Peritoneal dialysis (PD) is a form of renal replacement therapy for which peritonitis is a major cause of technique failure. Patients who require removal of the Tenckhoff catheter likely experience a worse outcome than those who can be treated conservatively. The former experience more severe infection (peritonitis) requiring surgery and its subsequent complications. Early identification of patients likely to require surgical treatment could therefore be of value. The present study examined the risk factors that determine removal of the PD catheter in bacterial peritonitis.
Methods
Study patients (on PD for end-stage renal disease) came from a tertiary care center in South India. At our institute, a program of PD was initiated in 1998. Data from patients on PD who experienced bacterial peritonitis from January 2005 to June 2011 were prospectively collected using a pre-defined form. Demographic, clinical, and laboratory values were collected. The definitions of peritonitis, refractory peritonitis, recurrent peritonitis, and relapsing peritonitis followed the recommendations published in 2005 by the Ad Hoc Advisory Committee on Peritoneal Dialysis-Related Infections of the International Society for Peritoneal Dialysis (1). Patients with fungal, mycobacterial, polymicrobial, and culture-negative peritonitis were excluded from the study.
Empiric treatment of peritonitis (before the availability of peritoneal fluid culture results) used amikacin (2 mg/kg intraperitoneally daily) and vancomycin (15 mg/kg intraperitoneally every 4 days). After the sensitivity report was obtained, treatment was switched (if necessary) to a specific antibiotic. In refractory peritonitis, the Tenckhoff catheter was removed on day 5 or 6, depending on the availability of an operating theater.
Clinical and laboratory parameters were compared be tween the catheter-removal and conservative-treatment groups using the Fisher exact test or the t-test, depending on the variable type. Univariate and multivariate analyses were also performed. The InStat 3 software (GraphPad Software, La Jolla, CA, USA) was used for the statistical analyses.
Results
Between program start in 1998 and April 2011, 550 end-stage renal disease patients were initiated on continuous ambulatory PD (CAPD) at our institute. A follow-up period of more than 6 months was available in 327 patients (59.5%) and 3 - 6 months in 42 patients (7.6%).
The 256 CAPD patients treated between 2005 and June 2011 experienced 131 episodes of bacterial peritonitis. The cumulative follow-up for those 256 patients was 5399 months. Hence, the incidence of bacterial peritonitis was 1 episode in 41.2 months.
During the same period, 65 culture-negative peritonitis episodes occurred, accounting for 33.16% of all peritonitis episodes (n = 196). The catheter was removed in 29 patients (44.61%) with culture-negative peritonitis.
Of the 131 episodes of bacterial peritonitis, 67 episodes (51.1%) resulted in removal of the Tenckhoff catheter because of refractory peritonitis. Peritonitis was treated effectively in 64 patients (48.9%). The two groups—patients whose catheters were removed and patients whose peritonitis was successfully treated—did not differ in age, the proportions of men or of patients with diabetes, and mean duration of PD before the first episode of peritonitis.
Table 1 shows the characteristics of the patients at presentation with peritonitis. The causes of peritonitis were Pseudomonas aeruginosa (n = 32), Escherichia coli (n = 26), Acinetobacter baumannii (n = 14), Klebsiella pneumoniae (n = 15), Staphylococcus aureus (n = 23), coagulase-negative Staphylococcus (n = 11), and Enterococcus faecalis (n = 10). Infection with P. aeruginosa was a found to have a significant influence on catheter removal: the catheter was removed in 25 patients (37.31%) and retained in 7 (10.93%; p = 0.0025; relative risk: 1.842; 95% confidence interval: 1.373 to 2.470).
TABLE 1.
Characteristics of the Patients
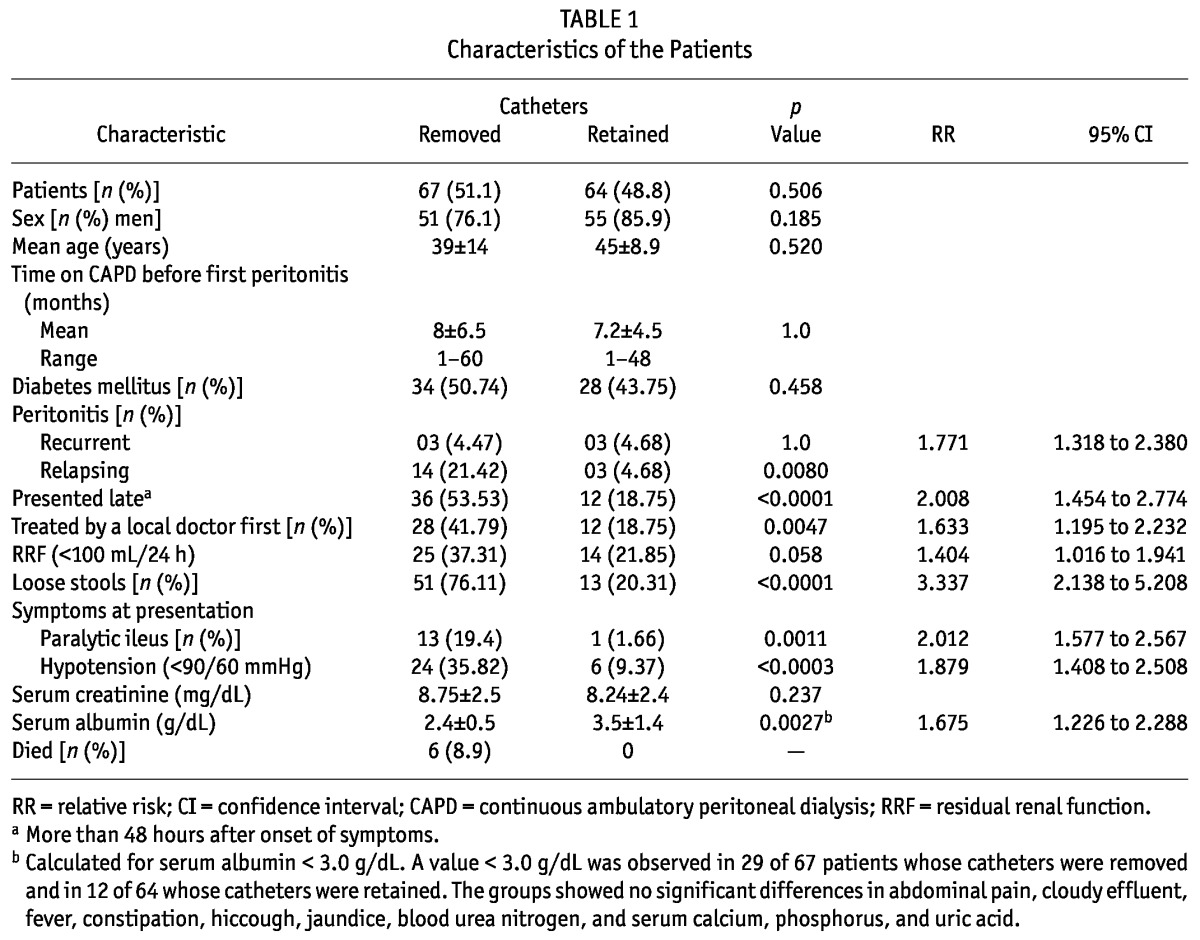
There were 17 episodes of relapsing peritonitis. The causative organisms were A. baumannii (n = 4), P. aeruginosa (n = 3), and E. faecalis (n = 1); the remaining episodes were culture-negative (n = 9).
The median leukocyte counts in effluent on days 1, 2, 3, 4, 5, and 6 in patients whose catheter was removed were 860/μL, 1970/μL, 2560/μL, 3480/μL, 3930/μL, 4360/μL respectively. Median leukocyte counts on the same days in patients whose catheter was retained were 940/μL, 1410/μL, 1480/μL, 1240/μL, 930/μL, and 310/μL respectively. We also found that, in the patients whose catheter was retained after effective treatment of peritonitis, reduction in the effluent leukocyte count appeared from day 4 of antibiotic therapy. The ratio of the median effluent leukocyte count from day 4 to day 3 was less than 1; in patients in whom the catheter was eventually removed, the same ratio remained higher than 1.
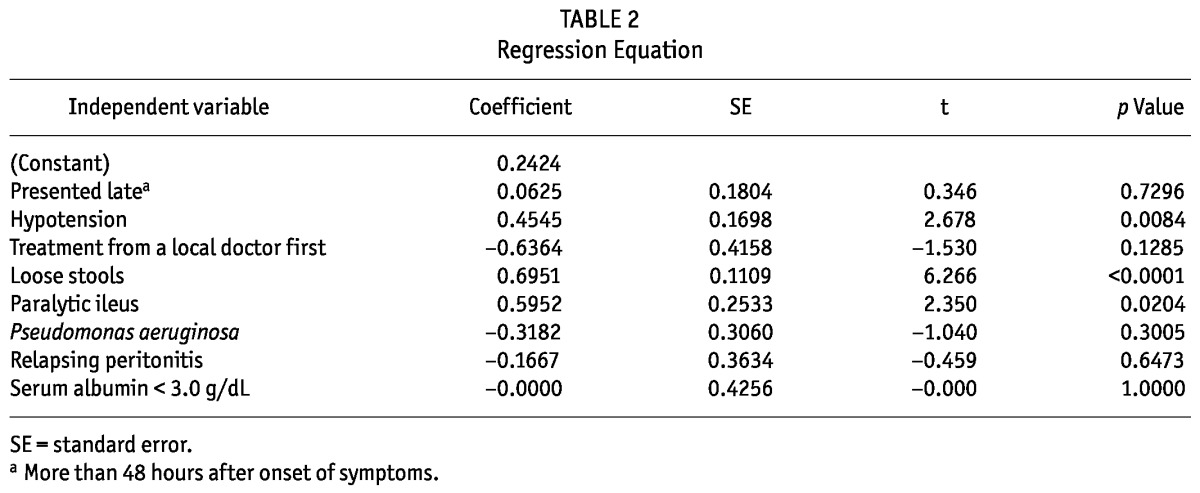
On univariate analysis, significant risk factors for removal of the catheter were relapsing peritonitis (p = 0.0080), presentation more than 48 hours after onset of peritonitis (p < 0.0001), treatment given by a local doctor (p = 0.0047), loose stools (p < 0.0001), paralytic ileus (p = 0.0011), hypotension (p < 0.0003), serum albumin less than 3.0 g/dL (p < 0.002), and peritonitis caused by P. aeruginosa (p = 0.0120). On multivariate analysis (Table 2), hypotension, loose stools, and paralytic ileus were identified as risk factors.
TABLE 2.
Regression Equation
Discussion
Removal of the Tenckhoff catheter in peritonitis should not be delayed beyond 5 days of peritonitis that is not responding to therapy (1). Removal protects the peritoneum for future reinitiation of PD. Resumption of PD after severe peritonitis was only 51% in one study (2). But in that study, catheters were removed on day 10 in patients who had not responded to antibiotics. For successful resumption of PD, early removal of the catheter is required, and for early catheter removal, the risk factors determining removal should be known.
The present study originated from an institute in a developing country in which conditions are conducive to CAPD peritonitis. Malnutrition, overcrowded families, high climatic temperatures, limited water supply, and a low level of literacy contribute to infections in developing countries. Our results should therefore not be generalized to other contexts.
Although 78.9% of CAPD patients were on regular follow-up, 48 patients (36.6%) presented to us 48 hours or more after onset of symptoms because our institute is located at one corner of the state. For the same reason, 40 patients (30.5%) received treatment from a local doctor. Both of the foregoing factors were significant risk factors in univariate analysis. In only 5 of the 40 patients (12.5%) seen by a local doctor were combinations of cefazolin, ceftazidime, or amikacin and vancomycin started by that local physician. Internet and mobile telephone aid in treating peritonitis has been used to some extent at some institutes in developing countries (3). Extensive use of this form of communication technology might lead to the disappearance of those two risk factors.
Fever was found in only in 13 of 131 patients (9.9%) in the present study, being mainly a symptom of tuberculous peritonitis (4). Loose stools, hypotension, and paralytic ileus were significant risk factors identified on multivariate analysis for catheter loss. The first two risk factors reflect systemic manifestations of abdominal infection. Hypotension was also identified as a risk factor for morbidity and mortality in peritonitis from other causes (5). Paralytic ileus might contribute to morbidity because of immobilization and its attendant pulmonary complications, lack of nutrition, and increased catabolism (6).
A low level of serum albumin predicted catheter removal (7,8). Although the precise reason for the association of hypoalbuminemia with technique failure is unclear, the suggestion has been made that serum albumin is an inverse acute-phase reactant (8), and the association was attributed to the underlying malnutrition (7).
It generally was believed that relapsing peritonitis episodes are more severe than recurrent ones (9); most experts would recommend catheter removal for relapsing peritonitis, but recurrent peritonitis is frequently treated without catheter removal (9). However, Szeto et al. (10) found that recurrent peritonitis episodes had the worst primary response rate, the greatest incidence of catheter removal, and the greatest mortality. The reason proposed for those phenomena was that the recent antibiotic therapy might have disturbed the gastrointestinal flora and provoked transmural migration of bowel organisms to the peritoneal cavity, resulting in recurrent peritonitis (11). The authors opined that the peritonitis episodes prone to relapse (that is, those caused by S. aureus and Pseudomonas species) are now more aggressively treated than they were 10 or 15 years ago, and that the clinical behavior of relapsing peritonitis episodes under contemporary treatment guidelines might be different from that observed in a decade or more ago (11). In the present study, culture-negative peritonitis was dominant in relapse, rendering treatment difficult, with resultant loss of the catheter.
Three studies (12-14) in the past examined the risk factors predicting removal of the catheter in refractory peritonitis. All three were retrospective analyses. In one study (14), although the catheter was said to have been removed for abdominal pain or persistent cloudy peritoneal fluid with more than 50 cells/μL for more than 3 days after the initiation of antibiotic therapy, the exact day of removal was not given. In the other two studies (12,13), the timing of catheter removal was not given. In the three studies, the catheter was removed in 68 patients (13), 64 patients (14), and 73 patients (12). The risk factors identified for catheter removal in the studies were an effluent cell count exceeding 100/μL for more than 5 days and a PD duration greater than 2.4 years (12); polymicrobial peritonitis, low serum albumin, duration of peritonitis more than 8.7 days, concomitant exit-site and tunnel infection, and catastrophic intra-abdominal events (13); and a patient age of more than 61 years, South Asian ethnicity, peritonitis duration greater than 7.5 days, and peritonitis caused by Pseudomonas, E. coli, and Enterobacter species (14). A drawback of these previous studies was that catheter removal occurred after 5 days of peritonitis. The main risk factors for catheter removal mentioned in the studies were those that appeared on day 4 or 5 of peritonitis. Such risk factors obviously could not help predict early removal of the Tenckhoff catheter and facilitate re-initiation of PD. Our study examined risk factors present on days 0 and 1 of peritonitis that might determine catheter outcome.
We found a trend in the effluent leukocyte count in patients who had a response to antibiotics and who eventually retained their catheter. The ratio of the median effluent leukocyte count from day 4 to day 3 was less than 1 in patients who responded to antibiotics. Recently, Chow et al. (15) demonstrated a significant association: an effluent leukocyte count of 1090/μL or greater on day 3 carried a risk for catheter loss and death that was increased by a factor of 9. In the present study, the median effluent cell count in our patients was greater than 1000/μL on day 3, even in patients who retained their catheter; however, on day 4, the median effluent leukocyte count was less than the highest leukocyte counts in the first 3 days.
Conclusions
The present study highlights risk factors present on day 0 and 1 of a bacterial peritonitis episode for removal of the peritoneal catheter. With this new knowledge, it might be possible to decide on catheter removal even before day 5 of peritonitis.
Disclosures
No author has a financial conflict of interest.
References
- 1. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [Erratum in: Perit Dial Int 2011; 31:512] [PubMed] [Google Scholar]
- 2. Szeto CC, Chow KM, Wong TYH, Leung CB, Wang AYM, Lui SF, et al. Feasibility of resuming peritoneal dialysis after severe peritonitis and Tenckhoff catheter removal. J Am Soc Nephrol 2002; 13:1040–5 [DOI] [PubMed] [Google Scholar]
- 3. Nayak KS, Prabhu MV, Sinoj KA, Subhramanyam SV, Sridhar G. Peritoneal dialysis in developing countries. Contrib Nephrol 2009; 163:270–7 [DOI] [PubMed] [Google Scholar]
- 4. Akpolat T. Tuberculous peritonitis. Perit Dial Int 2009; 29(Suppl 2):S166–9 [PubMed] [Google Scholar]
- 5. Tan KK, Bang SL, Sim R. Surgery for small bowel perforation in an Asian population: predictors of morbidity and mortality. J Gastrointest Surg 2010; 14:493–9 [DOI] [PubMed] [Google Scholar]
- 6. Livingston EH, Passaro EP. Postoperative ileus. Dig Dis Sci 1990; 35:121–32 [DOI] [PubMed] [Google Scholar]
- 7. Blake PG, Flowerdew G, Blake RM, Oreopoulos DG. Serum albumin in patients on continuous ambulatory peritoneal dialysis—predictors and correlations with outcomes. J Am Soc Nephrol 1993; 3:1501–7 [DOI] [PubMed] [Google Scholar]
- 8. Gulati S, Stephens D, Balfe JA, Secker D, Harvey E, Balfe JW. Children with hypoalbuminemia on continuous peritoneal dialysis are at risk for technique failure. Kidney Int 2001; 59:2361–7 [DOI] [PubMed] [Google Scholar]
- 9. Bonadio TL, Diaz-Buxo JA. Definition of recurrent peritonitis. Perit Dial Int 2007; 27:716–18 [PubMed] [Google Scholar]
- 10. Szeto CC, Kwan BCH, Chow KM, Law MC, Pang WF, Chung KY, et al. Recurrent and relapsing peritonitis: causative organisms and response to treatment. Am J Kidney Dis 2009; 54:702–10 [DOI] [PubMed] [Google Scholar]
- 11. Berg RD, Wommack E, Deitch EA. Immunosuppression and intestinal bacterial overgrowth synergistically promote bacterial translocation. Arch Surg 1988; 123:1359–64 [DOI] [PubMed] [Google Scholar]
- 12. Krishnan M, Thodis E, Ikonomopoulos D, Vidgen E, Chu M, Bargman JM, et al. Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int 2002; 22:573–81 [PubMed] [Google Scholar]
- 13. Yang CY, Chen TW, Lin YP, Lin CC, Ng YY, Yang WC, et al. Determinants of catheter loss following continuous ambulatory peritoneal dialysis peritonitis. Perit Dial Int 2008; 28:361–70 [PubMed] [Google Scholar]
- 14. Choi P, Nemati E, Banerjee A, Preston E, Levy J, Brown E. Peritoneal dialysis catheter removal for acute peritonitis: a retrospective analysis of factors associated with catheter removal and prolonged postoperative hospitalization. Am J Kidney Dis 2004; 43:103–11 [DOI] [PubMed] [Google Scholar]
- 15. Chow KM, Szeto CC, Cheung KK, Leung CB, Wong SS, Law MC, et al. Predictive value of dialysate cell counts in peritonitis complicating peritoneal dialysis. Clin J Am Soc Nephrol 2006; 1:768–73 [DOI] [PubMed] [Google Scholar]


