Abstract
Objectives
To determine the potential association between HPV infection and the squamous cell component of urothelial carcinoma (UC) of the bladder and to validate p16 overexpression as a surrogate marker for HPV infection in these cancers among Koreans.
Methods
We analyzed the presence of HPV infection using an HPV-DNA chip and the expression of p16 using immunohistochemistry in 47 subjects between July 2001 and March 2011. The study group (n = 35) included patients with squamous differentiation of UC of the bladder. The control group (n = 12) included patients with squamous metaplasia of the bladder.
Results
Baseline characteristics of control and study groups were similar. HPV DNA detection rates were approximately 2-fold higher in the study than the control group (17.1% [6/35] versus 8.3% [1/12], respectively), but the difference was not statistically significant. P16 overexpression was detected in 16/35 (45.7%) study group and 1/12 (8.3%) control group samples (p = 0.034). Both HPV-positivity and p16 overexpression were present in 3/35 (8.8%) study group samples, but none of the control group (p = 0.295). In the study group, the percentage of HPV-positive cases who were non-smokers was 2-fold higher than the percentage of HPV-negative cases who were non-smokers (66.7% [4/6] versus 31.0% [9/29], respectively); however, statistical significance was not achieved due to the small sample size.
Conclusions
HPV infection may be associated with UC of the bladder with squamous differentiation, especially in non-smokers. However, p16 expression does not appear to be a strong surrogate marker for evidence of HPV infection in this type of cancer.
Introduction
Bladder cancer (BC) accounts for 3.2–4% of all cancers worldwide and approximately 90% of BC are urothelial carcinoma (UC). Although UC of the bladder often has focal squamous differentiation, it is differentiated from squamous cell carcinoma (SCC) of bladder, which contains solely keratin-forming carcinoma cells. BC composed of mixed urothelial and squamous phenotypes is called UC with squamous differentiation (UC/SCC) [1].
Among the known carcinogens of UC of the bladder, cigarette smoking is the most well-characterized risk factor. It is responsible for nearly half of BCs [2]. Other risk factors for BC include occupational exposure to a number of aromatic amines; infection-related carcinogenesis, such as schistosomal infection; and large doses of certain drugs, including cyclophosphamide and phenacetin [3]. However, a large number of BCs remain unexplained.
Recently, the role of viruses has received considerable attention as potential carcinogens, especially human papilloma virus (HPV). HPV is a small, circular, double-stranded DNA virus that infects stratified squamous epithelium and has been estimated linked to almost 10% of all cancers worldwide, especially a subset of SCCs of the vulva, penis, anus, and oropharynx [4]–[7]. Among the different HPV types, certain types, such as HPV-16 and HPV-18, have an established etiological role in development of anogenital cancers. Although HPV-16 and/or -18 genomic sequences were also identified in the urinary tract of female patients with recurrent and persistent urethritis and cystitis [8], [9], a number of studies have investigated the possibility that HPV infection is a risk factor contributing to UC of the bladder; however, their results have conflicted, so no definitive conclusions are possible [10]–[13].
A related issue involves the possibility that p16 protein is overexpressed in UC of the bladder. The expression of p16 is well known to be associated with high-risk HPV infection in cervical cancer and head and neck cancer and it has been suggested as a qualified surrogate marker for the identification of biologically active HPV infection [14]–[16]. However, despite many recent studies focusing on the SCC component of UC of the bladder, it remains unclear whether overexpression of p16 protein is associated with oncogenesis of UC of bladder [5], [10], [17]–[19].
Therefore, the aim of the present study was to determine the presence of HPV infection and to validate the possibility of p16 overexpression as a surrogate marker for HPV infection in UC/SCC of the bladder in Koreans smokers and non-smokers.
Materials and Methods
Following approval of the study by our Institutional Review Board, 47 patients were selected from the archives of the hospital from July 2001 to March 2011 (IRB No. NCCNCS-12-643). All patients provided verbal informed consent. The written consent was not obtained because the IRB of National Cancer Center approved to exempt the written consent procedure. We examined paraffin-embedded tissue samples obtained during transurethral resection of the bladder or cystectomy. The study group consisted of 35 patients with mixed UC/SCC of the bladder and the control group consisted of 12 patients with squamous metaplasia of the bladder. Of the study group patients, 33 had evidence of mixed UC/SCC and two had extensive SCC with a history of UC. The UC of the bladder specimens were classified according to the World Health Organization (WHO) and International Society of Urological pathology (ISUP) grading criteria and staged according to the TNM 2010 version. None of the patients had known or suspected histories of Schistosoma heamatobium infection, uterine cervix SCC, or head and neck SCC.
A representative SCC component of each paraffin block sample was selected based on the presence of adequate quality and quantity of extracted DNA. DNA was extracted using Qiagen BioRobot M48 workstation (Qiagen, CA) and the quality and quantity of DNA was measured using Nanodrop ND 1000 (NanoDrop Technologies, Wilmington, DE). The presence of HPV infection was analyzed using an HPV-DNA chip. HPV genotyping was performed as per the manufacturer's protocol using a polymerase chain reaction (PCR)-based DNA microarray system (Greencross, Gyeonggi, Korea), consisting of multiple probes of the HPV L1 sequence [20]. These probes included specific probes for high-risk HPV types (HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, and 68) and low-risk HPV types (HPV-6, 11, 34, 40, 42, 43, 44, 54, and 70). Consensus PCR products were hybridized to the probes on the chip, scanned (Scanner GX, PerkinElmer, MA), and then analyzed. PCR amplification was performed using 10 μL purified DNA.
Immunohistochemistry (IHC) staining of p16 protein was performed using specific E6H4 antibodies (DAKO, Carpinteria, CA). IHC p16 expression was scored using a semiquantitative composite scoring system as follows: (1) staining intensity, defined as 0 for negative, 1+ for weak, 2+ for moderate, and 3+ for strong; (2) positive area, defined as the (10 X) fraction of stained tumor cells in the entire tumor; and (3) expression score, defined as the staining intensity multiplied by the positive area. The highest possible score was 30. Overexpression of p16 was defined as a score >20 (Figure 1).
Figure 1. Histological findings and expression patterns of p16.
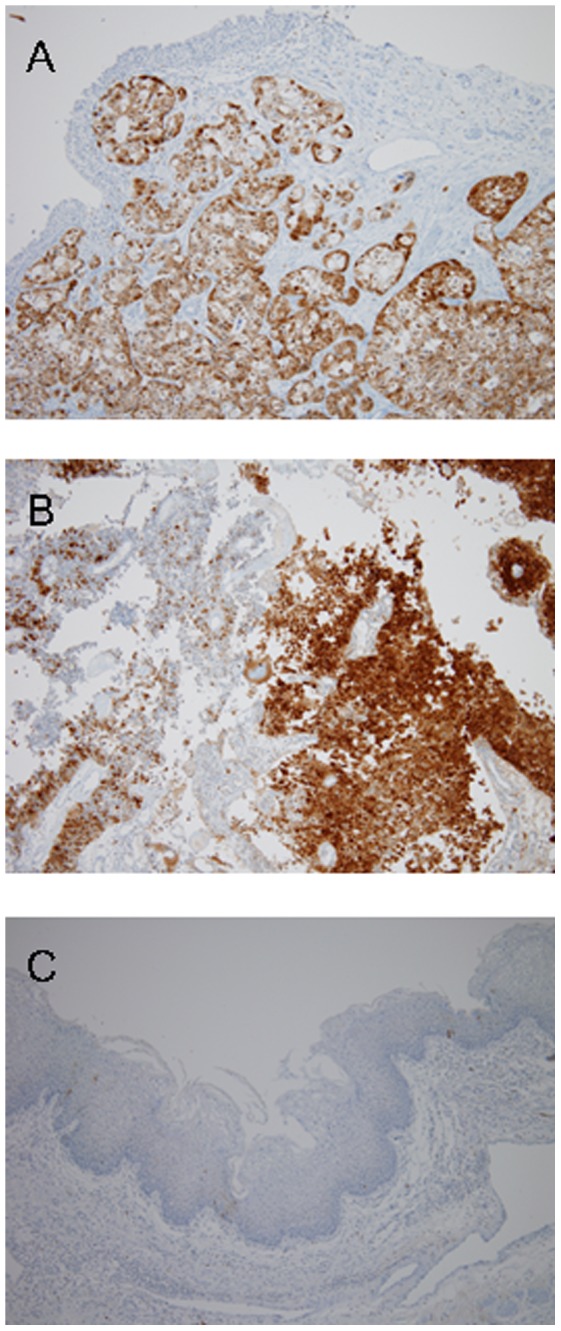
A: The squamous cell carcinoma components are located beneath the urothelial lining. The tumor cells are strongly positive for p16 (x100). B: This case is a mixed papillary urothelial carcinoma (left upper) and squamous cell carcinoma. The squamous cell carcinoma component is strong positive for p16. C: Squamous metaplasia shows no expression of p16.
The relationships between the presence of HPV infection and clinicopathological parameters were assessed using Fisher's exact tests and Mann-Whitney tests. All statistical analyses were performed using STATA (release 9.2, STATA Inc, Tex, USA). Results were considered statistically significant if the two-sided p value was <0.05.
Results
Clinicopathological characteristics of the 35 UC/SCC study group patients and 12 squamous metaplasia control group patients are shown in Table 1. No demographic differences were observed between the study and control groups, including age (71.2±7.7 vs. 66.5±10.1 years, p = 0.246), male to female ratio (82.9% vs. 66.7%, p = 0.251), and smoking history (62.9% vs. 41.7%, p = 0.311). In the study group, 4 (12.1%) patients had low-grade carcinoma and 29 (87.9%) had high-grade. For the remaining 2 patients, the available samples (obtained by transurethral resection of the bladder) contained only an SCC component, thereby prohibiting the assignment of a grade.
Table 1. Demographic and histopathological characteristics of the study group and control group.
| Study group | Control group | p-value | |
| No. of patients | 35 | 12 | |
| Age (years) | 71.2±7.7 | 66.5±10.1 | 0.247 |
| Sex | |||
| Male | 29 (82.9%) | 8 (66.7%) | 0.251 |
| Female | 6 (17.1%) | 4 (33.3%) | |
| Stage | |||
| NMIBC | 8 (22.9%) | NA | NA |
| MIBC | 17 (48.6%) | NA | NA |
| MBC | 10 (28.6%) | NA | NA |
| Grade | |||
| Low | 4 (12.1%) | NA | NA |
| High | 29 (87.9%) | NA | NA |
| NA | 2 | NA | NA |
| Smoking history | 0.311 | ||
| Smoker or Ex- smoker | 22 (62.9%) | 5 (41.7%) | |
| Non-smoker | 13 (37.1%) | 7 (58.3%) |
NMIBC: non-muscle invasive bladder cancer.
MIBC: muscle invasive bladder cancer.
MBC: metastatic bladder cancer.
NA: not applicable.
HPV DNA was detected in 6 of the 35 (17.1%) study group samples, and 1 of the 12 (8.3%) control group samples (Table 2). All HPVs were type 18, except for 1 study group sample, which was type 35. Overexpression of p16 was detected in 16 (45.7%) study group samples and 1 (8.3%) control group sample (Table 2). Both HPV positivity and p16 overexpression were present in only 3 (8.8%) study group samples and no control group sample.
Table 2. Detection of human papillomavirus and overexpression of p16 in the study group and control group.
| Study group | Control group | p-value | |
| No. of patients | 35 | 12 | |
| HPV+ | 6 (17.1%) | 1 (8.3%) | 0.659 |
| P16+ | 16 (45.7%) | 1 (8.3%) | 0.034 |
| HPV+/P16+ | 3 (8.6%) | 0 (0.0%) | 0.295 |
HPV: human papillomavirus virus.
HPV+: detection of HPV.
P16+: overexpression of p16.
For the study group, there were no differences in age, sex, stage, or grade between HPV-negative and HPV-positive cases. Of the 29 HPV-negative cases, 20 (69.0%) were smokers or ex-smokers, whereas of the 6 HPV-positive cases, only 2 (33.3%) were smokers or ex-smokers. For smokers or ex-smokers, the percentage with HPV-negative BC was approximately 2-fold higher than the percentage with HPV-positive BC. By contrast, the BC samples of non-smokers demonstrated an approximately 2-fold higher percentage of HPV-positivity than HPV-negativity (Table 3).
Table 3. Demographic and histopathological characteristics according to detection of human papillomavirus in the study group.
| Study group (N = 35) | p-value | ||
| HPV- (n = 29) | HPV+ (n = 6) | ||
| Age (years) | 71.4±7.9 | 70.0±6.5 | 0.718 |
| Sex | 0.973 | ||
| Male | 24 (82.8%) | 5 (93.3%) | |
| Female | 5 (17.2%) | 1 (16.7%) | |
| Stage | 0.220 | ||
| NMIBC | 6 (17.0%) | 2 (5.8%) | |
| MIBC | 16 (45.7%) | 1 (2.9%) | |
| MBC | 7 (20.0%) | 3 (8.6%) | |
| Grade | 0.571 | ||
| Low | 3 (11.1%) | 1 (16.7%) | |
| High | 24 (88.9%) | 5 (83.3%) | |
| NA | 2 | 0 | |
| Smoking history | 0.286 | ||
| Positive+ | 20 (69.0%) | 2 (33.3%) | |
| Negative | 9 (31.0%) | 4 (66.7%) | |
NMIBC: non-muscle invasive bladder cancer.
MIBC: muscle invasive bladder cancer.
MBC: metastatic bladder cancer.
NA: not applicable.
, Positive included with all of the present and ex-smokers.
Discussion
In addition to cigarette smoking, occupational exposure to aromatic amines, and use of specific drugs, such as cyclophosphamide and phenacetin, as known risk factors of BC, HPV infection has also been suggested as a potential causative agent of UC of the bladder [21], [22]. The prevalence of HPV in UC of the bladder reported in previous studies has varied from 0% to 81.3% [23]–[25]. The disparity is probably due to sampling problems, contamination, sensitivity of the detection systems, and geographic variation [26].
Youshya et al. [21]. reported that 60% of patients were positive for HPV L1 capsid protein expression by immunostaining, even though PCR using consensus GP5+/6+ primers failed to detect HPV DNA. The suggested reason for the disparity of HPV detection rates was the antibodies that were utilized and the sensitivity of analysis for detection of DNAs [27]. In this study, we utilized the HPV DNA chip microarray system. The HPV oligonucleotide microarray system is a newly developed biotechnology that can be applied in clinical practice for detection and genotyping of HPV. The HPV-DNA chip microarray has shown higher sensitivity for detection of HPV DNA than PCR alone in tissue block [28], [29].
The HPV has an affinity for squamous cells and its association with human SCC of the uterine cervix, head and neck, and anus has been well established [7]. In the current study, the detection rate of HPV DNA was 2-fold higher in the study group with mixed UC/SCC of the bladder (17.5%) than the control group with squamous dysplasia (8.3%). However, we could not confirm a statistically significant increase in risk of HPV infection in the study group, likely because of the relatively small sample size (Table 2). Our findings are consistent with other studies in which HPV DNA was detected in both UC of the bladder and SCC of the bladder [30].
Although HPV infection has been associated with BC in many studies, it is important to realize that such an association is not equivalent to causation. None of the previous studies have proven the existence of a direct link between HPV infection and carcinogenesis in BC, although the possibility of a causal role has been suggested because of the similarity to cervical and head and neck cancers, for which a causal relationship has been established [19] [31], [32]. To address this issue, we tried to validate p16 overexpression as a surrogate marker for active HPV infection in BC; however, we were unable to detect a strong association between p16 overexpression and HPV infection in UC/SCC of the bladder(Table 2).
HPV encodes two oncoproteins: E6 and E7 [33]. E6 protein binds to wild type (wt) p53, thus triggering ubiquitin-mediated degradation of p53 or directly inactivating wt-p53 by complex formation [34], [35]. E7 binds to pRb and releases the E2F transcription factor, which subsequently facilitates cell proliferation. P16 is a cyclin-dependent kinase (CDK) inhibitor that blocks CDK4-mediated phosphorylation of pRb and subsequent G1 to S progression. HPV-infected cells overexpress p16 in an attempt to compensate for the E7-induced loss of pRb. Because of these negative feedback mechanisms between pRb and p16, overexpression of p16 is now often used as a surrogate marker of HPV oncogene expression. In this study, p16 was overexpressed in 45.7% of UC/SCC samples; however, both HPV-positivity and p16 overexpression was present in only 8.6%. This finding thereby shows that p16 expression might not be a strong surrogate marker for evidence of HPV infection in UC/SCC, which is consistent with the results of Alexander et al. [10].
Additional findings of interest in this study were the types of HPV and the higher percentage of non-smokers in the HPV-positive group than in the HPV-negative group. Almost all HPV-positive samples in the study group were type 18. The sole exception was a sample with HPV type 35, which has been considered a variant of HPV 16 and which has not been previously isolated from the bladder [36]. As it is well known that the HPV types 16 and 18 are categorized as high risky HPV for a leading cause of cancer among various HPV types [7], [16], [37], [38], our result with HPV types 18 and 35 might be supported our hypothesis of HPV infection in association with the carcinogenesis of bladder cancer. Although the increased likelihood of HPV-positivity in non-smokers did not reach statistical significance because of the small sample size, this finding suggests that HPV infection might further be associated with the development of mixed UC/SCC of urinary bladder, especially in non-smokers because of its twice greater the percentage of HPV positivity than that of smokers (p = 0.286, Table 3).
This study had some limitations. A retrospective study with a relatively small number of samples limited its statistical power and potential bias. The serology to HPV suggested by Badawi could not be performed because of the study design, even though the antibody response (immunoglobulin [Ig]G) to L1 capsids could be used as a marker of cumulative HPV exposures [9]. The control group had squamous metaplasia, whereas a control group with normal urothelium may have been more appropriate to show a strong association between HPV infection and carcinogenesis of UC/SCC. However, this study is significant in determination of the relationship of HPV infection with prevalence of BC as one of causative factors in carcinogenesis of bladder and this is the first paper describing the clinical importance of the possible association between HPV infection and bladder cancer in non-smokers.
Conclusion
Based on the findings of this study, HPV infection appears to be associated with the development of mixed UC/SCC of the urinary bladder, especially in non-smokers among Koreans. However, the role of HPV infection in the development of this type of cancer may not be as significant as for SCC of the uterine cervix or the head and neck. Additionally, p16 expression does not appear to be a strong surrogate marker for evidence of HPV infection in UC of the bladder with squamous differentiation.
Funding Statement
This work was supported by a grant from the National Cancer Center (NCC-1110510), Republic of Korea. However, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Grignon DJ, El-Bolkainy MN, Schmitz-Drager BJ, Simon R, Tyoznski JE (2004) Squamous cell carcinoma; Eble JN SG, Epstein JI, Sesterhenn IA, editor. Lyon,France: IARC Press. 3 p. [Google Scholar]
- 2. Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC (2011) Association between smoking and risk of bladder cancer among men and women. JAMA 306: 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silverman DT, Hartge P, Morrison AS, Devesa SS (1992) Epidemiology of bladder cancer. Hematol Oncol Clin North Am 6: 1–30. [PubMed] [Google Scholar]
- 4. Parkin DM, Bray F (2006) Chapter 2: The burden of HPV-related cancers. Vaccine 24 Suppl 3S3/11–25. [DOI] [PubMed] [Google Scholar]
- 5. Blochin EB, Park KJ, Tickoo SK, Reuter VE, Al-Ahmadie H (2012) Urothelial carcinoma with prominent squamous differentiation in the setting of neurogenic bladder: role of human papillomavirus infection. Mod Pathol 25: 1534–1542. [DOI] [PubMed] [Google Scholar]
- 6. Shigehara K, Sasagawa T, Doorbar J, Kawaguchi S, Kobori Y, et al. (2011) Etiological role of human papillomavirus infection for inverted papilloma of the bladder. J Med Virol 83: 277–285. [DOI] [PubMed] [Google Scholar]
- 7. zur Hausen H (2009) Papillomaviruses in the causation of human cancers - a brief historical account. Virology 384: 260–265. [DOI] [PubMed] [Google Scholar]
- 8. Agliano AM, Gazzaniga P, Cervigni M, Gradilone A, Napolitano M, et al. (1994) Detection of human papillomavirus type 16 DNA sequences in paraffin-embedded tissues from the female urinary tract. Urol Int 52: 208–212. [DOI] [PubMed] [Google Scholar]
- 9. Badawi H, Ahmed H, Ismail A, Diab M, Moubarak M, et al. (2008) Role of human papillomavirus types 16, 18, and 52 in recurrent cystitis and urinary bladder cancer among Egyptian patients. Medscape J Med 10: 232. [PMC free article] [PubMed] [Google Scholar]
- 10. Alexander RE, Hu Y, Kum JB, Montironi R, Lopez-Beltran A, et al. (2012) p16 expression is not associated with human papillomavirus in urinary bladder squamous cell carcinoma. Mod Pathol 25: 1526–1533. [DOI] [PubMed] [Google Scholar]
- 11. Moonen PM, Bakkers JM, Kiemeney LA, Schalken JA, Melchers WJ, et al. (2007) Human papilloma virus DNA and p53 mutation analysis on bladder washes in relation to clinical outcome of bladder cancer. Eur Urol 52: 464–468. [DOI] [PubMed] [Google Scholar]
- 12. Helal Tel A, Fadel MT, El-Sayed NK (2006) Human papilloma virus and p53 expression in bladder cancer in Egypt: relationship to schistosomiasis and clinicopathologic factors. Pathol Oncol Res 12: 173–178. [DOI] [PubMed] [Google Scholar]
- 13. Gutierrez J, Jimenez A, de Dios Luna J, Soto MJ, Sorlozano A (2006) Meta-analysis of studies analyzing the relationship between bladder cancer and infection by human papillomavirus. J Urol 176: 2474–2481 discussion 2481. [DOI] [PubMed] [Google Scholar]
- 14.Cai T, Mazzoli S, Mondaini N, Bartoletti R (2008) Re: Paula M.J. Moonen, Judith M.J.E. Bakkers, Lambertus A.L.M. Kiemenay, et al. Human papilloma virus DNA and p53 mutation analysis on bladder washes in relation to clinical outcome of bladder cancer. Eur Urol 2007;52: :464–9. Eur Urol 53: 858–859; author reply 859. [DOI] [PubMed] [Google Scholar]
- 15. Polesel J, Gheit T, Talamini R, Shahzad N, Lenardon O, et al. (2012) Urinary human polyomavirus and papillomavirus infection and bladder cancer risk. Br J Cancer 106: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Nichols MA, Shay JW, Xiong Y (1994) Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res 54: 6078–6082. [PubMed] [Google Scholar]
- 17. Shaker OG, Hammam OA, Wishahi MM (2013) Is there a correlation between HPV and urinary bladder carcinoma? Biomed Pharmacother 67: 183–191. [DOI] [PubMed] [Google Scholar]
- 18. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, et al. (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189: 12–19. [DOI] [PubMed] [Google Scholar]
- 19.Abol-Enein H (2008) Infection: is it a cause of bladder cancer? Scand J Urol Nephrol Suppl: 79–84. [DOI] [PubMed]
- 20. Choi YD, Jung WW, Nam JH, Choi HS, Park CS (2005) Detection of HPV genotypes in cervical lesions by the HPV DNA Chip and sequencing. Gynecol Oncol 98: 369–375. [DOI] [PubMed] [Google Scholar]
- 21. Youshya S, Purdie K, Breuer J, Proby C, Sheaf MT, et al. (2005) Does human papillomavirus play a role in the development of bladder transitional cell carcinoma? A comparison of PCR and immunohistochemical analysis. J Clin Pathol 58: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiwanitkit V (2005) Urinary bladder carcinoma and human papilloma virus infection, an appraisal of risk. Asian Pac J Cancer Prev 6: 217–218. [PubMed] [Google Scholar]
- 23. Khaled HM, Raafat A, Mokhtar N, Zekri AR, Gaballah H (2001) Human papilloma virus infection and overexpression of p53 protein in bilharzial bladder cancer. Tumori 87: 256–261. [DOI] [PubMed] [Google Scholar]
- 24. Lopez-Beltran A, Escudero AL (1997) Human papillomavirus and bladder cancer. Biomed Pharmacother 51: 252–257. [DOI] [PubMed] [Google Scholar]
- 25. Jimenez-Pacheco A, Exposito-Ruiz M, Arrabal-Polo MA, Lopez-Luque AJ (2012) Meta-analysis of studies analyzing the role of human papillomavirus in the development of bladder carcinoma. Korean J Urol 53: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinestel J, Cronauer MV, Muller J, Al Ghazal A, Skowronek P, et al. (2013) Overexpression of p16(INK4a) in Urothelial Carcinoma In Situ Is a Marker for MAPK-Mediated Epithelial-Mesenchymal Transition but Is Not Related to Human Papillomavirus Infection. PLoS One 8: e65189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sano T, Sakurai S, Fukuda T, Nakajima T (1995) Unsuccessful effort to detect human papillomavirus DNA in urinary bladder cancers by the polymerase chain reaction and in situ hybridization. Pathol Int 45: 506–512. [DOI] [PubMed] [Google Scholar]
- 28. Um TH, Lee EH, Chi HS, Kim JW, Hong YJ, et al. (2011) Comparison of HPV genotyping assays and Hybrid Capture 2 for detection of high-risk HPV in cervical specimens. Ann Clin Lab Sci 41: 48–55. [PubMed] [Google Scholar]
- 29. Kim CJ, Jeong JK, Park M, Park TS, Park TC, et al. (2003) HPV oligonucleotide microarray-based detection of HPV genotypes in cervical neoplastic lesions. Gynecol Oncol 89: 210–217. [DOI] [PubMed] [Google Scholar]
- 30. Anwar K, Naiki H, Nakakuki K, Inuzuka M (1992) High frequency of human papillomavirus infection in carcinoma of the urinary bladder. Cancer 70: 1967–1973. [DOI] [PubMed] [Google Scholar]
- 31. Boucher NR, Anderson JB (1997) Human papillomavirus and bladder cancer. Int Urogynecol J Pelvic Floor Dysfunct 8: 354–357. [DOI] [PubMed] [Google Scholar]
- 32. Cai T, Mazzoli S, Meacci F, Nesi G, Geppetti P, et al. (2011) Human papillomavirus and non-muscle invasive urothelial bladder cancer: potential relationship from a pilot study. Oncol Rep 25: 485–489. [DOI] [PubMed] [Google Scholar]
- 33. Lehoux M, D'Abramo CM, Archambault J (2009) Molecular mechanisms of human papillomavirus-induced carcinogenesis. Public Health Genomics 12: 268–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buitrago-Perez A, Garaulet G, Vazquez-Carballo A, Paramio JM, Garcia-Escudero R (2009) Molecular Signature of HPV-Induced Carcinogenesis: pRb, p53 and Gene Expression Profiling. Curr Genomics 10: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 36. Chen Z, Schiffman M, Herrero R, Desalle R, Anastos K, et al. (2011) Evolution and taxonomic classification of human papillomavirus 16 (HPV16)-related variant genomes: HPV31, HPV33, HPV35, HPV52, HPV58 and HPV67. PLoS One 6: e20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doxtader EE, Katzenstein AL (2012) The relationship between p16 expression and high-risk human papillomavirus infection in squamous cell carcinomas from sites other than uterine cervix: a study of 137 cases. Hum Pathol 43: 327–332. [DOI] [PubMed] [Google Scholar]
- 38. Bussu F, Sali M, Gallus R, Vellone VG, Zannoni GF, et al. (2013) HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker? Br J Cancer 108: 1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]


