Abstract
Background
Adherence to guidelines is associated with improved outcomes of patients with acute coronary syndrome (ACS). Clinical registries developed to assess quality of care at discharge often do not collect the reasons for non-prescription for proven efficacious preventive medication in Continental Europe. In a prospective cohort of patients hospitalized for an ACS, we aimed at measuring the rate of recommended treatment at discharge, using pre-specified quality indicators recommended in cardiologic guidelines and including systematic collection of reasons for non-prescription for preventive medications.
Methods
In a prospective cohort with 1260 patients hospitalized for ACS, we measured the rate of recommended treatment at discharge in 4 academic centers in Switzerland. Performance measures for medication at discharge were pre-specified according to guidelines, systematically collected for all patients and included in a centralized database.
Results
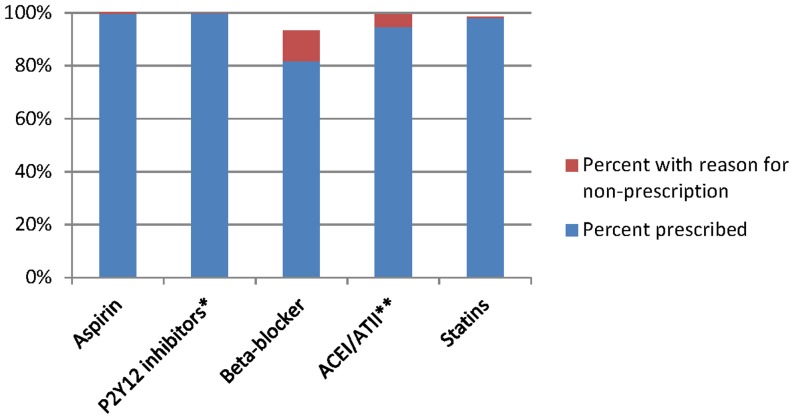
Six hundred and eighty eight patients(54.6%) were discharged with a main diagnosis of STEMI, 491(39%) of NSTEMI and 81(6.4%) of unstable angina. Mean age was 64 years and 21.3% were women. 94.6% were prescribed angiotensin converting enzyme inhibitors/angiotensin II receptor blockers at discharge when only considering raw prescription rates, but increased to 99.5% when including reasons non-prescription. For statins, rates increased from 98% to 98.6% when including reasons for non-prescription and for beta-blockers, from 82% to 93%. For aspirin, rates further increased from 99.4% to 100% and from to 99.8% to 100% for P2Y12 inhibitors.
Conclusions
We found a very high adherence to ACS guidelines for drug prescriptions at discharge when including reasons for non-prescription to drug therapy. For beta-blockers, prescription rates were suboptimal, even after taking into account reason for non-prescription. In an era of improving quality of care to achieve 100% prescription rates at discharge unless contra-indicated, pre-specification of reasons for non-prescription for cardiovascular preventive medication permits to identify remaining gaps in quality of care at discharge.
Trial Registration
ClinicalTrials.gov NCT01000701
Introduction
Cardiovascular disease remains the leading cause of death in adults in the United States (US) and in Europe. Acute coronary syndrome (ACS) is the most frequent cause leading to myocardial infarction, heart failure, and sudden death [1]. In-hospital initiation of evidence-based cardiovascular medication has been shown to improve long-term drug adherence and clinical outcomes [2], [3], [4].
Systematic monitoring of performance and annual report cards on quality of care, such as the US Healthcare Effectiveness Data and Information Set (HEDIS) [5], and financial incentives to improve quality are not implemented in Switzerland. Current clinical registries such as the NCDR ACTION Registry-GWTG (National Cardiovascular Data Registry (NCDR) ACC's Acute Coronary Treatment and Intervention Outcomes (ACTION) Registry- Get With the Guidelines (GWTG)) Network, a voluntary participation registry of patients admitted with ACS in the USA, the data collection to determine the rate of prescription of recommended treatment at discharge includes a box to systematically measure if the treatment was contraindicated [6]. Current clinical registries in Europe such as the FAST-MI registry [7], [8], or the APTOR registry [9], do not collect the reasons for non-prescription. A recent report on quality at discharge in Switzerland for patients discharged after a ST-elevation myocardial infarction (STEMI) has shown an improvement in quality of care over the last 15 years, but still suboptimal prescription rates of recommended therapies at discharge [10], [11], [12]. However, given that reasons for non-prescription were not collected, it is unknown if differences are due to remaining gaps in quality of care of if they are due to the absence of reporting on the reasons for non-prescription.
We aimed at measuring the rate of recommended treatment at discharge for patients hospitalized for an ACS in 4 university hospitals in Switzerland, using pre-specified quality indicator recommended in cardiologic guidelines in a centralized database, and including systematic collection of reason for non-prescription for preventive medication.
Methods
Study setting and participants
The SPUM-ACS (Special Program University Medicine-Acute Coronary Syndromes) research network was established in 2008 and collects data since 2009 on a prospective cohort of patients hospitalized for an ACS in 4 university medical centers in Switzerland (University hospital of Bern (BE), Geneva (GE), Lausanne (LA) and Zürich (ZH)) [13], [14]. We prospectively included patients hospitalized from September 2009 to October 2010, aged >18 years, hospitalized within 72 hours after pain onset with the main diagnosis of ACS. ACS was defined as patients with symptoms comparable with angina pectoris (chest pain, dyspnea) and at least one of the following characteristics: ST-segment elevation or depression, T inversion or dynamic ECG changes, evidence of positive Troponin and known coronary heart disease (status after myocardial infarction, bypass surgery or PTCA) [15]. The final ACS diagnosis was classified as follows: STEMI (ST-segment elevation myocardial infarction or NSTEMI non ST-segment elevation myocardial infarction or unstable angina. Patients were included in the catheterization laboratory in two participating hospitals (ZH and BE) and additionally while on ward in two participating hospitals (LA and GE). In order to allow comparison with other databases [6], [16], we report on data of patients who were discharged alive from each hospital.
Ethics statement
The study protocol was approved by the institutional review board of all participating centers; namely, the Ethics Committee on Clinical Research of the University of Lausanne, the Ethics Committee of the Department for Internal Medicine and Community Medicine of the University Hospital of Geneva, the Cantonal Ethics Committee (KEK) of the Canton of Bern, and the Cantonal Ethics Committee (KEK) of the Canton of Zurich. All patients provided written, informed consent.
Data collection and endpoints
Clinical data for the patients included in the SPUM-ACS study were collected by trained nurses and medical doctors on standardized, web-based case report forms and stored in a central database (Cardiobase, Clinical Trial Unit, and Department of Cardiology, Bern University Hospital, Switzerland, and 2 mT, Ulm, Germany). Data abstracted were (1) demographic characteristics such as age, sex, race, education and (2) medical history, such as previous coronary heart disease (CHD), stroke, renal failure requiring dialysis, valvular heart disease, congestive heart failure (CHF) as well as hypercholesterolemia, hypertension and diabetes. We further collected administrative data of the hospital stay (length of stay, direct transfer to a peripheral hospital or to inpatient cardiac rehabilitation) and prescription of recommended medication at discharge such as aspirin, P2Y12 inhibitors (clopidogrel, prasugrel or ticagrelor), beta-blockers, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers (ACEI/ATII) and statins.
Performance measures
Performance measures for medication at discharge were pre-specified, systematically collected for all patients and included in the centralized database. They were based on the ACC/AHA 2008 performance measures for adults with STEMI and NSTEMI and included the following pre-specified reasons for non-prescription [17]: for all medications, the reason “other reason documented by physician” and “patient refusal” were included. The reason in full text was entered when the reason “other reason documented by physician“ was selected. Additionally, pre-specified reasons for aspirin were: “active bleeding during hospital stay”, “coumadin/warfarin prescribed at discharge” and “aspirin allergy”; for ACEI/ATII reasons were: “Moderate or severe aortic stenosis” and “ACEI and/or ATII allergy”; for beta-blockers, reasons were: “beta-blocker allergy” and “second- or third-degree atrio-ventricular heart block”. We also included the reason “Bradycardia (heart rate <60/min) on day of discharge” given the frequency of this reported reason by physicians in some centers and despite its absence of recognition an acceptable reason for non-prescription in guidelines [17]. Patients who had “to be introduced later” as the reason for non-prescription of beta-blockers and who had been discharged home directly, were coded as not having been prescribed the recommended medication. We systematically collected information if patients had been offered a specialized smoking cessation intervention in 2 university hospitals (LA, GE). We did not collect information on brief smoking cessation counseling interventions that may have taken place during the hospital stay. In the US, smoking cessation counseling was systematically monitored as part of a pay-for-performance scheme rewarding hospitals for providing smoking cessation intervention. However, recent analyses in the US documented that hospitals were able to “game the system,” with scores approaching 100% on the tobacco-treatment measure [18], prompting the National Quality Forum to abandon tobacco-use intervention as a quality measure [19].
Statistical analyses
Frequencies, means with standard deviations (SDs), medians with interquartile ranges (IQR) were used when appropriate, as were chi2 tests, Fisher's exact test, Wilcoxon rank sum test and ANOVA for bivariate analyses. Statistical significance was set at 0.05. All analyses were performed using STATA version 12 (StataCorp, College Station, Texas).
Results
Patients characteristics
A total of 1260 patients with a main diagnosis of ACS were discharged from 4 university hospitals from September 2009 to October 2010 (Table 1). 688 patients (54.6%) were discharged with a main diagnosis of STEMI, 491 (39%) of NSTEMI and 81 (6.4%) of unstable angina. Mean age was 64 years and 21.3% were women. 22% had had a previous CHD and 38.2% were current smokers. Median length of stay for patients directly transferred home was 5.5 days for patients with STEMI, 3.7 among participants with NSTEMI and 2 among patients with unstable angina. 541 (43%) were discharged home after the hospital stay, 190 (15%) were directly transferred to a stationary cardiac rehabilitation facility and 529 (42%) to a peripheral hospital (Table 1).
Table 1. Baseline characteristics of the participants to the study hospitalized for an acute coronary syndrome in 4 academic centers in Switzerland from September 2009 to October 2010.
| Overall N = 1260 | Unstable Angina N = 81 | NSTEMI N = 491 | STEMI N = 688 | |
| Demographic variables | ||||
| Age, y (mean ± SD) | 64±12 | 67±12 | 65±13 | 62±12 |
| - <50 years, N (col. %) | 183 (14.5) | 7 (8.6) | 65 (13.4) | 111 (16.3) |
| - 50 to <65 years, N (col. %) | 496 (39.4) | 25 (30.9) | 181 (36.9) | 290 (42.0) |
| - 65 to 80 years, N (col. %) | 438 (34.8) | 36 (44.4) | 177 (36.1) | 225 (32.7) |
| - >80 years, N (col. %) | 143 (11.4) | 13 (16.1) | 68 (13.9) | 62 (9.0) |
| Female, N (%) | 268 (21.3) | 16 (19.8) | 114 (23.2) | 138 (20.1) |
| Race, N (%) | ||||
| - Caucasian | 1189 (94.6) | 76 (93.8) | 461 (94.4) | 652 (94.8) |
| - Black | 5 (.4) | 0 (.0) | 2 (.4) | 3 (.4) |
| - Asian | 7 (.6) | 2 (2.5) | 1 (.2) | 4 (.6) |
| - Other | 59 (4.7) | 3 (3.7) | 27 (5.5) | 29 (4.2) |
| Education* | ||||
| - Lower than apprenticeship, N (col. %) | 230 (20.4) | 21 (26.6) | 103 (23.8) | 106 (17.2) |
| - Apprenticeship or vocational school, N (col. %) | 599 (53.6) | 39 (49.4) | 214 (49.5) | 346 (56.9) |
| - High School or university graduation, N (col. %) | 300 (26.6) | 19 (24.1) | 115 (26.6) | 166 (26.8) |
| Clinical history | ||||
| Previous hypercholesterolemia†, N (%) | 742 (58.9) | 62 (76.5) | 327 (66.7) | 353 (51.3) |
| Previous hypertension†, N (%) | 742 (58.9) | 62 (76.5) | 327 (66.7) | 353 (51.3) |
| Previous diabetes†, N (%) | 227 (18.3) | 16 (19.8) | 109 (22.2) | 102 (14.8) |
| Previous CHD, N (%) | 276 (22.0) | 51 (63.0) | 135 (27.6) | 90 (13.1) |
| - Previous PCI, N (%) | 209 (16.6) | 42 (51.9) | 99 (20.3) | 68 (9.9) |
| - Previous CABG, N (%) | 74 (5.9) | 15 (18.5) | 37 (7.6) | 22 (3.2) |
| Previous stroke, N (%) | 36 (2.9) | 3 (3.7) | 16 (3.4) | 17 (2.5) |
| Previous renal failure requiring dialysis, N (%) | 8 (.7) | 1 (1.2) | 5 (1.1) | 2 (.3) |
| Previous valvular heart disease, N (%) | 32 (2.5) | 1 (1.2) | 23 (4.6) | 8 (1.2) |
| Anthropomorphic variables | ||||
| Obesity (BMI≥30 kg/m2) | 268 (21.6) | 20 (25) | 121 (24.9) | 127 (18.8) |
| Behavioral variables | ||||
| Current smoker, N (%) | 480 (38.2) | 23 (28.4) | 170 (34.6) | 287 (41.9) |
| Clinical variables | ||||
| Left ventricular function, mean (± SD) | 51.5 (±11.4) | 55.7 (±10.1) | 54.7 (±11.3) | 48.9 (±10.9) |
| - Left ventricular dysfunction (LVEF≤40%), N (%) | 220 (20.1) | 6 (9.2) | 58 (13.7) | 156 (25.7) |
| Hospital stay | ||||
| Coronary Revascularization | ||||
| - Overall revascularization, N (%) | 1170 (92.8) | 56 (69.7) | 439 (89.4) | 675 (98.1) |
| * PCI, N (%) | 1115 (88.4) | 52 (64.2) | 402 (81.9) | 661 (96.1) |
| * CABG, N (%) | 55 (4.4) | 4 (4.9) | 37 (7.5) | 14 (2.0) |
| Destination at discharge, N (%) | ||||
| - Home | 541 (42.9) | 62 (76.4) | 231 (47.1) | 248 (36.0) |
| - Direct transfer to inpatient cardiac rehabilitation | 190 (15.1) | 6 (7.4) | 100 (20.4) | 84 (12.2) |
| - Transfer to peripheral hospital | 529 (42.0) | 13 (16.1) | 160 (32.6) | 356 (51.7) |
| Length of stay, median (Q1,Q3), in days | ||||
| - For patients directly discharged home | 4.4 (2.3, 7) | 2 (1, 5) | 3.7 (1.9, 6.1) | 5.5 (4.0, 7.2) |
| - For patients transferred to peripheral hospital | 1 (.5, 1.5) | 1 (.5, 1.5) | 1 (.5, 1.5) | 1 (.5, 1.5) |
N, number of participants; BMI, body mass index; CABG, coronary artery by-pass graft; CHD, coronary heart disease; CR: cardiac rehabilitation; LVEF: Left ventricular ejection fraction; CHF, congestive heart failure; NSTEMI: Non ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; Q1: first quartile; Q3; third quartile; STEMI: ST-segment elevation myocardial infarction.
*38 participants with missing information on education status or who refused to disclose their education status.
Previous hypercholesterolemia, hypertension and diabetes based on self-report by patients or previous treatment by preventive medication specific to the hypercholesterolemia, hypertension or diabetes.
Prescription rates at discharge
For patients with a left ventricular ejection fraction (LVEF) of ≤40%, the rate of patients hospitalized for ACS who were prescribed ACEI/ATII at discharge was 94.6%. However, when including reasons non-prescription, 99.6% were prescribed ACEI/ATII or had a documented reason for non-prescription at discharge. The rate of patients hospitalized for ACS who were discharged on statins increased from 98% to 98.6% when including reasons for non-prescription. For beta-blockers, rates increased from 82% to 93% and for aspirin, from 99.4% to 100%. For patients that had had a PCI-stent treatment, rates further increase from 99.8% to 100% for P2Y12 inhibitor or had a documented reason for non-prescription and 99.9% on dual antiplatelet therapy (Table 2, Figure 1).147 patients discharged home directly or to another facility had a documented reason for non-prescription for beta-blockers, 8 for aspirin, 11 for ACEI/ATII and 7 for statins (Table 3). The most commonly reported reason for non-prescription for beta blockers was bradycardia (n = 62), defined as a heart rate of <60 beats/minute on the day of discharge. Sixty out of 1260 were discharged without the recommended treatment and without any documented contra-indication. Considering those discharged home directly, the type of ACS was associated with lower rate of treatment at discharge. 7 (11%) participants with UA did not receive the recommended treatment at discharge, 20(9%) with NSTEMI and 7(3%) with STEMI (p = <0.001 for the comparison between STEMI and UA or NSTEMI).
Table 2. Documented Treatment at Discharge for participants hospitalized for an acute coronary syndrome in 4 academic centers Switzerland from Sept 2009 to October 2010.
| Overall N = 1,260 | Unstable angina N = 81 | NSTEMI N = 491 | STEMI N = 688 | |
| Aspirin, % including reasons for not prescribing (NP) | 100% | 100% | 100% | 100% |
| - % prescribed regardless of reasons for NP | 99.4% | 98.8% | 99% | 99.7% |
| - Reason for NP documented, N/N eligible | 8/1260 | 1/81 | 5/491 | 2/688 |
| P2Y12 inhibitors if PCI-stent treatment, % including reasons for NP | 99.9% | 100% | 99.7% | 100% |
| - % prescribed regardless of reasons for NP | 99.8% | 100% | 99.5% | 100% |
| - Reason for NP documented, N/N eligible | 1/1066 | 0/47 | 1/379 | 0/640 |
| Dual antiplatelet therapy (DAPT) if PCI-stent treatment, % including reasons for NP | 99.9% | 100% | 99.7% | 100% |
| Beta-blockers, % including reasons for NP | 93.3% | 87.7% | 90.4% | 95.9% |
| - % prescribed regardless of reasons for NP | 81.7% | 76.5% | 83.3% | 81.1% |
| - Reason for NP documented, N/N eligible | 147/1260 | 11/81 | 45/491 | 91/688 |
| AT II antagonist/ACE inhibitors (LVEF≤40%), % including reasons for NP | 99.5% | 83.3% | 100% | 100% |
| - % prescribed regardless of reasons for NP | 94.6% | 83.3% | 87.9% | 97.4% |
| - Reason for NP documented, N/N eligible | 11/220 | 0/6 | 7/58 | 4/156 |
| Statins, % including reasons for NP | 98.6% | 96.3% | 98.0% | 99.3% |
| - % prescribed regardless of reasons for NP | 98.0% | 95.1% | 97.4% | 98.8% |
| - Reason for NP documented, N/N eligible | 7/1260 | 1/81 | 3/491 | 3/688 |
| Concomitant documentation of Aspirin, Statin, Beta-blockers and AT II antagonist/ACE inhibitors, % * | 95.2% | 88.9% | 94.3% | 96.7% |
| Nitrate documentation, % | 6.9% | 18.5% | 9.6% | 3.6% |
DAPT: Dual antiplatelet therapy; N, number of participants; STEMI: ST-segment elevation myocardial infarction; NP: non-prescription; NSTEMI: Non ST-segment elevation myocardial infarction; LVEF, left ventricular ejection fraction.
* Concomitant prescription at discharge unless contra-indicated or not indicated for aspirin, clopidogrel/prasugrel or ticagrelor if PCI-stent treatment, beta-blocker, statin, ACEI if EF≤40%. When participants transferred to peripheral hospital, beta-blocker and ACEI/ATII coded as not applicable.
Prescription rates according to guidelines taking into account reported indications reasons for not prescribing medication at discharge.
Figure 1. Percent of participants with recommended treatment at discharge taking into account reported reasons for non-prescription.
Abbreviations: P2Y12 inhibitors: clopidogrel, prasugrel or ticagrelor; ACEI/ATII : Angiotensin converting enzyme inhibitor/angiotensin II receptor blockers. * P2Y12 inhibitors if PCI-stent treatment (n = 1066). ** ACEI/ATII inhibitors if left ventricular ejection fraction (LVEF) ≤40% (n = 220).
Table 3. Documented reasons for not prescribing recommended cardiovascular medication at discharge.
| Documented reasons for not prescribing medication in patients discharged home, in cardiac rehabilitation or to another facility | Documented reasons for not prescribing medication in patients discharged home directly | |
| Aspirin | 8 | 3 |
| - Active bleeding during hospital stay | 0 | 0 |
| - Coumadin/warfarin prescribed at discharge | 5 | 2 |
| - Aspirin allergy | 3 | 1 |
| - Other reason documented by physician for not prescribing | 0 | 0 |
| - Patient refusal | 0 | 0 |
| - Introduced later (in peripheral hospital) | 0 | NA |
| - Other reason | 0 | 0 |
| Beta-blocker (only patients not transferred in peripheral hospital considered) | 147 | 41 |
| - Beta-blocker allergy | 2 | 2 |
| - Second- or third-degree atrio-ventricular heart block | 8 | 4 |
| - Bradycardia (heart rate <60/min) on day of discharge | 62 | 25 |
| - Hypotension | 10 | 3 |
| - Asthma or COPD | 1 | 1 |
| - Other reason documented by physician for not prescribing | 17 | 6 |
| - Patient refusal | 0 | 0 |
| - Introduced later (in peripheral hospital) | 47 | NA † |
| - Other reason | 0 | 0 |
| ACEI/ATII (only patients not transferred in peripheral hospital and with LVEF≤40% considered) | 11 | 1 |
| - Moderate or severe aortic stenosis | 0 | 0 |
| - ACEI or ATII allergy | 1 | 0 |
| - Other reason documented by physician for not prescribing | 1 | 0 |
| - Renal failure | 4 | 1 |
| - Hypotension | 1 | 0 |
| - Patient refusal | 0 | 0 |
| - Introduced later (in peripheral hospital) | 4 | NA |
| - Other reason | 0 | 0 |
| Statins | 7 | 2 |
| - Statin medication allergy | 0 | 0 |
| - Reason documented by physician for not prescribing | 3 | 0 |
| - Statin intolerance | 3 | 2 |
| - Patient refusal | 0 | 0 |
| - Introduced later (in peripheral hospital) | 1 | NA |
| - Other reason | 0 | 0 |
ACEI: Angiotensin Converting Enzyme Inhibitor; ATII: Angiotensin II receptor blockers; NA: Not applicable; NR: not reported.
6 patients discharged home directly and who had “to be introduced later” as the reason for not prescription were coded as not having been prescribed the recommended medication.
33% of smoking participants were offered a specialized smoking cessation intervention in 2 university hospitals (GE, LA).
Discussion
We found high adherence to ACS guidelines for drug prescriptions when including reasons for non-prescription to drug therapy. For beta-blockers, prescription rates were suboptimal, even after taking into account reason for non-prescription. In addition, bradycardia was often reported as a reason for non-prescription despite its absence from recommended reasons for non-prescription in guidelines. The prescription rate of recommended treatments were between 100% and 99% for antiplatelet therapy, statin therapy and ACEI/ATII for patients with LVEF≤40% after taking into account pre-specified and documented reason for non-prescription, suggesting that the optimal threshold has been achieved for these medications. Despite the proven benefits of dedicated smoking cessation interventions, only 33% of smokers received such an intervention.
In countries with systematic performance monitoring such as the US, an improvement of the recommended discharge medication has been reported [20]. The NCDR ACTION Registry-GWTG Network showed prescription rates for discharge therapies according to percentiles of performance after exclusion of participants with reasons for non-prescription for each medication. Hospitals in the top 10% of performance achieved prescription rates of 99% for aspirin and beta-blockers, 86% for P2Y12 inhibitors, 93% for ACEI/ATII and 94% for statins [6]. These results suggest that Swiss university hospitals would be within the top 10% hospitals in the US for aspirin, P2Y12 inhibitors, ACEI/ATII and statins. For beta-blockers however, the rate of prescription ranged below the top 10%, even after including documented reasons for non-prescription. Various quality improvement strategies have taken place in Switzerland within the last decade at both regional and national level [21]. However, none of these quality improvement strategies included financial incentives to improve quality of care or public reporting of prescription rate at discharge. The improvement in quality of care at discharge might be due to these quality improvement strategies.
Comparative data on quality of care at discharge taking into account reasons for non-prescription to medication is limited in Switzerland. The only study which considered reasons for non-prescription published so far was a retrospective chart review by trained medical doctors which selected patients hospitalized for a main diagnosis of acute myocardial infarction (AMI) (NSTEMI and STEMI) in three out of the four academic medical centers included in our study (BE, GE, LA) [16]. Patients transferred to another hospital for inpatient care or who expired during the hospital stay were excluded. Criteria for recommended medication at discharge in 1999 were derived from the US Cooperative Cardiovascular Project and adapted to the local context [16], [22]. Statins at discharge and attendance to cardiac rehabilitation were not abstracted. Patients in 1999 had similar baseline characteristics, except a higher age (mean age 68.2 vs. 63.6), higher proportion of women (35% vs. 18%) and previous CHD (36% vs. 22%). Comparing 2009–2010 data to the 1999 data, the prescription rate of patients discharged at home after a NSTEMI or STEMI increased from 91% in 1999 to 100% in 2009–2010 for aspirin and from 81% to 94% for beta-blockers. In patients with a left ventricular ejection fraction (LVEF) of less than 40%, the rates increased from 79% to 100% for ACEI/ATII (p<0.001).
Data from a registry in Switzerland (AMIS Plus) on patients with STEMI suggested that 84.2% were discharged on a P2Y12 inhibitor, 96% on aspirin, 89% on an ACEI/ATII, 91.7% on statins and 79.2% on beta-blockers in 2011, but report on quality data was on a voluntary basis and reasons for non-prescription were not reported [10], [11]. Quality at discharge for patients with STEMI has been reported in France showing high prescription rates of evidence based medication [8]. 95% were discharged on aspirin, 84% on beta-blockers and 75% on ACEI. However, reason for non-prescription to prescription medication was not reported. In an era of targets of prescription rates close to 100% unless contra-indicated, pre-specification of the reasons for non-prescription within the data collection forms permits to identify the remaining gaps in quality at discharge. The 100% prescription rates observed for aspirin and P2Y12 inhibitor obviously suggest that no further increase in quality can be achieved for the prescription rates of these medications, however, it permitted us to identify that beta-blockers might still be underutilized and that reasons for non-prescription such as bradycardia, which is not recognized as a contra-indication by current guidelines needs to be improved.
ESC and AHA Guidelines recommend the adoption of dedicated smoking cessation program in each hospital [23], [24]. Only 33% of smokers received dedicated smoking cessation interventions during the hospital stay. The beneficial effect of a systematic high intensity smoking cessation intervention to all smokers is currently assessed in the participating hospitals.
Potential limitations
These data are derived from university hospitals and might not represent patients hospitalized for an ACS in other hospital in Switzerland. Compared to the AMIS+, a national registry in academic and non-academic centers accounting for 78 out of 106 hospitals treating ACS in Switzerland, the mean age of participants with STEMI was similar in both men and women, but was lower than in other registries in Europe, and rates of revascularization were higher [11], [25], [26]. These differences in mean age and rate of catheterization might be due to the fact that patients were essentially included in the catheterization laboratory in two participating hospitals. Those patients undergoing catheterization are known to be younger than the total number of patients with ACS [27]. Elderly ACS patients and those not undergoing catheterization have been shown to be less likely to receive evidence-based therapies [27], [28]. In these patients, who may have more comorbidities, the adherence to guidelines could be still suboptimal even including the reasons for not prescription. We urge for careful comparison of the reported rates of treatment according to guidelines at discharge in our studied sample with other registries. The aim of this study was not to report on the quality of care for all patients in Switzerland, but to determine the importance of collecting reasons for non-prescription in databases to detect remaining gaps in quality of care in a sample of participants with high rate of recommended treatment at discharge. We found an association between the type of ACS and prescription of recommended treatment, including reasons for non-prescription. These results should be carefully interpreted due to the low number of participants without recommended treatment at discharge. Rates of referral to stationary cardiac rehabilitation were based on information at discharge. In Switzerland, both stationary cardiac rehabilitation and ambulatory cardiac rehabilitation are available. The reported rate of direct transfer to and stationary cardiac rehabilitation facility from the hospital does not reflect the overall attendance rate to cardiac rehabilitation in Switzerland. Future analyses should explore the attendance rate to cardiac rehabilitation using data at discharge and follow-up.
Conclusions
We found a found high adherence to ACS guidelines for drug prescriptions when including reasons for non-prescription to drug therapy. Achieved rates of prescribed medication at discharge were above 99% for Aspirin, P2Y12 inhibitors, ACEI/ATII and statins. Prescription rates for beta-blockers taking into account reasons for non-prescription were lower at 94%. In an era of improving quality of care to achieve 100% prescription rates at discharge unless contra-indicated, pre-specification of reasons for non-prescription for cardiovascular preventive medication within clinical registries permits to identify remaining gaps in quality of care at discharge.
Funding Statement
The SPUM-ACS cohort is supported by the Swiss National Science Foundation (SNSF 33CM30-124112, Inflammation and acute coronary syndromes (ACS) – Novel strategies for prevention and clinical management). The specific report on quality of care at discharge is supported by a grant from the Department of University Medicine and Community Care (DUMSC) of the University of Lausanne, Switzerland and the Swiss Heart Foundation. The authors acknowledge the cooperation of all participating centers, practicing physicians, referring doctors and institutions. Dr. Auer and Dr Rodondi's research on cardiovascular prevention is supported by grants from the Swiss Heart Foundation. Dr Auer's research on cardiovascular prevention is additionally supported by a grant for prospective researchers from the Swiss National Science Foundation PBLAP3-136774, the Société Académique Vaudoise and the SICPA Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. (2012) Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125: e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butler J, Arbogast PG, BeLue R, Daugherty J, Jain MK, et al. (2002) Outpatient adherence to beta-blocker therapy after acute myocardial infarction. J Am Coll Cardiol 40: 1589–1595. [DOI] [PubMed] [Google Scholar]
- 3. Smith CS, Cannon CP, McCabe CH, Murphy SA, Bentley J, et al. (2005) Early initiation of lipid-lowering therapy for acute coronary syndromes improves compliance with guideline recommendations: observations from the Orbofiban in Patients with Unstable Coronary Syndromes (OPUS-TIMI 16) trial. Am Heart J 149: 444–450. [DOI] [PubMed] [Google Scholar]
- 4. Setoguchi S, Glynn RJ, Avorn J, Mittleman MA, Levin R, et al. (2008) Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10-year trend analysis. J Am Coll Cardiol 51: 1247–1254. [DOI] [PubMed] [Google Scholar]
- 5.HEDIS Health Plan Employer Data and Information Set. Washington, DC: National Committee for Quality Insurance (NCQA) website Available: http://www.ncqa.org/. Accessed 2014 March 6.
- 6. Peterson ED, Roe MT, Chen AY, Fonarow GC, Lytle BL, et al. (2010) The NCDR ACTION Registry-GWTG: transforming contemporary acute myocardial infarction clinical care. Heart 96: 1798–1802. [DOI] [PubMed] [Google Scholar]
- 7. Ferrieres J, Bataille V, Leclercq F, Geslin P, Ruidavets JB, et al. (2009) Patterns of statin prescription in acute myocardial infarction: the French registry of Acute ST-elevation or non-ST-elevation Myocardial Infarction (FAST-MI). Atherosclerosis 204: 491–496. [DOI] [PubMed] [Google Scholar]
- 8. Belle L, Labarere J, Fourny M, Drouet E, Mulak G, et al. (2012) Quality of care for myocardial infarction at academic and nonacademic hospitals. Am J Med 125: 365–373. [DOI] [PubMed] [Google Scholar]
- 9. Zeymer U, James S, Berkenboom G, Mohacsi A, Iniguez A, et al. (2012) Differences in the use of guideline-recommended therapies among 14 European countries in patients with acute coronary syndromes undergoing PCI. Eur J Prev Cardiol [DOI] [PubMed] [Google Scholar]
- 10. Radovanovic D, Urban P, Simon R, Schmidli M, Maggiorini M, et al. (2010) Outcome of patients with acute coronary syndrome in hospitals of different sizes. A report from the AMIS Plus Registry. Swiss Med Wkly 140: 314–322. [DOI] [PubMed] [Google Scholar]
- 11. Radovanovic D, Nallamothu BK, Seifert B, Bertel O, Eberli F, et al. (2012) Temporal trends in treatment of ST-elevation myocardial infarction among men and women in Switzerland between 1997 and 2011. European Heart Journal Acute Cardiovascular Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wieser S, Ra Themann I, De B, Eichler K, Pletscher M, et al. (2012) Cost of acute coronary syndrome in Switzerland in 2008. Swiss Med Wkly 142: 0. [DOI] [PubMed] [Google Scholar]
- 13. Matter CM, Klingenberg R, Templin C, Altwegg L, Räber L, et al. (2010) Inflammation and acute coronary syndromes (ACS) – a clinical research network funded by the Swiss National Science Foundation. Kardiovaskuläre Medizin 13: 31–34. [Google Scholar]
- 14. Carballo D, Auer R, Carballo S, Windecker S, Matter C, et al. (2010) [A Swiss multicentric project to improve the prevention of cardiovascular event recurrence after acute coronary syndromes]. Rev Med Suisse 6: 518–524. [PubMed] [Google Scholar]
- 15. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, et al. (2007) Universal definition of myocardial infarction. Circulation 116: 2634–2653. [DOI] [PubMed] [Google Scholar]
- 16. Luthi JC, McClellan WM, Flanders WD, Pitts SR, Burnand B (2005) Variations in the quality of care of patients with acute myocardial infarction among Swiss university hospitals. Int J Qual Health Care 17: 229–234. [DOI] [PubMed] [Google Scholar]
- 17. Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, et al. (2008) ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to develop performance measures for ST-elevation and non-ST-elevation myocardial infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation 118: 2596–2648. [DOI] [PubMed] [Google Scholar]
- 18. Levy DE, Kang R, Vogeli CS, Rigotti NA (2011) Smoking cessation advice rates in US hospitals. Arch Intern Med 171: 1682–1684. [DOI] [PubMed] [Google Scholar]
- 19. Fiore MC, Goplerud E, Schroeder SA (2012) The Joint Commission's New Tobacco-Cessation Measures — Will Hospitals Do the Right Thing? New England Journal of Medicine 366: 1172–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jernberg T, Johanson P, Held C, Svennblad B, Lindbäck J, et al. (2011) Association Between Adoption of Evidence-Based Treatment and Survival for Patients With ST-Elevation Myocardial Infarction. JAMA: The Journal of the American Medical Association 305: 1677–1684. [DOI] [PubMed] [Google Scholar]
- 21. Luthi JC, McClellan WM, Flanders WD, Pitts S, Burnand B (2002) Quality of health care surveillance systems: review and implementation in the Swiss setting. Swiss Med Wkly 132: 461–469. [DOI] [PubMed] [Google Scholar]
- 22. Marciniak TA, Ellerbeck EF, Radford MJ, Kresowik TF, Gold JA, et al. (1998) Improving the Quality of Care for Medicare Patients With Acute Myocardial Infarction: Results From the Cooperative Cardiovascular Project. JAMA 279: 1351–1357. [DOI] [PubMed] [Google Scholar]
- 23. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, et al. (2011) ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 32: 2999–3054. [DOI] [PubMed] [Google Scholar]
- 24. Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, et al. (2012) ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J 33: 2569–2619. [DOI] [PubMed] [Google Scholar]
- 25. McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, et al. (2011) Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med 124: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bajaj RR, Goodman SG, Yan RT, Bagnall AJ, Gyenes G, et al. (2013) Treatment and outcomes of patients with suspected acute coronary syndromes in relation to initial diagnostic impressions (insights from the Canadian Global Registry of Acute Coronary Events [GRACE] and Canadian Registry of Acute Coronary Events [CANRACE]). Am J Cardiol 111: 202–207. [DOI] [PubMed] [Google Scholar]
- 27. Alexander KP, Newby LK, Bhapkar MV, White HD, Hochman JS, et al. (2006) International variation in invasive care of the elderly with acute coronary syndromes. Eur Heart J 27: 1558–1564. [DOI] [PubMed] [Google Scholar]
- 28. Avezum A, Makdisse M, Spencer F, Gore JM, Fox KA, et al. (2005) Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE). Am Heart J 149: 67–73. [DOI] [PubMed] [Google Scholar]