Coagulase-negative staphylococci (CNS) are a frequent cause of peritonitis in peritoneal dialysis (PD) patients and can result in relapsing peritonitis because of biofilm formation (1). In addition to standard antibiotics, thrombolytics and rifampin have been used as adjuvant therapy to penetrate the CNS biofilm (2). However, the International Society for Peritoneal Dialysis (ISPD) Advisory Committee on Peritonitis Management recommends removing the catheter in patients with relapsing and refractory peritonitis and focusing on preservation of the peritoneum rather than on saving the peritoneal catheter (3).
We carried out a quality improvement project to investigate the relationship between variabilities in our current practice for the management of CNS-related peritonitis and the frequency of suspected biofilm-associated relapse.
Methods
In our unit, peritonitis is tracked prospectively, and we reviewed the records of all patients with a first episode of CNS peritonitis at the Ottawa Hospital from January 2009 to August 2012. We excluded patients with coexisting tunnel infection, those whose cultures grew organisms other than CNS, and those who had CNS-associated peritonitis before 2009.
Based on standard ISPD definitions, outcomes recorded were complete recovery, refractory peritonitis, relapsing peritonitis, repeat peritonitis, and technique failure (3). Our primary outcome of interest was the combination of relapsing and early repeat peritonitis, which we defined as a second episode of CNS peritonitis within 3 months of discontinuing antibiotics for an earlier episode. The 3-month period was chosen because of the known epidemiology of CNS peritonitis and the high risk for a second episode with the same organism within that period (4).
The standard protocol for empiric treatment of peritonitis in our unit is cefazolin (1.5 - 2 g) and ceftazidime (1.5 - 2 g), with adjustment of antibiotic dosing based on culture and sensitivity results. For patients with methicillin-resistant CNS (or a history of penicillin allergy), vancomycin is given intraperitoneally at an initial dose of 15 - 20 mg/kg every 3 - 4 days, with the subsequent dose and frequency being adjusted to maintain a target serum level of 15 - 20 mg/L. Trough serum vancomycin is checked once weekly during therapy. Incident CNS peritonitis is treated for 2 weeks per the ISPD recommendations. Relapsed and early repeat CNS peritonitis are variably treated with catheter removal or a second course of antibiotics, often accompanied by either or both of rifampin and a single instillation of tissue plasminogen activator (tPA) into the catheter.
From the prospective database, we abstracted clinical management data, including duration and dose of antibiotics used, mean serum trough levels in patients treated with vancomycin, and use of tPA among patients with relapsed or repeat peritonitis. The duration of therapy was calculated from the first to the last antibiotic dose, whether cefazolin or vancomycin was used.
Results
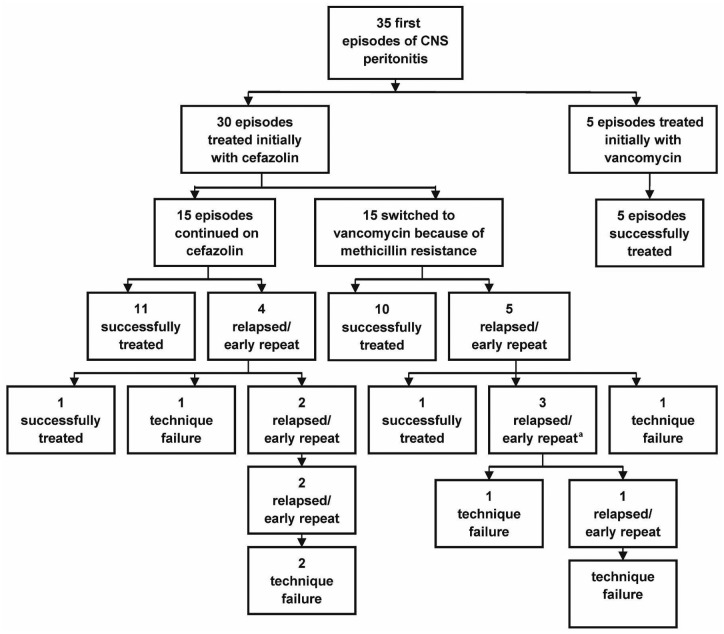
Between January 2009 and August 2012, the overall peritonitis rate at our center was 1 episode in 33 patient-months, with CNS peritonitis accounting for 34% of identified cases. Records showed that 58 episodes of CNS peritonitis occurred in 35 PD patients. Of the 35 first CNS peritonitis episodes, 15 involved methicillin-resistant strains (43%). As shown in Figure 1, 5 patients (14%) were allergic to penicillin or cephalosporin and were initially treated with vancomycin, with no relapses. Of the 30 patients initially treated with cefazolin, 15 (50%) were subsequently switched to vancomycin because of methicillin-resistance. All 35 patients responded to initial treatment, but 7 (20%) relapsed and 2 (6%) experienced an early repeat, for a combined total of 9 patients (26%). Among the 9 patients experiencing relapse or early repeat after their first episode of CNS peritonitis, technique survival was only 33%. In 5 patients who were free of relapse or early repeat, 6 late repeat episodes subsequently occurred, none of which was associated with technique failure or subsequent relapses (not shown in Figure 1).
Figure 1 —
Flow diagram of the peritonitis episodes (excludes 6 late repeat infections). a One of these patients continues on chronic suppressive antibiotics. CNS = coagulase-negative Staphylococcus.
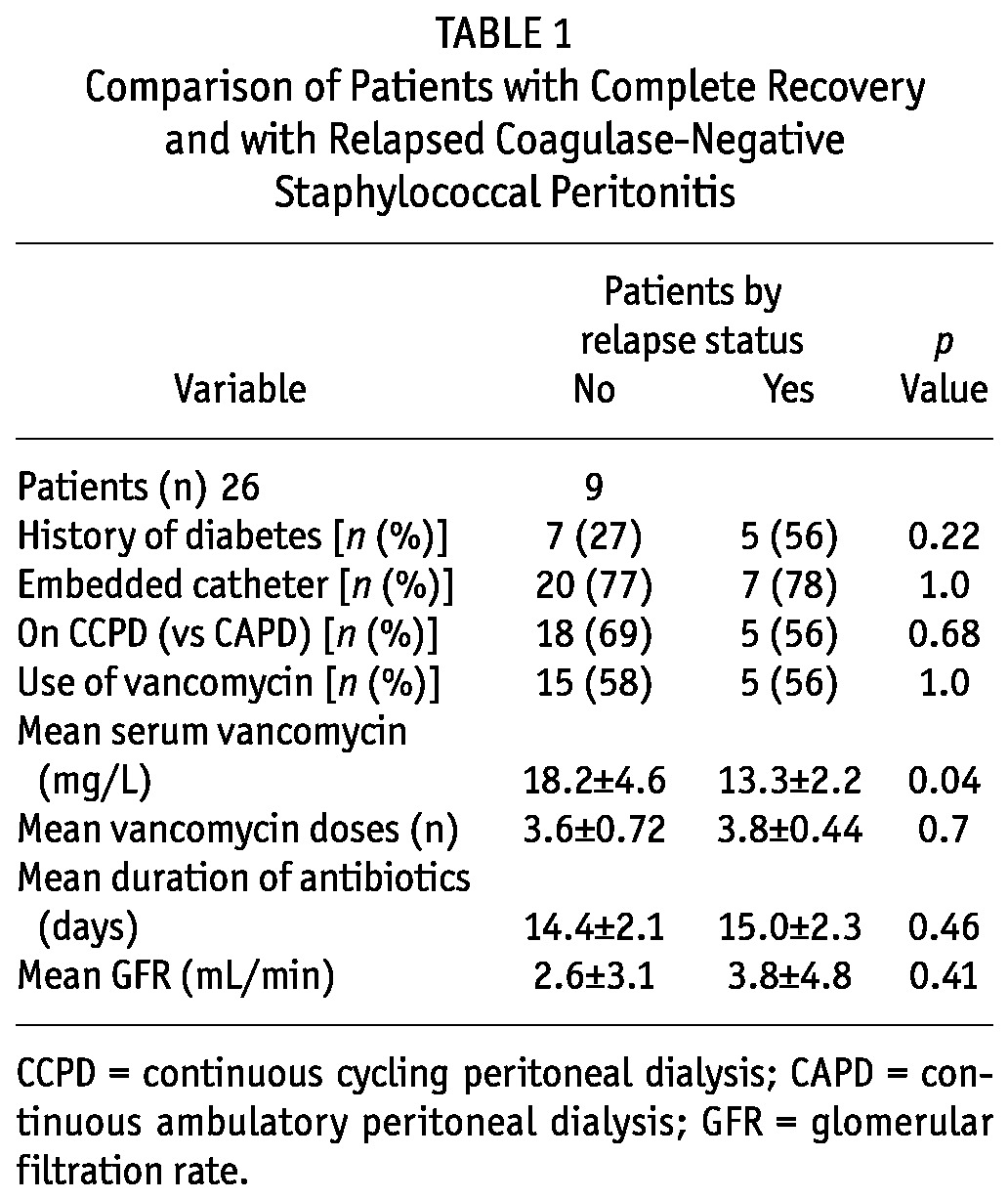
When patients with relapse or early repeat were compared with patients not experiencing those events, we observed no differences in the background rate of diabetes, the method of catheter insertion (embedded vs standard), the duration of antibiotics, use of vancomycin, or baseline glomerular filtration rate (Table 1). As the table shows, the mean trough serum vancomycin was higher in the group without relapse (18.2 ± 4.6 mg/L vs 13.3 ± 2.2 mg/L, p = 0.04). No relapses occurred in patients with a mean serum trough vancomycin of 16 mg/L or higher. The lowest mean serum vancomycin level in the relapse group was 13.04 ± 2.3 mg/L; it was 16.2 ± 3.7 mg/L in the non-relapse group (p = 0.088).
TABLE 1.
Comparison of Patients with Complete Recovery and with Relapsed Coagulase-Negative Staphylococcal Peritonitis
Exploratory analysis of the 20 patients treated with vancomycin for their first peritonitis episode revealed that 15 patients were switched to vancomycin because of methicillin-resistant strains, and 5 patients in that group (33%) relapsed. By contrast, none of the 5 patients receiving initial treatment with vancomycin relapsed (p = 0.136). Compared with patients who were converted to vancomycin because of methicillin resistance, those who were treated with vancomycin from the start tended to receive a higher number of vancomycin doses (4.2 ± 0.8 vs 3.5 ± 0.5, p = 0.046). Considering only the 15 patients with methicillin resistance, those who relapsed had a lower serum vancomycin level (13.3 ± 2.2 mg/L vs 18.8 ± 5 mg/L, p = 0.036), resembling the level in the entire group.
For the second episodes of peritonitis in the relapse and early repeat group, 8 of 9 patients were treated with vancomycin. Mean duration of therapy was 27 days, and the average serum level was 19.7 ± 4.4 mg/L. Oral rifampicin therapy (300 - 600 mg daily for 2 weeks) was used in 3 patients, and 2 of the 3 received concomitant tPA instillation into the PD catheter. Of the 9 patients with relapse or early repeat, 2 demonstrated evidence of complete recovery during an average follow-up of 24 months. The treating nephrologist deemed another 2 patients to have technique failure, and their PD catheters were removed. The remaining 5 patients—including the 2 who received adjuvant tPA and rifampin—subsequently developed a third episode of relapsing or early repeat peritonitis. Of those 5, 4 ultimately experienced technique failure; the 5th was treated with long-term suppressive antibiotics because he refused transfer to hemodialysis.
Discussion
Our study suggests that, despite a low background rate of peritonitis, significant technique failure is associated with CNS peritonitis. Patients who remained peritonitis-free for 3 months after completion of therapy for a CNS peritonitis tended to do very well, even if they experienced a late repeat episode of CNS peritonitis. Those who had a relapse or early repeat episode of CNS peritonitis also had a very high rate of technique failure (67%), even with a prolonged course of vancomycin in which high serum levels were attained and, in many cases, with the use of tPA and rifampin as adjuvant therapy.
The strong association of lower serum vancomycin levels during treatment of incident episodes of CNS peritonitis with the subsequent risk of relapse or early repeat peritonitis is potentially of significance. The mean serum trough level among patients without relapse or early repeat was 18 mg/L, higher than the level that would be achieved by following the ISPD recommendation to dose vancomycin when the trough level falls below 15 mg/L (3).
Data from Szeto and colleagues demonstrate that the incidence of a second CNS peritonitis remains high up to 3 months after the first incident episode, and then stabilizes. They suggest that, rather than being re-infections, these early failures are likely biofilm-associated; if they were re-infections, the rate of the new infections should be more constant over time (4). For that reason, we grouped those infections for analysis. Also, in asymptomatic patients with persistently positive effluent cultures, treatment with rifampin and urokinase after the dialysate has cleared has been shown to result in a high rate of effluent sterilization (2). Our group does not routinely monitor effluent cultures after the effluent has cleared, but that approach might potentially provide an avenue for reducing our high rate of relapse.
Our major novel finding is that serum vancomycin levels higher than those recommended in guidelines for the treatment of incident CNS peritonitis are associated with a lower rate of relapse and early repeat episodes. High vancomycin levels during treatment for second or subsequent episodes were not protective, suggesting that if a CNS biofilm becomes established during an incident peritonitis episode, then subsequent therapy is unlikely to be successful. Patients with a higher glomerular filtration rate tended to have lower serum vancomycin levels, which might explain some of the interindividual variability in those levels. Relapses in patients treated with vancomycin seemed to be more frequent in those who were initially treated with cefazolin and subsequently treated with vancomycin after sensitivities were available and methicillin resistance was confirmed. Although the results were not statistically significant (p = 0.136) because of the small number of patients in that group, our observations do raise the possibility that a delay in treatment might also have played a role. In addition, that group received fewer doses of vancomycin. Nevertheless, all vancomycin-treated patients who relapsed had mean serum trough levels of 16 mg/L or less.
The ISPD recommendation for vancomycin dosing seems to be based on a study by Mulhern and colleagues (5), who examined the relationship between rates of relapse of gram-positive peritonitis and trough serum vancomycin in a population of 31 patients who were treated with once-weekly intravenous vancomycin for 4 weeks. They found no relapses among patients with trough serum vancomycin levels greater than 12 mg/L; however, 64% of patients with lower levels experienced relapse. Surprisingly, no studies have investigated correlations between risk for relapse in gram-positive peritonitis and serum trough levels of vancomycin after intraperitoneal administration for the more conventional 2- or 3-week course. Thus, although our retrospective study is limited by relatively small numbers, it provides the best evidence to date for an actual target trough vancomycin level in the treatment of CNS peritonitis.
We did not note any symptoms vancomycin toxicity in our population, despite many patients having relatively high trough levels. Some data suggest that newer preparations of vancomycin are less nephrotoxic, having a risk of approximately 5% when vancomycin is used alone and when the trough level reaches 40 - 65 mg/L, much higher than in our current series (6).
Conclusions
We found that the rate of relapsed and early repeat CNS peritonitis is similar for methicillin-sensitive CNS treated with cefazolin and methicillin-resistant CNS treated with vancomycin. We also found considerable interindividual variability in serum vancomycin levels, and we recommend that serum trough levels be maintained above 15 mg/L, with repeat dosing when the trough level falls below 20 mg/L for patients with an incident CNS peritonitis.
Disclosures
The authors have no financial conflicts of interest to report. There was no external financial support for this study.
References
- 1. Nessim SJ, Nisenbaum R, Bargman JM, Jassal SV. Microbiology of peritonitis in peritoneal dialysis patients with multiple episodes. Perit Dial Int 2012; 32:316–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Demoulin N, Goffin E. Intraperitoneal urokinase and oral rifampicin for persisting asymptomatic dialysate infection following acute coagulase-negative Staphylococcus peritonitis. Perit Dial Int 2009; 29:548–53 [PubMed] [Google Scholar]
- 3. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [Erratum in: Perit Dial Int 2011; 31:512] [DOI] [PubMed] [Google Scholar]
- 4. Szeto CC, Kwan BC, Chow KM, Lau MF, Law MC, Chung KY, et al. Coagulase negative staphylococcal peritonitis in peritoneal dialysis patients: review of 232 consecutive cases. Clin J Am Soc Nephrol 2008; 3:91–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulhern JG, Braden GL, O’Shea MH, Madden RL, Lipkowitz GS, Germain MJ. Trough serum vancomycin levels predict the relapse of gram-positive peritonitis in peritoneal dialysis patients. Am J Kidney Dis 1995; 25:611–15 [DOI] [PubMed] [Google Scholar]
- 6. Farber BF, Moellering RC., Jr Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 1983; 23:138–14 [DOI] [PMC free article] [PubMed] [Google Scholar]