Abstract
Objective
Overweight early in life may contribute to cardiovascular disease mortality through progression to later life obesity or through a cumulative effect of excess weight. Few studies have investigated the relationship between body mass index (BMI) before middle age and cardiovascular disease mortality in women. Using the Child Health and Development Studies cohort of 11,006 pregnant women recruited between 1959 and 1967, we tested the hypothesis that higher self-reported pre-pregnancy BMI is associated with increased stroke and coronary heart disease mortality.
Design and Methods
Cause of death was assessed annually from enrollment through 2007 by linking with California Vital Status Records. We calculated Cox proportional hazards ratios for cause-specific mortality for each BMI category.
Results
Median follow-up was 37 years with 1,839 participant deaths at a mean age of 64.1 years. At higher levels of BMI, participants were older, had higher prevalence of co-morbid conditions, higher parity, and lower family income. In adjusted models, women with higher pre-pregnancy BMI had increased coronary heart disease mortality compared to those with normal BMI. Women who were underweight, overweight, or obese had higher all-cause mortality. Sensitivity analyses confirmed these results.
Conclusions
Pre-pregnancy BMI has a monotonic association with coronary heart disease mortality and a j-shaped association with non-cardiovascular mortality.
Keywords: Body mass index, Mortality, Coronary disease, Stroke, Women
Overweight and obesity are highly prevalent with a large public health impact.(1) Increasingly, evidence suggests that patterns of weight and body mass index (BMI) are formed early in life,(2) and that these patterns may begin influencing disease risk, particularly vascular disease, as early as adolescence or even childhood.(3, 4, 5) Several studies have suggested that overweight and obesity are risk factors for premature death, (6, 7, 8)particularly among younger people compared with older adults.(6, 9, 10, 11) The increased mortality risk may be attributable to higher total weight gain with age among those who are overweight early in life compared with those whose weight gain begins later in life. Additionally, early life overweight may inflict a larger cumulative exposure to adiposity and its associated disease risks. (4) However, evidence for the impact of obesity on mortality in younger adults is limited because few studies have investigated the relationship between BMI in adults younger than middle age and cause-specific mortality.(11, 12, 13) Most studies of younger adults have had relatively short follow up periods, ending before the period of peak risk for cardiovascular events and mortality, especially for women who have events even later in life than men.(9, 11, 13, 14, 15)
While women generally experience cardiovascular disease later in life than men, there is some evidence that morbidity and mortality from coronary heart disease (CHD) is increasing in younger women, (16, 17) possibly due to the increasing prevalence of obesity among women.(18) Even fewer studies have observed the relationship between obesity among women of reproductive age and mortality,(13) which may be of particular importance given the potential impact of pregnancy on weight and cardiovascular health.(19) Pregnancy complications, often associated with obesity,(20) are important indicators of vascular and metabolic health and later life risk.(21, 22) Furthermore, pregnancy is a period of relatively high health care utilization and contact with medical professionals, making this an opportune time for educational messaging and/or interventions, whereby obese women or those with a history of these pregnancy complications could be targeted for cardiovascular disease prevention before middle age.(23)
Our objective was to examine the association between pre-pregnancy BMI and cause-specific mortality in parous women of reproductive age over almost 40 years of follow up in the Child Health and Development Studies cohort. We hypothesized that pre-pregnancy overweight and obesity would be strongly associated with increased cardiovascular and all-cause mortality.
MATERIALS AND METHODS
Study Population and Sample Selection
The Child Health and Development Studies (CHDS) enrolled 15,528 pregnant women from the Kaiser Permanente Health Plan in California between 1959 and 1967.(24) Details of the study design, methodology, and cohort characteristics have been reported elsewhere.(24). A cohort of racially and economically diverse women were enrolled early in pregnancy. Participants could have multiple pregnancies included in the CHDS and were either nulliparous or multiparous when first enrolled. We excluded participants with a previous history of heart disease including congenital heart defects. The sample for this study includes 11,006 women with vital status data and pre-pregnancy body mass index available for the first study pregnancy.
IRB approval for follow up was provided by the Public Health Institute in Oakland (phi.org) and all participants provided full consent.
Data collection
Deaths were assessed annually in the CHDS by linking study participants to the California Department of Motor Vehicles and the California Vital Status Records.(25) Cause of death was determined from death certificates through 2007 and classified using International Classification of Diseases (ICD 9 and 10) codes. Death from stroke included the following: ICD10 I60–I69, ICD9 430-438. Coronary heart disease death included: ICD10 I20–25, ICD9 410-414. Cancer mortality included: ICD10 C00-C48, ICD9 140-239. Death from respiratory disease included: ICD10 J00-J99, ICD9 460-519. Mortality from endocrine or metabolic disease included: ICD10 E00-E90, ICD9 240-279.
The enrollment interview included demographics, reproductive history, and self-reported pre-pregnancy weight and height. Once enrolled, participants attended study-related prenatal examinations frequently during pregnancy, where weight and height were measured using a standard protocol.(24) We calculated pre-pregnancy BMI at enrollment using self-reported pre-pregnancy weight and measured height at enrollment. BMI was categorized using the NIH/WHO criteria: underweight (BMI < 18.5), normal weight (18.5 ≤ BMI < 25.0), overweight (25.0 ≤ BMI < 30.0), obese (BMI ≤ 30.0). We calculated pregnancy weight gain as the first measured weight during pregnancy subtracted from the last weight measured before delivery.
Other variables include socio-demographic factors, smoking status, and reproductive history. Oral contraceptive use was self-reported as use immediately before the current study pregnancy. Annual family income was collected as ten categories representing ranges of income from less than $2,500 to greater than or equal to $15,000. We dichotomized income categories into less than or greater than or equal to $10,000. To increase interpretability, annual family income was converted into modern buying power by multiplying the $10,000 cut off value by the percent increase in consumer price index from 1960 to 2010, using data from the United States Department of Labor Bureau of Labor Statistics.(26) The percent change in consumer price index between 1960 and 2010 was 7.367%,(26) converting $10,000 in 1960 into $73,600 in 2010 buying power. This value is similar to the median household income in Alameda County in 2010 ($70,821).(27) Medical conditions such as pre-existing hypertension, diabetes, and kidney disease were determined from pre-pregnancy medical chart extraction performed by trained medical abstracters for the six months prior to enrollment.(24)
Statistical Analysis
For each BMI category, we calculated the mean and standard error or percentage of baseline characteristics. We calculated Cox proportional hazards ratios (HR) and 95% confidence intervals (CI) for cardiovascular disease mortality by BMI category. We also calculated hazards ratios for mortality from cancer, respiratory disease, and metabolic disease as a comparison. Participants were censored at death or last known date of residence in California. Survival models were nested on an age-adjusted analysis, first including socio-demographic adjustment then adding adjustment for smoking and reproductive history variables, and finally including pre-pregnancy co-morbidities. We also adjusted for pregnancy weight gain to determine whether the association with pre-pregnancy BMI could be explained by correlations with gestational weight gain. We created Kaplan-Meier curves for specific cardiovascular causes of death, including stroke and CHD, for normal BMI and BMI ≥ 25 (overweight and obese).
Sensitivity Analyses
We conducted several sensitivity analyses to assess for bias. First, we addressed confounding through three levels of adjustment. We adjusted only for age, followed by adding a set of socio-economic factors as the main analysis, and finally by adding smoking, pre-pregnancy medical diagnoses, and reproductive factors. Second, to address the possibility of regression dilution bias and regression to the mean from a single measurement of BMI, we re-analyzed the data using the average of pre-pregnancy BMI collected across repeat study pregnancies as the exposure in the 2,927 (26.6%) women who had more than one study pregnancy. Third, we addressed “reverse causality”, in this case the confounding effect of illness related weight loss on the association between BMI and mortality, in two ways. Separately, we excluded: deaths that occurred in the first five years of follow-up, deaths that occurred in the first ten years of follow-up, and all smokers from the analysis. Fourth, to address the possibility of misclassification of BMI category due to self-report we calculated the agreement between pre-pregnancy BMI from self-reported weight and early pregnancy BMI from earliest measured weight in the first trimester. Fifth, since pre-pregnancy BMI was missing for 23.6% (n = 3,397) of participants, we assessed bias from missing BMI data in two different ways. We used early pregnancy BMI calculated with weight measured in the first trimester to fill missing self-reported pre-pregnancy BMI. We also used multiple imputation to fill missing BMI values. For the multiple imputation to predict the missing BMI, we included the earliest BMI measured in pregnancy, timing of BMI assessment by gestation, age at enrollment, education, income, parity, smoking status, and pre-existing diabetes. Finally, to assess bias due to interaction with age at enrollment and adolescent pregnancy, specifically among the women in the underweight category, we excluded women less than 18 years of age.
All analyses were performed using Stata 11.(28)
RESULTS
At enrollment, 11, 006 participants had a mean age of 26.9 years (range: 14-47), 66% were Caucasian, and 24% were African American. Deaths occurred in 1,839 participants (16.7% of study sample) after a median follow-up of 37 years. Mean age at death was 64.1 years. The most frequent causes of death, in order, were CHD, stroke, cancer, respiratory disease, and metabolic/endocrine disorders (12.3%, 6.0%, 3.2%, 2.2%, 1.3% of deaths respectively). Table 1 shows that participants with higher BMI at baseline had higher prevalence of pre-pregnancy co-morbid conditions, higher age and parity, and lower family income. The underweight group was generally younger than the other groups and was overrepresented by adolescent participants.
Table 1.
Baseline characteristics* of the sample of 11,006 Child Health and Development Studies participants at enrollment by pre-pregnancy body mass index category
| Variable |
Underweight BMI < 18.5 |
Normal weight 18.5 ≥ BMI <25.0 |
Overweight 25.0 ≥ BMI<30.0 |
Obese 30.0 ≥ BMI |
P-value for
difference ‡ |
|---|---|---|---|---|---|
| N | 983 | 8,379 | 1,258 | 386 | |
| BMI (kg/m2) | 17.6 (0.02) | 21.3 (0.02) | 26.94 (0.04) | 33.7 (0.19) | |
| Age at enrollment (years) | 25.2 (0.18) | 26.5 (0.07) | 28.4 (0.19) | 29.3 (0.34) | <0.001 |
| Age at death (years) | 61.7 (0.87) | 63.2 (0.38) | 65.7 (0.64) | 64.3 (0.89) | <0.001 |
| Race | <0.001 | ||||
| White | 65.7 | 69.8 | 49.4 | 47.4 | |
| Black | 21.1 | 21.2 | 41.8 | 45.6 | |
| Other | 13.2 | 9.0 | 8.8 | 6.9 | |
| Education | <0.001 | ||||
| < High school degree (%) | 18.6 | 15.1 | 27.4 | 32.7 | |
| Completed high school (%) | 39.4 | 37.2 | 41.2 | 40.8 | |
| College matriculation (%) | 42.0 | 47.7 | 31.4 | 26.5 | |
| Annual family income ≥ $73,600 (%)§ | 19.2 | 20.4 | 16.6 | 12.2 | <0.001 |
| Age at first menstruation (years) | 12.9 (0.06) | 12.58 (0.02) | 12.4 (0.05) | 12.31 (0.09) | <0.001 |
| Parity at enrollment (# prior pregnancies) | 1.01 (0.04) | 1.22 (0.02) | 2.00 (0.06) | 2.48 (0.12) | <0.001 |
| Parity at study end (# total pregnancies) | 1.23 (0.04) | 1.50 (0.02) | 2.37 (0.06) | 2.86 (0.12) | <0.001 |
| Oral Contraceptive Use (%)∥ | 5.2 | 5.0 | 5.0 | 3.1 | 0.32 |
| Pre-pregnancy Hypertension (%)# | 0.6 | 1.1 | 3.3 | 9.3 | <0.001 |
| Pre-pregnancy Diabetes (%)# | 0.4 | 0.8 | 1.4 | 3.4 | <0.001 |
| Pre-pregnancy Kidney Disease (%)# | 1.9 | 1.8 | 1.8 | 1.3 | 0.84 |
| Smoking | 0.015 | ||||
| Never smoker (%) | 42.7 | 46.8 | 50.8 | 53.4 | |
| Current smoker (%) | 43.0 | 34.6 | 32.2 | 30.2 | |
| Past smoker (%) | 14.3 | 18.6 | 16.9 | 16.4 |
Mean (SE) or percentages
All data collected at enrollment in early pregnancy unless otherwise stated
P-value for T-Test or Pearson Chi-Square test.
$73,600 in 2010 buying power. This equates to $10,000 in 1960 based on the percent increase in consumer price index from 1960 to 2010.
OC use is most recent before current pregnancy
Hypertension, diabetes, and kidney disease are pre-pregnancy diagnosis from medical chart abstraction.
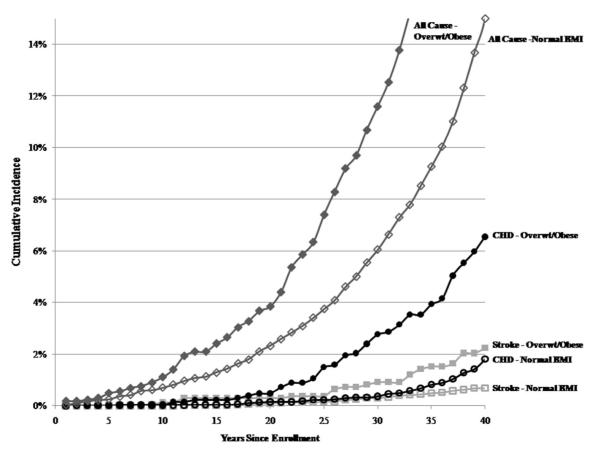
Figure 1 shows the Kaplan-Meier cumulative incidence of stroke, coronary heart disease, and all-cause mortality for normal weight compared to the combined group of participants who were overweight or obese. There is statistically significant separation between the curves for normal weight and overweight/obese groups for these causes of death.
Figure 1.
Kaplan-Meier cumulative incidence for cause-specific mortality by pre-pregnancy body mass index in the sample of 11,006 women from the Child Health and Development Studies
* For each cause of death, the curve for normal BMI is significantly different (P < 0.05) than the curve for overweight/obese.
Age-adjusted pre-pregnancy underweight, overweight, and obesity were significantly associated with increased risk for all cause mortality (Table 2). These associations persisted after all levels of adjustment. Compared with all cause mortality, CHD mortality exhibited a more linear association with BMI. CHD mortality was significantly higher for women who were overweight or obese prior to pregnancy. Estimates for stroke mortality risk exhibited a similar pattern to all-cause mortality, but did not reach age-adjusted statistical significance for the underweight group. None of the estimates for stroke reached statistical significance after further adjustment. All estimates persisted after adjustment for pregnancy weight gain.
Table 2.
Sensitivity analyses for body mass index and cause-specific mortality: Hazard ratios and 95% confidence intervals
| Analytic Approach | BMI category | Stroke | CHD | Non-Vascular * | All Cause | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR | CI | HR | CI | HR | CI | HR | CI | ||
| Age Adjusted Analysis | |||||||||
| # events | 110 | 226 | 1348 | 1836 | |||||
| Underweight | 1.62 | 0.85, 3.07 | 1.02 | 0.57, 1.80 | 1.36 | 1.13, 1.64 | 1.3 | 1.10, 1.53 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.64 | 1.01, 2.63 | 2.15 | 1.56, 2.96 | 1.29 | 1.11, 1.49 | 1.49 | 1.32, 1.68 | |
| Obese | 2.08 | 1.04, 4.20 | 4.02 | 2.70, 6.00 | 1.72 | 1.39, 2.14 | 2.16 | 1.83, 2.57 | |
| Demographically Adjusted Analysis † | |||||||||
| # events | 94 | 177 | 1082 | 1465 | |||||
| Underweight | 1.49 | 0.74, 3.02 | 1.08 | 0.58, 2.02 | 1.34 | 1.09, 1.65 | 1.27 | 1.05, 1.53 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.29 | 0.76, 2.21 | 1.84 | 1.28, 2.67 | 1.22 | 1.03, 1.44 | 1.35 | 1.17, 1.55 | |
| Obese | 1.43 | 0.64, 3.18 | 2.47 | 1.50, 4.06 | 1.54 | 1.20, 1.96 | 1.7 | 1.39, 2.07 | |
| Fully Adjusted Analysis ‡ | |||||||||
| # events | 60 | 130 | 789 | 1053 | |||||
| Underweight | 1.38 | 0.53, 3.59 | 1.09 | 0.52, 2.28 | 1.53 | 1.22, 1.94 | 1.43 | 1.15, 1.77 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.59 | 0.82, 3.08 | 1.39 | 0.89, 2.18 | 1.17 | 0.96, 1.43 | 1.27 | 1.08, 1.50 | |
| Obese | 1.56 | 0.57, 4.26 | 1.56 | 0.83, 2.92 | 1.31 | 0.96, 1.79 | 1.43 | 1.11, 1.83 | |
| Missing BMI filled with first trimester BMI § | |||||||||
| # events | 98 | 197 | 1174 | 1588 | |||||
| Underweight | 1.47 | 0.73, 2.96 | 1.08 | 0.60, 1.97 | 1.31 | 1.07, 1.60 | 1.25 | 1.05, 1.51 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.22 | 0.72, 2.06 | 1.77 | 1.25, 2.50 | 1.18 | 1.01, 1.39 | 1.33 | 1.16, 1.51 | |
| Obese | 1.44 | 0.68, 3.07 | 2.33 | 1.46, 3.72 | 1.58 | 1.26, 1.98 | 1.72 | 1.43, 2.07 | |
| Missing BMI multiply imputed ∥ | |||||||||
| # events | 151 | 312 | 1835 | 2499 | |||||
| Underweight | 1.32 | 0.66, 2.64 | 1.08 | 0.59, 1.99 | 1.24 | 1.01, 1.52 | 1.18 | 0.98, 1.41 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.23 | 0.75, 2.01 | 1.74 | 1.23, 2.45 | 1.17 | 1.00, 1.37 | 1.31 | 1.16, 1.49 | |
| Obese | 0.9 | 0.50, 1.62 | 1.67 | 1.17, 2.39 | 1.24 | 1.06, 1.45 | 1.28 | 1.12, 1.46 | |
| Average study BMI # | |||||||||
| # events | 100 | 198 | 1175 | 1593 | |||||
| Underweight | 1.61 | 0.83, 3.15 | 1.01 | 0.54, 1.87 | 1.25 | 1.02, 1.54 | 1.2 | 0.99, 1.44 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.06 | 0.61, 1.83 | 1.85 | 1.31, 2.63 | 1.21 | 1.03, 1.43 | 1.33 | 1.16, 1.52 | |
| Obese | 1.42 | 0.66, 3.005 | 2.5 | 1.56, 4.01 | 1.51 | 1.19, 1.93 | 1.65 | 1.36, 2.01 | |
| Excluding deaths in the first 5 years of follow up | |||||||||
| # events | 94 | 176 | 1064 | 1446 | |||||
| Underweight | 1.49 | 0.74, 3.02 | 1.09 | 0.58, 2.04 | 1.36 | 1.11, 1.68 | 1.29 | 1.07, 1.55 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.29 | 0.75, 2.21 | 1.86 | 1.29, 2.69 | 1.24 | 1.04, 1.47 | 1.37 | 1.19, 1.57 | |
| Obese | 1.43 | 0.64, 3.17 | 2.5 | 1.52, 4.11 | 1.5 | 1.17, 1.93 | 1.68 | 1.37, 2.06 | |
| Excluding deaths in the first 10 years of follow up | |||||||||
| # events | 93 | 174 | 1030 | 1405 | |||||
| Underweight | 1.49 | 0.74, 3.02 | 1.11 | 0.59, 2.08 | 1.36 | 1.10, 1.68 | 1.29 | 1.07, 1.56 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.22 | 0.71, 2.11 | 1.93 | 1.34, 2.80 | 1.23 | 1.04, 1.47 | 1.37 | 1.19, 1.58 | |
| Obese | 1.46 | 0.65, 3.24 | 2.61 | 1.59, 4.30 | 1.54 | 1.19, 1.98 | 1.73 | 1.41, 2.12 | |
| Excluding women < 18 years of age | |||||||||
| # events | 94 | 176 | 1069 | 1451 | |||||
| Underweight | 1.48 | 0.73, 3.01 | 1.09 | 0.59, 2.04 | 1.36 | 1.10, 1.67 | 1.28 | 1.06, 1.55 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.29 | 0.76, 2.21 | 1.87 | 1.29, 2.69 | 1.22 | 1.03, 1.44 | 1.35 | 1.18, 1.56 | |
| Obese | 1.42 | 0.64, 3.17 | 2.5 | 1.52, 4.11 | 1.56 | 1.22, 1.99 | 1.72 | 1.41, 2.10 | |
| Never Smokers | |||||||||
| # events | 44 | 63 | 389 | 534 | |||||
| Underweight | 1.32 | 0.39, 4.40 | 1.04 | 0.32, 3.40 | 1.16 | 0.79, 1.70 | 1.07 | 0.76, 1.52 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.44 | 0.68, 3.05 | 2.23 | 1.24, 4.03 | 1.37 | 1.05, 1.79 | 1.54 | 1.24, 1.91 | |
| Obese | 1.86 | 0.69, 4.97 | 2.87 | 1.33, 6.16 | 1.84 | 1.28, 2.65 | 1.98 | 1.47, 2.67 | |
| Current Smokers | |||||||||
| # events | 34 | 97 | 518 | 701 | |||||
| Underweight | 1.6 | 0.55, 4.67 | 0.92 | 0.42, 2.02 | 1.33 | 1.01, 1.74 | 1.27 | 1.00, 1.62 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.34 | 0.52, 3.43 | 1.73 | 1.04, 2.88 | 1.15 | 0.89, 1.49 | 1.3 | 1.05, 1.61 | |
| Obese | 0.76 | 0.10, 5.72 | 1.96 | 0.88, 4.35 | 1.32 | 0.87, 2.00 | 1.57 | 1.13, 2.19 | |
| Previous Smokers | |||||||||
| # events | 16 | 17 | 171 | 226 | |||||
| Underweight | 3.6 | 0.75, 17.38 | 2.04 | 0.25, 16.55 | 1.27 | 0.66, 2.43 | 1.33 | 0.75, 2.36 | |
| Normal | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Overweight | 1.25 | 0.31, 4.96 | 1.5 | 0.38, 5.92 | 1.22 | 0.80, 1.87 | 1.23 | 0.86, 1.76 | |
| Obese | 1.07 | 0.13, 9.00 | 6.05 | 1.65, 22.13 | 1.91 | 1.08, 3.36 | 1.91 | 1.18, 3.09 | |
Abbreviations: BMI (body mass index), CI (confidence interval), CHD (coronary heart disease), HR (hazard ratio)
Non-vascular causes of death include all causes of death that are not cardiovascular in nature.
Reported analysis is from the Demographically adjusted Survival model: mortality=age at enrollment +underweight+overweight+obese +education+annual family income+African American
This model is used for all analyses except where otherwise stated
Fully adjusted survival model: mortality= demographically adjusted model + smoking+age at first menstruation+parity+oral contraceptive use+hypertension+diabetes+kidney disease
Where self reported pre-pregnancy weight was unavailable, we used measured weight in the first trimester to calculate BMI
Multiple imputation model: pre-pregnancy BMI= first measured BMI +gestational weight in days of measured BMI+ measured height+ age at enrollment+ parity at enrollment+ education+ annual family income+ smoking status+ diagnosis of diabetes +oral contraceptive use before most recent pregnancy
Average study BMI includes pre-pregnancy BMI averaged across all pregnancies recorded in the CHDS
Our sample had 46 cancer deaths and there was no significant association between BMI category and cancer mortality: HR for underweight 1.82 (95% CI: 0.78, 4.23), overweight 1.27 (0.58, 2.78), and obese 0.43 (0.06, 3.15). There were 32 deaths due to respiratory disease and being overweight was significantly associated with increased respiratory mortality (HR 2.39 (1.04, 5.46). There were insufficient events in obese individuals to assess the association with respiratory disease. With only 20 deaths due to endocrine or metabolic disease, there were not enough events for an underweight estimate, but we identified a statistically significant association between obesity and metabolic mortality: HR for overweight 1.45 (0.42, 5.01), and obese 8.17 (2.91, 23.0).
The relationship between BMI and all-cause mortality was similar to that for mortality from causes other than CVD, cancer, respiratory disease, or metabolic disease: HR of underweight 1.31 (1.05,1.63), overweight 1.17 (0.97, 1.39), and obese 1.52 (1.17, 1.97).
The association between BMI and cause-specific mortality was not qualitatively or statistically different for African American compared with Caucasian participants. Similarly, we did not detect a significant interaction between BMI and age in the mortality association.
Sensitivity Analyses
Table 2 shows the results of different modeling strategies and sensitivity analyses. Adjustment for confounding from socio-economic factors slightly attenuated the results. Estimates were further attenuated and less likely to be significant after further adjustment for parity, age at menarche, oral contraceptive use, smoking, pre-existing diabetes, hypertension, and kidney problems. In analyses to account for regression dilution bias, the results were not appreciably changed by using the exposure defined as pre-pregnancy BMI averaged across all included study pregnancies. Few deaths (n = 19) occurred in the first five years of follow-up and excluding them from the analysis to adjust for confounding by illness related weight loss did not significantly change the hazard ratio estimates. Similarly, excluding deaths in the first 10 years (n = 60) also did not alter the estimates, nor did excluding current or previous smokers. In an assessment of misclassification, pre-pregnancy BMI based on self-reported weight had high agreement with BMI based on measured first trimester weight (Kappa=0.68 (0.67, 0.71)). Different techniques for reducing the level of missing BMI values, including multiple imputation, produced similar results as well (Table 2). Finally, mortality estimates were unchanged when we excluded women less than 18 years of age at the time of pregnancy.
DISCUSSION
In the CHDS pregnancy cohort, women who were overweight or obese prior to pregnancy had a significantly higher risk of CHD mortality compared with normal weight women after an average of 37 years of follow-up. Women with a pre-pregnancy BMI in the underweight, overweight or obese BMI categories had increased risk of both non-cardiovascular and all-cause mortality. These results were robust to a number of different sensitivity analyses assessing for biases.
Our results are consistent with prior studies describing the association between BMI and mortality in women and enhances our understanding of this association at younger ages. Similar to other published studies, we found a significant j-shaped association between BMI and all-cause mortality, with increased risk at both lower and higher BMI categories(6, 8, 29, 30). We also confirmed a significant linear increase in CHD mortality risk in women with higher BMI.(8) Our results are also consistent with prior studies that have included women younger than middle age.(12, 13, 14, 31) Our study contributes evidence to only four studies, to our knowledge, that examined the prospective association between BMI in adolescent or young adult women and mortality.
The other evidence about BMI in younger women comes from two studies using data from the Nurses’ Health Study from Manson and colleagues and van Dam and colleagues,(14, 31) a study from Bjørge and colleagues that appears to build on the work by Engeland and colleagues in the Norwegian health surveys,(12, 32) and a National Health Interview Survey study by Ma and colleagues. (13) The most notable difference between these studies and ours is the all-cause mortality results. While these prior studies found an association with overweight and obesity, they did not find association in the underweight group.(12, 13, 14, 31)
Our study supports evidence that being underweight, similar to being obese, is also associated with higher mortality. While this result is not consistent with other studies in young adults,(12, 13, 14, 31) it does support what has been shown in older adults. Given the skepticism around the results in older adults, we further explored this relationship in our dataset using sensitivity analyses. Since some evidence suggests that pregnant adolescents are more likely to become overweight or obese in adulthood(33, 34) and because the underweight category disproportionately included adolescents, we excluded them from the analysis. We also excluded smokers, who may be underweight but have higher mortality, and assessed whether women in the underweight group had more pre-pregnancy co-morbid illnesses, as being underweight could be marker for poor health status. None of these additional analyses provided an explanation for our results. Despite the rapidly decreasing prevalence of underweight in the US and similar countries,(1) if shown to have a causal basis, this association may play an important role in the mortality of countries with greater levels of underweight.
The contrasts between our study and findings from the Nurses’ Health Study, the Norwegian health surveys, and the National Health Interview Survey may be explained by two aspects of the differences in follow-up. First, the average ages of death in the NHIS and Norwegian cohorts were approximately 40 years compared to 64 years in the CHDS, because the NHIS and Norwegian cohorts ended follow-up when women were still relatively young. Ending follow-up before the peak age of risk may have changed the distribution of causes of death across the three cohorts. The shifted distribution of CVD and respiratory disease to older women, who had follow-up data available in CHDS, but not the other cohorts,could have influenced the all-cause mortality estimates. If the causes of death most common for younger women such as accidents are less affected by BMI than those for older women such as heart disease, then the estimates for all-cause mortality from a study that ends follow-up in middle age may underestimate the association between BMI and all-cause mortality for the majority of women. Second, by ending follow-up well before the age of peak risk, studies may underestimate the strength of the association between BMI and mortality in women.(13) This is a particular concern for studies that examined BMI in younger women but did not have the long follow up duration needed to reach disease-specific peak risk.(12, 13, 14, 31) Specifically for women who’s young adult BMI is an indication of a lifelong BMI trajectory or other cumulative factors that may put them at risk later in life, studies with shorter follow-up may not assess the full association between BMI and mortality. Our study further adds to the body of literature examining BMI in young women and cause-specific mortality by providing rare evidence from follow up into the age of peak risk, using robust methods to account for bias.
While the increased risk of mortality from obesity is accepted, controversy remains about the relationship between overweight and mortality.(7, 35) For example, since McGee et al. did not find an association between overweight and all-cause mortality, they suggest that the overweight category may be less important.(7) In contrast, our findings, and those from other studies of younger women,(12, 13, 14, 31) support evidence of a significantly increased all-cause mortality risk from being overweight. While McGee et al. did not find such an association with their main meta-analysis, they did find significant heterogeneity among the included studies, but did not examine the effects of differing follow-up times on the BMI/mortality association. Lewis et al. suggest that some of the heterogeneity with regards to a positive association between overweight and mortality can be ascribed to studies with inadequate statistical power resulting from follow-up times that are too short and have insufficient event numbers.(36)
We suggest a different reason for the heterogeneity, based on McGee et al. finding a significant association between overweight and CHD mortality, consistent with our results. Ma et al. found that different distributions in cause of death between men and women helped explained the sex differences in National Health Interview Survey all-cause mortality.(13) Similarly, in studies with a lower proportion of deaths from CHD, including cohorts still too young to be at peak risk, overweight may contribute less to overall mortality. Studies with a different mean age at final follow-up will therefore have different distributions for cause-specific mortality that may explain the heterogeneity in estimates of all-cause mortality.
This study has several limitations. First, pre-pregnancy weight was self-reported, however, similar to previous literature,(37) we found that the BMI calculated from self-reported weight had high agreement with the BMI calculated from measured weight early in pregnancy. Misclassification of self-reported BMI is likely to be away from the extremes and would lead to underestimation of the association with mortality. Second, post-pregnancy exposures such as lifestyle factors and the development of CVD risk factors and diagnoses were not measured in this study. For example, the development of hypertension following pregnancy may be associated with elevated pre-pregnancy BMI, but may be an even more important independent predictor of CVD mortality. These intermediate factors on the pathway between overweight/obese and CVD mortality may be important to assess in the life course trajectory, but may not need to be adjusted for in models assessing the association between BMI and mortality.(36) Third, the average age in 2008 for women in the cohort was 72 years, which is the mean age at first stroke for women.(15) This may be the reason why there were only a small number of stroke deaths (n = 151), which limited the precision for mortality estimates, especially in the underweight group. Furthermore, the lack of stroke events did not allow for subgroup analysis by stroke type. Fourth, missing data on BMI limited the effective sample size and missing data on other covariates may limit the interpretability of adjusted estimates. Fifth, the use of ICD coded mortality data from the California Vital Status Records may have misclassified specific causes of death. This misclassification could lead to underestimates of the cause-specific hazards ratios, but should not affect estimates for all-cause mortality.
This study has a number of strengths. The most significant strength was the long follow-up duration that began with BMI measured pre-pregnancy in early adulthood and continued into the peak ages for cardiovascular risk for women. Since most prior studies did not begin recruitment until middle age or later, this is a useful contribution. Similarly, the large cohort and long follow up duration provided a sufficient number of events to investigate cause-specific mortality in addition to combined mortality. The large number of available covariates enabled sensitivity and subgroup analyses that supported the main results. While this study is limited by only having BMI measured at one point during the lifespan, using pre-pregnancy BMI offers some advantages over using one measurement before adulthood or after middle age. Weight trajectory has mostly stabilized by the reproductive years and is well correlated with pregnancy weight gain and post-pregnancy weight retention. (3, 19) Finally, this is a study of young adults, who are less likely to experience frailty or sudden unintentional weight loss and less likely to die early in follow up. This minimizes the potential for reverse causation and survivor bias, (13) two main reasons to suspect that the risk of mortality from overweight and obesity might be underestimated.(38, 39, 40)
Pregnancy provides a rare opportunity to approach younger women with health messages and behavior change interventions.(22) More specifically, pregnancy can be a “powerful teachable moment” for weight control.(23) The importance of pregnancy and weight changes during the reproductive years on setting BMI trajectories,(19) combined with the increased involvement with health systems and potential for behavior change make the pre, peri, and post-partum window an opportune time to address the negative health effects of overweight and obesity in young women. This study provides evidence that BMI in the reproductive years may play a role in later life mortality risk in women and reinforces the need for healthy weight interventions around the time of pregnancy.
In conclusion, our study found that pre-pregnancy BMI is associated with all-cause and CHD mortality risk, possibly through the early development of BMI trajectories or a cumulative effect of excess adiposity. These results support the hypothesis that the influence of overweight and obesity on health begins early in life and persists as women age. This study further supports the need for interventions that focus on weight loss or maintenance in younger women.
What is already known about this subject.
Weight gain during pregnancy and retention afterwards are important predictors of weight in middle age women.
Obesity in middle aged women is associated with risk for cardiovascular disease and mortality.
What this study adds
Pre-pregnancy overweight and obesity are significantly associated with higher coronary heart disease mortality in women.
Pre-pregnancy underweight, overweight, and obesity are significantly associated with higher all-cause mortality in women.
Acknowledgments
MMC designed the study, conducted analyses, wrote and edited the manuscript. CAMA and JC contributed interpretation of analyses and edited the manuscript. WB helped design the study, wrote and edited the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.
We would like to thank the staff and participants of the Child Health and Development Studies for their valuable contributions. We would specifically like to acknowledge Piera Cirillo and Nickilou Krigbaum for sharing their expertise with the CHDS datasets.
Funding Sources: Morgana Mongraw-Chaffin was supported by National Heart, Lung, and Blood Institute grant 5T32HL007024 and by National Institute of Diabetes and Digestive and Kidney Diseases grant 5T32DK062707. Dr. Wendy Bennett is supported by a career development award from the National Heart, Lung, and Blood Institute, 5K23HL098476– 02
Abbreviations
- BMI
Body mass index
- CVD
Cardiovascular disease
- CHDS
Child Health and Development Studies
- CI
Confidence interval
- CHD
Coronary heart disease
- HR
Hazard ratio
- ICD
International Classification of Diseases
- NHIS
National Health Interview Survey
Footnotes
Competing Interests: the authors have no competing interests.
REFERENCES
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. Jama. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Guo S, Huang C, Maynard L, Demerath E, Towne B, Chumlea W, et al. Body mass index during childhood, adolescence and young adulthood in relation to adult overweight and adiposity: the Fels Longitudinal Study. International Journal of Obesity. 2000;24:1628–1635. doi: 10.1038/sj.ijo.0801461. [DOI] [PubMed] [Google Scholar]
- 3.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. The new england journal of medicine. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 4.Owen C, Whincup P, Orfei L, Chou Q-A, Rudnicka A, Wathern A, et al. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. International Journal of Obesity. 2009;33:866–877. doi: 10.1038/ijo.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Look HC. Childhood Obesity, Other Cardiovascular Risk Factors, and Premature Death. New England Journal of Medicine. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 10.Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 11.Park HS, Song Y-M, Cho S-I. Obesity has a greater impact on cardiovascular mortality in younger men than in older men among non-smoking Koreans. International Journal of Epidemiology. 2006;35:181–187. doi: 10.1093/ije/dyi213. [DOI] [PubMed] [Google Scholar]
- 12.Bjørge T, Engeland A, Tverdal A, Smith GD. Body Mass Index in Adolescence in Relation to Cause-specific Mortality: A Follow-up of 230,000 Norwegian Adolescents. American Journal of Epidemiology. 2008;168:30–37. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 13.Ma J, Flanders WD, Ward EM, Jemal A. Body Mass Index in Young Adulthood and Premature Death: Analyses of the US National Health Interview Survey Linked Mortality Files. American Journal of Epidemiology. 2011;174:934–944. doi: 10.1093/aje/kwr169. [DOI] [PubMed] [Google Scholar]
- 14.van Dam RM, Willett WC, Manson JE, Hu FB. The Relationship between Overweight in Adolescence and Premature Death in Women. Annals of Internal Medicine. 2006;145:91–97. doi: 10.7326/0003-4819-145-2-200607180-00006. [DOI] [PubMed] [Google Scholar]
- 15.Appelros P, Stegmayr B, Terént A. Sex Differences in Stroke Epidemiology : A Systematic Review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 16.Roger VL, Go AS, Donald M, Lloyd-Jones EJB, Berry JD, Borden WB, Bravata DM, et al. Heart Disease and Stroke Statistics--2012 Update : A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. Journal of the American College of Cardiology. 2011;57:1404–1423. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford E, Ajani U, Croft J, Critchley J, Labarthe D, Kottke T, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980- 2000. New England Journal of Medicine. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 19.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36:317–332. ix. doi: 10.1016/j.ogc.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nodine PM, Hastings-Tolsma M. Maternal Obesity: Improving Pregnancy Outcomes. The American journal of maternal child nursing. 2012;37:110–115. doi: 10.1097/NMC.0b013e3182430296. [DOI] [PubMed] [Google Scholar]
- 21.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? British Medical Journal. 2002;325:157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pina IL. Cardiovascular disease in women: challenge of the middle years. Cardiol Rev. 2011;19:71–75. doi: 10.1097/CRD.0b013e318209c233. [DOI] [PubMed] [Google Scholar]
- 23.Phelan S. Pregnancy: a “teachable moment” for weight control and obesity prevention. Am J Obstet Gynecol. 2010;202:135–e131138. doi: 10.1016/j.ajog.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Berg B, Christianson R, Oechsli F. The California child health and development studies of the school of public health, University of California at Berkeley. Paediatr Perinat Epidemiol. 1988;2:265–282. doi: 10.1111/j.1365-3016.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 25.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and Cardiovascular Disease Death. Prospective Evidence From theChild Health and Development Studies Cohort. Hypertension. 2010;56:166–171. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United States Department of Labor: Bureau of Labor Statistics. CPI Inflation Calculator. 2012.
- 27.United States Census Bureau . State & County QuickFacts: Alameda County. California: [Last Revised: Thursday, 06-Dec-2012 [cited 2013 1-27-2013]]. [Google Scholar]
- 28.StataCorp . Stata Statistical Software: Release 11. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- 29.Song X, Pitkaniemi J, Gao W, Heine RJ, Pyorala K, Soderberg S, et al. Relationship between body mass index and mortality among Europeans. Eur J Clin Nutr. 2012;66:156–165. doi: 10.1038/ejcn.2011.145. [DOI] [PubMed] [Google Scholar]
- 30.Sasazuki S, Inoue M, Tsuji I, Sugawara Y, Tamakoshi A, Matsuo K, et al. Body Mass Index and Mortality From All Causes and Major Causes in Japanese: Results of a Pooled Analysis of 7 Large-Scale Cohort Studies. Journal of Epidemiology. 2011;21:417–430. doi: 10.2188/jea.JE20100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 32.Engeland A, Bjørge T, Søgaard AJ, Tverdal A. Body Mass Index in Adolescence in Relation to Total Mortality: 32-Year Follow-up of 227,000 Norwegian Boys and Girls. American Journal of Epidemiology. 2003;157:517–523. doi: 10.1093/aje/kwf219. [DOI] [PubMed] [Google Scholar]
- 33.Gunderson EP, Striegel-Moore R, Schreiber G, Hudes M, Biro F, Daniels S, et al. Longitudinal study of growth and adiposity in parous compared with nulligravid adolescents. Arch Pediatr Adolesc Med. 2009;163:349–356. doi: 10.1001/archpediatrics.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hediger ML, Scholl TO, Schall JI. Implications of the Camden Study of adolescent pregnancy: interactions among maternal growth, nutritional status, and body composition. Ann N Y Acad Sci. 1997;817:281–291. doi: 10.1111/j.1749-6632.1997.tb48214.x. [DOI] [PubMed] [Google Scholar]
- 35.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. Journal of the American Medical Association. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis CE, McTigue KM, Burke LE, Poirier P, Eckel RH, Howard BV, et al. Mortality, health outcomes, and body mass index in the overweight range: a science advisory from the American Heart Association. Circulation. 2009;119:3263–3271. doi: 10.1161/CIRCULATIONAHA.109.192574. [DOI] [PubMed] [Google Scholar]
- 37.Phelan S, Phipps MG, Abrams B, Darroch F, Schaffner A, RR W. Randomized trial of a behavioral intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. American Journal of Clinical Nutrition. 2011;93:772–779. doi: 10.3945/ajcn.110.005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg JA. Correcting Biases in Estimates of Mortality Attributable to Obesity. Obesity. 2006;14:2071–2079. doi: 10.1038/oby.2006.242. [DOI] [PubMed] [Google Scholar]
- 39.Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse Causation and Illness-related Weight Loss in Observational Studies of Body Weight and Mortality. American Journal of Epidemiology. 2010;173:1–9. doi: 10.1093/aje/kwq341. [DOI] [PubMed] [Google Scholar]
- 40.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]