts with a tomato atypical receptor-like kinase and TFT1. Plant Cell 21: 1305–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scholze H, Boch J (2011) TAL effectors are remote controls for gene activation. Curr Opin Microbiol 14: 47–53. [DOI] [PubMed] [Google Scholar]
- 21. Yang B, Sugio A, White FF (2006) Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc Natl Acad Sci USA 103: 10503–10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furutani A, Takaoka M, Sanada H, Noguchi Y, Oku T, et al. (2009) Identification of novel type III secretion effectors in Xanthomonas oryzae pv. oryzae . Mol Plant-Microbe Interact 22: 96–106. [DOI] [PubMed] [Google Scholar]
- 23. Jiang J, Zou H, Li Y, Chen G (2009) Expression of the hrcC, hrpE and hpa3 genes is not regulated by the hrpG and hrpX genes in a rice pathogen Xanthomonas oryzae pv. oryzicola . Wei Sheng Wu Xue Bao 49: 1018–1025. [PubMed] [Google Scholar]
- 24. Li Y-R, Che Y-Z, Zou H-S, Cui Y-P, Guo W, et al. (2011) Hpa2 required by HrpF to translocate Xanthomonas oryzae transcriptional activator-like effectors into rice for pathogenicity. Appl Environ Microbiol 77: 3809–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brito B, Marenda M, Barberis P, Boucher C, Genin S (1999) prhJ and hrpG, two new components of the plant signal-dependent regulatory cascade controlled by PrhA in Ralstonia solanacearum . Mol Microbiol 31: 237–251. [DOI] [PubMed] [Google Scholar]
- 26. Koebnik R, Krueger A, Thieme F, Urban A, Bonas U (2006) Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J Bacteriol 188: 7652–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noel L, Thieme F, Nennstiel D, Bonas U (2002) Two novel type III-secreted proteins of Xanthomonas campestris pv. vesicatoria are encoded within the hrp pathogenicity island. J Bacteriol 184: 1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsuge S, Nakayama T, Terashima S, Ochiai H, Furutani A, et al. (2006) Gene involved in transcriptional activation of the hrp regulatory gene hrpG in Xanthomonas oryzae pv. oryzae . J Bacteriol 188: 4158–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Furutani A, Nakayama T, Ochiai H, Kaku H, Kubo Y, et al. (2006) Identification of novel HrpXo regulons preceded by two cis-acting elements, a plant-inducible promoter box and a-10 box-like sequence, from the genome database of Xanthomonas oryzae pv. oryzae . FEMS Microbiol Lett 259: 133–141. [DOI] [PubMed] [Google Scholar]
- 30. Cunnac S, Occhialini A, Barberis P, Boucher C, Genin S (2004) Inventory and functional analysis of the large Hrp regulon in Ralstonia solanacearum: identification of novel effector proteins translocated to plant host cells through the type III secretion system. Mol Microbiol 53: 115–128. [DOI] [PubMed] [Google Scholar]
- 31. Schechter LM, Vencato M, Jordan KL, Schneider SE, Schneider DJ, et al. (2006) Multiple approaches to a complete inventory of Pseudomonas syringae pv. tomato DC3000 type III secretion system effector proteins. Mol Plant-Microbe Interact 19: 1180–1192. [DOI] [PubMed] [Google Scholar]
- 32. Tampakaki AP, Fadouloglou VE, Gazi AD, Panopoulos NJ, Kokkinidis M (2004) Conserved features of type III secretion. Cell Microbiol 6: 805–816. [DOI] [PubMed] [Google Scholar]
- 33. Schechter LM, Roberts KA, Jamir Y, Alfano JR, Collmer A (2004) Pseudomonas syringae type III secretion system targeting signals and novel effectors studied with a Cya translocation reporter. J Bacteriol 186: 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robin GP, Ortiz E, Szurek B, Brizard JP, Koebnik R (2013) Comparative proteomics reveal new HrpX-regulated proteins of Xanthomonas oryzae pv. oryzae . J Proteomics Available: http://dxdoiorg/101016/jjprot201304010. [DOI] [PubMed] [Google Scholar]
- 35. Casper-Lindley C, Dahlbeck D, Clark ET, Staskawicz BJ (2002) Direct biochemical evidence for type III secretion-dependent translocation of the AvrBs2 effector protein into plant cells. Proc Natl Acad Sci USA 99: 8336–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chao N-X, Wei K, Chen C, Meng Q-L, Tang D-J, et al. (2008) The rsmA-like gene rsmA(Xcc) of Xanthomonas campestris pv. campestris is involved in the control of various cellular processes, including pathogenesis. Mol Plant-Microbe Interact 21: 411–423. [DOI] [PubMed] [Google Scholar]
- 37. Huang D-L, Tang D-J, Liao Q, Li X-Q, He Y-Q, et al. (2009) The Zur of Xanthomonas campestris is involved in hypersensitive response and positively regulates the expression of the hrp cluster via hrpX but not hrpG . Mol Plant-Microbe Interact 22: 321–329. [DOI] [PubMed] [Google Scholar]
- 38. Jiang W, Jiang B-L, Xu R-Q, Huang J-D, Wei H-Y, et al. (2009) Identification of six type III effector genes with the PIP box in Xanthomonas campestris pv. campestris and five of them contribute individually to full pathogenicity. Mol Plant-Microbe Interact 22: 1401–1411. [DOI] [PubMed] [Google Scholar]
- 39. Wei K, Tang D-J, He Y-Q, Feng J-X, Jiang B-L, et al. (2007) HpaR, a putative marR family transcriptional regulator, is positively controlled by HrpG and HrpX and involved in the pathogenesis, hypersensitive response, and extracellular protease production of Xanthomonas campestris pathovar campestris . J Bacteriol 189: 2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang S-S, He Y-Q, Xu L-M, Chen B-W, Jiang B-L, et al. (2008) A putative colR XC1049-colS XC1050 two-component signal transduction system in Xanthomonas campestris positively regulates hrpC and hrpE operons and is involved in virulence, the hypersensitive response and tolerance to various stresses. Res Microbiol 159: 569–578. [DOI] [PubMed] [Google Scholar]
- 41. Roden JA, Belt B, Ross JB, Tachibana T, Vargas J, et al. (2004) A genetic screen to isolate type III effectors translocated into pepper cells during Xanthomonas infection. Proc Natl Acad Sci USA 101: 16624–16629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oku T, Alvarez AM, Kado CI (1995) Conservation of the hypersensitivity-pathogenicity regulatory gene hrpX of Xanthomonas campestris and X. oryzae . DNA Seq 5: 245–249. [DOI] [PubMed] [Google Scholar]
- 43. Wengelnik K, Bonas U (1996) HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria . J Bacteriol 178: 3462–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo Y, Figueiredo F, Jones J, Wang N (2011) HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri . Mol Plant-Microbe Interact 24: 649–661. [DOI] [PubMed] [Google Scholar]
- 45. Yamazaki A, Hirata H, Tsuyumu S (2008) HrpG regulates type II secretory proteins in Xanthomonas axonopodis pv. citri . J Gen Plant Pathol 74: 138–150. [Google Scholar]
- 46.Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual, 2nd edn. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 47. Guo W, Cai LL, Zou HS, Ma WX, Liu XL, et al. (2012) Ketoglutarate transport protein KgtP is secreted through the type III secretion system and contributes to virulence in Xanthomonas oryzae pv. oryzae . Appl Environ Microbiol 78: 5672–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiao Y, Li Y, Liu Z, Xiang Y, Chen G (2007) Establishment of the hrp-inducing systems for the expression of the hrp genes of Xanthomonas oryzae pv. oryzicola . Wei Sheng Wu Xue Bao 47: 396–401. [PubMed] [Google Scholar]
- 49. Venkatesh B, Babujee L, Liu H, Hedley P, Fujikawa T, et al. (2006) The Erwinia chrysanthemi 3937 PhoQ sensor kinase regulates several virulence determinants. J Bacteriol 188: 3088–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stushnoff C, Ducreux LJ, Hancock RD, Hedley PE, Holm DG, et al. (2010) Flavonoid profiling and transcriptome analysis reveals new gene–metabolite correlations in tubers of Solanum tuberosum L. J Exp Bot 61: 1225–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turner PE (2004) Phenotypic plasticity in bacterial plasmids. Genetics 167: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lian H-L, He S-B, Zhang Y-C, Zhu D-M, Zhang J-Y, et al. (2011) Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Develop 25: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protocol 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 54. Pieretti I, Royer M, Barbe V, Carrere S, Koebnik R, et al. (2009) The complete genome sequence of Xanthomonas albilineans provides new insights into the reductive genome evolution of the xylem-limited Xanthomonadaceae . BMC Genomics 10: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin T, Wöhner R, Hummel S, Willmitzer L, Frommer WB, et al.. (1992) The GUS reporter system as a tool to study plant gene expression. In: Gallagher Se, editor. GUS protocols: using the GUS gene as a reporter of gene expression pp. 23–43. New York: Academic Press. [Google Scholar]
- 56. Shen YP, Zou LF, Li YR, Zou HS, Liu XL, et al. (2012) Xoryp_08180 of Xanthomonas oryzae pv. oryzicola, encoding a hypothetical protein, is regulated by HrpG and HrpX and required for full virulence in rice. J Integrative Agric 11: 600–610. [Google Scholar]
- 57. Zou LF, Li YR, Chen GY (2011) A non-marker mutagenesis strategy to generate poly-hrp gene mutants in the rice pathogen Xanthomonas oryzae pv. oryzicola . Agric Sci China 10: 1139–1150. [Google Scholar]
- 58. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 59. Furutani A, Tsuge S, Oku T, Tsuno K, Inoue Y, et al. (2003) Hpa1 secretion via type III secretion system in Xanthomonas oryzae pv. oryzae . J Gen Plant Pathol 69: 271–275. [Google Scholar]
- 60. McNally RR, Toth IK, Cock PJA, Pritchard L, Hedley PE, et al. (2012) Genetic characterization of the HrpL regulon of the fire blight pathogen Erwinia amylovora reveals novel virulence factors. Mol Plant Pathol 13: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seo YS, Sriariyanun M, Wang L, Pfeiff J, Phetsom J, et al. (2008) A two-genome microarray for the rice pathogens Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola and its use in the discovery of a difference in their regulation of hrp genes. BMC Microbiol 8: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Y, Zou L, Zhou D, Chen G (2009) Key roles of hrpE gene of Xanthomonas oryzae pv. oryzicola in formation of Hrp pilus and pathogenicity in rice. Acta Phytopathologica Sinica 39: 392–398. [Google Scholar]
- 63. Song CF, Yang B (2010) Mutagenesis of 18 type III effectors reveals virulence function of XopZ(PXO99) in Xanthomonas oryzae pv. oryzae . Mol Plant-Microbe Interact 23: 893–902. [DOI] [PubMed] [Google Scholar]
- 64. Kamdar HV, Kamoun S, Kado CI (1993) Restoration of pathogenicity of avirulent Xanthomonas oryzae pv. oryzae and X. campestris pathovars by reciprocal complementation with the hrpXo and hrpXc genes and identification of HrpX function by sequence analyses. J Bacteriol 175: 2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qian G, Zhou Y, Zhao Y, Song Z, Wang S, et al. (2013) Proteomic analysis reveals novel extracellular virulence-associated proteins and functions regulated by the diffusible signal factor (DSF) in Xanthomonas oryzae pv. oryzicola . J Proteome Res [DOI] [PubMed] [Google Scholar]
- 66. Axtell MJ, Chisholm ST, Dahlbeck D, Staskawicz BJ (2003) Genetic and molecular evidence that the Pseudomonas syringae type III effector protein AvrRpt2 is a cysteine protease. Mol Microbiol 49: 1537–1546. [DOI] [PubMed] [Google Scholar]
- 67. Hotson A, Chosed R, Shu H, Orth K, Mudgett MB (2003) Xanthomonas type III effector XopD targets SUMO-conjugated proteins in planta. Mol Microbiol 50: 377–389. [DOI] [PubMed] [Google Scholar]
- 68. Hotson A, Mudgett MB (2004) Cysteine proteases in phytopathogenic bacteria: identification of plant targets and activation of innate immunity. Curr Opin Plant Biol 7: 384–390. [DOI] [PubMed] [Google Scholar]
- 69. Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL (2003) Arabidopsis RIN4 Is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112: 379–389. [DOI] [PubMed] [Google Scholar]
- 70. Roden J, Eardley L, Hotson A, Cao Y, Mudgett MB (2004) Characterization of the Xanthomonas AvrXv4 effector, a SUMO protease translocated into plant cells. Mol Plant-Microbe Interact 17: 633–643. [DOI] [PubMed] [Google Scholar]
- 71. Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, et al. (2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301: 1230–1233. [DOI] [PubMed] [Google Scholar]
- 72. Bernhardt R (2006) Cytochromes P450 as versatile biocatalysts. J Biotechnol 124: 128–145. [DOI] [PubMed] [Google Scholar]
- 73. Thieme F, Szczesny R, Urban A, Kirchner O, Hause G, et al. (2007) New type III effectors from Xanthomonas campestris pv. vesicatoria trigger plant reactions dependent on a conserved N-myristoylation motif. Mol Plant-Microbe Interact 20: 1250–1261. [DOI] [PubMed] [Google Scholar]
- 74. Cui Y, Zou L, Zou H, Li Y, Zakria M, et al. (2013) HrpE3 is a type III effector protein required for full virulence of Xanthomonas oryzae pv. oryzicola in rice. Mol Plant Pathol 14: 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sory MP, Cornelis GR (1994) Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol 14: 583–594. [DOI] [PubMed] [Google Scholar]
- 76. Mukaihara T, Tamura N, Iwabuchi M (2010) Genome-wide identification of a large repertoire of Ralstonia solanacearum type III effector proteins by a new functional screen. Mol Plant-Microbe Interact 23: 251–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File containing supporting Figure S1 and Tables S1–S3.
(DOC)