Abstract
Vitamin D3 is made in the skin from 7-dehydrocholesterol under the influence of UV light. Vitamin D2 (ergocalciferol) is derived from the plant sterol ergosterol. Vitamin D is metabolized first to 25 hydroxyvitamin D (25OHD), then to the hormonal form 1,25-dihydroxyvitamin D (1,25(OH)2D). CYP2R1 is the most important 25-hydroxylase; CYP27B1 is the key 1-hydroxylase. Both 25OHD and 1,25(OH)2D are catabolized by CYP24A1. 1,25(OH)2D is the ligand for the vitamin D receptor (VDR), a transcription factor, binding to sites in the DNA called vitamin D response elements (VDREs). There are thousands of these binding sites regulating hundreds of genes in a cell-specific fashion. VDR-regulated transcription is dependent on comodulators, the profile of which is also cell specific. Analogs of 1,25(OH)2D are being developed to target specific diseases with minimal side effects. This review will examine these different aspects of vitamin D metabolism, mechanism of action, and clinical application.
With the finding of the vitamin D receptor (VDR) in nearly every tissue and the more recent discovery of thousands of VDR binding sites throughout the genome controlling hundreds of genes, the interest in vitamin D and its impact on multiple biologic processes has accelerated tremendously as evidenced by the thousands of publications each year for the past several years. These observations have spawned a major effort to develop vitamin D analogs that can separate the effects of the active metabolite 1,25-dihydroxyvitamin D (1,25(OH)2D) on calcium and phosphate homeostasis from its effects on these other biologic processes and, in particular, to target just one such process. For some circumstances, this has been achieved. For example, calcipotriol and 22-oxa calcitriol (OCT) are approved for the treatment of psoriasis; paricalcitol, doxercalciferol, and falecalcitriol are approved for secondary hyperparathyroidism (nota bene: OCT and falecalcitriol are approved for use only in Japan). The mechanisms by which these analogs achieve relative specificity for the application for which they have been approved are several, including their affinity for the major vitamin D transport protein in blood (vitamin D binding protein [DBP]), their metabolism either as prodrug activation or rates of catabolism, their affinity for the VDR, and their ability to influence VDR transcriptional activity through effects on retinoid X receptor (RXR) heterodimerization and/or comodulator recruitment. Thus, to understand the future of vitamin D with respect to clinical applications, it is necessary to understand aspects of vitamin D metabolism and mechanisms of action that can be manipulated to facilitate tissue-specific clinical applications. Although for the most part we are not yet at the point of tissue-specific application, a good start has been made. In this review, I have had to be selective, so my apologies in advance to those investigators whose work I have not cited.
Vitamin D Production
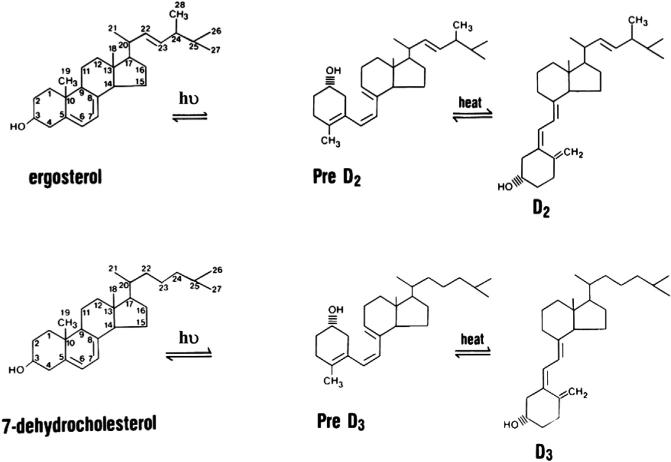
The production of vitamin D3 (D3) in the skin is not an enzymatic process (Figure 1). D3 (cholecalciferol) is produced from 7-dehydrocholesterol (7-DHC) through a two-step process in which the B ring is broken by UV light (spectrum 280–320 UVB) radiation from the sun, forming pre-D3 that isomerizes to D3 in a thermo-sensitive but noncatalytic process. Both UVB intensity and skin pigmentation level contribute to the rate of D3 formation (Holick et al., 1980). Melanin in the skin blocks UVB from reaching 7-DHC, thus limiting D3 production, as do clothing and sun-screen. The intensity of UVB from sunlight varies according to season and latitude, so the further one lives from the equator, the less time of the year one can rely on solar exposure to produce D3 (Webb et al., 1989). Vitamin D can also be obtained from the diet. Most foods with the exception of fatty fish contain little vitamin D unless fortified. The vitamin D in fish is D3, whereas that used for fortification is often D2 (ergocalciferol). D2 is produced by UVB irradiation of the ergosterol in plants and fungi (e.g., mushrooms). It differs from D3 in having a double bond between C22 and C23 and a methyl group at C24 in the side chain. D2 can be considered the first vitamin D analog. These differences from D3 in the side chain lower its affinity for DBP resulting in faster clearance from the circulation, limit its conversion to 25 hydroxyvitamin D (25OHD) by at least some of the 25-hydroxylases to be described, and alter its catabolism by the 24-hydroxyase (CYP24A1) (Houghton and Vieth, 2006; Hollis, 1984; Horst et al., 1986). Therefore, unless given daily, D2 supplementation does not result in as high a blood level of 25OHD as comparable amounts of D3 (Tripkovic et al., 2012). On the other hand, 1,25(OH)2D2 and 1,25(OH)2D3 have comparable affinities for the VDR (Hollis, 1984).
Figure 1. The Production and Metabolism of D2 and D3.
D3 is produced in the skin from 7-DHC in a nonenzymatic process in which the B ring is broken by UVB radiation, and the pre-D3 formed isomerizes to D3 in a thermo-sensitive process. D3 is converted to 25OHD3 in the liver and elsewhere by a number of enzymes of which CYP2R1 is the most important. The regulation of this step is modest at best. The kidney and other tissues metabolize 25OHD to the active metabolite 1,25(OH)2D3 or the first step in the catabolic process 24,25(OH)2D3. The enzymes responsible, CYP27B1 and CYP24A1, respectively, are tightly controlled. Although the regulation differs in different tissues, in the kidney, CYP27B1 is stimulated by PTH and inhibited by FGF23 and high calcium (Ca) and phosphate (P). The regulation of CYP24A1 is just the opposite. 1,25(OH)2D3 also regulates its own production directly and by inhibiting PTH production, stimulating FGF23 production, and inducing CYP24A1.
Vitamin D Metabolism
The three main steps in vitamin D metabolism, 25-hydroxylation, 1α-hydroxylation, and 24-hydroxylation are all performed by cytochrome P450 mixed-function oxidases (CYPs). These enzymes are located either in the endoplasmic reticulum (ER) (e.g., CYP2R1) or in the mitochondria (e.g., CYP27A1, CYP27B1, and CYP24A1). The electron donor for the ER enzymes is the reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent P450 reductase. The electron donor chain for the mitochondrial enzymes is comprised of ferredoxin and ferredoxin reductase. These are not specific for a given CYP—specificity lies within the CYP. Although of the CYPs involved in vitamin D metabolism, only CYP2R1 and CYP24A1 have been crystallized, it is likely that these enzymes contain a number of common structural features. These include 12 helices (A–L) and loops and a common prosthetic group, namely the iron-containing protoporphyrin IX (heme) linked to the thiolate of cysteine. The I helix runs through the center of the enzyme above the heme where a thr(ser) and asp(glu) pair is essential for catalytic activity (Sugimoto and Shiro, 2012). CYP2R1, like other microsomal CYPs, contains two extra helices that appear to form a substrate channel in the bilayer of the ER (Sugimoto and Shiro, 2012). The B′ helix serves as a gate, closing on substrate binding. Whether a similar substrate channel exists for the mitochondrial CYPs is not clear.
25-hydroxylase
The liver has been established as the major if not sole source of 25OHD production from vitamin D. Initial studies of the hepatic 25-hydroxlase found activity in both the mitochondrial and microsomal fractions, and subsequent studies have demonstrated a number of CYPs with 25-hydroxylase activity. CYP27A1 is the only mitochondrial 25-hydroxylase. It was initially identified as a sterol 27-hydroxylase involved in bile acid synthesis. This CYP is widely distributed in the body, not just in the liver. However, CYP27A1 does not 25-hydroxylate D2. Moreover, when it is deleted in the mouse, blood levels of 25OHD actually increase (Zhu et al., 2013), and inactivating mutations in humans cause cerebrotendinous xanthomatosis with abnormal bile and cholesterol metabolism, but not rickets (Moghadasian, 2004). Subsequently, CYP2R1 was identified in the microsomal fraction of mouse liver (Cheng et al., 2003). This enzyme 25-hydroxylates both D2 and D3 with comparable kinetics, unlike CYP27A1. Its expression is primarily in the liver and testes. CYP2R1 expression is increased in the CYP27A1 null mouse, likely explaining the increased blood levels of 25OHD in the CYP27A1 null mouse. When CYP2R1 is deleted from mice, blood levels of 25OHD fall more than 50%, but not to zero (Zhu et al., 2013). Even the double deletion of CYP2R1 and CYP27A1 does not reduce the blood level of 25OHD to zero and actually has little impact on blood levels of calcium and phosphate (Zhu et al., 2013), suggesting compensation by other enzymes with 25-hydroxylase activity. In humans, a leu99pro mutation in CYP2R1 has been found in a Nigerian with severe bone disease associated with biochemical evidence of rickets including a low 25OHD but normal 1,25(OH)2D (Cheng et al., 2004). When tested in vitro, this mutation profoundly decreased the activity of CYP2R1 (Cheng et al., 2004). However, given the prevalence of rickets in this population, these findings do not rule out the role for other 25-hydroxylases. CYP3A4, the major drug-metabolizing enzyme preferentially located in liver and intestine, has 25-hydroxylase activity (Gupta et al., 2004). CYP3A4 prefers 1αOHD to 25OHD as substrate. CYP2J3 is expressed in rat liver and has 25-hydroxylase activity, but its human homolog, CYP2J2, has less such 25-hydroxylase activity, is located primarily in the heart, and appears to function mainly as an arachidonic acid epooxygenase (Zhu and DeLuca, 2012). The human homolog of CYP2D25, initially isolated from pig liver and kidney (Postlind et al., 1997), does not have substantial 25-hydroxylase activity (Hosseinpour and Wikvall, 2000). CYP2C11 is expressed in the liver of male rats. It has 25-hydroxylase activity for D3 and D2 as well as the 1OHD analogs but is better known for its hydroxylations of testosterone (Rahmaniyan et al., 2005). It is not clear if there is a human homolog. Although some studies have indicated regulation for some of these 25-hydroxylases, in general, regulation of vitamin D 25-hydroxylation is not a major consideration, and circulating levels of 25OHD are a useful marker of vitamin D nutrition. Thus, CYP2R1 appears to be the major 25-hydroxylase, but other enzymes have 25-hydroxylase activity that may affect levels of 25OHD within a given tissue and/or contribute to the circulating levels of 25OHD.
1α-hydroxylase
The kidney is the major if not the sole source of circulating levels of 1,25(OH)2D. Unlike 25-hydroxylation, there is only one enzyme recognized to have 25OHD 1α-hydroxylase activity, and that is CYP27B1. This enzyme was cloned and sequenced by four different groups in the same year (Fu et al., 1997; Shinki et al., 1997; Takeyama et al., 1997; St-Arnaud et al., 1997). Mutations within the gene have been shown to underlie the condition of pseudovitamin D deficiency caused by inadequate 1,25(OH)2D production (Fu et al., 1997; Shinki et al., 1997; Takeyama et al., 1997; St-Arnaud et al., 1997). CYP27B1 has a high degree of homology with the other mitochondrial enzymes involved with vitamin D metabolism: CYP27A1 and CYP24A1. Although the kidney is the main source of circulating 1,25(OH)2D, a number of other tissues also express the enzyme, and the regulation of the extrarenal CYP27B1 differs from that of the renal CYP27B1 (review in Bikle, 2010). Examples include the epithelial cells in the skin, lungs, breast, intestine, and prostate; endocrine glands including the parathyroid gland (PTG), pancreatic islets, thyroid, testes, ovary, and placenta; cells of the immune system including macrophages, and T and B lymphocytes and dendritic cells (DCs); osteoblasts and chondrocytes; and a variety of tumors derived from these cells. Unlike the hepatic 25-hydroxylases, the renal 1α-hydroxylase is tightly regulated primarily by three hormones: parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), and 1,25(OH)2D itself. PTH stimulates whereas FGF23 and 1,25(OH)2D inhibit CYP27B1. Elevated calcium suppresses CYP27B1 primarily via suppression of PTH; elevated phosphate suppresses CYP27B1 primarily by stimulating FGF23, although these ions can have direct effects on renal CYP27B1 on their own (Bikle and Rasmussen, 1975; Bikle et al., 1975). The stimulation by PTH involves cyclic AMP (cAMP) (Rost et al., 1981), and consensus cAMP response elements are found in the proximal promoter of the CYP27B1 gene. However, the precise mechanism of stimulation is not clear and may involve the transcription factor C/EBPβ acting as an inhibitor of the orphan receptor NR4A2 (Zierold et al., 2007). Similarly, the precise mechanism by which FGF23 inhibits CYP27B1 remains unclear. FGF23 signals through select FGF receptors only in the presence of the coreceptor Klotho. This signaling activates the mitogen-activated protein kinase (MAPK) cascade, but its role in CYP27B1 expression remains unclear (Urakawa et al., 2006). 1,25(OH)2D limits CYP27B1 activity by inhibiting PTH and increasing FGF23 production as well as reducing 1,25(OH)2D levels by inducing the catalytic enzyme CYP24A1. However, 1,25(OH)2D3 also directly inhibits CYP27B1 expression in the kidney through a complex mechanism involving VDR and a vitamin D inhibitory receptor (VDIR) that brings both histone deacetylases (HDACs) and DNA methyl transferases to the promoter of CYP27B1 inhibiting its transcription (Kim et al., 2007).
Regulation of extrarenal CYP27B1 differs. Most attention regarding regulation of extrarenal CYP27B1 has focused on keratinocytes and macrophages. Keratinocytes respond to PTH with increased 1,25(OH)2D3 production, but these cells do not have the classic PTH receptor and do not respond to cAMP (Bikle et al., 1986). The mechanism by which PTH stimulates 1,25(OH)2D production in these cells remains unclear. However, using a CYP27B1 promoter/luciferase reporter assay, Flanagan et al. (2003) demonstrated that PTH stimulated expression in a kidney cell line but not in keratinocytes, suggesting that the effect of PTH may be posttranscriptional. The effect of FGF23 on keratinocyte CYP27B1 expression or function has not been reported. Unlike the kidney, 1,25(OH)2D does not directly affect CYP27B1 expression in keratinocytes. Rather, 1,25(OH)2D regulates its own levels in the keratinocyte by inducing CYP24A1, the catabolic enzyme for 1,25(OH)2D3 (Xie et al., 2002). Tumor necrosis factor α (TNF-α) (Bikle et al., 1991) and interferon γ (IFN-γ) (Bikle et al., 1989), on the other hand, are potent inducers of CYP27B1 activity in the keratinocyte.
The production of 1,25(OH)2D by circulating monocytes can be stimulated by IFN and other cytokines including TNF, inter-leukin-1 (IL-1), and IL-2 (Gyetko et al., 1993) signaling through the JAK/STAT, p38 MAPK, and NFκB pathways (Adams and Gacad, 1985; Pryke et al., 1990; Gyetko et al., 1993; Stoffels et al., 2006). Although PTH has not been shown to alter macrophage CYP27B1 activity, FGF23 has recently been shown to be inhibitory (Bacchetta et al., 2013). In parathyroid cells, however, FGF23 has been found to stimulate CYP27B1 expression (Krajisnik et al., 2007), so its actions are not always inhibitory. CYP24A1 induction and/or function in macrophages in response to 1,25(OH)2D is blunted and does not provide the safety valve found in keratinocytes (Adams and Gacad, 1985). The mechanism appears to involve the expression of a truncated form of CYP24, which includes the substrate binding domain, but not the mitochondrial targeting sequence. This truncated form is postulated to act as a dominant-negative form of CYP24A1, binding 1,25(OH)2D within the cytoplasm and preventing its catabolism (Ren et al., 2005).
24-hydroxylase
CYP24A1 is the only established 24-hydroxylase involved with vitamin D metabolism. This enzyme has both 24-hydroxylase and 23-hydroxylase activity, the ratio of which is species dependent (Jones et al., 2012). The enzyme in humans has both capabilities, but the rat enzyme is primarily a 24-hydroxylase (Sakaki et al., 2000). Mutating ala 326 to gly 326 in the human CYP24A1 shifts the profile from one favoring 24-hydroxyation to one favoring 23-hydroxylation (Prosser et al., 2007). Other mutagenesis studies in combination with the known crystal structure of CYP24A1 have provided an excellent understanding of the substrate binding pocket as recently reviewed by Jones et al. (2012). The 24-hydroxylase pathway results in the biologically inactive calcitroic acid, whereas the 23-hydroxylase pathway ends up producing the biologically active 1,25-26,23 lactone. All steps are performed by one enzyme (Sakaki et al., 2000). 1,25(OH)2D is the preferred substrate relative to 25OHD, but both are 24-hydroxylated. 1,24,25(OH)3D has substantial affinity for the VDR and therefore has biological activity. There may be a physiologic role for 24,25(OH)2D in the growth plate in that both 1,25(OH)2D and 24,25(OH)2D appear to be required for optimal endochondral bone formation (Plachot et al., 1982), and evidence for a specific receptor for 24,25(OH)2D in chondrocytes is gaining strength (R. St-Arnaud, personal communication). Deletion of CYP24A1, thus eliminating all 24-hydroxylated metabolites of vitamin D, results in defective mineralization of intramembranous (not endochondral) bone (St-Arnaud et al., 2000). However, crossing this mouse with one lacking the VDR corrects the mineralization defect (St-Arnaud et al., 2000), indicating that it is the high circulating 1,25(OH)2D not the lack of 24,25(OH)2D that causes the phenotype. Furthermore, inactivating mutations in CYP24A1 have recently been found in children with idiopathic infantile hypercalcemia who present with severe hypercalcemia, hypercalciuria, and nephrocalcinosis, decreased PTH, low 24,25(OH)2D, and inappropriately normal-to-high 1,25(OH)2D (Schlingmann et al., 2011). Thus, it appears that the primary function of CYP24A1 is to prevent the accumulation of toxic levels of 1,25(OH)2D and 25OHD.
Regulation of CYP24A1is the reciprocal of that of CYP27B1 at least in the kidney. In essentially all cells in which it is expressed, CYP24A1 is strongly induced by 1,25(OH)2D and often serves as a marker of 1,25(OH)2D response in that cell. The promoter of CYP24A1 contains two vitamin D response elements (VDREs) around 150 and 250 bp upstream of the transcriptional start site to which VDR/RXR bind (Zierold et al., 1995) as well as sites for the transcription factors Ets-1 and C/EBPβ. More recently, chromatin immunoprecipitation (ChIP)-chip data have identified sites 50–70 kb downstream of the human CYP24A1 gene to which H4 acetylases and RNA polymerase II are recruited and that play a role in 1,25(OH)2D induction of CYP24A1 (Meyer et al., 2010a). PTH attenuates the induction of CYP24A1 by 1,25(OH)2D at least in part by increasing the degradation of CYP24A1 mRNA through the cAMP/PKA pathway (Zierold et al., 2000, 2001). However, in osteoblasts, PTH enhances 1,25(OH)2D induction of CYP24A1 transcription through the same cAMP/PKA pathway (Armbrecht et al., 1998), illustrating the fact that regulation of these vitamin D-metabolizing enzymes is cell specific. FGF23 increases CYP24A1 expression in the kidney, but the mechanism is unclear (Perwad et al., 2005), and its effect on CYP24A1 expression in other tissues responding to FGF23 has not been studied to my knowledge.
Given that the principal role of CYP24A1 is to control levels of 1,25(OH)2D within tissues and that a number of malignancies have increased CYP24A1 expression (Anderson et al., 2006), efforts have been made to develop inhibitors of CYP24A1 to increase endogenous 1,25(OH)2D levels in tumors in hopes of increasing the antiproliferative/prodifferentiating effects of 1,25(OH)2D in those cells. A number of azoles such as ketoconazole inhibit CYP24A1 activity but with little specificity with respect to other CYPs including CYP27B1. VID-400, an imidazole derivative, was developed and shown to have a 40-fold selectivity for CYP24A1 over CYP27B1 at least in keratinocytes (Schuster et al., 2001). More recently, CTA091, a vitamin D analog with a 24(S)-NH phenyl sulfoximine D-ring side chain, has been developed with no VDR binding but that is highly selective for CYP24A1 inhibition (Posner et al., 2010). Other vitamin D analogs such as CTA018 have both VDR agonist activity and selective antagonist activity for CYP24A1 expression (Posner et al., 2010). CTA018 is in phase II of a clinical trial for secondary hyperparathyroidism (Cytochroma), but not for cancer.
3-epimerase
3-epimerase (3-epi) activity was first identified in the keratinocyte where it produced the 3-epi form of 1,25(OH)2D (Reddy et al., 2001). It has also been identified in a number of other cells such as colon cancer cells (Caco2), parathyroid cells, osteo-blasts, hepatocyte-derived cells (HepG2), but not in the kidney (Bailey et al., 2013). The enzyme per se has not yet been purified and sequenced, so it is not clear that one gene product is involved. The 3-epi isomerizes the C-3 hydroxy group of the A ring of all natural vitamin D metabolites from the α to β orientation. This does not restrict the action of CYP27B1 or CYP24A1. However, the C-3 epimer of 25OHD has reduced binding to DBP relative to 25OHD, and the C-3 epimer of 1,25(OH)2D has reduced affinity for the VDR relative to 1,25(OH)2D, thus reducing its transcriptional activity and most biologic effects (Kamao et al., 2004). Surprisingly, however, it is equipotent to 1,25(OH)2D with respect to PTH suppression (Brown et al., 1999). Clinically, interest in the C-3 epimerase arises because the C-3 epimer of 25OHD (or 1,25(OH)2D) is not distinguished from 25OHD (or 1,25(OH)2D) by liquid chromatography-mass spectroscopy without special chromatographic methods to separate the epimers prior to mass spectroscopy. Thus, the measurement of 25OHD using standard mass spectroscopic procedures results in a value increased above true levels to the extent that the sample contains the C-3 epimer. Immunoassays by and large do not recognize the C-3 epimer and so are not affected. This issue is particularly important in assessing 25OHD levels in infants where levels of the C-3 epimer of 25OHD can equal or exceed that of 25OHD (Bailey et al., 2013). However, levels in adults can also be substantial (Bailey et al., 2013). Given that the C-3 epimer does have biologic activity and that the epimers can be separated prior to mass spectroscopy, there may be justification for measuring both epimers to provide a more complete picture of vitamin D status at least in future research protocols or when assessing infant samples. At this point, it is not clear whether the cost/benefit of such additional effort justifies its application to adult samples measured routinely.
CYP11A1
Recently, an alternative pathway for vitamin D activation at least in keratinocytes has been identified, namely 20-hydroxylation of vitamin D by CYP11A1, the side-chain cleavage enzyme essential for steroidogenesis (Slominski et al., 2010). The product, 20OHD, or its metabolite, 20,23(OH)2D, appears to have activity similar to 1,25(OH)2D, at least for some functions. Whether this pathway can explain the differences in phenotype between animals and humans lacking VDR compared to those lacking CYP27B1 remains to be seen.
Vitamin D Mechanism of Action
Genomic actions are reviewed in Pike and Meyer (2010) and Haussler et al. (2011). All genomic actions of 1,25(OH)2D are mediated by the VDR. VDR is a transcription factor and member of the steroid hormone nuclear receptor family. It is comprised of three domains: the N-terminal DNA binding domain with two zinc fingers that bind to the grooves of the DNA at discrete sites (VDREs), the C-terminal ligand binding domain, and the hinge region binding these two domains together. The ligand binding domain structure has been solved by x-ray crystallography (Rochel et al., 2000). It is comprised of 12 helices. The terminal helix serves as a gating mechanism closing around the incorporated ligand and forming an interface for coactivators as well as facilitating the interaction of VDR with its heterodimer partner, generally RXR. Although there is substantial variability in the sequence of VDREs, most of those with the highest affinity for VDR are direct repeats of hexanucleotides with a spacing of 3 nt between the half sites, a motif called a DR3. VDR binding to its VDRE then recruits coregulatory complexes required for its genomic activity. These complexes can be both gene and cell specific, enabling the selectivity of 1,25(OH)2D action from cell type to cell type. These complexes include a subunit that directly binds to the VDR generally through an LXXLL motif along with a number of subunits that contain enzyme activity such as histone acetyl transferases (coactivators such as the SRC family) or deacetylases (corepressors such as SMRT and NCoR), methyl transferases and demethylases, ATPase-containing nucleosomal-remodeling activity (SWI/SNF), and links to RNA polymerase II (Mediator complex).
The newer techniques of microarray, ChIP-chip, and ChIP-seq have markedly expanded our understanding of vitamin D mechanism of action at the genomic level. For example, in the mouse osteoblast, 1,200 VDR binding sites were found under basal (i.e., no 1,25(OH)2D) conditions, whereas 8,000 sites were observed following 1,25(OH)2D administration (Meyer et al., 2010b). In a separate study with human lymphoblastoid cell lines treated with 1,25(OH)2D, 2,776 VDR binding sites were found altering the expression of 229 genes (Ramagopalan et al., 2010). The profile of VDR binding sites and genes activated varies from cell to cell with some albeit far from total overlap especially when comparing results with different time courses of 1,25(OH)2D exposure (Carlberg et al., 2012). Moreover, these VDR binding sites can be anywhere in the genome, often many thousands of base pairs away from the gene being regulated. These sites are generally found associated with binding sites for other transcription factors. In osteoblasts, these include RUNX2, C/EBPα, and C/EBPβ, among others (Zella et al., 2010; Meyer et al., 2012). These sites often demonstrate a distinct epigenetic histone signature involving methylation and/or acetylation of lysines within H3 and H4 (Ernst et al., 2011). In their recent review, Pike and Meyer (2010) enunciated six principles of VDR/RXR action on target genomes: “1) the number of VDR binding sites on the genome is cell type-specific; 2) the active transcription unit is predominantly, but not exclusively, the VDR/RXR heterodimer; 3) VDR binding sites are predominantly, but not exclusively, classic hexamer half-sites separated by 3 base pairs; 4) enhancers are located promoter-proximal (near), promoter distal (far) or a combination thereof, relative to transcriptional start sites: many enhancers are located in clusters hundreds of kilobases from their target genes; 5) enhancers are modular in nature, containing binding sites for a number of different transcription factors; 6) enhancers that populate a genome are cell type-unique and highly dynamic.”
Nongenomic Actions
1,25(OH)2D also exerts effects that are too rapid to involve a genomic action. The first of these that was identified involved the rapid stimulation of intestinal calcium transport in a vitamin D replete chick, called transcaltachia (Norman et al., 1997). Analogs of 1,25(OH)2D with little genomic activity were comparable in function to 1,25(OH)2D with respect to transcaltachia. Other examples emerged including effects on the chondrocytes in the growth plate (Norman et al., 1997) and keratinocytes in the skin (Sequeira et al., 2012). Identification of the receptor for 1,25(OH)2D has focused on the VDR itself albeit in a different configuration to enable binding by nongenomic VDR agonists (Mizwicki and Norman, 2009) and membrane-associated rapid response steroid binding protein (MARRS), also known as ERp57/GRp58/ERp60 (Nemere et al., 2004). These receptors are located in the membrane within caveolae/lipid rafts (Huhtakangas et al., 2004) where they are poised to activate kinases, phosphatases, and ion channels. The crystallographic form of VDR would indicate that it can accommodate only agonists with a 6-s-trans configuration, yet those agonists specific for the rapid responses are in a 6-s-cis configuration. However, a model of the VDR has been proposed with an alternative ligand pocket that can accommodate the 6-s-cis analogs (Mizwicki and Norman, 2009). Crystallographic evidence for this configuration has not been obtained. In the three examples mentioned above, both the MARRS and VDRs have been implicated, and in the skin, both receptors were found to be involved in the same study examining photoprotection (Sequeira et al., 2012).
Thus, the panoply of pathways now known to be regulated by 1,25(OH)2D opens up a large selection of targets for clinical application, with the proviso that functional selectivity can be achieved to match what the cell-specific genomic selectivity would seem to promise. This realization has spawned great interest in developing 1,25(OH)2D analogs to do just that.
Vitamin D Analogs
Thousands of analogs have been synthesized (reviews in Jones, 2010; Brown et al., 1999; Brown and Slatopolsky, 2008) (Figure 2). The earliest analogs were prodrugs requiring further metabolism to be active. Such drugs include D2, 1αOHD3 (alpha-calcidol) and 1αOHD2 (doxercalciferol), and dihydrotachysterol (DHT). Alphacalcidol is approved in Europe and Japan for the treatment of osteoporosis. Doxercalciferol is approved in the USA for the treatment of secondary hyperparathyroidism. DHT is no longer used clinically. As noted earlier, D2 like D3 undergoes 25-hydroxylation and 1α-hydroxylation to become active. Different 25-hydroxylases distinguish between D2 and D3 (i.e., D2 is a poor substrate for CYP27A1 but is an equivalent substrate for CYP2R1). CYP27B1 does not distinguish between the two forms of 25OHD, but 1,25(OH)2D2 is metabolized differently by CYP24A1 than 1,25(OH)2D3 because of the methyl group in C24 and the double bond between C22 and C23. Moreover, D2 and its metabolites have lower affinity for DBP so are cleared faster from the bloodstream. Similar differences would be expected for 1αOHD3 and 1αOHD2 that must undergo 25-hydroxylation to be active. DHT, when 25-hydroxylated, was thought to be biologically active because the 3β OH group assumed a pseudo 1α position, but subsequent studies have shown 1α-hydroxylation of the 25OHDHT, which enhances its biologic activity (Qaw et al., 1993).
Figure 2. Clinically Used Analogs of 1,25(OH)2.
The structures of various vitamin D analogs currently in use clinically.
An early analog of 1,25(OH)2D is 26,27 F6-1,25(OH)2D3 (falecalcitriol), which is currently approved for use in osteoporosis, secondary hyperparathyroidism, and hypoparathyroidism in Japan. The fluoride components in the side chain reduce its metabolism because the 23-hydroxylated metabolite resists further metabolism but retains biologic activity (Imanishi et al., 1999). However, falecalcitriol is not selective for the PTG or bone. Other analogs were designed to reduce the affinity for DBP, increasing clearance, with the hope of reducing the impact on intestinal calcium transport and bone resorption. Calcipotriol has a C22-C23 double bond, a C24 hydroxyl group, and a cyclo-propane ring. This drug has seen success in the treatment of psoriasis in numerous countries, in part because it can be applied topically to the involved skin and undergo substantial metabolism in the skin limiting its access to the circulation. Calcipotriol has also been used in cancer trials. OCT (maxacalcitol) has an oxygen group instead of carbon at the 22 position. It is approved for psoriasis and secondary hyperparathyroidism in Japan. It was found to be substantially less hypercalcemic than 1,25(OH)2D while retaining substantial ability to suppress PTH secretion (Brown et al., 1993) at least in part because of the rapid clearance of the drug from the circulation limiting its accumulation in the intestine. 19-nor-1α 25(OH)2D2 (paricalcitol) lacks the C19 methylene group in ring A of 1,25(OH)2D2. It is approved for secondary hyperparathyroidism in chronic kidney disease (CKD). It has substantially less hypercalcemic effect relative to its inhibition of PTH secretion (Slatopolsky et al., 1995) and blocks PTG hyperplasia. Furthermore, paricalcitol appeared to have a lower risk of vascular calcification than 1,25(OH)2D3 (Cardús et al., 2007) or doxercalciferol (Mizobuchi et al., 2007). The reason for the selective effect of paricalcitol is not clear because its clearance from the bloodstream is comparable to that of 1,25(OH)2D (Brown et al., 2000). ED-71 (eldecalcitol) has a 2-hydroxypropoxy group in the A ring. It is nearing approval for use in osteoporosis in Japan because it restores bone mass with minimal effect on serum calcium levels (Matsumoto and Kubodera, 2007). Unlike the other analogs discussed, ED-71 has a higher affinity for DBP with a longer half-life in blood that may contribute to its selective effect on bone (Nishii et al., 1993). 2-methylene-19 nor (20S)-1,25(OH)2D3 (2MD) likewise appears to be specific for bone formation (Shevde et al., 2002) but is no longer in clinical trials. BXL628 (Crescioli et al., 2004; Adorini et al., 2007) combines fluorination, 16-ene and 23-yne double bonds, 26,27 homologation, and 20-epimerization, modifications found singly in other analogs with potent antiproliferative activity. It is undergoing clinical trials for prostate hypertrophy, cancer, and prostatitis. In addition to modifications of the basic vitamin D structure, high-throughput screening has been used to identify nonsecosteroid VDR modulators, of which LY2109866 is one example (Ma et al., 2006). This class of compounds has not yet been tested clinically to my knowledge.
Mechanisms invoked to explain the relative specificity of these analogs other than their pharmacokinetic properties have been examined. One surprising result from crystallographic data is that different analogs did not appear to alter the configuration of the VDR (Rochel et al., 2000; Tocchini-Valentini et al., 2001). That said, different analogs led to different profiles with respect to proteolytic digestion of the VDR/agonist complex (Peleg et al., 1995). Moreover, different analogs appear to affect the recruitment of different coactivator complexes differently, with higher affinity of VDR for these coactivators with analogs with super agonist properties (Eelen et al., 2006). Examples include two 14-epi analogs (TX527 and TX522) that are ten times as potent as 1,25(OH)2D with respect to their antiproliferative actions with 50–400 times lower calcemic effects; these drugs have comparable affinity as 1,25(OH)2D for the VDR with comparable impact on VDR/RXR binding to VDREs (Verlinden et al., 2001) but induced stronger interactions between VDR and the SRC coactivators (Eelen et al., 2006). Other examples include the analog 2MD, which induced stronger interaction among VDR, SRC2, and MED1 (Yamamoto et al., 2003), and OCT, which induced stronger binding between VDR and SRC2 (Takeyama et al., 1999).
Clinical Applications
The literature assessing the relationship of vitamin D adequacy to human disease is vast, and attempts to summarize it in a few short paragraphs is not feasible. However, there are several points that can be made about some of the clinical applications that have received the most study.
The Skeleton
There is little controversy that adequate vitamin D is necessary to prevent rickets and osteomalacia. More controversy exists with respect to the role of vitamin D in the prevention of osteoporosis and fractures (Bikle, 2012a). However, a meta-analysis of a number of randomized controlled trials demonstrated a positive dose-response relationship between vitamin D supplementation and fracture prevention (Bischoff-Ferrari et al., 2009). At least part of the protection could be attributed to a vitamin D-related reduction in falls (Murad et al., 2011). It is not settled whether this beneficial action of vitamin D on bone is due solely to the ability of vitamin D via 1,25(OH)2D to provide adequate levels of calcium and phosphate from the diet by promoting their intestinal absorption, or whether 1,25(OH)2D also exerts a direct action on cartilage and bone to promote normal skeletal development and turnover. Mice and humans lacking a functional VDR or CYP27B1 develop rickets, but this can be prevented by a diet high in calcium and lactose (rescue diet) to enhance calcium absorption or with infusions of calcium and phosphate. Moreover, expressing the VDR only in the intestine of a VDR null mouse prevents the rickets from developing (Xue and Fleet, 2009). However, longer-term studies demonstrated that in the CYP27B1 null or CYP27B1/VDR double null mouse raised on the rescue diet to normalize serum calcium, phosphate, and PTH, osteopenia and defective osteoblast function were demonstrated (Panda et al., 2004). Thus, vitamin D appears to have direct and indirect effects on bone development and remodeling, important clinically to prevent rickets in the developing skeleton and osteoporosis and fractures in the aging skeleton. The major controversy regarding the latter is what level of vitamin D is sufficient.
The PTG
The inverse relationship between circulating 25OHD levels (but not 1,25(OH)2D levels) and PTH levels is well established, but the degree of variability in the population with respect to this relationship is large. Nevertheless, PTH levels are a useful marker for vitamin D sufficiency, and maintaining adequate levels of 25OHD in the blood will reduce the risk for PTG hyperplasia and elevated PTH secretion with its potential deleterious effects on bone. The PTG expresses both the VDR and CYP27B1. The PTH gene contains a negative response element for 1,25(OH)2D /VDR (Demay et al., 1992). Most likely, part of the inverse relationship between 25OHD (but not 1,25(OH)2D) and PTH is due to the ability of the PTG to produce its own 1,25(OH)2D. 1,25(OH)2D also induces the calcium-sensing receptor in the PTG making the PTG more sensitive to suppression by calcium as well as 1,25(OH)2D. In CKD, the PTG becomes less sensitive to both 1,25(OH)2D and calcium as levels of their respective receptors decrease resulting in secondary hyperparathyroidism. Several analogs of 1,25(OH)2D and 1,25(OH)2D itself are approved for the treatment of secondary hyperparathyroidism in CKD, in particular doxercalciferol, paricalcitol, falecalcitriol, and maxacalcitol (the latter two only in Japan). These analogs consistently reduce PTH levels with an acceptable increase in serum calcium. The benefits of these analogs on mortality in CKD (generally cardiovascular) have consistently been found in observational studies (Duranton et al., 2013). As discussed below, this may reflect not only an effect of these vitamin D analogs on the PTG but also an effect on the cardiovascular system.
The Skin
The use of the 1,25(OH)2D analogs calcipotriol and maxacalcitol for the treatment of the hyperproliferative skin disease psoriasis represents another approved clinical application outside of the skeleton for vitamin D and its analogs. Psoriasis is a disorder with hyperproliferation and decreased or abnormal differentiation driven by an abnormal immunologic component. The successful use of 1,25(OH)2D and several of its analogs is likely due to their ability to inhibit the proliferation, stimulate the differentiation, and suppress the immune activity associated with this disease (Bikle, 2012b). Nonmelanoma skin cancer also represents a condition of increased proliferation and decreased differentiation of keratinocytes. Mice lacking the VDR in their keratinocytes are predisposed to UVB and chemically induced skin cancer (Teichert et al., 2011), and topical application of 1,25(OH)2D appears to be photoprotective (Mason and Reich-rath, 2013). However, this potential has not been examined clinically.
Obesity, Diabetes Mellitus, and Metabolic Syndrome
25OHD levels are typically lower in obese individuals who are more likely to develop diabetes mellitus and the metabolic syndrome. Adipocytes express the VDR, and 1,25(OH)2D promotes increased lipogenesis and decreased lipolysis (Shi et al., 2001). The pancreatic β cell expresses the VDR, and 1,25(OH)2D promotes insulin secretion (Norman et al., 1980). Moreover, vitamin D deficiency is associated with insulin resistance (Kayaniyil et al., 2010). Clinical trials in individuals with diabetes mellitus or who are prediabetic suggest a benefit from vitamin D administration with respect to improving or preventing the development of frank diabetes (Mitri et al., 2011; Pittas et al., 2007), but longer and larger randomized clinical trials are required.
Cancer
The data from animal and cell culture studies are very promising that 1,25(OH)2D or its analogs can prevent cancer development or retard its progress/metastasis once developed (Bikle, 2004). The mechanisms by which 1,25(OH)2D can suppress tumor development are numerous and in many cases cell specific. These include inhibition of proliferation by blocking elements of the cell cycle or interference with signaling by growth factors, inducing apoptosis, stimulation of DNA damage repair, prevention of tumor angiogenesis, and inhibition of metastasis. However, most of the clinical data stem from observational studies. These studies consistently show a likely benefit for vitamin D supplementation in colon and breast cancer, but randomized clinical trial data of sufficient size and duration with sufficient doses of vitamin D to be definitive are lacking (Chung et al., 2011; Manson et al., 2011). Development of an analog with tissue specificity relative to effects on calcium absorption/bone resorption would enhance the chances of success in treating malignancies.
Cardiovascular Disease
The VDR and CYP27B1 are expressed in the heart, both in the myocytes and in the fibroblasts (Chen et al., 2008). 1,25(OH)2D and its analogs suppress markers of cardiac hypertrophy, and deletion of the VDR specifically from the heart results in hypertrophy (Chen et al., 2011; Gardner et al., 2013). VDR and CYP27B1 null mice are also hypertensive with increased production of renin from kidneys and heart resulting in increased circulating angiotensin II levels (Zhou et al., 2008). The increase in reninangiotensin may contribute to the acceleration of atherosclerosis observed in VDR null mice (Szeto et al., 2012). Severe vitamin D deficiency in humans is associated with cardiomyopathy (Uysal et al., 1999), and in a number of large epidemiologic studies, the association of increased cardiovascular disease (CVD) risk with reductions in 25OHD levels has been found (Brøndum-Jacobsen et al., 2012). However, to date, no large randomized clinical trials have been performed specifically designed to test the role of vitamin D or any of its analogs in the prevention/treatment of CVD, and the results from fracture studies with CVD as a secondary outcome have not been compelling.
Immune Function
The immune system is comprised of two distinct but interacting types of immunity: innate and adaptive. The innate immune response involves the activation of Toll-like receptors (TLRs) in polymorphonuclear cells (PMNs), monocytes, and macrophages as well as in a number of epithelial cells. TLRs are an extended family of host noncatalytic transmembrane pathogen-recognition receptors that interact with specific membrane patterns (pathogen-associated molecular pattern [PAMP]) shed by infectious agents that trigger the innate immune response in the host. Activation of TLRs leads to the induction of antimicrobial peptides (AMPs) such as cathelicidin and reactive oxygen species (ROS), which kill the organism. The expression of cathelicidin is induced by 1,25(OH)2D in both myeloid and epithelial cells (Gombart et al., 2005). Stimulation of TLR2 by a lipopeptide from an infectious organism such as M. tuberculosis in macrophages (Liu et al., 2006) results in increased expression of CYP27B1 and VDR, which in the presence of adequate substrate (25OHD), results in the induction of cathelicidin. Thus, adequate levels of vitamin D promote the innate immune response. The adaptive immune response is initiated by cells specialized in antigen presentation, DCs and macrophages in particular, activating the cells responsible for subsequent antigen recognition, the T and B lymphocytes. Importantly, the type of T cell activated, CD4 or CD8, or within the helper T cell class Th1, Th2, Th17, Treg, and subtle variations of those, is dependent on the context in which the antigen is presented by which cell and in what environment. Systemic factors such as vitamin D influence this process. Vitamin D in general exerts an inhibitory action on the adaptive immune system. 1,25(OH)2D decreases the maturation of DCs decreasing their ability to present antigen and so activate T cells (van Etten and Mathieu, 2005). Furthermore, by suppressing IL-12 production, important for Th1 development, and IL-23 and IL-6 production important for Th17 development and function, 1,25(OH)2D inhibits the development of Th1 cells capable of producing IFN-γ and IL-2, and Th17 cells producing IL-17 (Daniel et al., 2008). Clinically, there are no approved vitamin D drugs for immune modulation. However, the association of tuberculosis with vitamin D deficiency is well known (Ustianowski et al., 2005), but adequately powered randomized clinical trial data showing efficacy with vitamin D supplementation are lacking (Martineau, 2012). Animal studies demonstrating the benefit of 1,25(OH)2D and its analogs in the treatment of autoimmune diseases (Adorini and Penna, 2008) and as adjuncts to immunosuppressants following transplantation procedures (van Etten et al., 2000) are also compelling, but as for the treatment of infections, randomized clinical trial data are lacking.
Summary and Conclusions
Vitamin D, whether produced in the skin from 7-DHC or absorbed from the diet, must be activated first to 25OHD and then to its active form 1,25(OH)2D. The production of vitamin D is not enzymatic but depends on UVB. The 25-hydroxylation of vitamin D can be accomplished by a number of enzymes, but the most important appears to be CYP2R1. CYP27B1 is the only enzyme responsible for the subsequent 1α-hydroxylation to 1,25(OH)2D. The renal CYP27B1 is likely responsible for most of the circulating 1,25(OH)2D, but CYP27B1 is found in a number of other tissues where 1,25(OH)2D is likely to serve a paracrine/autocrine function. Control of extrarenal CYP27B1 differs from renal CYP27B1. CYP24A1 is responsible for the catabolism of both 25OHD and 1,25(OH)2D, although some data suggest that 24,25(OH)2D and certainly 1,24,25(OH)3D have biologic activity. CYP24A1 is found in nearly every cell expressing VDR. 1,25(OH)2D is the major biologically active metabolite, the hormonal form of vitamin D. It is a steroid hormone binding to its nuclear hormone receptor VDR. VDR typically as a heterodimer with RXR binds to specific sites in the genome (VDREs) to activate or in some cases suppress transcription. Hundreds of genes and thousands of VDRE sites have been identified. Given the widespread expression of VDR and CYP27B1, there is great interest in identifying means to target specific cells with analogs that do not also increase intestinal calcium absorption and/or bone resorption. This effort has been partially successful, and analogs have been developed for the treatment of hyperproliferative skin diseases, hyperparathyroidism, and osteoporosis. But for many of the potential applications including the treatment/prevention of cancer, CVD, infections, and autoimmune diseases, solid data from randomized clinical trials are lacking despite promising epidemiologic data and animal studies.
ACKNOWLEDGMENTS
The author acknowledges the administrative support of Aaminah Khan and the financial support from National Institutes of Health grant R01 AR050023, the Department of Defense CA 110338, and a VA Merit Review.
REFERENCES
- Adams JS, Gacad MA. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J. Exp. Med. 1985;161:755–765. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat. Clin. Pract. Rheumatol. 2008;4:404–412. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- Adorini L, Penna G, Amuchastegui S, Cossetti C, Aquilano F, Mariani R, Fibbi B, Morelli A, Uskokovic M, Colli E, Maggi M. Inhibition of prostate growth and inflammation by the vitamin D receptor agonist BXL-628 (elocalcitol). J. Steroid Biochem. Mol. Biol. 2007;103:689–693. doi: 10.1016/j.jsbmb.2006.12.065. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother. Pharmacol. 2006;57:234–240. doi: 10.1007/s00280-005-0059-7. [DOI] [PubMed] [Google Scholar]
- Armbrecht HJ, Hodam TL, Boltz MA, Partridge NC, Brown AJ, Kumar VB. Induction of the vitamin D 24-hydroxylase (CYP24) by 1,25-dihydroxyvitamin D3 is regulated by parathyroid hormone in UMR106 osteoblastic cells. Endocrinology. 1998;139:3375–3381. doi: 10.1210/endo.139.8.6134. [DOI] [PubMed] [Google Scholar]
- Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, Adams JS, Salusky IB, Hewison M. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. J. Bone Miner. Res. 2013;28:46–55. doi: 10.1002/jbmr.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D, Veljkovic K, Yazdanpanah M, Adeli K. Analytical measurement and clinical relevance of vitamin D(3) C3-epimer. Clin. Biochem. 2013;46:190–196. doi: 10.1016/j.clinbiochem.2012.10.037. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D and skin cancer. J. Nutr. 2004;134(12, Suppl):3472S–3478S. doi: 10.1093/jn/134.12.3472S. [DOI] [PubMed] [Google Scholar]
- Bikle D. Extrarenal synthesis of 1,25-dihydroxyvitamin D and its health implications. In: Holick MF, editor. Nutrition and Health: Vitamin D. Humana Press; New York: 2010. pp. 277–295. [Google Scholar]
- Bikle DD. Vitamin D and bone. Curr. Osteoporos. Rep. 2012a;10:151–159. doi: 10.1007/s11914-012-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012b;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Rasmussen H. The ionic control of 1,25-dihydroxyvitamin D3 production in isolated chick renal tubules. J. Clin. Invest. 1975;55:292–298. doi: 10.1172/JCI107932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Murphy EW, Rasmussen H. The ionic control of 1,25-dihydroxyvitamin D3 synthesis in isolated chick renal mitochondria. The role of calcium as influenced by inorganic phosphate and hydrogen-ion. J. Clin. Invest. 1975;55:299–304. doi: 10.1172/JCI107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J. Clin. Invest. 1986;78:557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Pillai S, Gee E, Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124:655–660. doi: 10.1210/endo-124-2-655. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Pillai S, Gee E, Hincenbergs M. Tumor necrosis factor-alpha regulation of 1,25-dihydroxyvitamin D production by human keratinocytes. Endocrinology. 1991;129:33–38. doi: 10.1210/endo-129-1-33. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch. Intern. Med. 2009;169:51–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- Brøndum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocar-dial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler. Thromb. Vasc. Biol. 2012;32:2794–2802. doi: 10.1161/ATVBAHA.112.248039. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Slatopolsky E. Vitamin D analogs: therapeutic applications and mechanisms for selectivity. Mol. Aspects Med. 2008;29:433–452. doi: 10.1016/j.mam.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Finch J, Grieff M, Ritter C, Kubodera N, Nishii Y, Slatopolsky E. The mechanism for the disparate actions of calcitriol and 22-oxacalcitriol in the intestine. Endocrinology. 1993;133:1158–1164. doi: 10.1210/endo.133.3.8396012. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Ritter C, Slatopolsky E, Muralidharan KR, Okamura WH, Reddy GS. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J. Cell. Biochem. 1999;73:106–113. [PubMed] [Google Scholar]
- Brown AJ, Finch J, Takahashi F, Slatopolsky E. Calcemic activity of 19-Nor-1,25(OH)(2)D(2) decreases with duration of treatment. J. Am. Soc. Nephrol. 2000;11:2088–2094. doi: 10.1681/ASN.V11112088. [DOI] [PubMed] [Google Scholar]
- Cardús A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J. Bone Miner. Res. 2007;22:860–866. doi: 10.1359/jbmr.070305. [DOI] [PubMed] [Google Scholar]
- Carlberg C, Seuter S, Heikkinen S. The first genome-wide view of vitamin D receptor locations and their mechanistic implications. Anticancer Res. 2012;32:271–282. [PubMed] [Google Scholar]
- Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, Law CS, Gardner DG. Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension. 2008;52:1106–1112. doi: 10.1161/HYPERTENSIONAHA.108.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124:1838–1847. doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxilase. J. Biol. Chem. 2003;278:38084–38093. doi: 10.1074/jbc.M307028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl. Acad. Sci. USA. 2004;101:7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2011;155:827–838. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- Crescioli C, Ferruzzi P, Caporali A, Scaltriti M, Bettuzzi S, Mancina R, Gelmini S, Serio M, Villari D, Vannelli GB, et al. Inhibition of prostate cell growth by BXL-628, a calcitriol analogue selected for a phase II clinical trial in patients with benign prostate hyperplasia. Eur. J. Endocrinol. 2004;150:591–603. doi: 10.1530/eje.0.1500591. [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM. Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA. 1992;89:8097–8101. doi: 10.1073/pnas.89.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am. J. Nephrol. 2013;37:239–248. doi: 10.1159/000346846. [DOI] [PubMed] [Google Scholar]
- Eelen G, Verlinden L, De Clercq P, Vandewalle M, Bouillon R, Verstuyf A. Vitamin D analogs and coactivators. Anticancer Res. 2006;26(4A):2717–2721. [PubMed] [Google Scholar]
- Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JN, Wang L, Tangpricha V, Reichrath J, Chen TC, Holick MF. Regulation of the 25-hydroxyvitamin D-1alpha-hydroxylase gene and its splice variant. Recent Results Cancer Res. 2003;164:157–167. doi: 10.1007/978-3-642-55580-0_12. [DOI] [PubMed] [Google Scholar]
- Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, Portale AA. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol. Endocrinol. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- Gardner DG, Chen S, Glenn DJ. Vitamin D and the heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R969–R977. doi: 10.1152/ajpregu.00322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- Gupta RP, Hollis BW, Patel SB, Patrick KS, Bell NH. CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J. Bone Miner. Res. 2004;19:680–688. doi: 10.1359/JBMR.0301257. [DOI] [PubMed] [Google Scholar]
- Gyetko MR, Hsu CH, Wilkinson CC, Patel S, Young E. Monocyte 1 alpha-hydroxylase regulation: induction by inflammatory cytokines and suppression by dexamethasone and uremia toxin. J. Leukoc. Biol. 1993;54:17–22. doi: 10.1002/jlb.54.1.17. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT, Jr., Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J. Steroid Biochem. 1984;21:81–86. doi: 10.1016/0022-4731(84)90063-3. [DOI] [PubMed] [Google Scholar]
- Horst RL, Reinhardt TA, Ramberg CF, Koszewski NJ, Napoli JL. 24-Hydroxylation of 1,25-dihydroxyergocalciferol. An unambiguous deactivation process. J. Biol. Chem. 1986;261:9250–9256. [PubMed] [Google Scholar]
- Hosseinpour F, Wikvall K. Porcine microsomal vitamin D(3) 25-hydroxylase (CYP2D25). Catalytic properties, tissue distribution, and comparison with human CYP2D6. J. Biol. Chem. 2000;275:34650–34655. doi: 10.1074/jbc.M004185200. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am. J. Clin. Nutr. 2006;84:694–697. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol. Endocrinol. 2004;18:2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- Imanishi Y, Inaba M, Seki H, Koyama H, Nishizawa Y, Morii H, Otani S. Increased biological potency of hexafluorinated analogs of 1,25-dihydroxyvitamin D3 on bovine parathyroid cells. J. Steroid Biochem. Mol. Biol. 1999;70:243–248. doi: 10.1016/s0960-0760(99)00112-0. [DOI] [PubMed] [Google Scholar]
- Jones G. Vitamin D analogs. Endocrinol. Metab. Clin. North Am. 2010;39:447–472. doi: 10.1016/j.ecl.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Ozono K, Kubodera N, Reddy GS, Okano T. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J. Biol. Chem. 2004;279:15897–15907. doi: 10.1074/jbc.M311473200. [DOI] [PubMed] [Google Scholar]
- Kayaniyil S, Vieth R, Retnakaran R, Knight JA, Qi Y, Gerstein HC, Perkins BA, Harris SB, Zinman B, Hanley AJ. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33:1379–1381. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Fujiki R, Kitagawa H, Kato S. 1alpha,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene. Mol. Cell. Endocrinol. 2007;265-266:168–173. doi: 10.1016/j.mce.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Ma Y, Khalifa B, Yee YK, Lu J, Memezawa A, Savkur RS, Yamamoto Y, Chintalacharuvu SR, Yamaoka K, Stayrook KR, et al. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J. Clin. Invest. 2006;116:892–904. doi: 10.1172/JCI25901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer—ready for prime time? N. Engl. J. Med. 2011;364:1385–1387. doi: 10.1056/NEJMp1102022. [DOI] [PubMed] [Google Scholar]
- Martineau AR. Old wine in new bottles: vitamin D in the treatment and prevention of tuberculosis. Proc. Nutr. Soc. 2012;71:84–89. doi: 10.1017/S0029665111003326. [DOI] [PubMed] [Google Scholar]
- Mason RS, Reichrath J. Sunlight vitamin D and skin cancer. Anticancer. Agents Med. Chem. 2013;13:83–97. [PubMed] [Google Scholar]
- Matsumoto T, Kubodera N. ED-71, a new active vitamin D3, increases bone mineral density regardless of serum 25(OH)D levels in osteoporotic subjects. J. Steroid Biochem. Mol. Biol. 2007;103:584–586. doi: 10.1016/j.jsbmb.2006.12.088. [DOI] [PubMed] [Google Scholar]
- Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J. Biol. Chem. 2010a;285:15599–15610. doi: 10.1074/jbc.M110.119958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MB, Goetsch PD, Pike JW. Genome-wide analysis of the VDR/RXR cistrome in osteoblast cells provides new mechanistic insight into the actions of the vitamin D hormone. J. Steroid Biochem. Mol. Biol. 2010b;121:136–141. doi: 10.1016/j.jsbmb.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MB, Goetsch PD, Pike JW. VDR/RXR and TCF4/β-catenin cistromes in colonic cells of colorectal tumor origin: impact on c-FOS and c-MYC gene expression. Mol. Endocrinol. 2012;26:37–51. doi: 10.1210/me.2011-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 2011;94:486–494. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi M, Finch JL, Martin DR, Slatopolsky E. Differential effects of vitamin D receptor activators on vascular calcification in uremic rats. Kidney Int. 2007;72:709–715. doi: 10.1038/sj.ki.5002406. [DOI] [PubMed] [Google Scholar]
- Mizwicki MT, Norman AW. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci. Signal. 2009;2:re4. doi: 10.1126/scisignal.275re4. [DOI] [PubMed] [Google Scholar]
- Moghadasian MH. Cerebrotendinous xanthomatosis: clinical course, genotypes and metabolic backgrounds. Clin. Invest. Med. 2004;27:42–50. [PubMed] [Google Scholar]
- Murad MH, Elamin KB, Abu Elnour NO, Elamin MB, Alkatib AA, Fatourechi MM, Almandoz JP, Mullan RJ, Lane MA, Liu H, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2011;96:2997–3006. doi: 10.1210/jc.2011-1193. [DOI] [PubMed] [Google Scholar]
- Nemere I, Farach-Carson MC, Rohe B, Sterling TM, Norman AW, Boyan BD, Safford SE. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc. Natl. Acad. Sci. USA. 2004;101:7392–7397. doi: 10.1073/pnas.0402207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishii Y, Sato K, Kobayashi T. The development of vitamin D3 analogues for the treatment of osteoporosis. Osteoporos. Int. 1993;3(Suppl 1):190–193. doi: 10.1007/BF01621903. [DOI] [PubMed] [Google Scholar]
- Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- Norman AW, Okamura WH, Hammond MW, Bishop JE, Dormanen MC, Bouillon R, van Baelen H, Ridall AL, Daane E, Khoury R, Farach-Carson MC. Comparison of 6-s-cis- and 6-s-trans-locked analogs of 1alpha,25-dihydroxyvitamin D3 indicates that the 6-s-cis conformation is preferred for rapid nongenomic biological responses and that neither 6-scis- nor 6-s-trans-locked analogs are preferred for genomic biological responses. Mol. Endocrinol. 1997;11:1518–1531. doi: 10.1210/mend.11.10.9993. [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J. Biol. Chem. 2004;279:16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- Peleg S, Sastry M, Collins ED, Bishop JE, Norman AW. Distinct conformational changes induced by 20-epi analogues of 1 alpha,25-dihydroxyvitamin D3 are associated with enhanced activation of the vitamin D receptor. J. Biol. Chem. 1995;270:10551–10558. doi: 10.1074/jbc.270.18.10551. [DOI] [PubMed] [Google Scholar]
- Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol. Metab. Clin. North Am. 2010;39:255–269. doi: 10.1016/j.ecl.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachot JJ, Du Bois MB, Halpern S, Cournot-Witmer G, Garabedian M, Balsan S. In vitro action of 1,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol on matrix organization and mineral distribution in rabbit growth plate. Metab. Bone Dis. Relat. Res. 1982;4:135–142. doi: 10.1016/0221-8747(82)90027-3. [DOI] [PubMed] [Google Scholar]
- Posner GH, Helvig C, Cuerrier D, Collop D, Kharebov A, Ryder K, Epps T, Petkovich M. Vitamin D analogues targeting CYP24 in chronic kidney disease. J. Steroid Biochem. Mol. Biol. 2010;121:13–19. doi: 10.1016/j.jsbmb.2010.03.065. [DOI] [PubMed] [Google Scholar]
- Postlind H, Axén E, Bergman T, Wikvall K. Cloning, structure, and expression of a cDNA encoding vitamin D3 25-hydroxylase. Biochem. Biophys. Res. Commun. 1997;241:491–497. doi: 10.1006/bbrc.1997.7551. [DOI] [PubMed] [Google Scholar]
- Prosser DE, Kaufmann M, O'Leary B, Byford V, Jones G. Single A326G mutation converts human CYP24A1 from 25-OH-D3-24-hydroxylase into -23-hydroxylase, generating 1alpha,25-(OH)2D3-26,23-lactone. Proc. Natl. Acad. Sci. USA. 2007;104:12673–12678. doi: 10.1073/pnas.0702093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryke AM, Duggan C, White CP, Posen S, Mason RS. Tumor necrosis factor-alpha induces vitamin D-1-hydroxylase activity in normal human alveolar macrophages. J. Cell. Physiol. 1990;142:652–656. doi: 10.1002/jcp.1041420327. [DOI] [PubMed] [Google Scholar]
- Qaw F, Calverley MJ, Schroeder NJ, Trafford DJ, Makin HL, Jones G. In vivo metabolism of the vitamin D analog, dihydrotachysterol. Evidence for formation of 1 alpha,25- and 1 beta,25-dihydroxy-dihydrotachysterol metabolites and studies of their biological activity. J. Biol. Chem. 1993;268:282–292. [PubMed] [Google Scholar]
- Rahmaniyan M, Patrick K, Bell NH. Characterization of recombinant CYP2C11: a vitamin D 25-hydroxylase and 24-hydroxylase. Am. J. Physiol. Endocrinol. Metab. 2005;288:E753–E760. doi: 10.1152/ajpendo.00201.2004. [DOI] [PubMed] [Google Scholar]
- Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GS, Muralidharan KR, Okamura WH, Tserng KY, McLane JA. Metabolism of 1alpha,25-dihydroxyvitamin D(3) and its C-3 epimer 1alpha,25-dihydroxy-3-epi-vitamin D(3) in neonatal human keratinocytes. Steroids. 2001;66:441–450. doi: 10.1016/s0039-128x(00)00228-2. [DOI] [PubMed] [Google Scholar]
- Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J. Biol. Chem. 2005;280:20604–20611. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell. 2000;5:173–179. doi: 10.1016/s1097-2765(00)80413-x. [DOI] [PubMed] [Google Scholar]
- Rost CR, Bikle DD, Kaplan RA. In vitro stimulation of 25-hydroxycholecalciferol 1 alpha-hydroxylation by parathyroid hormone in chick kidney slices: evidence for a role for adenosine 30,50-monophosphate. Endocrinology. 1981;108:1002–1006. doi: 10.1210/endo-108-3-1002. [DOI] [PubMed] [Google Scholar]
- Sakaki T, Sawada N, Komai K, Shiozawa S, Yamada S, Yamamoto K, Ohyama Y, Inouye K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur. J. Biochem. 2000;267:6158–6165. doi: 10.1046/j.1432-1327.2000.01680.x. [DOI] [PubMed] [Google Scholar]
- Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, Fehrenbach H, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 2011;365:410–421. doi: 10.1056/NEJMoa1103864. [DOI] [PubMed] [Google Scholar]
- Schuster I, Egger H, Bikle D, Herzig G, Reddy GS, Stuetz A, Stuetz P, Vorisek G. Selective inhibition of vitamin D hydroxylases in human keratinocytes. Steroids. 2001;66:409–422. doi: 10.1016/s0039-128x(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Sequeira VB, Rybchyn MS, Tongkao-On W, Gordon-Thomson C, Malloy PJ, Nemere I, Norman AW, Reeve VE, Halliday GM, Feldman D, Mason RS. The role of the vitamin D receptor and ERp57 in photoprotection by 1a,25-dihydroxyvitamin D3. Mol. Endocrinol. 2012;26:574–582. doi: 10.1210/me.2011-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevde NK, Plum LA, Clagett-Dame M, Yamamoto H, Pike JW, DeLuca HF. A potent analog of 1alpha,25-dihydroxyvitamin D3 selectively induces bone formation. Proc. Natl. Acad. Sci. USA. 2002;99:13487–13491. doi: 10.1073/pnas.202471299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1alpha,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001;15:2751–2753. doi: 10.1096/fj.01-0584fje. [DOI] [PubMed] [Google Scholar]
- Shinki T, Shimada H, Wakino S, Anazawa H, Hayashi M, Saruta T, De-Luca HF, Suda T. Cloning and expression of rat 25-hydroxyvitamin D3-1alpha-hydroxylase cDNA. Proc. Natl. Acad. Sci. USA. 1997;94:12920–12925. doi: 10.1073/pnas.94.24.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E, Finch J, Ritter C, Denda M, Morrissey J, Brown A, DeLuca H. A new analog of calcitriol, 19-nor-1,25-(OH)2D2, suppresses parathyroid hormone secretion in uremic rats in the absence of hyper-calcemia. Am. J. Kidney Dis. 1995;26:852–860. doi: 10.1016/0272-6386(95)90455-7. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Arnaud R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH. The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J. Bone Miner. Res. 1997;12:1552–1559. doi: 10.1359/jbmr.1997.12.10.1552. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Shiro Y. Diversity and substrate specificity in the structures of steroidogenic cytochrome P450 enzymes. Biol. Pharm. Bull. 2012;35:818–823. doi: 10.1248/bpb.35.818. [DOI] [PubMed] [Google Scholar]
- Szeto FL, Reardon CA, Yoon D, Wang Y, Wong KE, Chen Y, Kong J, Liu SQ, Thadhani R, Getz GS, Li YC. Vitamin D receptor signaling inhibits atherosclerosis in mice. Mol. Endocrinol. 2012;26:1091–1101. doi: 10.1210/me.2011-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- Takeyama K, Masuhiro Y, Fuse H, Endoh H, Murayama A, Kitanaka S, Suzawa M, Yanagisawa J, Kato S. Selective interaction of vitamin D receptor with transcriptional coactivators by a vitamin D analog. Mol. Cell. Biol. 1999;19:1049–1055. doi: 10.1128/mcb.19.2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Over-expression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J. Invest. Dermatol. 2011;131:2289–2297. doi: 10.1038/jid.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchini-Valentini G, Rochel N, Wurtz JM, Mitschler A, Moras D. Crystal structures of the vitamin D receptor complexed to superagonist 20-epi ligands. Proc. Natl. Acad. Sci. USA. 2001;98:5491–5496. doi: 10.1073/pnas.091018698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R, Lanham-New S. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2012;95:1357–1364. doi: 10.3945/ajcn.111.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- Ustianowski A, Shaffer R, Collin S, Wilkinson RJ, Davidson RN. Prevalence and associations of vitamin D deficiency in foreign-born persons with tuberculosis in London. J. Infect. 2005;50:432–437. doi: 10.1016/j.jinf.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Uysal S, Kalayci AG, Baysal K. Cardiac functions in children with vitamin D deficiency rickets. Pediatr. Cardiol. 1999;20:283–286. doi: 10.1007/s002469900464. [DOI] [PubMed] [Google Scholar]
- van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J. Steroid Biochem. Mol. Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- van Etten E, Branisteanu DD, Verstuyf A, Waer M, Bouillon R, Mathieu C. Analogs of 1,25-dihydroxyvitamin D3 as dose-reducing agents for classical immunosuppressants. Transplantation. 2000;69:1932–1942. doi: 10.1097/00007890-200005150-00032. [DOI] [PubMed] [Google Scholar]
- Verlinden L, Verstuyf A, Quack M, Van Camp M, Van Etten E, De Clercq P, Vandewalle M, Carlberg C, Bouillon R. Interaction of two novel 14-epivitamin D3 analogs with vitamin D3 receptor-retinoid X receptor heterodimers on vitamin D3 responsive elements. J. Bone Miner. Res. 2001;16:625–638. doi: 10.1359/jbmr.2001.16.4.625. [DOI] [PubMed] [Google Scholar]
- Webb AR, DeCosta BR, Holick MF. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J. Clin. Endocrinol. Metab. 1989;68:882–887. doi: 10.1210/jcem-68-5-882. [DOI] [PubMed] [Google Scholar]
- Xie Z, Munson SJ, Huang N, Portale AA, Miller WJ, Bikle DD. The mechanism of 1,25 dihydroxyvitamin D3 auto-regulation in keratinocytes. J. Biol. Chem. 2002;277:36987–36990. doi: 10.1074/jbc.M201404200. [DOI] [PubMed] [Google Scholar]
- Xue Y, Fleet JC. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136:1317–1327. doi: 10.1053/j.gastro.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Shevde NK, Warrier A, Plum LA, DeLuca HF, Pike JW. 2-Methylene-19-nor-(20S)-1,25-dihydroxyvitamin D3 potently stimulates gene-specific DNA binding of the vitamin D receptor in osteoblasts. J. Biol. Chem. 2003;278:31756–31765. doi: 10.1074/jbc.M304737200. [DOI] [PubMed] [Google Scholar]
- Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional enhancers regulate mouse and human vitamin D receptor gene transcription. Mol. Endocrinol. 2010;24:128–147. doi: 10.1210/me.2009-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- Zhu J, DeLuca HF. Vitamin D 25-hydroxylase—four decades of searching, are we there yet? Arch. Biochem. Biophys. 2012;523:30–36. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc. Natl. Acad. Sci. USA. 2013;110:15650–15655. doi: 10.1073/pnas.1315006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierold C, Darwish HM, DeLuca HF. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J. Biol. Chem. 1995;270:1675–1678. doi: 10.1074/jbc.270.4.1675. [DOI] [PubMed] [Google Scholar]
- Zierold C, Reinholz GG, Mings JA, Prahl JM, DeLuca HF. Regulation of the procine 1,25-dihydroxyvitamin D3-24-hydroxylase (CYP24) by 1,25-dihydroxyvitamin D3 and parathyroid hormone in AOK-B50 cells. Arch. Biochem. Biophys. 2000;381:323–327. doi: 10.1006/abbi.2000.1964. [DOI] [PubMed] [Google Scholar]
- Zierold C, Mings JA, DeLuca HF. Parathyroid hormone regulates 25-hydroxyvitamin D(3)-24-hydroxylase mRNA by altering its stability. Proc. Natl. Acad. Sci. USA. 2001;98:13572–13576. doi: 10.1073/pnas.241516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierold C, Nehring JA, DeLuca HF. Nuclear receptor 4A2 and C/EBPbeta regulate the parathyroid hormone-mediated transcriptional regulation of the 25-hydroxyvitamin D3-1alpha-hydroxylase. Arch. Biochem. Biophys. 2007;460:233–239. doi: 10.1016/j.abb.2006.11.028. [DOI] [PubMed] [Google Scholar]