Abstract
Vancomycin has been the antibiotic of choice in the treatment of methicillin-resistant Staphylococcus aureus infections for decades. But relatively recently, vancomycin-intermediate-susceptible S. aureus (VISA) have been reported. Phenotypically, VISA are characterized by thicker cell walls, requiring higher concentrations of vancomycin for inhibition of bacterial cell growth. Vancomycin-intermediate-susceptible S. aureus represent just the tip of the iceberg of an insidious loss of vancomycin susceptibility in staphylococci. Increasing proportions of S. aureus isolates have higher minimum inhibitory concentrations that are still within the officially susceptible range, a characteristic that is associated with treatment failure. The most important risk factor for decreased vancomycin susceptibility is in vivo selection pressure. To prevent the development of VISA, prolonged or inappropriate use of vancomycin and suboptimal vancomycin levels should be avoided. Trough serum vancomycin concentrations of 15 - 20 mg/L for intermittent dosing and plateau serum vancomycin concentrations of 20 - 25 mg/L for continuous infusions are therefore currently recommended. The widespread clinical application of these intensive dosing regimens has resulted in an increasing awareness of vancomycin-induced nephrotoxicity, which is especially relevant in patients whose renal function is already compromised. This narrow therapeutic-toxic window reinforces the use of rigorous dosing protocols. In hemodialysis, the use of a vancomycin dose calculator permits achievement of target concentrations in most patients. In peritoneal dialysis (PD), intermittent vancomycin dosing regimens often lead to low end-of-dwell concentrations. On the other hand, a continuous vancomycin dosing regimen after a loading dose offers the desired combination of high local levels without toxic systemic levels.
Keywords: Vancomycin, MRSA, VISA
Vancomycin was isolated in 1953 from a sample of dirt collected by a missionary in the interior jungle of Borneo. That sample contained the soil bacterium Amycolatopsis orientalis. At first, vancomycin was eclipsed by antibiotics for penicillinase-resistant organisms, which were thought to be more efficacious and less toxic; however, with the emergence of methicillin-resistant staphylococci, interest in vancomycin was revived. The molecule was termed “vancomycin” because it could “vanquish” resistant staphylococci.
In gram-negative bacteria, the cell wall contains only a thin peptidoglycan layer, but in gram-positive bacteria, the cell wall is thick and consists almost entirely of peptidoglycan (1). Peptidoglycan is a polymer of disaccharides (glycans) cross-linked by short chains of amino acids (peptides). The monomers are synthesized within the cell and assembled outside of the cell membrane by enzymes located within the membrane. The cross-linking between amino acids in various chains occurs with the help of transpeptidases, also known as penicillin-binding proteins. Methicillin-resistant Staphylococcus aureus (MRSA) have acquired the mecA gene, which encodes for an altered penicillin-binding protein 2a. The resulting enzyme is unaffected by methicillin and other beta-lactam antibiotics used for penicillinase-resistant organisms and continues to catalyze the transpeptidation reaction, enabling cell wall synthesis in the presence of antibiotics.
Vancomycin inhibits bacterial cell growth by binding to d-alanine-d-alanine residues of the monomers, thus preventing cross-linking during cell wall synthesis (Figure 1). The result is a weakened cell wall, which slows bacterial growth and eventually causes death from osmotic lysis, which most often occurs during cell division (3). Vancomycin can also bind to d-alanine-d-alanine residues in the already completed peptidoglycan layers, but that action does not affect bacterial cell growth (Figure 1). Vancomycin is considered a bactericidal antibiotic, but the slow nature of its cidal activity is an important limitation in clinical practice (4). In addition, the activity of vancomycin is inoculum-dependent (5). A large burden of bacteria and bacteria in the stationary growth phase or in an anaerobic environment impair the speed and extent of vancomycin’s bactericidal activity (4,6).
Figure 1 —
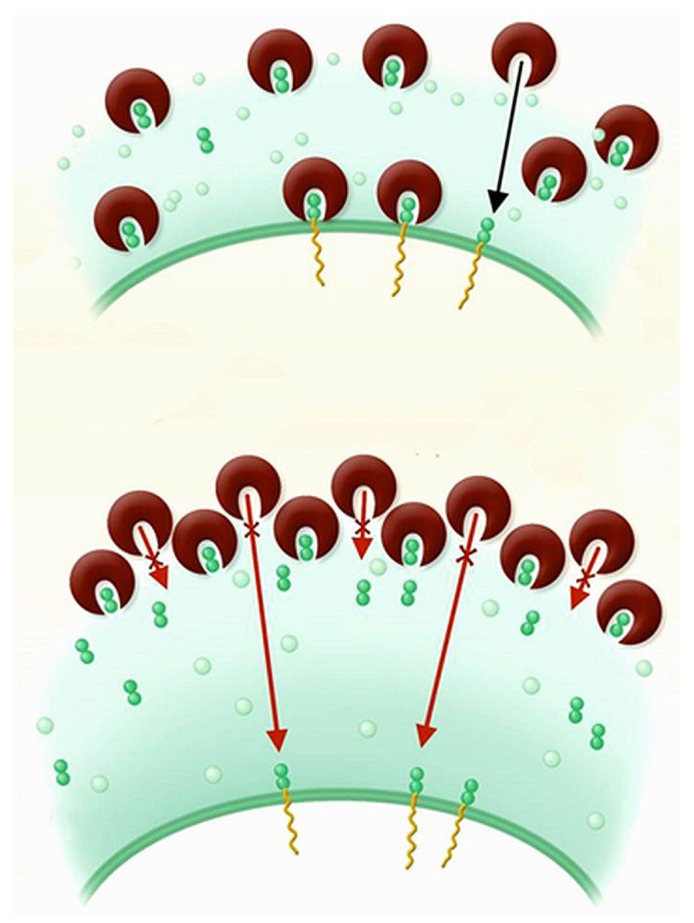
Vancomycin inhibits cell wall synthesis by binding to the terminal d-alanine-d-alanine residues of the cell wall precursors. It can also bind to the d-alanine-d-alanine residues in the already completed peptidoglycan layers, but that binding does not affect bacterial cell growth. Upper panel shows susceptible bacterium. Lower panel shows bacterium with reduced vancomycin susceptibility, characterized by a thickened cell wall with an increased number of d-alanine-d-alanine residues trapping vancomycin. Adapted from Lowy, 2003 (2), reproduced with permission of the American Society for Clinical Investigation.
Vancomycin Resistance
For a long time, vancomycin has been the most reliable agent in the treatment of MRSA infections. With most antibiotics, the first reports of resistance start to appear only a few years after their introduction. Vancomycin, however, has been used for decades with apparently unaffected clinical effectiveness. It was only in 1996 that the first MRSA to acquire vancomycin-intermediate resistance was isolated in Japan (7). Vancomycin-intermediate S. aureus (VISA) are characterized by a moderate reduction in their susceptibility to vancomycin. Phenotypically, they have a thickened cell wall with increased d-alanylation, trapping vancomycin in the already completed peptidoglycan layers (Figure 1). More of the antibiotic is therefore required to inhibit bacterial growth (2). On the molecular level, diverse mutations in a small number of staphylococcal regulatory genes have been identified (8).
Heterogenous VISA (hVISA) are strains of apparently susceptible staphylococci that contain subpopulations of resistant cells. Because their numbers fall below the detection limit of standard laboratory techniques, they are not routinely identified. More detailed testing with a higher inoculum and prolonged incubation using the population analysis profile technique is considered the “gold standard” for a diagnosis of hVISA (9). The true prevalence of this type of resistance is unknown, owing to the underreporting that follows from the inability of the clinical laboratory to detect these strains. Clinicians should consider the possibility of hVISA when confronted with clinical failure in an apparently vancomycin-sensitive MRSA infection.
The emergence of VISA and hVISA is most likely just the tip of the iceberg of an insidious loss of vancomycin susceptibility in staphylococci. Increasing proportions of MRSA isolates have a high minimal inhibitory concentration (MIC)—the concentration of an antibiotic that inhibits growth, usually in 90% of the inoculum—for vancomycin that nevertheless remains within the officially susceptible range, a phenomenon called “vancomycin MIC creep” (10-13). Because failures of vancomycin to cure MRSA infections with an intermediate or borderline susceptible MIC had been reported, the Clinical and Laboratory Standards Institute revised its criteria for susceptibility to vancomycin in 2006 (9). The MIC breakpoints for S. aureus were lowered to ≤2 mg/L from ≤4 mg/L for “susceptible,” to 4 - 8 mg/L from 8 - 16 mg/L for “intermediate,” and to ≥16 mg/L from ≥32 mg/L for “resistant.”
The mechanism of resistance in VISA is profoundly distinct from that of vancomycin-resistant enterococci, imparted by the enterococcal van gene. The vanA gene converts d-alanine-d-alanine residues to d-alanine-d-lactate, which decreases the binding affinity for vancomycin, resulting in a MIC of more than 512 mg/L. In 2002, the first full-level vancomycin-resistant S. aureus (VRSA) was documented in the United States (14). That strain was shown to carry a van gene, suggesting that the resistance was acquired through the exchange of genetic material between vancomycin-resistant enterococci and S. aureus. Only 34 strains of VRSA (1 in Pakistan, 4 in Iran, 13 in the United States, and 16 in India) have been reported so far, most of which were discovered in dialysis patients (15,16).
Clinical Importance of Waning Vancomycin Susceptibility
Few well-controlled prospective clinical studies have been performed to determine the impact of hVISA and VISA on treatment outcomes in patients. In an observational study of 53 MRSA bacteremias, 9.4% were identified as hVISA by population analysis profiling (17). The patients were more likely to have a high bacterial load, vancomycin treatment failure, and initially low serum vancomycin levels (17). In another study of 268 MRSA bacteremias, 37.7% were characterized as hVISA; those infections occurred especially in patients who had undergone prior vancomycin therapy and were using immunosuppressive drugs (18). Although overall mortality was similar for hVISA and vancomycin-susceptible S. aureus, hVISA was associated with persistent bacteremia when initial trough levels of vancomycin were less than 15 mg/L (18). A meta-analysis of 22 studies reported that a high vancomycin MIC (≥1.5 mg/L vs <1.5 mg/L) was associated with higher attributed mortality and treatment failure (19). Taken together, MRSA isolates with MICs on the high end but still in the officially sensitive range are less likely to be treated successfully with vancomycin.
Risk Factors for Decreased Vancomycin Susceptibility
Identified risk factors for VISA are treatment with hemodialysis or PD, diabetes, prior prolonged or repeated vancomycin exposure, and suboptimal vancomycin concentrations (6). The common denominator of these factors is in vivo selection pressure. In vitro studies showed that hVISA or VISA emerge when S. aureus isolates with a downregulated or defective agr locus are exposed to suboptimal vancomycin concentrations (20,21). Infectious S. aureus clones contain subpopulations with lower sensitivity to vancomycin. Vancomycin creates a selection pressure that favors the outgrowth of rare vancomycin-resistant clones leading to hVISA, and eventually, with continued exposure, to a uniform population of VISA clones (22,23).
Recent studies have revealed that mutations in the staphylococcal regulatory genes that lead to the VISA phenotype might be associated with increased resistance to the innate immune system response (8). In addition, there appears to be a reduced expression of virulence genes, producing clinical persistence rather than aggressive disease (8). Thus, VISA combines antibiotic resistance with expression of a phenotype characterized by a persistent, often biofilm-associated, growth modus.
To prevent the development of VISA, prolonged or inappropriate use of vancomycin should be avoided. In infections that are foreign body-related or that contain death space (for example, abscesses or sequesters in osteomyelitis), adequate surgical intervention and removal of foreign material is mandatory. Theoretically, when using vancomycin, the pharmacologic target that assures microbiologic effectiveness is a ratio of more than 400 for the area under the 24-hour concentration curve divided by the MIC (4,6). That target is hard to achieve when the MIC exceeds 1.5 - 2 mg/L (6). In 2009, the American Society of Health System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists issued joint guidelines that translated the target into a recommendation for a total trough serum vancomycin concentration of 15 - 20 mg/L (6). When vancomycin is administered as a continuous infusion, plateau levels of 20 - 25 mg/L are recommended (4). Thus far, only limited data on the effect of these more intensive dosing regimens are available, and they suggest that maintenance of higher vancomycin trough values improves clinical outcome (24).
The Pyrrhic Victory
The toxicity of early formulations of vancomycin was well-recognized and attributed to impurities associated with the production process, but vancomycin produced with modern methods has been considered to be associated with infrequent, if any, nephrotoxicity. However, after widespread adoption of the more intensive vancomycin dosing regimens, reports of nephrotoxicity are increasingly emerging.
A systematic review and meta-analysis of intermittent vancomycin dosing regimens identified trough values in excess of 15 mg/L and longer duration of vancomycin administration as independent risk factors for nephrotoxicity (25,26). Overall, a trough value in excess of 15 mg/L (relative to a trough value below 15 mg/L) was associated with an increased odds ratio of 2.67 for nephrotoxicity (25). Extraction of data from four studies that allowed more detailed analysis (24,27-29) revealed a trough-toxicity gradient, with the greatest risk in patients achieving troughs in excess of 20 mg/L. Nephrotoxicity appeared to be largely reversible after discontinuation and required temporary dialysis in only 3% of cases (25).
A meta-analysis comparing intermittent and continuous vancomycin dosing found that, for equal daily exposure (area under the 24-hour concentration curve), continuous infusion was associated with similar efficacy but lower drug-related nephrotoxicity (26). Those findings suggest that high peak levels might be the most toxic to kidney, and that continuous infusions might be the preferred mode of administration to preserve residual renal function (4,26). The impact of vancomycin exposure in general and of the higher dosing regimens in particular on residual renal function in hemodialysis or PD patients has not been systematically examined. However, the special vulnerability of patients with already compromised renal function to vancomycin-induced renal failure is well-recognized (30). Repercussions of vancomycin overdosing on residual renal function can therefore be expected, and nephrologists should intently monitor vancomycin levels in their patients.
Vancomycin Dosing in Hemodialysis
When vancomycin is given at each dialysis session, the levels follow a triphasic course (31,32). Administration at the end of dialysis is followed by a rapid redistribution phase. Thereafter, levels decline steadily in the interdialytic interval, with a half-life of 100 - 200 hours, depending on the degree of residual renal function (31,32). Finally, intradialytic clearance of vancomycin reduces vancomycin levels by 30% - 46%.
Several dosing regimens have been proposed in the literature, varying from 500 mg after each dialysis session (33) or during each dialysis session (34), to a trough-guided algorithm (35). Those regimens result in subtherapeutic levels in most patients (31), especially given current treatment targets. In addition, several issues remain unresolved. How much vancomycin should be given before the long interdialytic interval? What should be done when body weight is below or above average? Should vancomycin be given during or after dialysis?
To handle those difficulties, a vancomycin dose calculator was developed (36). When fed 3 easily obtainable variables (vancomycin level at the start of dialysis, body weight, number of days to the next dialysis session), the calculator determines the dose to be administered at the end of the current dialysis session to obtain a trough level of 15 - 20 mg/L at the start of the next dialysis session. The rate of the vancomycin infusion is 15 mg/min, and the timing is such that the end of the infusion coincides with the end of the dialysis session. A weight-based loading dose should be given, adjusted for the number of days to the next dialysis session: 15 mg/kg for 1 day, 25 mg/kg for 2 days, 35 mg/kg for 3 days. Using the calculator, 78% of values were within target and major under- or overdosing was avoided (36).
Vancomycin Dosing in Peritoneal Dialysis
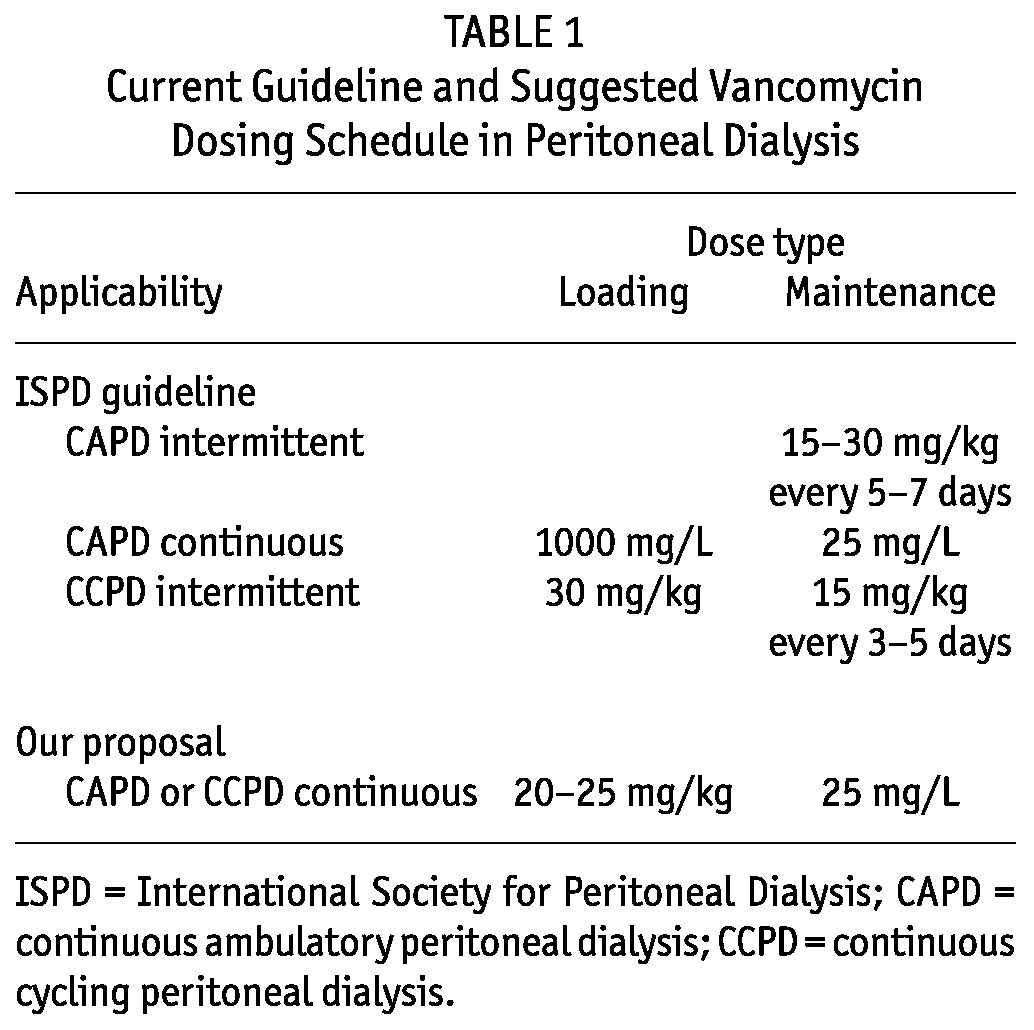
In PD peritonitis, the infection involves the first few cell layers of the mesothelium and is generally confined to the peritoneal cavity, although transient bacteriemia can occur. Intraperitoneal administration of antibiotics is therefore not only convenient, but also offers the unique opportunity to achieve the highest levels of antibiotic at the site of infection. The International Society for Peritoneal Dialysis (ISPD) guidelines for vancomycin administration (Table 1) advance an intermittent regimen (15 - 30 mg/kg every 5 - 7 days) and a continuous regimen (1000 mg/L loading dose with 25 mg/L maintenance dose) for continuous ambulatory PD (CAPD), and an intermittent regimen (30 mg/kg loading dose, 15 mg/kg maintenance dose every 3-5 days) for continuous cycling PD (CCPD) (37).
TABLE 1.
Current Guideline and Suggested Vancomycin Dosing Schedule in Peritoneal Dialysis
In gram-positive peritonitis in children, continuous and intermittent administration of vancomycin or teicoplanin were equally efficacious, with similar primary success (95%) and relapse (21%) rates (38). Another small study reported clinical resolution in all patients treated with either a continuous or an intermittent vancomycin regimen, with similar low recurrence rates (39). However, a more recent and larger study in adult S. aureus peritonitis reported less optimistic cure rates (40). The overall primary response rate was 87.8%, with a complete cure rate of 74.3%. However, MRSA peritonitis had a significantly lower primary response rate (64.4% vs 93.0%) and complete cure rate (60.0% vs 77.5%) than methicillin-sensitive S. aureus peritonitis (40). Notably, only 46% of patients with MRSA peritonitis and 51% of methicillin-sensitive S. aureus peritonitis had a complete cure without the need for catheter removal and without relapsed, recurrent, or repeat peritonitis. In almost one third of patients achieving a complete cure, repeat peritonitis developed, more than half of which occurred within 3 months of completion of therapy (40). Vancomycin was included in the initial antibiotic regimen and was given intermittently every 5 days (40). In another study of gram-positive peritonitis, relapses developed in 9 of 14 peritonitis episodes with a mean vancomycin trough level of less than 12 mg/L; no relapses occurred in 17 episodes with a trough level greater than 12 mg/L (40). A low initial vancomycin trough level was the only predictor of subsequent relapsed peritonitis (41).
These mixed clinical outcomes data warrant scrutiny of the guidelines for vancomycin administration presented by the ISPD. Vancomycin has a molecular weight of approximately 1500 Da (30). After intraperitoneal administration, 30% - 70% of the dose will be absorbed into the systemic circulation at the end of a 4- to 6-hour dwell, regardless of whether the peritoneal membrane is inflamed (42-50). Those data provide support for the recommendation that antibiotic-containing dialysate must dwell at least 4 hours to ensure an adequate antibiotic reservoir in the body (37). During subsequent antibiotic-free dialysate exchanges, vancomycin will move in the opposite direction, back into the peritoneal cavity (Figure 2). Dialysate-to-plasma ratios of vancomycin have been reported, measuring 0.18 ± 0.10 at 45 minutes, 0.22 ± 0.11 at 90 minutes, 0.40 ± 0.14 at 6 hours, and 0.45 ± 0.20 at 8 hours (49). No correlation between peritoneal equilibration test category and dialysate-to-plasma vancomycin ratio was found, but the number of patients might have been too small to observe a relation (49). Because of the slow transfer rate of vancomycin from blood to dialysate, end-of-dwell effluent concentrations can be very low, particularly when short exchange times— as in automated PD (APD)—are applied (Figure 2).
Figure 2 —
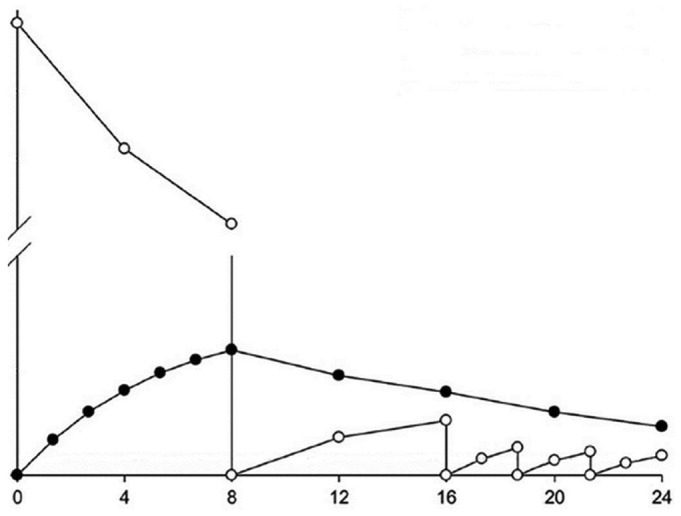
Hypothetical course of intraperitoneal (open circles) and serum (filled circles) vancomycin concentrations after a single intraperitoneal dose, followed by antibiotic-free dwells (1 eight-hour dwell and 3 subsequent short dwells).
In CAPD, administration of vancomycin at a dose of 30 mg/kg, which is at the high end of the dose recommended in the ISPD guidelines, yields serum trough levels of 15 ± 3.6 mg/L at 72 hours (45), which is at the low end of current treatment targets (6). More importantly, end-of-dwell dialysate levels already decline to 4.7 ± 2.5 mg/L after 48 hours (45). A 5-day delay in the subsequent dose will thus lead to subtherapeutic vancomycin levels at the site of infection. Administration of vancomycin at a dose of 15 mg/kg intravenously, which is more or less the equivalent of 30 mg/kg intraperitoneal (IP) administration, taking into account the bioavailability of 30% - 70%, resulted in low end-of-dwell levels in most CCPD patients (51). Based on the observations from that study, the dose required in CCPD to obtain adequate local levels was calculated to be 35 mg/kg IP on day 1 and 15 mg/kg IP daily thereafter (51). It can easily be inferred that those dosing regimens will be associated with very high systemic levels, potentially at the cost of residual renal function.
A factor that further complicates the use of intermittent vancomycin dosing regimens is the major, but hardly predictable, impact of residual renal function on vancomycin clearance. The ISPD Advisory Committee on Peritonitis Management recommends a 25% increase in antibiotic dose in non-anuric patients. In an observational study of PD peritonitis, the mean vancomycin dose given on day 1 was 2.00 ± 0.02 g for anuric and 2.35 ± 0.02 g for non-anuric patients on CAPD, and 2.00 ± 0.04 g for anuric and 2.36 ± 0.03 g for non-anuric patients on CCPD (52). A substantial number of patients had a day 5 vancomycin serum level below 12 mg/L, for which the subsequent vancomycin dose was increased by 500 mg. Despite incremental dosing, the proportion of non-anuric patients that continued to have a low vancomycin level on day 10 was 21% for CAPD and 25% for CCPD (52).
Taken together, the evidence reveals that intermittent vancomycin dosing regimens often lead to inadequate end-of-dwell concentrations, especially with the use of short dwells and in non-anuric patients. We contend that a continuous vancomycin dosing regimen offers the desired combination of high local levels and low systemic exposure. The loading dose should be weight-based (for instance, 20 - 25 mg/kg) to obtain a systemic level of more than 15 mg/L in all patients, including those with non-standard body weights (Table 1). This systemic reservoir serves two purposes: it covers incidental bacteriemia and minimizes the concentration gradient between the peritoneal cavity and the circulation to prevent vancomycin absorption from the peritoneal cavity. Subsequently, vancomycin is administered in each dwell at a dose of 25 mg/L (Table 1). With this regimen, little or no checking of serum concentrations is required. In patients with important residual renal function, serum concentrations can decline rapidly because of renal clearance of vancomycin, thus resulting in more rapid absorption of vancomycin from the peritoneal cavity. In such patients, a check of the serum concentration after 3 - 5 days, followed by incremental dosing, might be helpful. The stability of vancomycin dissolved in diverse types of peritoneal dialysate is at least 24 hours at room temperature and at least 6 days at 4°C (53).
A factor unique to PD-related peritonitis that has been unaccounted for by most studies, is the effect of the dialysate itself on the antibacterial activity of vancomycin. The mechanism of action of vancomycin requires that bacteria are in the exponential growth phase if the drug is to be effective. Dianeal (Baxter Healthcare Corporation, Deerfield, IL, USA) is known to be bacteriostatic (54). In vitro studies have demonstrated that the activity of vancomycin is negatively affected in the setting of dialysate-induced growth suppression (54).
Conclusions
In the past few years, clinicians have been confronted with an insidious loss of the clinical effectiveness of vancomycin. Nonetheless, it appears that VISA are not intrinsically more virulent, but rather lead to vancomycin treatment failure associated with more prolonged infection and extended hospitalization and treatment. Development of VISA might simply reflect a sequence of adaptive responses of the organism under increased vancomycin selection pressure. Optimizing vancomycin therapy is therefore mandatory.
In PD, intermittent antibiotic dosing regimens have been promulgated because of convenience and a reduced risk of accidental contamination of the system by the patient. However, the risk of subtherapeutic local levels is high. We therefore favor reversion to continuous therapy for the combined advantage of high local concentrations and low systemic exposure in an era of reduced vancomycin susceptibility, and for use of a technique in which preservation of residual renal function is of importance.
Disclosures
The authors have no financial conflicts to disclose.
References
- 1. Vandecasteele SJ, Boelaert JR, De Vriese AS. Staphylococcus aureus infections in hemodialysis: what a nephrologist should know. Clin J Am Soc Nephrol 2009;4:1388–400 [DOI] [PubMed] [Google Scholar]
- 2. Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest 2003; 111:1265–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cosgrove SE, Carroll KC, Perl TM. Staphylococcus aureus with reduced susceptibility to vancomycin. Clin Infect Dis 2004; 39:539–45 [DOI] [PubMed] [Google Scholar]
- 4. Vandecasteele SJ, De Vriese AS, Tacconelli E. The pharmacokinetics and pharmacodynamics of vancomycin in clinical practice: evidence and uncertainties. J Antimicrob Chemother 2013; 68:743–8 [DOI] [PubMed] [Google Scholar]
- 5. LaPlante KL, Rybak MJ. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2004; 48:4665–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98 [Erratum in: Am J Health Syst Pharm 2009; 66:887] [DOI] [PubMed] [Google Scholar]
- 7. Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 1997; 40:135–6 [DOI] [PubMed] [Google Scholar]
- 8. Howden BP, Peleg AY, Stinear TP. The evolution of vancomycin intermediate Staphylococcus aureus (VISA) and heterogenous-VISA. Infect Genet Evol 2013; S1567-1348(13)00136-6 [DOI] [PubMed] [Google Scholar]
- 9. Tenover FC, Moellering RC., Jr The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007; 44:1208–15 [DOI] [PubMed] [Google Scholar]
- 10. Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sader HS, Fey PD, Limaye AP, Madinger N, Pankey G, Rahal J, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother 2009; 53:4127–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lubin AS, Snydman DR, Ruthazer R, Bide P, Golan Y. Predicting high vancomycin minimum inhibitory concentration in methicillin resistant Staphylococcus aureus bloodstream infections. Clin Infect Dis 2011; 52:997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kehrmann J, Kaase M, Szabados F, Gatermann SG, Buer J, Rath PM, et al. Vancomycin MIC creep in MRSA blood culture isolates from Germany: a regional problem? Eur J Clin Microbiol Infect Dis 2011; 30:677–83 [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep 2002; 51:565–7 [PubMed] [Google Scholar]
- 15. Gould IM. VRSA-doomsday superbug or damp squib? Lancet Infect Dis 2010; 10:816–18 [DOI] [PubMed] [Google Scholar]
- 16. Moravvej Z, Estaji F, Askari E, Solhjou K, Naderi Nasab M, Saadat S. Update on the global number of vancomycin-resistant Staphylococcus aureus (VRSA) strains. Int J Antimicrob Agents 2013; 42:370–1 [DOI] [PubMed] [Google Scholar]
- 17. Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–51 [DOI] [PubMed] [Google Scholar]
- 18. Park KH, Kim ES, Kim HS, Park SJ, Bang KM, Park HJ, et al. Comparison of the clinical features, bacterial genotypes and outcomes of patients with bacteraemia due to heteroresistant vancomycin-intermediate Staphylococcus aureus and vancomycin-susceptible S. aureus. J Antimicrob Chemother 2012; 67:1843–9 [DOI] [PubMed] [Google Scholar]
- 19. van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 2012; 54:755–71 [DOI] [PubMed] [Google Scholar]
- 20. Sakoulas G, Eliopoulos GM, Moellering RC, Jr, Novick RP, Venkataraman L, Wennersten C, et al. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J Infect Dis 2003; 187:929–38 [DOI] [PubMed] [Google Scholar]
- 21. Sakoulas G, Eliopoulos GM, Fowler VG, Jr, Moellering RC, Jr, Novick RP, Lucindo N, et al. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother 2005; 49:2687–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Hal SJ, Steen JA, Espedido BA, Grimmond SM, Cooper MA, Holden MT, et al. In vivo evolution of antimicrobial resistance in a series of Staphylococcus aureus patient isolates: the entire picture or a cautionary tale? J Antimicrob Chemother 2014; 69:363–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis 2011; 52:975–81 [DOI] [PubMed] [Google Scholar]
- 25. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 2013; 57:734–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. Continuous versus intermittent infusion of vancomycin for the treatment of gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother 2012; 67:17–24 [DOI] [PubMed] [Google Scholar]
- 27. Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, et al. on behalf of the Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Study Group. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP database. Clin Ther 2012; 34:149–57 [DOI] [PubMed] [Google Scholar]
- 28. Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 2009; 49:507–14 [DOI] [PubMed] [Google Scholar]
- 29. Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012; 54:621–9 [DOI] [PubMed] [Google Scholar]
- 30. Vandecasteele SJ, De Vriese AS. Recent changes in vancomycin use in renal failure. Kidney Int 2010; 77:760–4 [DOI] [PubMed] [Google Scholar]
- 31. Vandecasteele SJ, De Vriese AS. Vancomycin dosing in patients on intermittent hemodialysis. Semin Dial 2011; 24:50–5 [DOI] [PubMed] [Google Scholar]
- 32. Pallotta KE, Manley HJ. Vancomycin use in patients requiring hemodialysis: a literature review. Semin Dial 2008; 21:63–70 [DOI] [PubMed] [Google Scholar]
- 33. Barth RH, DeVincenzo N. Use of vancomycin in high-flux hemodialysis: experience with 130 courses of therapy. Kidney Int 1996; 50:929–36 [DOI] [PubMed] [Google Scholar]
- 34. Ariano RE, Fine A, Sitar DS, Rexrode S, Zelenitsky SA. Adequacy of a vancomycin dosing regimen in patients receiving high-flux hemodialysis. Am J Kidney Dis 2005; 46:681–7 [DOI] [PubMed] [Google Scholar]
- 35. Pai AB, Pai MP. Vancomycin dosing in high flux hemodialysis: a limited-sampling algorithm. Am J Health Syst Pharm 2004; 61:1812–16 [DOI] [PubMed] [Google Scholar]
- 36. Vandecasteele SJ, De Bacquer D, De Vriese AS. Implementation of a dose calculator for vancomycin to achieve target trough levels of 15-20 microg/mL in persons undergoing hemodialysis. Clin Infect Dis 2011; 53:124–9 [DOI] [PubMed] [Google Scholar]
- 37. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. on behalf of the International Society for Peritoneal Dialysis. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [Erratum in: Perit Dial Int 2011; 31:512] [DOI] [PubMed] [Google Scholar]
- 38. Schaefer F, Klaus G, Müller-Wiefel DE, Mehls O. Intermittent versus continuous intraperitoneal glycopeptide/ceftazidime treatment in children with peritoneal dialysis-associated peritonitis. The Mid-European Pediatric Peritoneal Dialysis Study Group (MEPPS). J Am Soc Nephrol 1999; 10:136–45 [DOI] [PubMed] [Google Scholar]
- 39. Boyce NW, Wood C, Thomson NM, Kerr P, Atkins RC. Intraperitoneal (IP) vancomycin therapy for CAPD peritonitis— a prospective, randomized comparison of intermittent v continuous therapy. Am J Kidney Dis 1988; 12:304–6 [DOI] [PubMed] [Google Scholar]
- 40. Szeto CC, Chow KM, Kwan BC, Law MC, Chung KY, Yu S, et al. Staphylococcus aureus peritonitis complicates peritoneal dialysis: review of 245 consecutive cases. Clin J Am Soc Nephrol 2007; 2:245–51 [DOI] [PubMed] [Google Scholar]
- 41. Mulhern JG, Braden GL, O’Shea MH, Madden RL, Lipkowitz GS, Germain MJ. Trough serum vancomycin levels predict the relapse of gram-positive peritonitis in peritoneal dialysis patients. Am J Kidney Dis 1995; 25:611–15 [DOI] [PubMed] [Google Scholar]
- 42. Pancorbo S, Comty C. Peritoneal transport of vancomycin in 4 patients undergoing continuous ambulatory peritoneal dialysis. Nephron 1982; 31:37–9 [DOI] [PubMed] [Google Scholar]
- 43. Bunke CM, Aronoff GR, Brier ME, Sloan RS, Luft FC. Vancomycin kinetics during continuous ambulatory peritoneal dialysis. Clin Pharmacol Ther 1983; 34:631–7 [DOI] [PubMed] [Google Scholar]
- 44. Rogge MC, Johnson CA, Zimmerman SW, Welling PG. Vancomycin disposition during continuous ambulatory peritoneal dialysis: a pharmacokinetic analysis of peritoneal drug transport. Antimicrob Agents Chemother 1985; 27:578–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morse GD, Farolino DF, Apicella MA, Walshe JJ. Comparative study of intraperitoneal and intravenous vancomycin pharmacokinetics during continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother 1987; 31:173–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bastani B, Spyker DA, Westervelt FB., Jr Peritoneal absorption of vancomycin during and after resolution of peritonitis in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 1988; 8:135–136 [Google Scholar]
- 47. Neal D, Bailie GR. Clearance from dialysate and equilibration of intraperitoneal vancomycin in continuous ambulatory peritoneal dialysis. Clin Pharmacokinet 1990; 18:485–90 [DOI] [PubMed] [Google Scholar]
- 48. Bailie GR, Eisele G, Venezia RA, Yocum D, Hollister A. Prediction of serum vancomycin concentrations following intraperitoneal loading doses in continuous ambulatory peritoneal dialysis patients with peritonitis. Clin Pharmacokinet 1992; 22:298–307 [DOI] [PubMed] [Google Scholar]
- 49. Blowey DL, Warady BA, Abdel-Rahman S, Frye RF, Manley HJ. Vancomycin disposition following intraperitoneal administration in children receiving peritoneal dialysis. Perit Dial Int 2007; 27:79–85 [PubMed] [Google Scholar]
- 50. Montañés Pauls B, Almiñana MA, Casabó Alós VG. Vancomycin pharmacokinetics during continuous ambulatory peritoneal dialysis in patients with peritonitis. Eur J Pharm Sci 2011; 43:212–16 [DOI] [PubMed] [Google Scholar]
- 51. Manley HJ, Bailie GR, Frye RF, McGoldrick MD. Intravenous vancomycin pharmacokinetics in automated peritoneal dialysis patients. Perit Dial Int 2001; 21:378–85 [PubMed] [Google Scholar]
- 52. Blunden M, Zeitlin D, Ashman N, Fan SL. Single UK centre experience on the treatment of PD peritonitis—antibiotic levels and outcomes. Nephrol Dial Transplant 2007; 22:1714–19 [DOI] [PubMed] [Google Scholar]
- 53. de Vin F, Rutherford P, Faict D. Intraperitoneal administration of drugs in peritoneal dialysis patients: a review of compatibility and guidance for clinical use. Perit Dial Int 2009; 29:5–15 [PubMed] [Google Scholar]
- 54. Hermsen ED, Hovde LB, Hotchkiss JR, Rotschafer JC. Increased killing of staphylococci and streptococci by daptomycin compared with cefazolin and vancomycin in an in vitro peritoneal dialysate model. Antimicrob Agents Chemother 2003; 47:3764–7 [DOI] [PMC free article] [PubMed] [Google Scholar]


