Abstract
The Somatic Embryogenesis Receptor Kinase 3 (SERK3)/Brassinosteroid (BR) Insensitive 1-Associated Kinase 1 (BAK1) is required for pattern-triggered immunity (PTI) in Arabidopsis thaliana and Nicotiana benthamiana. Tomato (Solanum lycopersicum) has three SlSERK members. Two of them exhibit particularly high levels of sequence similarity to AtSERK3 and, therefore, were named SlSERK3A and SlSERK3B. To characterize a role for SlSERK3A and SlSERK3B in defense, we suppressed each gene individually or co-silenced both using virus-induced gene silencing (VIGS) in the tomato cv. Moneymaker. Co-silencing SlSERK3A and SlSERK3B resulted in spontaneous necrotic lesions and reduced sensitivity to exogenous BR treatment. Silencing either SlSERK3A or SlSERK3B resulted in enhanced susceptibility to root knot-nematode and to non-pathogenic Pseudomonas syringae pv. tomato (Pst) DC3000 hrcC indicating that both SlSERK3s are positive regulators of defense. Interestingly, silencing SlSERK3B, but not SlSERK3A, resulted in enhanced susceptibility to the pathogenic strain Pst DC3000 indicating distinct roles for these two SlSERK3 paralogs. SlSERK3A and SlSERK3B are active kinases, localized to the plasma membrane, and interact in vivo with the Flagellin Sensing 2 receptor in a flg22-dependent manner. Complementation of the Atserk3/bak1-4 mutant with either SlSERK3A or SlSERK3B partially rescued the mutant phenotype. Thus, SlSERK3A and SlSERK3B are likely to constitute tomato orthologs of BAK1.
Introduction
Innate immunity is the genetically determined and inheritable ability of any given host organisms to discriminate between self or non-self and activate defense responses against attempted microbial or pest/parasite infection. Plants utilize a multilayered immune system to protect themselves from invading pathogens or pests. One of the first layers of plant active defense is the ability of the host to sense microbes by perceiving microbe-associated molecular patterns (MAMPs). This type of recognition is mediated by pattern recognition receptors (PRRs) present at the cell surface and triggers a resistance response known pattern-triggered immunity (PTI) [1]–[3]. MAMP perception elicits a variety of defense responses including phosphorylation and dephosphorylation of proteins, production of reactive oxygen species (ROS), callose deposition and defense gene expression [4], [5]. Microbial pathogens evolved effectors to suppress PTI. In return, plant evolved resistance (R) proteins that recognize effectors direct or indirect and activate effector-triggered immunity (ETI) [1]. Frequently, ETI responses are dependent on the defense hormone salicylic acid (SA).
Root-knot nematodes (RKN; Meloidogyne spp.) are sedentary endoparasites of great agricultural importance. RKN are obligate biotrophs, penetrate the host roots behind the root cap and move towards the vascular cylinder where they initiate feeding on the cytoplasm of live cells and develop an elaborate feeding site known as giant cells. Cells around the feeding site undergo hyperplasia and hypertrophy resulting in the formation of galls, root symptoms associated with this group of nematodes [6]. Nematode salivary secretions have been implicated in development and maintenance of the feeding site [7]. Once feeding is initiated, RKN become sedentary and mature females lay eggs in gelatinous sacs protruding on the root surface. Although no information exists about how nematodes induce PTI, host defense responses against RKN are similar to biotrophic microbial pathogens and resistance to this pest is mediated by classical R gene responses frequently associated with cell death [8], [9].
Receptor like kinases (RLKs) are among the well characterized PRRs. Common features of the RLKs are the presence of an N-terminal signal sequence, an extracellular domain that varies in structure, a single membrane-spanning region, and a cytoplasmic protein kinase catalytic domain. RLKs with leucine-rich repeat (LRR)-containing extracellular domains comprise the largest subfamily of transmembrane RLKs in plants with over 200 members in Arabidopsis thaliana (Arabidopsis) [10], [11].
The LRR-RLK FLS2 (FLAGELLIN SENSING 2 (FLS2), belonging to LRR-RLK subfamily XII, was first identified in Arabidopsis by its ability to perceive the bacterial flagellin including the minimal epitope flg22 [12]. Responsiveness to flg22 is shared by members of all major clades of higher plants indicating that the PRR for this bacterial epitope is evolutionarily ancient and critical for antibacterial immunity. Interestingly, Arabidopsis fls2 mutant plants, compromised in flg22 perception, are more susceptible to the bacterial pathogen Pseudomonas syringae pv. tomato (Pst) DC3000 only when spray inoculated and not when syringe infiltrated [13]. In contrast, Fls2-silenced Nicotiana benthamiana plants were more susceptible to both virulent and nonpathogenic Pst strains when syringe infiltrated [14], [15]. Besides N. benthamiana, orthologs of FLS2 have been identified in several plant species including tomato (Solanum lycopersicum) [16].
In Arabidopsis, the SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) family consists of five LRR-RLKs belonging to subfamily II that share the presence of five LRRs in their extracellular domain [17]. These SERK family members play diverse roles in male sporogenesis, brassinosteroid (BR) response, PTI and cell death control [18]. The best-studied member of this family is AtSERK3. This kinase was independently identified as the BRASSINOSTEROID INSENSITIVE1 (BRI1)-ASSOCIATED KINASE1 (BAK1) in a genetic screen for suppressors of a weak bri1 phenotype [19] as well as a BRI1 interacting protein in a yeast two-hybrid screen [20]. In addition, BAK1 directly interacts with BRI1 in vivo and the BAK1-BRI1 hetero-oligomers initiate BR-induced downstream signaling [21]. bak1 null mutant plants display reduced sensitivity to BRs and reduced root growth inhibition by BR compared to wild type plants [19], [22]. Additional members of the family, AtSERK1, AtSERK2 and AtSERK4/BKK1 (BAK1-like 1), have also been implicated in BR signaling in a partially redundant role with BAK1 [18], [22], [23]. BAK1 also controls innate immunity independent from its function in BR signaling [24]–[28]. In combination with BKK1, BAK1 regulates a cell-death signaling pathway as bak1 bkk1 null double mutants display a dwarf phenotype, spontaneous cell death and seedling lethality [29]. In addition, both BAK1 and BKK1 contribute to basal disease resistance to the hemibiotrophic pathogen Pst and the biotrophic oomycete pathogen Hyaloperonospora arabidopsidis [28].
BAK1 forms flg22-induced complexes with FLS2, directly interacts with FLS2 and recognizes the C-terminus of the FLS2-bound flg22 [30]. bak1 null mutants exhibit reduced flg22-responses including production of ROS, activation of mitogen-activated protein kinases (MAPK) and induction of defense genes indicating a role for this kinase in FLS2-mediated PTI [24], [25], [28]. BAK1 also forms complexes with additional PRRs and is required for responses triggered by a number of MAMPs from bacteria, fungi and oomycetes as well as signals generated from abiotic stresses such as cold shock and damage-associated molecular patterns indicating its role as a master regulator of stress responses [31].
In Solanaceous plants, SERK3 homologs have been characterized from N. benthamiana, N. attenuata and tomato. In N. benthamiana, two AtSERK3/BAK1 homologs, NbSERK3A and NbSERK3B were identified [32] while a single homolog NaBAK1 has been reported from N. attenuata [33]. The entire tomato SERK family members have been identified [34], [35]. However, unlike Arabidopsis, tomato has only three SERKs (SlSERK) members. These were named based on their phylogenetic relationship to the Arabidopsis SERKs as SlSERK1, SlSERK3A and SlSERK3B [34]. Interestingly, SlSERK1 is required for potato aphid (Macrosiphum euphorbiae) resistance mediated by the presumed cytoplasmically localized nucleotide-binding (NB)-LRR R protein Mi-1, indicating a role for LRR-RLK in NB-LRR-mediated ETI [34]. Surprisingly, SlSERK1 is not required for Mi-1-mediated resistance to RKN suggesting distinct recognition processes or signaling responses for aphids and nematodes.
Here, we describe the functional characterization of the remaining two SlSERKs, SlSERK3A and SlSERK3B, and their role in PTI to a bacterial pathogen and RKN. Using virus-induced gene silencing targeting SlSERK3A and SlSERK3B individually or combined revealed overlapping and unique roles for these SlSERK3 paralogs in plant defense, cell death control and BR response. In addition, we show that both SlSERK3A and SlSERK3B co-immunoprecipitate with SlFLS2 and partially complement the bak1-4 null mutant.
Results
Molecular structure of SlSERK3A and SlSERK3B
The protein coding sequence (CDS) of SlSERK3A (1,848 bp) and SlSERK3B (1,854 bp) and their chromosome localization (chromosome 10 and 1, respectively) have been reported earlier [34]. The genomic sequences of both SlSERK3A and SlSERK3B were obtained from tomato cv. Moneymaker by amplifying overlapping regions based on cDNA sequences. Sequence analysis indicated that SlSERK3A genomic (KC261564) sequence is 10,874 bp in length while the SlSERK3B genomic (KC261565) sequences is 7,965 bp. As predicted, SlSERK3A and SlSERK3B contain 11 exons (Figure S1). The predicted proteins of SlSERK3A (616 amino acids, 68.28 kD) and SlSERK3B (618 amino acids, 68.27 kD) have domains characteristic of SERK proteins including a signal peptide (with a putative cleavage site between amino acids 24 and 25 for SlSERK3A or amino acids 29 and 30 for SlSERK3B), a LRR N-terminal domain followed by four successive LRR domains, a Pro-rich region including a SPP motif, a single membrane-spanning domain and 11 conserved subdomains of a putative Ser/Thr protein kinase, followed by a short C-terminal (CT) tail [35] (Figure S2). Similar to BAK1, BKK1 and AtSERK5, both SlSERK3A and SlSERK3B lack the LRR-CT domain present in AtSERK1 and AtSERK2. The levels of protein sequence identity of SlSERK3A with N. benthamiana SERK3s and BAK1 and BKK1 proteins are: NbSERK3A (96%), NbSERK3B (96%), AtSERK3/BAK1 (84%) and AtSERK4/BKK1 (78%); while those of SlSERK3B are: NbSERK3A (91%), NbSERK3B (89%), AtSERK3/BAK1 (85%) and AtSERK4/BKK1 (77%) (Figure S2).
SlSERK3A and SlSERK3B are localized at the plasma membrane (PM)
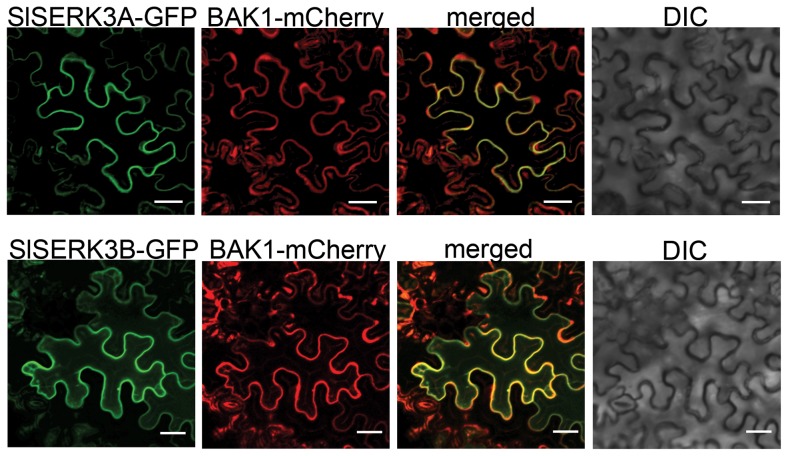
Analysis of SlSERK3A and SlSERK3B protein sequences and their hydrophobicity profiles predicted a single transmembrane (TM) helix between the receptor-like part and the kinase domain, suggesting that SlSERK3A and SlSERK3B are TM proteins that are likely anchored to the PM, analogous to other SERK proteins [19], [34]–[37]. The subcellular localization of SlSERK3A and SlSERK3B was determined in vivo using translational fusions to green fluorescent protein (GFP) expressed by the p35S-SlSERK3A-GFP and p35S-SlSERK3B-GFP constructs. Confocal microscopy of N. benthamiana leaves transiently expressing these constructs in combination with the p35S-BAK1-mCherry construct, revealed that SlSERK3A-GFP and SlSERK3B-GFP are localized at a similar location as BAK1-mCherry mainly at the PM (Figure 1).
Figure 1. SlSERK3A and SlSERK3B co-localize with BAK1 at the plasma membrane (PM).
Agrobacterium-mediated transient expression of SlSERK3A-GFP or SlSERK3A-GFP with BAK1-mCherry in Nicotiana benthamiana leaves. Localization of PM-associated BAK1-mCherry was compared with that of SlSERK3A and SlSERK3B (merged). Differential interference contrast (DIC) image. Leaf epidermal cells were imaged by confocal microscopy 72 h after infiltration with Agrobacterium. Bar = 20 µm.
SlSERK3A and SlSERK3B are active protein kinases
The presence of an Arg-Asp (RD) motif at the catalytic site in kinase subdomain VI and the conserved DFG motif in the kinase subdomain VII indicate that the LRR-containing SlSERK3A and SlSERK3B belong to the RD kinase LRR type-II subfamily of plant RLKs [38]. Comparison of the individual kinase subdomains of SlSERK3A and SlSERK3B with AtSERKs revealed that the critical catalytic loop, which comprises a short stretch of residues in the kinase subdomain VI, is conserved. Recently, it has been shown that the cytoplasmic domains (CD) of SlSERK3B to have kinase activity with the ability to autophosphorylate and transphosphorylate kinase inactive SlBRI1-CD [39]. To test whether SlSERK3A is also an active kinase, the CD of SlSERK3A (residues 263 to 615), including the juxtamembrane, kinase domain and C-terminal parts, was produced in a heterologous system as GST-fusion proteins (GST-SlSERK3A). As a control, the CD of SlSERK3B (residues 259 to 616) was also produced as a GST fusion protein (GST-SlSERK3B). We also developed the respective kinase-dead mutant variants SlSERK3A* CD (D418N) and SlSERK3B* CD (D420N), by introducing point mutations in the kinase catalytic loop based on a BAK1 kinase dead mutant [40], as GST- fusion proteins.
Purified proteins were subjected in vitro to an auto-phosphorylation assay as well as a trans-phosphorylation assay using the artificial substrate myelin basic protein (MBP). Analysis of the GST fusion proteins by SDS PAGE showed that both CD domains (66.32 and 67.64 kD) were soluble, and migrated as single bands at their predicted molecular masses (Figure 2, lower panel). A band corresponding to each of the auto-phosphorylated SlSERK3A and SlSERK3B proteins was observed when the kinase domains were used alone or in combination with MBP (Figure 2). In the presence of the wild type kinase domains, a phosphorylated MBP band was observed. As expected, both auto-phosphorylation and trans-phosphorylation of MPB were abolished by the kinase dead mutants of each SlSERK3* CD (Figure 2). Although kinase activity, both auto-phosphorylation and trans-phosphorylation, is stronger for SlSERK3A CD compared to SlSERK3B CD (Figure 2), this pattern of kinase activity was not consistently observed in replicated experiments. Taken together these results indicate that similar to SlSERK3B, SlSERK3A is also an active kinase catalyzing in vitro both auto- and trans-phosphorylation.
Figure 2. SlSERK3A and SlSERK3B are active protein kinases.
Auto-phosphorylation and trans-phosphorylation of MBP were tested in vitro using freshly expressed and purified GST-tagged fusion proteins corresponding to the cytoplasmic domain of both SlSERK3A and SlSERK3B and their respective kinase dead mutants, SlSERK3A* CD (D418N) and SlSERK3B* CD (D420N). Proteins were fractionated on 12% SDS-PAGE. Coomassie blue stained and dried gel, lower panel; radiolabeled bands were revealed by autoradiography, upper panel. This experiment was repeated twice.
SlSERK3A and SlSERK3B control cell death
To assess the functional roles of SlSERK3A and SlSERK3B, we developed gene-specific silencing constructs able to suppress SlSERK3A or SlSERK3B transcripts using virus-induced gene silencing (VIGS). A third construct was developed to co-silence both SlSERK3A and SlSERK3B (Figure S3). The target specificities of the VIGS constructs in tomato were confirmed using quantitative RT-PCR (Figure 3A and Figure S4A). Co-silencing both SlSERK3A and SlSERK3B in tomato cultivar Moneymaker resulted in plants exhibiting reduced growth (Figure 3B) and spontaneous cell death in leaves (Figure 3C). Silencing SlSERK3A also reduced plant growth albeit to a lesser degree than the co-silenced plants, while silencing SlSERK3B did not have any obvious effect on plant growth (Figure 3B). Silencing either SlSERK3A or SlSERK3B individually did not result in spontaneous cell death (Figure S4B).
Figure 3. SlSERK3A and SlSERK3B co-silenced plants are compromised in cell death control and BR sensitivity.
(A) Transcript levels of VIGS-silenced genes were evaluated using qRT-PCR. Tomato cv. Moneymaker plants treated with TRV empty vector (TRV), TRV-SlSERK3A, TRV-SlSERK3B or TRV-SlSERK3AB were evaluated. Expression was normalized against UBI3. Two independent samples were analyzed per construct. Values are average ± SE of three technical replicates. *P<0.05 significant difference from TRV (two-sample t-test). Experiment was repeated three times with similar results. (B) Phenotype of individually silenced SlSERK3A, SlSERK3B and co-silenced plants. (C) Cell death lesions in SlSERK3A and SlSERK3B co-silenced tomato leaflets. (D) Defense and senescence-related SlPRIa, SlPR2, SlPR5, and SlACS2 gene regulation in SlSERK3A or SlSERK3B silenced and co-silenced plants. Transcript levels were evaluated using qRT-PCR normalized against SlUBI3. Values are average ± SE (n = 3). * indicates significance difference from TRV at P<0.05 (two-sample t-test). Experiment was repeated twice with similar results. (E) Aniline blue-stained tomato leaf discs. Callose accumulation was detected near the edges of leaf patches showing cell-death in co-silenced SlSERK3A and SlSERK3B plants and TRV control. Leaves treated with 1 µM flg22 for 24 h were used as control. (F) Leaflets of tomato plants silenced for SlSERK3A, SlSERK3B or co-silenced and TRV control were treated with 10 µM BL for 12 h for SlCPD expression evaluation. Transcript levels were evaluated using qRT-PCR normalized against SlUBI3. Values are average ± SE (n = 3). *P<0.05 and **P<0.01 indicate significant difference from the respective –BL control (two-sample t-test). This experiment was repeated twice with similar results.
To investigate the molecular mechanism leading to the cell death phenotype in the co-silenced plants, we examined expression of the defense and senescence-related genes SlPR1b1, SlPR2, SlPR5, and SlACS2 ([41]; Table S1). Expression of all four genes was upregulated in SlSERK3A SlSERK3B co-silenced leaves (Figure 3D). This overall expression is similar to transcript patterns for the respective Arabidopsis orthologs reported for bak1-4 bkk1-1 double mutant [29]. Strikingly, expression of none of these four genes was upregulated in plants individually silenced for SlSERK3A or SlSERK3B (Figure 3D). Tomato leaflets individually silenced for SlSERK3A or SlSERK3B or co-silenced, were further evaluated for callose deposition a known cell death-associated defense response. Aniline blue staining of individually silenced leaflets did not reveal callose deposition (Figure S4C), while callose deposits were detected in co-silenced leaflets in areas near tissues exhibiting cell death (Figure 3E). Co-silenced leaflets were also evaluated for cell-death associated H2O2 accumulation. In similar regions near dead tissues, H2O2 accumulation was detected as brown spots using 3,3′-diamino benzidine (DAB) staining (Figure S5A). Taken together, these results indicate that SlSERK3A and SlSERK3B have a redundant function in suppressing cell death. Because of the spontaneous nature of the cell death phenotype, co-silenced plants were not included in defense related experiments.
SlSERK3A and SlSERK3B co-silenced plants were smaller in overall stature compared to TRV-empty vector (TRV) control plants (Figure 3B). The semi-dwarf stature suggested BR-deficiency or -response in these plants. In Arabidopsis, expression of the CPD gene, involved in BR biosynthesis, is downregulated by BR treatment and this downregulation is compromised in the bri1 mutant as well as in most double and triple mutants of bak1 with other Atserks but not in any single Atserk mutant [22], [42]. To assess whether SlSERK3 silenced plants were affected in BR response, SlCPD gene expression was evaluated in plants treated or untreated with BR. Basal SlCPD transcript levels were similar in TRV control plants and plants individually silenced for SlSERK3A or SlSERK3B (Figure 3F). However, a reduction in SlCPD transcript levels was observed in SlSERK3A and SlSERK3B co-silenced plants suggesting that SlSERK3A or SlSERK3B are required for the basal expression of SlCPD (Figure 3F). Similar to Arabidopsis, exogenous application of BR downregulated SlCPD transcript levels in tomato TRV control plants (Figure 3F). In addition, downregulation of SlCPD transcript levels in response to BR was not affected in tomato plants individually silenced for SlSERK3A or SlSERK3B (Figure 3F and Figure S5B). In contrast, downregulation of SlCPD transcript levels was greatly compromised in the SlSERK3A and SlSERK3B co-silenced plants treated with BR (Figure 3F and Figure S5B) suggesting a redundant function for these two paralogs in BR signaling.
SlSERK3A and SlSERK3B are required for disease resistance
To investigate a possible role for a single SlSERK3 gene in disease resistance, we evaluated SlSERK3A or SlSERK3B silenced plants for resistance to the tomato pathogen Pst DC3000 and its nonpathogenic hrcC mutant derivative Pst DC3000 hrcC. To develop a control, we targeted the tomato flagellin receptor SlFLS2 (Figure S3) [16] for silencing in tomato and vacuum infiltrated the silenced plants (Figure S6) with Pst DC3000 hrcC and Pst DC3000. Silencing SlFLS2 enhanced the growth of both Pst DC3000 hrcC and Pst DC3000 relative to TRV control plants (Figure 4A and 4B). Importantly, silencing either SlSERK3A or SlSERK3B (Figure S4A) also enhanced growth of Pst DC3000 hrcC indicating non-redundant roles for SlSERK3s in PTI against non-pathogenic Pst (Figure 4A). Interestingly, silencing SlSERK3B and not SlSERK3A resulted in enhanced Pst DC3000 growth suggesting an additional role for SlSERK3B in bacterial defense that may be distinct from its role in PTI against the non-pathogenic Pst strain (Figure 4B).
Figure 4. SlSERK3A and SlSERK3B are involved in PTI in tomato.
Five-week-old tomato plants cv. Moneymaker silenced for SlFLS2, SlSERK3A or SlSERK3B and TRV control were used. (A) and (B) Plants were vacuum infiltrated with Pst DC3000 hrcC or Pst DC3000 and bacterial counts were performed at 0 and 3 days post infiltration (dpi). Results are average (±) SE (n = 5). Letters above the graphs denote significance difference at P<0.01 (ANOVA Tukey HSD test). These experiments were repeated twice with similar results. (C) Plants were infected with 1,000 J2 each and evaluated 6 weeks later. No FLS2 silenced plants were used in this assay. Results are average (±) SE (n = 9). Letters above the graphs denote significance difference at P<0.05 (ANOVA Tukey HSD test). This experiment was repeated once with similar results. (D) Leaf samples were floated on water overnight. ROS burst was measured as relative light units (RLUs) emitted in a luminol-based assay within 15 min after 1 µM flg22 treatment. Values are average ± SE (n = 4). * indicates statistically significant difference from TRV at P<0.05 (two-sample t-test). This experiment was repeated once. (E) TRV-treated plants were vacuum infiltrated with Pst DC3000 hrcC and harvested 6 h later. Expression was evaluated using qRT-PCR normalized against SlUBI3. Values are average ± SE (n = 3). *P<0.05 and ** P<0.001 significant difference from TRV (two-sample t-test). This experiment was repeated twice with similar results.
Root-knot nematodes are serious tomato pests and no information exists on PTI for this group of pests. We wondered whether resistance to nematodes might also involve PTI and the likely requirement for the master PTI regulator SERK3. To address this, we infected tomato plants silenced for SlSERK3A or SlSERK3B with Meloidogyne incognita infective-stage juveniles and evaluated the roots for nematode infection and reproduction. Root weights of tomato plants silenced for either SlSERK3A or SlSERK3B (Figure S7A) were similar to TRV control plants (Figure S7B). Interestingly, plants silenced for either SlSERK3A or SlSERK3B exhibited enhanced susceptibility to RKN compared to TRV control indicating a likely role for PTI in RKN resistance (Figure 4C). As reported earlier [43], VIGS in tomato roots was patchy (Figure S7A) suggesting that the enhanced susceptibility values reported in this assay are likely an underestimate.
SlSERK3A and SlSERK3B are required for flg22-triggered immunity
To further characterize the role of SlSERK3s in bacterial defense, we evaluated ROS production in SlSERK3A-, SlSERK3B- or SlFLS2-silenced plants. Tomato silenced for SlSERK3A or SlSERK3B were severely reduced in flg22-triggered ROS production, similar to SlFLS2 silenced plants, consistent with their role as positive regulators of bacterial PTI (Figure 4D). To confirm attenuation of PTI in SlSERK3-silenced plants, expression of known PTI marker genes [15], [44] was investigated. Transcripts of both SlPTI5 and SlWRKY28 were upregulated within 6 h after Pst DC3000 hrcC treatment in TRV-treated leaves (Figure 4E). In contrast, silencing SlFLS2, SlSERK3A or SlSERK3B severely reduced this up-regulation of both genes (Figure 4E). The observed attenuation of ROS production and reduction in defense marker gene induction further confirmed the role of SlSERK3s in tomato PTI.
SlSERK3A and SlSERK3B form a flg22-induced complex with SlFLS2 in N. benthamiana
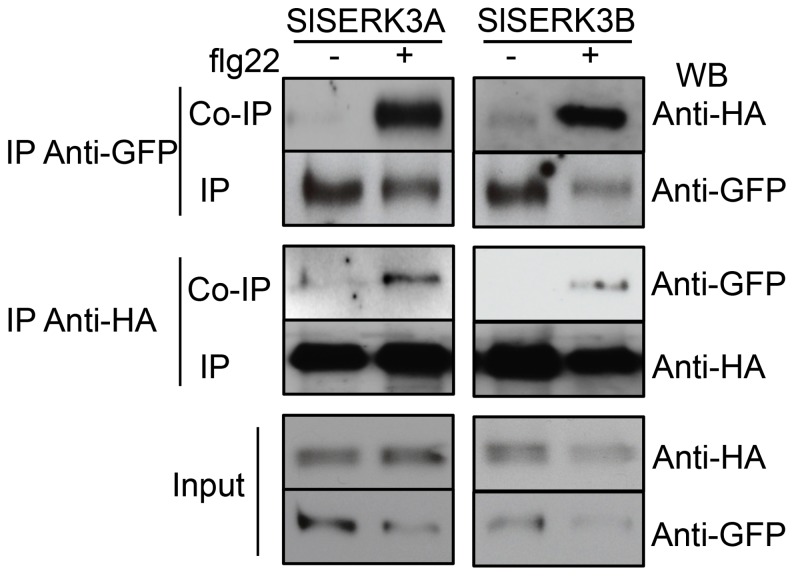
To test whether SlFLS2 heterodimerizes with SlSERK3A or SlSERK3B in vivo, we transiently co-expressed a SlFLS2-GFP fusion protein with either SlSERK3A-HA or SlSERK3B-HA fusion proteins in N. benthamiana for co-immunoprecipitation (Co-IP) experiments. Within 5 minutes after flg22-treatment, interactions between SlFLS2 and either SlSERK3A or SlSERK3B were detected by Co-IP with anti-GFP and immunoblotting with anti-HA, (Figure 5). Neither SlSERK3A or SlSERK3B were detected in the untreated anti-GFP immunoprecipitates (Figure 5). Reciprocal Co-IP using anti-HA to precipitate SlSERK3A or SlSERK3B and immunoblotting with anti-GFP, detected SlFLS2 only in flg22-treated samples (Figure 5). These results suggest flg22-induced complex formation between SlFLS2 and SlSERK3A or SlSERK3B consistent with the ligand dependency of the AtFLS2-BAK1 association [24], [25], [45], [46].
Figure 5. SlFLS2 co-immunoprecipitates with SlSERK3A and SlSERK3B.
Nicotiana benthamiana leaves transiently expressing SlSERK3A-HA or SlSERK3B-HA constructs and SlFLS2-GFP were elicited (+) or not (−) with 100 nM flg22 for 5 min. Total proteins (input) were subjected to reciprocal immunoprecipitation and immunoblotting. Immunoprecipitation with anti-GFP Protein A agarose beads (upper panel) or anti-HA (middle panel). This experiment was repeated once with similar results. WB: Western blot.
Heterologous expression of SlSERK3A and SlSERK3B
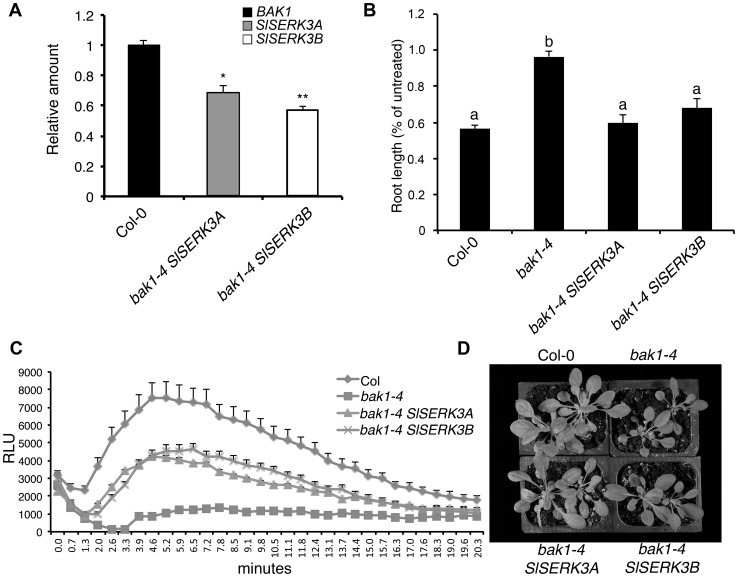
To determine whether SlSERK3A or SlSERK3B are the functional orthologs of BAK1, we performed complementation tests with the A. thaliana bak1-4 mutant. We introduced SlSERK3A or SlSERK3B expression constructs containing the Arabidopsis BAK1 promoter, into the bak1-4 null mutant background and developed stable transgenic plants. The bak1-4 mutant has reduced sensitivity to exogenous BR treatments and displays semi-dwarf phenotype when grown under short-day conditions [24]. Root growth assays showed that transgenic bak1-4 mutant plants expressing SlSERK3A or SlSERK3B (Figure 6A) exhibited restored wild-type sensitivity to exogenous BR treatment (Figure 6B and Figure S8). These complementation lines also exhibited an intermediate growth phenotype compared to wild type Col-0 and the bak1-4 mutant (Figure 6D). In addition, the complemented plants showed enhanced flg22-induced ROS production compared to the bak1-4 mutant albeit ROS levels were lower than in wild type Col-0 (Figure 6C).
Figure 6. SlSERK3A and SlSERK3B partially complemented the Arabidopsis bak1-4 null mutant.
(A) SlSERK3A and SlSERK3B transcript levels in transgenic bak1-4 plants expressing pBAK1-SlSERK3A (bak1-4 SlSERK3A) or pBAK1-SlSERK3B (bak1-4 SlSERK3B) were evaluated using qRT-PCR. Values are average ± SE (n = 3) normalized relative to AtActin and calibrated to expression of BAK1 in Col-0. *P<0.05 and ** P<0.001 significant difference from Col-0 (two-sample t-test). (B) Relative root growth of 9-day-old seedlings grown on medium with or without 1 nM BL. Root length is presented relative to untreated control for each genotype. Values are average ± SE (n = 50). Values were arcsine transformed for statistical analysis. Letters above the graphs denote significance difference at P<0.01 (ANOVA Tukey HSD test). This experiment was repeated twice with similar results. (C) Leaf discs were floated on water overnight. ROS burst was measured using a luminol-based assay within 25 min after 1 µM flg22 treatment. Values are average ± SE (n = 8). (D) A photo of representative short-day grown 4.5-week-old Arabidopsis plants of the indicated genotypes.
Discussion
In Solanaceae, SERK members have been identified in tomato, N. benthamiana and N. attenuata. However, it is not clear how many SERKs members Nicotiana species have. Only for tomato, all members of this family have been identified and unlike Arabidopsis, that has five members, tomato was found to have only three members [34]. In both tomato and N. benthamiana, two members have particularly high levels of sequence similarity to AtSERK3/BAK1 suggesting recent duplication events in the lineage of these solanaceous species. Although a role for NbSERK3 has been identified in microbial pathogen defense [25], [32], it is not clear which of the two NbSERK3 paralogs contribute to the resistance and whether the two members have redundant roles in defense. Similarly, SlSERK3 is required for the resistance to the vascular fungal pathogen Verticillum mediated by the receptor like protein (RLP) Ve1 and for defense responses induced by the fungal Ethylene-induced xylanase (Eix) mediated by RLP LeEix [47], [48]. In these tomato and N. benthamiana studies, VIGS was used to evaluate the defense related roles of SERK3 and because of the high level of sequence identity between the two SERK3 paralogs from each plant species, the VIGS constructs used are capable to silence both members. However, the specificity of silencing was not evaluated in these experiments, consequently, the specific function of the individual paralog remains unclear. In this work, we were able to specifically silence individual SlSERK3A and SlSERK3B and co-silenced them by designing VIGS constructs partially targeting the respective untranslated gene regions. This allowed us to dissect the contributions of each of these gene paralogs and identify common and distinct roles for them.
SlSERK3A silenced plants were smaller in size compared to TRV control or SlSERK3B silenced plants which could be due to pleiotropic effect on BR signaling. However, molecular data indicate that individually silenced SlSERK3A plants are not affected in BR signaling (Figure 3F). Although the reason for SlSERK3A silenced plant short stature is unclear, our data indicate that BR signaling is not affected at a detectable level in either SlSERK3A or SlSERK3B silenced plants.
Interestingly, vacuum infiltration of FLS2-silenced tomato plants with Pst resulted in significant increase in bacterial growth a similar phenotype seen in FLS2-silenced N. benthamiana plants [14], [15]. This is in contrast to Arabidopsis fls2 mutant on which, compared to wild-type plants, no bacterial growth difference was observed after syringe infiltration [13]. Lower bacterial growth was seen on the fls2 mutant only when bacteria were spray inoculated [13]. Our result with tomato, combined with that from N. benthamiana, indicates that flagellin perception by FLS2 in Solanaceae functions in the mesophyll cells while this perception in Arabidopsis is active in the guard cells. It remains to be determined whether FLS2 perception in Solanaceae functions also in the guard cells.
Our results showed that both SlSERK3 paralogs contributed to resistance against the non-pathogenic Pst DC3000 hrcC strain and to RKN, while only SlSERK3B promoted resistance against virulent Pst DC3000. This indicates that these two SERK3 members have evolved distinct immune related functions. The bacterial defense related role of SlSERK3B is similar to that of BAK1, as shown with the bak1-5 mutant, suggesting that this tomato SERK member is an authentic BAK1 ortholog [28]. However, no clear SlSERK3A Arabidopsis ortholog can be identified based only on its defense function in tomato. Although a role in bacterial defense has been demonstrated for AtSERK4/BKK1, the closest BAK1 paralog, this role is only detectable in the bak1-5 bkk1 double mutant infected by a weekly virulent coronatine defective Pst strain [28]. Thus, BKK1 appears only to play a minor role in bacterial defense, unlike SlSERK3A which strongly contributes to basal resistance against Pst DC3000 hrcC. Both BAK1 and BKK1 have non-redundant basal resistance functions against fungal and oomycete pathogens [28]. Although SERK3 paralogs have been implicated in fungal resistance in tomato it remains unclear which one of them contributes to this defense function because of the reasons stated above.
Recently the presence of PTI in roots was demonstrated using the well-known MAMPs chitin, flg22 and peptidoglycans and the immune responses to the latter two MAMPs were BAK1-dependent [49]. In addition, immunity function has been attributed to the bak1-4 Arabidopsis mutant to Verticillium indicating a role for BAK1 in basal defense to vascular pathogens [50]. Our results showing enhanced RKN susceptibility of SlSERK3A or SlSERK3B silenced plants indicate a role for SERK3 in resistance to RKN and the likely existence of PTI by nematode-associated molecular pattern(s). Silencing SlSERK3A resulted in reduced plant size and may be affected in BR signaling. However, it is unlikely that the enhanced RKN susceptibility is due to altered BR signaling as SlSERK3B silenced plants also exhibited enhanced RKN susceptibility but did not have altered plant size phenotype or are affected in BR signaling.
A number of nematode parasitism genes have been reported that play roles in virulence and suppression of host defenses [9], [51], [52]. However, no nematode-derived molecular patterns have been yet identified and it is difficult to speculate as to the nature of this pattern. Proteinaceous salivary secretions originating from esophageal gland cells have been implicated in nematode root invasion and migration as well as initiation and maintenance of their elaborate feeding sites [7]. Other sources of secretions from the nematode could be from sensory structures such as amphids or phasmids, or the excretory pore or the cuticle, none of which have been implicated in interactions with their hosts. Moreover, nematode penetration, feeding and secretion of cell wall degrading enzymes potentially produce damage-associated molecular patterns which could be the source of the nematode induced PTI. New research is needed to investigate nematode-induced PTI and to identify the nature of the nematode-associated molecular pattern(s) and its cognate PRR.
Our results showed that SlSERK3A and SlSERK3B have a redundant function in suppressing cell death. A similar function has been attributed to BAK1 and BKK1 [29], indicating that these SERK paralogs share similar cell death control functions in both Arabidopsis and tomato. However, co-silencing SERK3A and SERK3B in N. benthamiana does not result in cell death indicating this redundant cell death suppression function for two SERK members is not universal among plant species [32]. In Arabidopsis, it is speculated that these two SERK members suppress cell death through their interaction with the RLK, BIR1 (BAK1-Interacting Receptor Like Kinase 1) that possibly perceives an endogenous survival signal(s) [53]. As an alternative, it is discussed that SERK-associated PTI signaling complexes are guarded by R proteins [53], [54]. In the latter case, the absence of both BAK1 and BKK1 may constitutively activate R protein-mediated defense responses, including cell death. Such a scenario is supported by the fact that the cell death phenotype in the bak1 bkk1 double mutant is dependent on the defense hormone SA which is required for many R protein-dependent immune responses [29]. The constitutive activation of the SA-regulated gene, SlPR1b1 [55], we observed in SlSERK3A and SlSERK3B co-silenced plants suggests that the cell death phenotype in these tomato plants is also SA-regulated and could be triggered by an R protein. It remains to be seen whether an R protein guards SERK3-associated PTI signaling complexes in tomato.
SlSERK3A and SlSERK3B belong to the RD class of Ser/Thr kinases that share a conserved catalytic core. All AtSERK family members are active kinases and able to autophosphorylate in vitro [19], [56]. Similarly, both SlSERK3A and SlSERK3B are active kinases able to auto-phosphorylate and trans-phosphorylate MPB in vitro. Multiple Ser and Thr residues are auto-phosphorylated in SlSERK3B-CD only a subset correspond to auto-phosphorylated Arabidopsis BAK1-CD residues [39]. It remains to be seen whether these additional conserved residues are also auto-phosphorylated in SlSERK3A-CD.
As the single amino acid mutation that eliminated the kinase activity of BAK1 also eliminated the kinase activities of both SlSERK3A and SlSERK3B [40], our data showed that the catalytic kinase core between BAK1 and the two SlSERK3 paralogs are structurally and functionally conserved. Although the substrates of most SERK members are not well defined, it is well documented that BAK1 trans-phosphorylates a number of RLKs [19], [20], [40], [45], [57]. Recently it has been shown that SlSERK3B can trans-phosphorylate SlBRI [39]. Based on the high sequence similarity between the SlSERK3A and SlSERK3B catalytic domains, and their redundant role in BR signaling, we hypothesis that SlSERK3A can also trans-phosphorylate SlBRI. Since SlSERK3 is required for signaling and immunity mediated by the tomato RLP LeEix2 and Ve1, respectively, it is likely that SlSERK3A or SlSERK3B individually or together are capable of trans-phosphorylating multiple receptors in a manner similar to BAK1.
Both SlSERK3A and SlSERK3B formed flg22-dependent complex with SlFLS2. Several AtSERK members are able to heterodimerize with FLS2, albeit at variable levels of association, in ligand dependent manner [25], [28]. Further investigations should reveal whether SlSERK3A and SlSERK3B form a heterodimer in this interaction and whether SlFLS2 is also able to heterodimerize with SlSERK1.
The ability of SlSERK3A and SlSERK3B to form a ligand induced complex with SlFLS2 suggested a role for these two SERK paralogs in FLS2-dependent signaling. Indeed, both SlSERK3A and SlSERK3B have non-redundant functions in flg22-induced ROS production and activation of defense related genes. Among Arabidopsis SERK members, a similar function is only demonstrated for BAK1. Only bak1 single mutants are compromised in flg22-induced ROS and none of the remaining individual serk null mutants are impaired in ligand induced ROS production [24], [28]. A minor role in flg22-induced ROS production was uncovered for bkk1 in the bak1-5 bkk1 double mutant [28]. Taken together, this information indicates that SlSERK3A and SlSERK3B have BAK1-related functions and seem to be true orthologs of this Arabidopsis gene. Indeed, in complementation experiments either SlSERK3A or SlSERK3B partially rescued the bak1 mutant phenotype. The lack of full bak1 complementation is likely due to sequence divergence between these Arabidopsis and tomato orthologs.
Similar to Arabidopsis, the expression of SlCPD in tomato is down-regulated by exogenous application of BR via a presumed negative feedback mechanism [42]. The attenuation of SlCPD responsiveness to exogenous BR application in the SlSERK3A and SlSERK3B co-silenced plants, and not in the individual SlSERK3A or SlSERK3B silenced plants, indicates that SlSERK3A and SlSERK3B have redundant function in BR signaling. Since the SlSERK3A and SlSERK3B co-silenced plants had residual BR signaling competence, it suggests that the only other family member SlSERK1 also contributes to BR response. Based on CPD expression analysis in Arabidopsis, it is not clear the contribution of BKK1/SERK4 to BR signaling as BR effect on CPD expression have been analyzed in the double mutant bak1 serk1 or the triple mutant bak1 bkk1 serk1 and not in the double mutant bak1 bkk1 [22]. Nonetheless, our results show that SlSERK3A and SlSERK3B contribute to most of the BR effect on CPD expression in tomato.
In summary, our work provides functional characterization of SlSERK3A and SlSERK3B in an important crop and demonstrates differences and similarities in the role of BAK1 and SlSERK3A and SlSERK3B paralogs in immunity and BR signaling. This work also provides a foundation for future characterization of PTI against RKN in roots.
Materials and Methods
Plant material and growth conditions
One-week-old tomato (Solanum lycopersicum) cv. Moneymaker seedlings were transplanted into California mix II or sand. Plants were maintained in plant growth rooms at 24°C before VIGS treatment and then at 19°C until use in bioassays with a 16 h light and 8 h dark photoperiod. Nicotiana benthamiana plants were maintained in a plant growth room at 24°C at a similar photoperiod. Plants were fertilized biweekly with MiracleGro (Stern's MiracleGro). Arabidopsis thaliana (Arabidopsis) Col-0 and T-DNA insertion null mutant bak1-4 (SALK_116202) plants were grown in soil under fluorescent lights (10 h light and 14 h dark, 100 µEinstein/m2/s) at 22°C.
Virus-induced gene silencing (VIGS)
The TRV-SlSERK3A (contains 152 bp of the SlSERK3B gene (+1837 to +1988)), TRV-SlSERK3B [contains 174 bp of the SlSERK3B gene (+1844 to +2017)], TRV-SlSERK3AB [contains 178 bp of the SlSERK3B gene (+1289 to +1466)] and TRV-SlFLS2 [contains 111 bp of the SlFLS2 gene (+3460 to +1570)] were constructed by amplifying the desired fragments using gene-specific primers (Table S2) and tomato cv. Moneymaker cDNA, and recombining into Gateway compatible pDONR207 vector (Invitrogen) and finally into pTRV2. After sequence verification, constructs were transformed into A. tumefaciens strain GV3101.
VIGS was performed using the bipartite TRV (pTRV1 and pTRV2; [58]) vector in Agrobacterium tumefaciens and syringe infiltration (agroinfiltration) of 2-week-old tomato leaflets. Equal volumes (OD600 = 1) of A. tumefaciens pTRV1 and suspensions containing pTRV2-derived constructs, pTRV2 empty vector or TRV-PDS were mixed before infiltration [58].
Constructs
The coding sequences (CDS) of SlSERK3A and SlSERK3B were PCR amplified from tomato cDNA using the primers given in Table S2. The BAK1 promoter was amplified from Arabidopsis genomic DNA using primers listed in Table S1 and fused with the CDS of either SlSERK3A or SlSERK3B and cloned into pDONR207 (pBAK1-SlSERK3A and pBAK1-SlSERK3B). The CDS without stop of SlSERK3A, SlSERK3B and SlFLS2 were PCR amplified from tomato cDNA using primers listed in Table S1 and cloned into pDONR207. All resulting constructs were sequence verified.
pENTR207 (pBAK1-SlSERK3A) and pENTR207(pBAK1-SlSERK3B) were recombined into pEarleyGate303. pENTR207-SlFLS2, pENTR207-SlSERK3A and pENTR207-SlSERK3B were recombined into pEarleyGate103 generating C-terminal GFP-His-tag fusion constructs behind the 35S promoter. pENTR207-SlSERK3A and pENTR207-SlSERK3B were also recombined into pGWB14 generating C-terminal HA-tag fusion constructs behind the 35S promoter. All resulting constructs were sequence verified and transformed into A. tumefaciens strain GV3101.
The cytoplasmic domains (CD) of SlSERK3A and SlSERK3B were amplified from tomato cDNA using gene-specific primers (Table S2). Single point mutation variants of SlSERK3A CD (D418N) and SlSERK3B CD (D420N) were generated by PCR-based site-directed mutagenesis [65]. The amplified products were cloned into the pGEX4T-1 vector (Pharmacia) using EcoRI and NotI (NEB) to generate N-terminal GST fusion constructs. The resulting constructs were sequence verified.
Recombinant protein purification and in vitro phosphorylation assays
Recombinant fusion proteins were produced in Escherichia coli strain BL21 (DE3). Bacteria were induced with 0.5 mM isopropyl-b-D-1-thiogalactopyranoside (IPTG) at 30°C for 4 h and extracted with lysis buffer containing 1× PBS, 1 M DTT, 0.1M ATP and 1 tablet protease inhibitor cocktail (Roche) for 10 ml buffer. The soluble fraction was used to enrich for the fusion proteins. GST-tagged fusion proteins were enriched using glutathione-agarose beads (BD Biosciences) according to the manufactures protocol. The eluted fusion proteins were adjusted to the same concentration in 1× PBS and 10% glycerol and incubated in the kinase buffer immediately. The in vitro phosphorylation of each kinase (1 µg) with [γ−32P] ATP was assayed as described earlier [34].
RNA extraction and quantitative RT-PCR
RNA from leaves was extracted using TRIzol (Invitrogen) and treated with DNase I (New England Biolabs), while RNA from roots was extracted using hot phenol [59]. Five µg RNA was reverse-transcribed using SuperScript III reverse transcriptase (Invitrogen) and oligo-dT primer. For quantitative PCR, transcripts were amplified from 1 µl of a 5× diluted cDNA in a 15 µl reaction using gene-specific primers (Table S1) and iQ SYBR Green Supermix (Bio-Rad). The PCR amplification consisted of 3 min at 94°C, 40 cycles of 30 sec at 94°C, 30 sec at 58°C and 1 min at 72°C, 15 min at 72°C, followed by the generation of a dissociation curve. The generated threshold cycle (CT) was used to calculate transcript abundance relative to tomato Ubi gene as described previously [60]. DNase-treated RNA was used as template for control.
Bacterial virulence assay
To prepare bacterial inoculum, a lawn of Pseudomonas syringae pv. tomato (Pst) DC3000 or Pst DC3000 hrcC was grown overnight at 30°C on King's medium B plates with appropriate antibiotics. Cells were collected from plates with 10 mM MgCl2 and adjusted to the desired colony-forming units (CFU)/ml. Five-week-old tomato VIGS plants were vacuum infiltrated with bacterial suspension (Pst DC3000 104 CFU/ml and Pst DC3000 hrcC 5×104 CFU/ml). To assess bacterial titer, five 1 cm2 leaf discs were harvested and ground in 1 ml 10 mM MgCl2, diluted and plated [61].
Nematode virulence assay
Meloidogyne incognita was maintained on tomato cv. UC82B. Nematode eggs were extracted from infected roots in 0.5% NaOCl and eggs were hatched as described in Martinez de Illarduya et al. (2001) [62]. Three weeks after agroinfiltration, tomato roots were infected with freshly hatched 1000 infective-stage juveniles and maintained at 24°C. Six weeks later, roots were washed from soil particles, weighed and stained in 0.001% erioglaucine (Sigma). Individual roots were chopped into small pieces, mixed and egg masses were counted in two 10 g subsamples and the average calculated.
Oxidative burst assay
For tomato, one leaf sample (two 2 mm2 per sample) from four 5-week-old plants was dissected with a sharp blade. For Arabidopsis, one leaf disc (4 mm diameter) from eight 4-week-old plants was sampled. Samples were floated overnight in sterile water and water was replaced with a solution of 1.7 µg/ml luminol (Sigma) and 10 µg/ml horseradish peroxidase (Sigma) containing 1 µM flg22. Luminescence was captured using a multiplate reader (BMG LUMIstar Galaxy Luminometer or BertholdTech TriStar).
BL assays
For Arabidopsis root inhibition assays, sterilized seeds were vernalized at 4°C then sown on 1/2 MS medium supplemented with 1 nM epibrassinolide (BL) (Sigma) and 0.8% agar. Plates were incubated at 22°C, 16 h light and 8 h dark, 100 µEinstein/m2/s, for 9 days. Root length was measure for 50 seedlings per genotype and plotted as inhibition percentage compared with untreated roots [46].
For SlCPD expression analysis, tomato leaflets were syringe infiltrated with 10 µM BL 12 h before use.
Transient expression in N. benthamiana for microscopy and immunoprecipitation
Agrobacterium tumefaciens containing constructs pEARLEYGATE103-SlFLS2, pEARLEYGATE103-SlSERK3A, pEARLEYGATE103-SlSERK3B, pCAMBIA-AtBAK1-mCherry, pGWB14-SlSERK3A and pGWB14-SlSERK3B were grown overnight in LB medium supplemented with appropriate antibiotics. Cultures were resuspended in 10 mM MgCl2, 10 mM MES, and 150 µM acetosyringone to a final OD600 = 0.2 to 0.5. After 3 h induction, cultures were infiltrated into 3-week-old N. benthamiana leaves using a needleless syringe.
Microscopy
For localization, 35S-SlSERK3A-GFP or 35S-SlSERK3B-GFP (pEarleyGate103) and 35S-AtBAK1-mCherry (pCambia) proteins were transiently co-expressed in N. benthamiana leaves by agroinfiltration. Fluorescence was monitored 48 h later using a Leica SP2 Confocal microscope, with laser set at 488- and 563-nm to excite the GFP and mCherry, respectively, and images were collected through band emission filters at 500–530 and 600–630 nm, respectively.
For callose visualization, leaf discs were cleared using hot 95% ethanol, stained with 150 mM K2P04 (pH 9.5), 0.01% aniline blue for 2 h, and examined for UV fluorescence using Olympus BX51 microscope.
For H202 accumulation, leaf discs were vacuum infiltrating with 3,3′-diamaminobenzidine (DAB) as previously described [63]. Tissues were cleared with ethanol and examined under a bright-field microscope.
Co-immunoprecipitation and immunoblot analysis
Leaf samples were processed as described earlier [28]. Samples were centrifuged at 13000 g for 20 min at 4°C, adjusted to 2 mg/ml total protein concentration, and pretreated with Protein A-agarose (Roach) for 3 to 4 h. Immunoprecipitations were performed on 1.5 ml total protein by adding anti-HA (Santa Cruz; 1∶100) or anti-GFP (Roach; 1∶100) overnight at 4°C. After incubation with 20 µl protein A-agarose at 4°C for 3 to 4 h, beads were washed 4 times with Tris-buffered saline (TBS) containing 0.5% (v/v) ND-40, immunoprecipitates were analyzed by immunobloting.
Samples were electrophoresed on 8% SDS-acrylamide gels, transferred onto nitrocellulose membranes (BIO-RAD), blocked, incubated overnight with primary antibody [anti-GFP (Roach) 1∶5000; anti-HA-HRP (Santa Cruz) 1∶2000], and washed in TBST (TBS with 0.1% (w/v) Tween-20). For anti-GFP, blots were incubated with a secondary antibody anti-mouse-HRP [(Santa Cruz) 1∶5000]. Signals were visualized using chemiluminescent substrate (Thermo Scientific) before exposure to X-ray film.
Arabidopsis transgenic plants
Agrobacterium tumefaciens GV3101 containing pBAK1-SlSERK3A or pBAK1-SlSERK3B in pEARLEYGATE303 were transformed into the bak1-4 mutant by the floral-dip method [64].
Supporting Information
The SlSERK3 s have conserved SERK gene structure. SlSERK3A and SlSERK3B gene structures with introns and exons shown as lines and boxes, respectively. Areas of the proteins coded by each exon are indicated beneath the boxes. SP, signal peptide; LRR, leucine-rich repeat; LRRNT, LRR N-terminal domain; SPP, proline-rich region; TM, transmembrane; & kinase subdomains (I–XI).
(PPTX)
The characteristic domains of SERK proteins are conserved in Sl SERK3s. The deduced amino acid sequence of tomato SlSERK3s protein was aligned with the five Arabidopsis and two Nicotiana benthamiana SERK members. Conserved and most conserved amino acids residues are highlighted in black and grey, respectively. The protein domains are indicated below the sequences. Roman numerals indicate the position of the protein kinase catalytic subdomains. LRR, Leucine-rich repeat; LRRNT, LRR N-terminal domain. Double line in red indicate LRR C-terminal (LRRCT) domain. Single underline in black indicates the catalytic loop. Red star indicates the mutation to generate kinase dead mutants (D to N).
(PPTX)
Gene fragments used in VIGS. (A) Position of TRV-SlFLS2 VIGS fragment used for silencing relative to the full-length open reading frame (ORF). (B), Upper panel, position of TRV-SlSERK3A VIGS fragment used for silencing relative to the ORF. Lower panel, line-up of the TRV-SlSERK3A fragment with the corresponding region in SlSERK3B. (C) Upper panel, position of TRV-SlSERK3B VIGS fragment used for silencing relative to the ORF. Lower panel, line-up of the TRV-SlSERK3B fragment with the corresponding region in SlSERK3A. (D) Upper panel, position of TRV-SlSERK3AB VIGS fragment (originating from SlSERK3B) used for co-silencing SlSERK3A and SlSERK3B relative to their ORF. Lower panel, line-up of the TRV-SlSERK3AB fragment with the corresponding regions in SlSERK3A and SlSERK3B.
(PPTX)
Silencing individually SlSERK3A or SlSERK3B does not result in cell death. (A) Transcript levels of VIGS-silenced genes were evaluated using qRT-PCR. Additional samples (to those presented in Figure 3A) of tomato cv. Moneymaker plants (used in the bacterial screens) treated with TRV empty vector (TRV), TRV-SlSERK3A, TRV-SlSERK3B, and TRV-SlSERK3AB were evaluated. Expression was normalized against UBI3. Values are average ± SE of three technical replicates. *P<0.05 significant difference from TRV (two-sample t-test). (B) Tomato cv. Moneymaker leaflets from plants silenced with the indicated TRV constructs. Photos were taken 3 weeks after TRV treatment. (C) Aniline blue-stained tomato leaf discs. No callose deposits were detected in leaflets silenced for either SlSERK3A or SlSERK3B. Leaves treated with 1 mM flg22 for 24 h were used as control.
(PPTX)
Co-silencing SlSERK3A and SlSERK3B result in cell death and reduced BR sensitivity. (A) DAB-stained tomato leaf discs. Leaflets of tomato cv. Moneymaker plants co-silenced for SlSERK3A and SlSERK3B showing cell death and TRV empty vector (TRV) control were evaluated for H2O2 accumulation. (B) Leaflets of tomato cv. Moneymaker plants silenced for SlSERK3A, SlSERK3B or co-silenced and TRV control were evaluated for BR-sensitivity. Leaflets were infiltrated with 10 µM BL 12 h before use. Transcript levels of VIGS-silenced genes and SlCPD were evaluated using qRT-PCR normalized against UBI3. Values represent the average and ± SE of three biological replicates. *P<0.05 and **P<0.01 indicate significant difference from the respective – BL control (two-sample t-test). This experiment was repeated twice with similar results.
(PPTX)
SlFLS2 transcript levels in TRV- SlFLS2 treated plants. Transcript levels were evaluated in leaflets of tomato cv. Moneymaker silenced for SlFLS2 and TRV empty vector (TRV) control using qRT-PCR. Expression was normalized against UBI3. Four independent samples were analyzed per construct. Values are average ± SE of three technical replicates. *P<0.05 significant difference from TRV (two-sample t-test).
(PPTX)
SlSERK3A and SlSERK3B transcript levels in silenced roots and root weight. (A) Transcript levels of VIGS-silenced genes were evaluated using qRT-PCR. Tomato cv. Moneymaker plants, treated with TRV empty vector (TRV), TRV-SlSERK3A, or TRV-SlSERK3B, were evaluated. Expression was normalized against UBI3. A subsample from six different roots was analyzed per construct. This experiment was performed twice and data from both experiments are presented. Values are average ± SE of three technical replicates. *P<0.05 significant difference from TRV (two-sample t-test). (B) Root weight of RKN infected plants. Values are average (±) SE (n = 9) from a single experiment. No significance difference (ANOVA Tukey's HSD test) was observed in root weight.
(PPTX)
SlSERK3A and SlSERK3B complemented the Arabidopsis bak1-4 mutant BR-induced root length inhibition. Transgenic bak1-4 plants expressing pBAK1-SlSERK3A (bak1-4 SlSERK3A) or pBAK1-SlSERK3B (bak1-4 SlSERK3B) and bak1-4 mutant plants were evaluated for root growth. Nine-day-old Arabidopsis seedlings root grown on medium with (right panel) or without (left panel) 1 nM BL.
(PPTX)
List of primers used in qPCR.
(DOC)
List of primers used in cloning.
(DOC)
Acknowledgments
We thank Thomas Eulgem for comments on the manuscript, Cyril Zipfel and Wenbo Ma for helpful discussions. We are also grateful to Frank Takken for discussions and the BAK1-mCherry construct.
Funding Statement
This research was supported by the University of California Agricultural Experiment Station. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- 2. Zipfel C (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20: 10–16. [DOI] [PubMed] [Google Scholar]
- 3. Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406. [DOI] [PubMed] [Google Scholar]
- 4. van Loon LC, Rep M, Pieterse CM (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44: 135–162. [DOI] [PubMed] [Google Scholar]
- 5. Boller T, He SY (2009) Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324: 742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson VM, Kumar A (2006) Nematode resistance in plants: the battle underground. Trends Genet 22: 396–403. [DOI] [PubMed] [Google Scholar]
- 7. Davis EL, Hussey RS, Mitchum MG, Baum TJ (2008) Parasitism proteins in nematode-plant interactions. Curr Opin Plant Biol 11: 360–366. [DOI] [PubMed] [Google Scholar]
- 8.Kaloshian I, Desmond O, Atamian HS (2011) Disease resistance genes and defense responses during incompatible interactions. In: GGJ Jones, C Fenoll, editor editors. Genomics and Molecular Genetics of Plant-Nematode Interactions. Heidelberg: Springer. pp. 309–324. [Google Scholar]
- 9.Smant G, Jones J (2011) Suppression of plant defences by nematodes. In: GGJ Jones, C Fenoll, editor editors. Genomics and Molecular Genetics of Plant-Nematode Interactions. Heidelberg: Springer. pp. 273–286. [Google Scholar]
- 10. Shiu SH, Bleecker AB (2001) Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE 2001: re22. [DOI] [PubMed] [Google Scholar]
- 11. Torii KU (2004) Leucine-rich repeat receptor kinases in plants: structure, function, and signal transduction pathways. Int Rev Cytol 234: 1–46. [DOI] [PubMed] [Google Scholar]
- 12. Gomez-Gomez L, Boller T (2002) Flagellin perception: a paradigm for innate immunity. Trends Plant Sci 7: 251–256. [DOI] [PubMed] [Google Scholar]
- 13. Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, et al. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767. [DOI] [PubMed] [Google Scholar]
- 14. Hann DR, Rathjen JP (2007) Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana . Plant J 49: 607–618. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen HP, Chakravarthy S, Velasquez AC, McLane HL, Zeng L, et al. (2010) Methods to study PAMP-triggered immunity using tomato and Nicotiana benthamiana . Mol Plant-Microbe Interact 23: 991–999. [DOI] [PubMed] [Google Scholar]
- 16. Robatzek S, Bittel P, Chinchilla D, Kochner P, Felix G, et al. (2007) Molecular identification and characterization of the tomato flagellin receptor LeFLS2, an orthologue of Arabidopsis FLS2 exhibiting characteristically different perception specificities. Plant Mol Biol 64: 539–547. [DOI] [PubMed] [Google Scholar]
- 17. Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt ED, Boutilier K, et al. (2001) The Arabidopsis somatic embryogenesis receptor kinase 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127: 803–816. [PMC free article] [PubMed] [Google Scholar]
- 18. Albrecht C, Russinova E, Kemmerling B, Kwaaitaal M, de Vries SC (2008) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol 148: 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Wen J, Lease KA, Doke JT, Tax FE, et al. (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222. [DOI] [PubMed] [Google Scholar]
- 20. Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212. [DOI] [PubMed] [Google Scholar]
- 21. Bucherl CA, van Esse GW, Kruis A, Luchtenberg J, Westphal AH, et al. (2013) Visualization of BRI1 and BAK1(SERK3) membrane receptor heterooligomers during brassinosteroid signaling. Plant Physiol 162: 1911–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gou X, Yin H, He K, Du J, Yi J, et al. (2012) Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling. PLoS Genet 8: e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karlova R, Boeren S, Russinova E, Aker J, Vervoort J, et al. (2006) The Arabidopsis somatic embryogenesis receptor-like kinase1 protein complex includes brassinosteroid-insensitive1. Plant Cell 18: 626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, et al. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500. [DOI] [PubMed] [Google Scholar]
- 25. Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, et al. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, et al. (2007) The BRI1-Associated Kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol 17: 1116–1122. [DOI] [PubMed] [Google Scholar]
- 27. Shan L, He P, Li J, Heese A, Peck SC, et al. (2008) Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, et al. (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He K, Gou X, Yuan T, Lin H, Asami T, et al. (2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 30. Sun Y, Li L, Macho AP, Han Z, Hu Z, et al. (2013) Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342: 624–628. [DOI] [PubMed] [Google Scholar]
- 31. Monaghan J, Zipfel C (2012) Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol 15: 349–357. [DOI] [PubMed] [Google Scholar]
- 32. Chaparro-Garcia A, Wilkinson RC, Gimenez-Ibanez S, Findlay K, Coffey MD, et al. (2011) The receptor-like kinase SERK3/BAK1 is required for basal resistance against the late blight pathogen Phytophthora infestans in Nicotiana benthamiana . PLoS ONE 6: e16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang DH, Hettenhausen C, Baldwin IT, Wu J (2011) BAK1 regulates the accumulation of jasmonic acid and the levels of trypsin proteinase inhibitors in Nicotiana attenuata's responses to herbivory. J Exp Bot 62: 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mantelin S, Peng HC, Li B, Atamian HS, Takken FL, et al. (2011) The receptor-like kinase SlSERK1 is required for Mi-1-mediated resistance to potato aphids in tomato. Plant J 67: 459–471. [DOI] [PubMed] [Google Scholar]
- 35. Sakamoto T, Deguchi M, Brustolini OJ, Santos AA, Silva FF, et al. (2012) The tomato RLK superfamily: phylogeny and functional predictions about the role of the LRRII-RLK subfamily in antiviral defense. BMC Plant Biol 12: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah K, Gadella TW Jr, van Erp H, Hecht V, de Vries SC (2001) Subcellular localization and oligomerization of the Arabidopsis thaliana somatic embryogenesis receptor kinase 1 protein. J Mol Biol 309: 641–655. [DOI] [PubMed] [Google Scholar]
- 37. Russinova E, Borst JW, Kwaaitaal M, Cano-Delgado A, Yin Y, et al. (2004) Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell 16: 3216–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson LN, Noble ME, Owen DJ (1996) Active and inactive protein kinases: structural basis for regulation. Cell 85: 149–158. [DOI] [PubMed] [Google Scholar]
- 39. Bajwa VS, Wang X, Blackburn RK, Goshe MB, Mitra SK, et al. (2013) Identification and functional analysis of tomato BRI1 and BAK1 receptor kinase phosphorylation sites. Plant Physiol 163: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, et al. (2011) Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet 7: e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang X, Abel S, Keller JA, Shen NF, Theologis A (1992) The 1-aminocyclopropane-1-carboxylate synthase gene family of Arabidopsis thaliana . Proc Natl Acad Sci USA 89: 11046–11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mathur J, Molnar G, Fujioka S, Takatsuto S, Sakurai A, et al. (1998) Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J 14: 593–602. [DOI] [PubMed] [Google Scholar]
- 43. Bhattarai KK, Li Q, Liu Y, Dinesh-Kumar SP, Kaloshian I (2007) The MI-1-mediated pest resistance requires Hsp90 and Sgt1 . Plant Physiol 144: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim JG, Li X, Roden JA, Taylor KW, Aakre CD, et al. (2009) Xanthomonas T3S effector XopN suppresses PAMP-triggered immunity and interacts with a tomato atypical receptor-like kinase and TFT1. Plant Cell 21: 1305–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, et al. (2010) Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 26: 9444–9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ntoukakis V, Schwessinger B, Segonzac C, Zipfel C (2011) Cautionary notes on the use of c-terminal BAK1 fusion proteins for functional studies. Plant Cell 23: 3871–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fradin EF, Zhang Z, Juarez Ayala JC, Castroverde CD, Nazar RN, et al. (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1 . Plant Physiol 150: 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bar M, Sharfman M, Ron M, Avni A (2010) BAK1 is required for the attenuation of ethylene-inducing xylanase (Eix)-induced defense responses by the decoy receptor LeEix1. Plant J 63: 791–800. [DOI] [PubMed] [Google Scholar]
- 49. Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, et al. (2010) Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22: 973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fradin EF, Abd-El-Haliem A, Masini L, van den Berg GC, Joosten MH, et al. (2011) Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol 156: 2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haegeman A, Mantelin S, Jones JT, Gheysen G (2012) Functional roles of effectors of plant-parasitic nematodes. Gene 492: 19–31. [DOI] [PubMed] [Google Scholar]
- 52. Quentin M, Abad P, Favery B (2013) Plant parasitic nematode effectors target host defense and nuclear functions to establish feeding cells. Frontiers in Plant Science 4: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gao M, Wang X, Wang D, Xu F, Ding X, et al. (2009) Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6: 34–44. [DOI] [PubMed] [Google Scholar]
- 54. Wang Z, Meng P, Zhang X, Ren D, Yang S (2011) BON1 interacts with the protein kinases BIR1 and BAK1 in modulation of temperature-dependent plant growth and cell death in Arabidopsis. Plant J 67: 1081–1093. [DOI] [PubMed] [Google Scholar]
- 55. Tornero P, Gadea J, Conejero V, Vera P (1997) Two PR-1 genes from tomato are differentially regulated and reveal a novel mode of expression for a pathogenesis-related gene during the hypersensitive response and development. Mol Plant-Microbe Interact 10: 624–634. [DOI] [PubMed] [Google Scholar]
- 56. Karlova R, Boeren S, van Dongen W, Kwaaitaal M, Aker J, et al. (2009) Identification of in vitro phosphorylation sites in the Arabidopsis thaliana somatic embryogenesis receptor-like kinases. Proteomics 9: 368–379. [DOI] [PubMed] [Google Scholar]
- 57. Wang X, Kota U, He K, Blackburn K, Li J, et al. (2008) Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell 15: 220–235. [DOI] [PubMed] [Google Scholar]
- 58. Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP (2002) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30: 415–429. [DOI] [PubMed] [Google Scholar]
- 59. Verwoerd TC, Dekker BM, Hoekema A (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res 17: 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ginzinger DG (2002) Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol 30: 503–512. [DOI] [PubMed] [Google Scholar]
- 61. Anderson JC, Pascuzzi PE, Xiao F, Sessa G, Martin GB (2006) Host-mediated phosphorylation of type III effector AvrPto promotes Pseudomonas virulence and avirulence in tomato. Plant Cell 18: 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Martinez de Ilarduya O, Moore AE, Kaloshian I (2001) The tomato Rme1 locus is required for Mi-1-mediated resistance to root-knot nematodes and the potato aphid. Plant J 27: 417–425. [DOI] [PubMed] [Google Scholar]
- 63. Martinez de Ilarduya O, Xie Q, Kaloshian I (2003) Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol Plant-Microbe Interact 16: 699–708. [DOI] [PubMed] [Google Scholar]
- 64. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 65. Allemandou F, Nussberger J, Brunner HR, Brakch N (2003) Rapid site-directed mutagenesis using two-PCR-generated DNA fragments reproducing the plasmid template. J Biomed Biotechnol 2003: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The SlSERK3 s have conserved SERK gene structure. SlSERK3A and SlSERK3B gene structures with introns and exons shown as lines and boxes, respectively. Areas of the proteins coded by each exon are indicated beneath the boxes. SP, signal peptide; LRR, leucine-rich repeat; LRRNT, LRR N-terminal domain; SPP, proline-rich region; TM, transmembrane; & kinase subdomains (I–XI).
(PPTX)
The characteristic domains of SERK proteins are conserved in Sl SERK3s. The deduced amino acid sequence of tomato SlSERK3s protein was aligned with the five Arabidopsis and two Nicotiana benthamiana SERK members. Conserved and most conserved amino acids residues are highlighted in black and grey, respectively. The protein domains are indicated below the sequences. Roman numerals indicate the position of the protein kinase catalytic subdomains. LRR, Leucine-rich repeat; LRRNT, LRR N-terminal domain. Double line in red indicate LRR C-terminal (LRRCT) domain. Single underline in black indicates the catalytic loop. Red star indicates the mutation to generate kinase dead mutants (D to N).
(PPTX)
Gene fragments used in VIGS. (A) Position of TRV-SlFLS2 VIGS fragment used for silencing relative to the full-length open reading frame (ORF). (B), Upper panel, position of TRV-SlSERK3A VIGS fragment used for silencing relative to the ORF. Lower panel, line-up of the TRV-SlSERK3A fragment with the corresponding region in SlSERK3B. (C) Upper panel, position of TRV-SlSERK3B VIGS fragment used for silencing relative to the ORF. Lower panel, line-up of the TRV-SlSERK3B fragment with the corresponding region in SlSERK3A. (D) Upper panel, position of TRV-SlSERK3AB VIGS fragment (originating from SlSERK3B) used for co-silencing SlSERK3A and SlSERK3B relative to their ORF. Lower panel, line-up of the TRV-SlSERK3AB fragment with the corresponding regions in SlSERK3A and SlSERK3B.
(PPTX)
Silencing individually SlSERK3A or SlSERK3B does not result in cell death. (A) Transcript levels of VIGS-silenced genes were evaluated using qRT-PCR. Additional samples (to those presented in Figure 3A) of tomato cv. Moneymaker plants (used in the bacterial screens) treated with TRV empty vector (TRV), TRV-SlSERK3A, TRV-SlSERK3B, and TRV-SlSERK3AB were evaluated. Expression was normalized against UBI3. Values are average ± SE of three technical replicates. *P<0.05 significant difference from TRV (two-sample t-test). (B) Tomato cv. Moneymaker leaflets from plants silenced with the indicated TRV constructs. Photos were taken 3 weeks after TRV treatment. (C) Aniline blue-stained tomato leaf discs. No callose deposits were detected in leaflets silenced for either SlSERK3A or SlSERK3B. Leaves treated with 1 mM flg22 for 24 h were used as control.
(PPTX)
Co-silencing SlSERK3A and SlSERK3B result in cell death and reduced BR sensitivity. (A) DAB-stained tomato leaf discs. Leaflets of tomato cv. Moneymaker plants co-silenced for SlSERK3A and SlSERK3B showing cell death and TRV empty vector (TRV) control were evaluated for H2O2 accumulation. (B) Leaflets of tomato cv. Moneymaker plants silenced for SlSERK3A, SlSERK3B or co-silenced and TRV control were evaluated for BR-sensitivity. Leaflets were infiltrated with 10 µM BL 12 h before use. Transcript levels of VIGS-silenced genes and SlCPD were evaluated using qRT-PCR normalized against UBI3. Values represent the average and ± SE of three biological replicates. *P<0.05 and **P<0.01 indicate significant difference from the respective – BL control (two-sample t-test). This experiment was repeated twice with similar results.
(PPTX)
SlFLS2 transcript levels in TRV- SlFLS2 treated plants. Transcript levels were evaluated in leaflets of tomato cv. Moneymaker silenced for SlFLS2 and TRV empty vector (TRV) control using qRT-PCR. Expression was normalized against UBI3. Four independent samples were analyzed per construct. Values are average ± SE of three technical replicates. *P<0.05 significant difference from TRV (two-sample t-test).
(PPTX)
SlSERK3A and SlSERK3B transcript levels in silenced roots and root weight. (A) Transcript levels of VIGS-silenced genes were evaluated using qRT-PCR. Tomato cv. Moneymaker plants, treated with TRV empty vector (TRV), TRV-SlSERK3A, or TRV-SlSERK3B, were evaluated. Expression was normalized against UBI3. A subsample from six different roots was analyzed per construct. This experiment was performed twice and data from both experiments are presented. Values are average ± SE of three technical replicates. *P<0.05 significant difference from TRV (two-sample t-test). (B) Root weight of RKN infected plants. Values are average (±) SE (n = 9) from a single experiment. No significance difference (ANOVA Tukey's HSD test) was observed in root weight.
(PPTX)
SlSERK3A and SlSERK3B complemented the Arabidopsis bak1-4 mutant BR-induced root length inhibition. Transgenic bak1-4 plants expressing pBAK1-SlSERK3A (bak1-4 SlSERK3A) or pBAK1-SlSERK3B (bak1-4 SlSERK3B) and bak1-4 mutant plants were evaluated for root growth. Nine-day-old Arabidopsis seedlings root grown on medium with (right panel) or without (left panel) 1 nM BL.
(PPTX)
List of primers used in qPCR.
(DOC)
List of primers used in cloning.
(DOC)