Abstract
♦ Background: Peritoneal dialysis (PD) is an established treatment modality for end-stage renal disease (ESRD). Peritonitis remains a serious complication in PD patients and an important cause of drop-out from the program. Types of pathogens and their drug resistance patterns may determine the outcome of peritonitis. The present study was undertaken to determine the microbiology of peritonitis in PD patients, antibiotic resistance in commonly isolated bacterial pathogens and clinical outcomes.
♦ Method: We enrolled 211 patients with ESRD undergoing PD who developed peritonitis during 2002 to 2011. PD fluids were cultured and antibiotic susceptibility test of the bacterial isolates was performed.
♦ Result: A total of 303 peritonitis episodes with an overall incidence of 0.41 episodes per patient-year were recorded. Gram-positive, gram-negative, fungi, Mycobacterium tuberculosis and ≥ 2 organisms were isolated from 102 (33.7%), 89 (29.4%), 41 (13.5%), 11 (3.6%) and five (1.6%) episodes respectively; 55 (18.2%) episodes were culture negative. Coagulase-negative Staphylococcus spp. (CONS) was the most common isolate. Catheter loss and hospital admission in gram-negative peritonitis were significantly higher than in gram-positive peritonitis (36/89 (40.4%) vs 20/102 (19.6%), p < 0.001; and 56/89 (62.9%) vs 42/102 (41.2%), p = 0.004 respectively). Antibiotic susceptibility tests showed 54.3% of Enterobacteriaceae isolates were extended spectrum β-lactamase (ESBL) producers, 23.5% of Acinetobacter species and 11.5% of Pseudomonas aeruginosa were metallo-β-lactamase (MBL) producers; 15.4% of enterococci and 28.6% of staphylococci were resistant to vancomycin and methicillin respectively. Mortality was significantly higher in patients having peritonitis due to vancomycin-resistant enterococci, ESBL- and MBL-producing bacteria.
♦ Conclusion: Emerging antimicrobial resistance calls for prompt diagnosis and aggressive empiric therapy based on the local sensitivity data.
Keywords: Antibiotic resistance, etiology of peritonitis, mortality in peritonitis, peritoneal dialysis, outcome of peritonitis
Peritoneal dialysis (PD) has been an established treatment modality for end-stage renal disease (ESRD) for more than four decades. Approximately 10% to 15% of ESRD patients are on PD worldwide. Despite a dramatic reduction in peritonitis rate, it still remains a significant problem in PD and a major cause for switching from PD to hemodialysis. The most common organisms associated with PD peritonitis reported worldwide are coagulase-negative Staphylococcus spp. (CONS) and Staphylococcus aureus followed by streptococci, Enterobacteriaceae, non-fermenting gram-negative bacilli and gram-positive bacilli (1,2). However, in India, the spectrum of organisms associated with PD peritonitis showed a predominance of gram-negative organisms (3,4).
Peritonitis is an important cause of morbidity and mortality in PD patients. In the United States, 18% of the infection-related mortality in PD patients was due to peritonitis (5). Peritonitis was identified as a “contributing factor” in 16% of deaths of patients who were on PD (6). Other studies suggest that mortality can vary from 3.5% to 5% and it can be associated with specific organisms (1,7). In one retrospective Spanish study of 565 patients in 2005, mortality with fungal peritonitis was 28% followed by 19% due to enteric organisms and 15% due to S. aureus (8).
The International Society for Peritoneal Dialysis (ISPD) developed extensive guidelines for the treatment of peritonitis in PD patients (9). Empiric antibiotic therapy should cover both gram-positive and gram-negative organisms and selection should be center specific based on local sensitivity data of the major causative organisms. Cross-sectional studies on the causative organisms of peritonitis have been reported frequently (10,7). However, long-term studies on changes in the causative organisms of peritonitis and their antibiotic sensitivity are scarce. The present study was designed to study the microbiology of peritonitis in PD patients, resistance patterns of commonly isolated bacterial pathogens and eventual outcome of peritonitis.
Materials and Methods
A total of 211 PD patients who developed peritonitis from 2002 to 2011 were included in the study. During this study period, a total of 560 patients were enrolled in the PD program. For all patients, a double-cuffed straight Tenckhoff catheter was inserted by surgical technique and PD was started after a break-in period of 12 ± 4 days. All patients were started on a disconnect system using ultra-bag dianeal PD fluid (Baxter India, Manesar, India) with three 2-L exchanges daily. Dialysis prescription was changed according to individual requirements during follow-up. Diabetes, coronary artery disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure, malignancy, cardiac arrhythmias, chronic lung disease, and chronic liver disease were considered as comorbidities. The diagnosis of peritonitis was made when the patients fulfilled at least two of the following three criteria: i) signs and symptoms of peritonitis, ii) cloudy dialysate with white blood cell count of > 100/μL with more than 50% neutrophils and iii) demonstration of organism either by smear examination or by culture of peritoneal dialysate. Centrifuged dialysate was examined microscopically and cultured in automated BACTEC blood culture system (BD Biosciences, San Jose, CA, USA) following standard protocol (11). The isolated bacterial pathogens were identified and subjected to antibiotic sensitivity test following Clinical Laboratory Standards Institute (CLSI) guidelines (12). The antibiotic discs were procured from Hi-Media, Mumbai, India. For detection of ESBL production in Enterobacteriaceae, cefotaxime and cefotaxime + clavulanic acid, and ceftazidime and ceftazidime + clavulanic acid discs were used and the results were interpreted according to CLSI guidelines, 2010. For metallo-β-lactamase production in non-fermenters, meropenem and meropenem + EDTA discs were used following standard guidelines (13). For outcome analysis, polymicrobial, fungal, tubercular and sterile peritonitis were excluded because of their different outcomes from bacterial peritonitis. The outcomes were analyzed in terms of catheter loss, hospitalization, death within four weeks of peritonitis, and switch to permanent maintenance hemodialysis. Catheter loss was defined as catheter removal required for the resolution of peritonitis.
Statistical Analysis
Statistical analysis was performed using Fisher exact test with Yates correction and chi-square test for differences in proportions. Data were expressed as mean ± standard deviation. Statistical significance was defined at a p value of ≤ 0.05.
Results
Demography of the Study Subjects
During the study, 742.33 patient-years were followed up and the mean duration of PD therapy was 3.52 years. A total of 211 patients (mean age 51.26 ± 14.44 years; 150 male) developed 303 episodes of peritonitis (range: 1-6 episodes per patient) of which 12 episodes were relapsing peritonitis and 10 episodes were recurrent peritonitis. In all these patients, catheters were removed for the resolution of peritonitis. The overall peritonitis rate was 0.41 episodes/patient-year. Of these 211 patients, 114 had diabetes, 34 had cardiovascular comorbidities, two malignancies, and two chronic liver diseases with portal hypertension.
Causative Organisms of Peritonitis
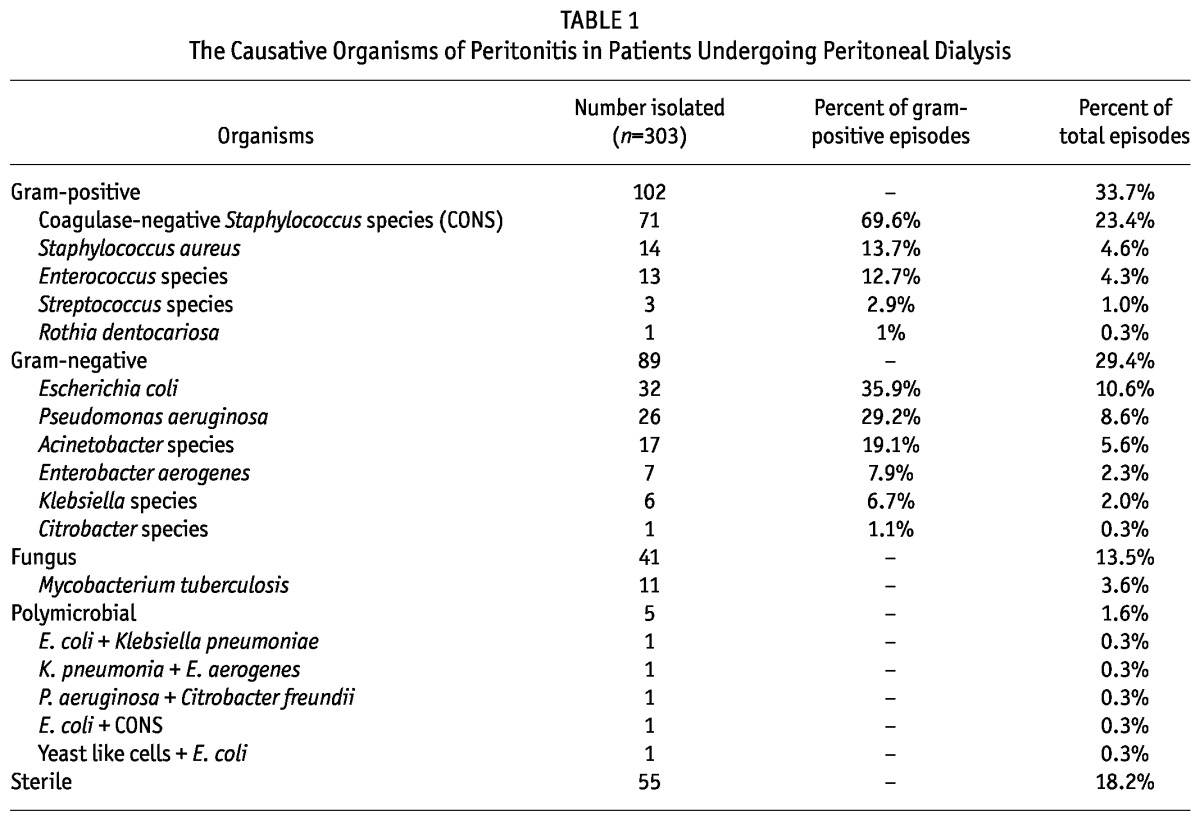
Gram-positive, gram-negative, fungi, Mycobacterium tuberculosis and ≥ 2 organisms were isolated from 102 (33.7%), 89 (29.4%), 41 (13.5%), 11 (3.6%) and five (1.6%) episodes respectively; 55 (18.2%) episodes were culture negative. The distribution of bacterial pathogens was as follows: CONS (71; 23.4%), Escherichia coli (32; 10.6%), Pseudomonas aeruginosa (26; 8.6%), Acinetobacter species (17; 5.6%), S. aureus (14; 4.6%) and Enterococcus species (13; 4.3%) (Table 1).
TABLE 1.
The Causative Organisms of Peritonitis in Patients Undergoing Peritoneal Dialysis
Antibiotic Sensitivity Patterns of Organisms
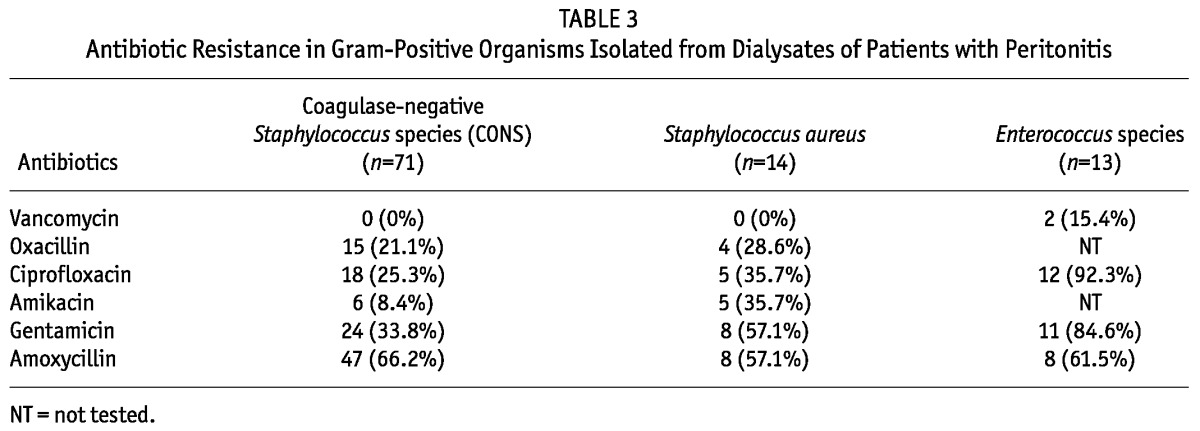
The antibiotic sensitivity patterns of the commonly isolated organisms are shown in Tables 2 and 3. It was observed that 54.3% of the Enterobacteriaceae isolates were resistant to third-generation cephalosporins and all of them were ESBL-producers. However, no carbapenem-resistant strain was detected in the Enterobacteriaceae family. Resistance to third-generation cephalosporins was very high in non-fermenters (Table 2). Carbapenem resistance in Acinetobacter species and P. aeruginosa was 23.5% and 11.5% respectively and all of them were MBL-producers. Amikacin resistance in Acinetobacter species and P. aeruginosa was also very high, 47% and 34.5% respectively. Among the gram-positive organisms isolated from PD fluid, 15.4% were vancomycin-resistant enterococci (VRE) and 28.6% were methicillin-resistant S. aureus. Of the 71 episodes of peritonitis caused by CONS, 15 (21.1%) were by methicillin-resistant strains.
TABLE 2.
Antibiotic Resistance in Gram-Negative Organisms Isolated from Dialysates of Patients with Peritonitis
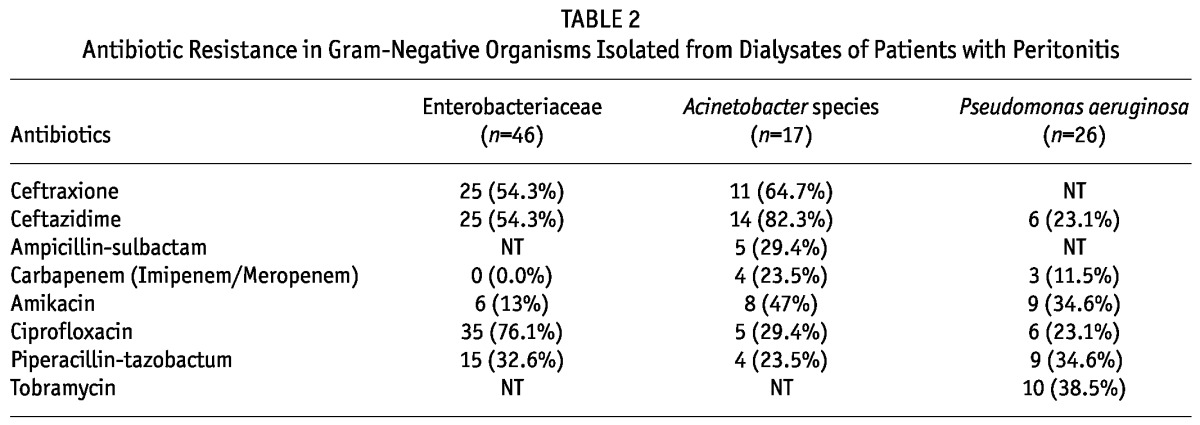
TABLE 3.
Antibiotic Resistance in Gram-Positive Organisms Isolated from Dialysates of Patients with Peritonitis
Outcome Analysis of Gram-Positive and Gram-Negative Peritonitis Episodes
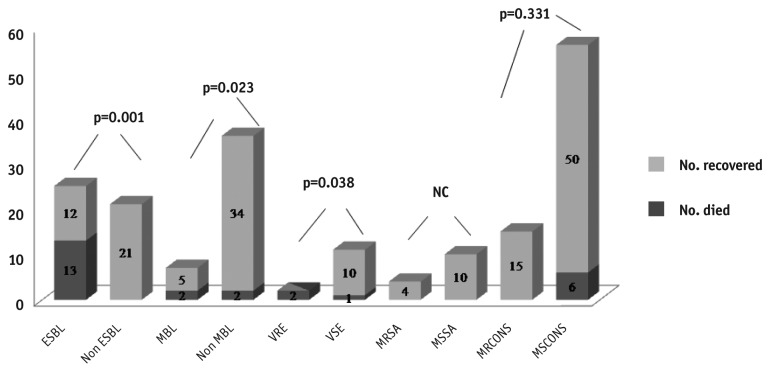
Catheter loss in gram-negative peritonitis was significantly higher than in gram-positive peritonitis (36/89 (40.4% vs 20/102 (19.6%); p < 0.001). The hospitalization rate required for management of peritonitis was also significantly higher for gram-negative than for gram-positive peritonitis (56/89 (62.9%) vs 42/102 (41.2%); p = 0.004). The trend of death within four weeks of peritonitis was also higher for gram-negative episodes (10.8%), although the difference was not statistically significant (Table 4). Overall, death was higher in patients with peritonitis due to E. coli, and catheter loss had significant association with CONS infection. Catheter loss and death due to peritonitis caused by different organisms are shown in Table 5. Mortality was significantly higher in patients having peritonitis due to VRE, and ESBL and MBL producers (Figure 1).
TABLE 4.
Comparison of Outcomes in Gram-Positive (n=102) and Gram-Negative (n=89) Peritonitis Episodes
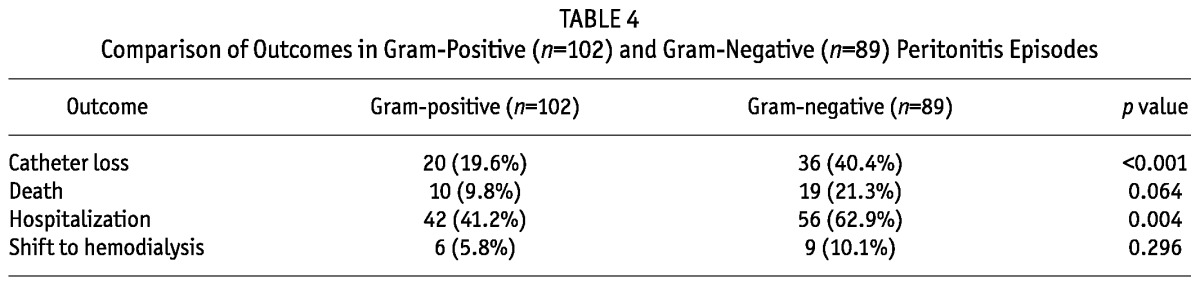
TABLE 5.
Catheter Loss and Peritonitis-Associated Death According to Causative Organism
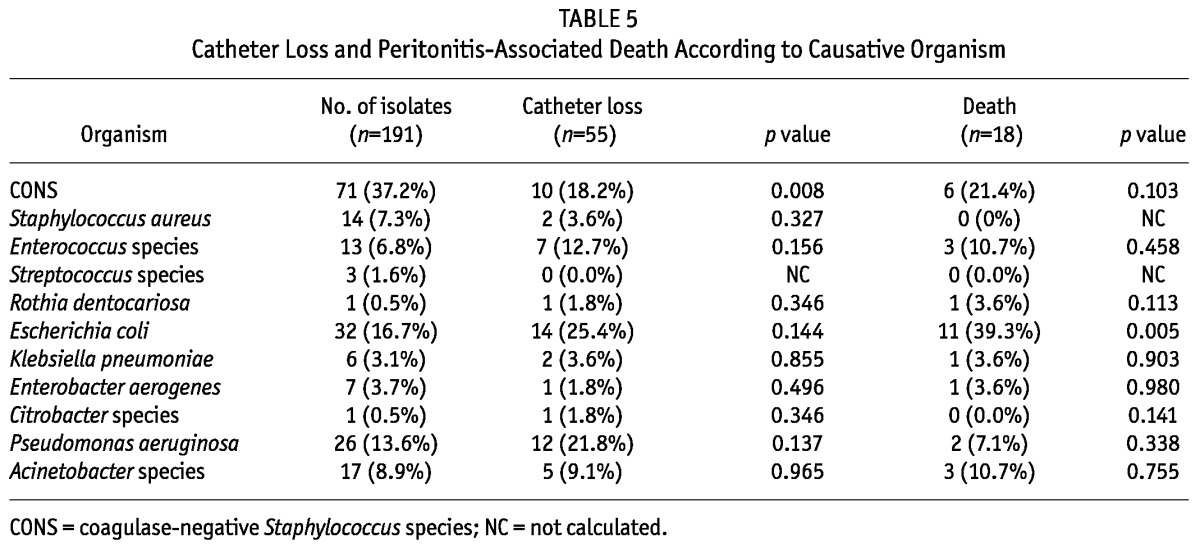
Figure 1 —
Death related to ESBL, MBL, VRE, MRSA, MSSA, MRCONS and MSCONS. NC = not calculated; ESBL = extended spectrum β-lactamase; MBL = metallo-β-lactamase; VRE = vancomycin-resistant enterococci; VSE = vancomycin-sensitive enterococci; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive Staphylococcus aureus; MRCONS = methicillin-resistant coagulase-negative Staphylococcus species; MSCONS = methicillin-sensitive coagulase-negative Staphylococcus species.
Discussion
Peritonitis in PD patients has been decreasing over the past years due to advances in PD technique (14-16), but it still remains a major cause of technique failure; in addition, it can also lead to death (9,14,16-18). The peritonitis rate at our center during the study period was 0.41 episodes per patient-year, which is comparable with other leading centers that reported approximately 0.5 episodes per patient-year (19,20). Currently, the incidence of peritonitis has further decreased to 0.33 episodes per patient-year; in some centers, the incidence is as low as 0.2 episodes per patient-year (21). Previously, in the year 2003, our center reported 0.63 episodes per patient-year (4). Hence the trend of peritonitis at our center is decreasing, possibly due to better patient counseling and improvement in PD technique. In the present study, the culture-positive rate was 81.8% and culture-negative rate was 18.2%. Earlier in 2003, our center reported 36.9% of culture-negative episodes and this decrease in culture-negative rate is due to improvement of microbiological culture technique. The present study showed that the rates of gram-positive and gram-negative peritonitis were almost equal (33.7% vs 29.4%) in contrast to our earlier study where a preponderance of gram-negative infections was reported (4). This shift might be related to better bacterial isolation technique since all the dialysates were cultured in BACTEC 9120 after at least 50 mL dialysate was centrifuged, thus increasing the isolation rate to 81.9%. There was a tremendous increase in the isolation rate of CONS (23.4% of total peritonitis episodes but 69.6% of all gram-positive episodes), possibly because of a better isolation technique. This is also concordant with reports from the developed world, where CONS was identified as the most common pathogen (1,22-23). In our earlier study, the isolation rate of CONS was low (13% of total peritonitis episodes) with an overall culture-positive rate of 63% (4). The reason might be that around one-third of the samples in the earlier study were processed in private laboratories where dialysates were neither centrifuged nor cultured in appropriate media and the culture-positive rate in private laboratories was very low (around 20%). However, we found a lesser isolation rate for S. aureus (4.6%) compared to the typical pattern of 10-15% in the developed world (23). In other Indian studies, culture-positive rates varied between 30% and 72% (3,24-25) and gram-negative bacteria were responsible for 60-66% of the episodes, with E. coli being the most common pathogen (3,24). We also observed fungal peritonitis in 13.5% of the episodes. Fungal peritonitis usually constitutes 2-15% of the episodes and it is a serious complication of PD with mortality ranging between 25-53% (26). Immediate catheter removal is indicated in fungal peritonitis, which is strictly being followed at our center.
The emergence of antibiotic-resistant bacteria is being increasingly reported and it has become a major public health problem. Systematic data on antibiotic susceptibility of pathogens isolated exclusively from PD-related infections are limited. Zelenitsky et al., in 2000, reported a significant increase in antibiotic resistance (20). In his study, the most dramatic increase in antibiotic resistance was seen among S. epidermidis. From 1991 and 1992 to 1997 and 1998, resistance to ciprofloxacin increased from 5.4% to 47.8% and resistance to methicillin increased from 18.9% to 73.9%. In our study, 54.3% of gram-negative bacteria were resistant to third-generation cephalosporin (ESBL producer) and 23.5% of Acinetobacter species and 11.5% of P. aeruginosa were MBL producers and resistant to carbapenem. Two different studies from India showed that resistance to third-generation cephalosporins in Enterobacteriaceae varied from 67.4% to 72.8% (24,27). A study from Bangladesh also found that gram-negative organisms were predominantly associated with peritonitis and around 50% of them were resistant to third-generation cephalosporins (28). Another study from Brazil reported that non-fermenter gram-negative bacteria showed a decline in ceftazidime susceptibility with only 25% resolution rate (29). Reporting on temporal changes in the susceptibility profile of gram-negative bacilli, Kim et al., in 2004, reported no changes in antimicrobial susceptibility to aminoglycosides, quinolones and imipenem for P. aeruginosa and E. coli (30). On the other hand, in the largest series study of Enterobacteriaceae peritonitis, Szeto et al., in 2006, observed an increase in resistance to gentamycin and netilmycin among E. coli and Klebsiella species, while resistance to ciprofloxacin and ceftazidime remained constant over time (31). In the present study, 15.6% of E. coli isolates were resistant to amikacin and 37.5% to gentamycin. Resistance to aminoglycosides in the current study was high in non-fermenters (47% in Acinetobacter species and 34.6% in P. aeruginosa). Aminoglycosides and ceftazidime are frequently recommended for gram-negative coverage in PD peritonitis. However, emerging resistance to third-generation cephalosporins and aminoglycosides may restrict their use in developing countries. Vancomycin-resistant enterococci accounted for 15.4% and 28.6% were methicillin-resistant staphylococci. Emerging vancomycin resistance in enterococci is also a major concern since this antibiotic is recommended for the empirical treatment of suspected gram-positive infections. Although there is a specific guideline for the empirical therapy, the local epidemiology and sensitivity pattern of the bacterial isolates should ideally guide the therapy and there should be a center-specific antibiotic policy for PD peritonitis.
In gram-negative peritonitis episodes, as compared with gram-positive episodes, the proportion of catheter loss (40.4% vs 19.6%, p = 0.001) and hospitalization (62.9% vs 41.2%, p = 0.004) were significantly higher. Death within four weeks of peritonitis was also more frequent in gram-negative episodes (21.3%) than in gram-positive episodes (9.8%) but the difference was not statistically significant. The above observations were similar to our earlier study (4). However, mortality was significantly higher in patients having peritonitis due to ESBL- and MBL-producing bacteria, and VRE. In an earlier study, the ESBL group had a mortality rate of 27.3% (32). Higher mortality in VRE, ESBL and MBL groups emphasizes the need for early detection of such resistant strains so that appropriate therapy can be initiated to have better therapeutic outcomes. Catheter loss was significantly higher in CONS infection. This might be due to biofilm formation by CONS that resulted in resolution failure. We also observed significantly higher mortality in E. coli-induced peritonitis. Kim et al., (2004) reported that catheter loss was highest among Pseudomonas-associated peritonitis but mortality was highest among Klebsiella-associated peritonitis (30). Poor outcomes in gram-negative peritonitis have also been observed in other studies (7,33).
It is evident from various studies and registry reports that the organisms responsible for peritonitis in PD patients and their antimicrobial sensitivity vary significantly from center to center even within similar geographic and socio-economic conditions. Therefore, there is a need for constant surveillance of emerging drug resistance to develop an antibiotic policy exclusively for PD-related infections based on local susceptibility of the isolated pathogens. Since the gram-negative infections with multidrug resistant pathogens are associated with higher mortality, their early detection in order to initiate appropriate therapy can lead to better clinical outcomes.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
The authors would like to thank the Indian Council of Medical Research (ICMR) for providing financial support (grant reference no. 5/4/7-2/2010-/NCD-II) for the study.
References
- 1. Munib S. Continuous ambulatory peritoneal dialysis. Gomal J Med Sci 2006; 4:82–5 [Google Scholar]
- 2. Flournoy DJ, Perryman FA, Qadri SMH. Growth of bacterial clinical isolates in continuous ambulatory peritoneal dialysis fluid. Perit Dial Int 1983; 3:144–5 [Google Scholar]
- 3. Jha V. Current status of PD in India: Current status and challenges. Perit Dial Int 2008; 28:36–41 [PubMed] [Google Scholar]
- 4. Prasad N, Gupta A, Sharma RK, Prasad KN, Gulati S, Sharma AP. Outcome of gram-positive and gram-negative peritonitis in patients on continuous ambulatory peritoneal dialysis: A single center experience. Perit Dial Int 2003; 23:144–8 [PubMed] [Google Scholar]
- 5. Bloembergen WE, Port FK. Epidemiological perspective on infections in chronic dialysis patients. Adv Ren Replace Ther 1996; 3:201–7 [DOI] [PubMed] [Google Scholar]
- 6. Fried LF, Bernardini J, Johnston JR, Piraino B. Peritonitis influences mortality in peritoneal dialysis patients. J Am Soc Nephrol 1996; 7:2176–82 [DOI] [PubMed] [Google Scholar]
- 7. Bunke CM, Brier ME, Golper TA. Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 peritonitis study. Kidney Int 1997; 52:524 [DOI] [PubMed] [Google Scholar]
- 8. Perez Fontan M, Rodriguez-Carmona A, Garcia-Naveiro R, Rosales M, Villaver P, Valdes F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005; 25:274 [PubMed] [Google Scholar]
- 9. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. ISPD Ad Hoc Advisory Committee. Peritoneal dialysis related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 10. Bernardini J, Holley JL, Johnston JR, Perlmutter JA, Piraino B. An analysis of ten-year trends in infections in adults on continuous ambulatory peritoneal dialysis (CAPD). Clin Nephrol 1991; 36:29–34 [PubMed] [Google Scholar]
- 11. Prasad KN. Diagnosis of peritonitis and exit site infection in patients on continuous ambulatory peritoneal dialysis. In: Indian Peritoneal Dialysis Practice Guidelines, Gupta A, Jha V, eds. Chennai, India: Peritoneal Dialysis Society of India, 2007, 55–62 [Google Scholar]
- 12. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twentieth informational supplement. Document M100-S20. Wayne PA: CLSI; 2010. [Google Scholar]
- 13. Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. Imipenem-EDTA disk method for differentiation of metallo beta-lactamase producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol 2002; 26:54–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krishnan M, Thodis E, Ikonomopoulos D, Vidgen E, Chu M, Bargman JM, et al. Predictor of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int 2002; 22:573–81 [PubMed] [Google Scholar]
- 15. Barretti P, Bastos KA, Dominguez J, Caramori JC. Peritonitis in Latin America. Perit Dial Int 2007; 27:332–9 [PubMed] [Google Scholar]
- 16. Stinghen AE, Barretti P, Pecoits-Filho R. Factors contributing to the differences in peritonitis rates between centers and regions. Perit Dial Int 2007; 27(2):281–5 [PubMed] [Google Scholar]
- 17. Voinescu CG, Khanna R. Peritonitis in peritoneal dialysis. Int J Artif Organs 2002; 25(4):249–60 [DOI] [PubMed] [Google Scholar]
- 18. Kavanagh D, Prescott GJ, Robert A. Peritoneal dialysis associated peritonitis in Scotland (1999-2002). Nephrol Dial Trans 2004; 19(10):2584–91 [DOI] [PubMed] [Google Scholar]
- 19. Korbet SM, Vonesh EF, Firanek CA. Peritonitis in an urban peritoneal dialysis program: an analysis of infecting pathogens. Am J Kidney Dis 1995; 26:47–53 [DOI] [PubMed] [Google Scholar]
- 20. Zelenitsky S, Barris L, Alfa M, Ariano R, Fine A, Harding G. Analysis of microbiological trends in peritoneal dialysis related peritonitis from 1991 to 1998. Am J Kid Dis 2000; 36:1009–13 [DOI] [PubMed] [Google Scholar]
- 21. Oreopoulos DG, Ossareh S, Thodis E. Peritoneal dialysis past, present and future. Int J Kid Dis 2008; 2:171–82 [PubMed] [Google Scholar]
- 22. Von Graevenitz A, Amsterdam D. Microbiological aspects of peritonitis associated with continuous ambulatory peritoneal dialysis. Clin Microbiol Rev 1992; 5:36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Troidle L, Finkelstein F. Treatment and outcome of CPD-associated peritonitis. Ann Clin Microbiol Antimicrob 2006; 5:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keithi-Reddy SR, Gupta KL, Jha V, Sud K, Singh SK, Kohli HS. Spectrum and sensitivity pattern of gram negative organism causing CAPD peritonitis in India. Perit Dial Int 2007; 27:205–7 [PubMed] [Google Scholar]
- 25. Sharma RK, Kumar J, Gupta A, Gulati S. Peritoneal infection in acute intermittent peritoneal dialysis. Renal Failure 2003; 25:975–80 [DOI] [PubMed] [Google Scholar]
- 26. Prasad KN, Prasad N, Gupta Amit, Sharma RK, Verma AK, Ayyagari A. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: a single centre Indian experience. J Infect 2004; 48:96–101 [DOI] [PubMed] [Google Scholar]
- 27. Gupta S, Muralidharan S, Gokulnath, Srinivasa H. Epidemiology of culture isolates from peritoneal dialysis peritonitis patients in southern India using an automated blood culture system to culture peritoneal dialysate. Nephrology (Carlton) 2011; 16(1):63–7 [DOI] [PubMed] [Google Scholar]
- 28. Iqbal MM, Sattar H, Islam MN, Ahmed AH, Rahman MH, Rashid HU, et al. Spectrum of organisms causing peritonitis in peritoneal dialysis patients—experience from Bangladesh. Adv Perit Dial 2008; 24:40–3 [PubMed] [Google Scholar]
- 29. Barretti P, Pereira D, Brasil MA, Cunha ML, Caramori J, Montelli A. Evolution of gram-negative bacilli susceptibility in peritoneal dialysis related peritonitis in Brazil: a single center’s experience over nine years. Perit Dial Int 2009; 29:230–3 [PubMed] [Google Scholar]
- 30. Kim DK, Yoo TH, Ryu DR, Xu ZG, Kim HJ, Choi KH, et al. Changes in causative organisms and their antimicrobial susceptibilities in CAPD peritonitis: a single center’s experience over one decade. Perit Dial Int 2004; 24:424–32 [PubMed] [Google Scholar]
- 31. Szeto CC, Chow VC, Chow KM, Lai RW, Chung KY, Leung CB, et al. Enterobacteriaceae peritonitis complicating peritoneal dialysis: a review of 210 consecutive cases. Kidney Int 2006; 69:1245–52 [DOI] [PubMed] [Google Scholar]
- 32. Yip T, Tse KC, Lam MF, Tang S, Li FK, Choy BY, et al. Risk factors and outcomes of extended-spectrum beta-lactamase producing E. coli peritonitis in CAPD patients. Perit Dial Int 2006; 26:191–7 [PubMed] [Google Scholar]
- 33. Troidle L, Gorban-Brennan N, Kliger A, Finkelstein F. Differing outcomes of gram-positive and gram-negative peritonitis. Am J Kidney Dis 1998; 32:623–8 [DOI] [PubMed] [Google Scholar]