Abstract
Objective
To evaluate the association between self-reported daily sitting time and the incidence of type II diabetes in a cohort of postmenopausal women.
Design and Methods
Women (N = 88,829) without diagnosed diabetes reported the number of hours spent sitting over a typical day. Incident cases of diabetes were identified annually by self-reported initiation of using oral medications or insulin for diabetes over 14.4 years follow-up.
Results
Each hour of sitting time was positively associated with increased risk of diabetes (Risk ratio (RR): 1.05; 95% confidence interval (CI): 1.02–1.08]. However, sitting time was only positively associated with incident diabetes in obese women. Obese women reporting sitting 8–11 (RR: 1.08; 95% CI 1.0–1.1), 12–15 (OR: 1.13; 95% CI 1.0–1.2), and ≥16 hours (OR: 1.25; 95% CI 1.0–1.5) hours per day had an increased risk of diabetes compared to women sitting ≤ 7 hours per day. These associations were adjusted for demographics, health conditions, behaviors (smoking, diet and alcohol intake) and family history of diabetes. Time performing moderate to vigorous intensity physical activity did not modify these associations.
Conclusion
Time spent sitting was independently associated with increased risk of diabetes diagnosis among obese women— a population already at high risk of the disease.
Keywords: sedentary, glucose control, overweight, glycemia
Introduction
Time spent in sedentary behaviors is extremely common among today’s population, with the majority of middle-age Americans spending over half their waking day (approximately 8 hours) engaged in sedentary pursuits (1). Physical inactivity and associated low level of energy expenditure have traditionally been considered a major risk factor for weight gain, by promoting positive energy balance (2). Public health recommendations have primarily focused on increasing levels of physical activity to achieve energy homeostasis and/or negative energy balance in order to obtain a variety of metabolic benefits that influence glucose regulation, weight maintenance and other health outcomes (3). The potential role that sustained engagement in sedentary activities, most commonly sitting (4), has in promoting metabolic dysregulation (e.g. Type II diabetes) is increasingly recognized. Importantly, recent findings suggest that metabolic dysregulation associated with prolonged sitting may confer adverse health effects even among individuals who are otherwise physically active (5, 6).
New evidence suggests that people exposed to prolonged sedentary time have adverse cardiometabolic risk factor profiles and elevated risk of cardiovascular disease (CVD) and even death. These findings are observed even after accounting for regular exercise habits and body weight status (7–9). Poor glucose control has been proposed as one potential physiological mechanism for the adverse health effects seen in people who lead excessively sedentary lifestyles (5, 6, 10). This is particularly true in women who have made a menopausal transition that is linked to central adiposity, diabetes and cardiovascular disease (11). However, while these previous studies have elucidated a potential mechanism that might link sedentary time with risks of CVD, mortality, and other outcomes including diabetes mellitus, reported findings are limited by their cross-sectional design. Understanding the temporal relationship between exposure to prolonged sedentary time and development of diseases such as diabetes is critical to clarifying whether the association is causal and to defining potential opportunities for enhancing existing strategies for disease prevention.
We conducted a longitudinal analysis in the Women’s Health Initiative Observational Study (WHI OS) to investigate the association between self-reported sitting time and incidence of diabetes mellitus. Based on extant literature (5, 6, 10), we hypothesized that women who reported longer sitting time would have a higher risk for developing diabetes. We also explored the modifying effects of physical activity and body mass—known risk factors for diabetes and likely to be connected to participating in sedentary behaviors (12).
Methods
Study population
The WHI OS is a prospective, multicenter observational study designed to address the major causes of illness and death in postmenopausal women (see the Acknowledgements section for a list of study investigators) (13). Women were screened out of the study due to the presence of any medical condition associated with predicted survival of less than three years, alcoholism, mental illness, or dementia. A total of 93,676 women aged 50–79 years were enrolled in the observational study at 40 clinical centers between 1994 and 1998. Women were excluded from the present analyses if, at baseline, they were taking pills or insulin for diabetes or had missing information about treatment status (n = 3890), or had missing data on the question that asked about daily sitting time (n = 867). An additional 579 women did not have information about diabetes status during follow-up. After women were excluded for these reasons, 88,250 women remained in the analysis. All women provided written informed consent, and the study protocol was approved by the institutional review board of each center.
Measurement of sitting time exposure
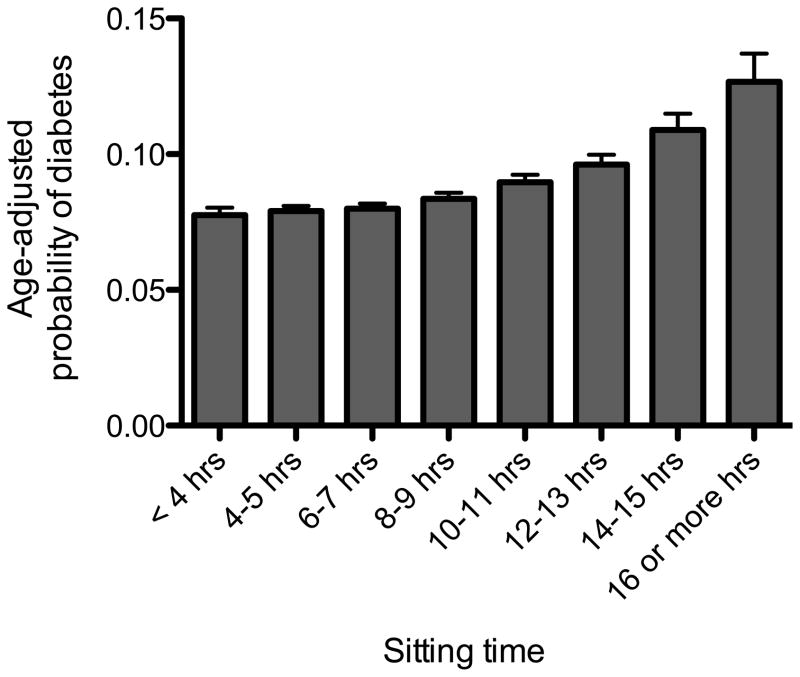
During the WHI OS baseline screening clinic visit women were asked to complete a self-administered questionnaire that asked about personal socioeconomic factors, medical history, physical activity, smoking, diet, and other behaviors. Amount of daily sitting time was measured by asking, “During a usual day and night, about how many hours do you spend sitting? Be sure to include the time you spend sitting at work, sitting at the table eating, driving or riding in a car or bus, and sitting up watching TV or talking.” Eight categories were available for women to choose: <4, 4–5, 6–7, 8–9, 10–11, 12–13, 14–15, and >16 hours. These categories were collapsed to four (≤7 hrs, 8–11 hrs, 12–15 hrs and 16 or more hours) categories after performing preliminary analyses that examined the association between the risk of diabetes across sitting time categories as seen in Figure 1. In separate studies, test-retest reliability has been high (Reliability Coefficient ~ 0.60–0.80) and correlation with monitor-based determination of sedentary time has been modest (Pearson Correlation Coefficient = 0.39) (14, 15).
Figure 1.
Age-adjusted probability of diabetes according to hours of sitting (p-value for trend < 0.001).
Measurement of diabetes
At baseline, participants were asked if a physician had ever told them that they had ‘sugar diabetes or high blood sugar’ when they were not pregnant. Follow-up questions were asked about age at diagnosis, hospitalization for diabetic coma, dietary treatment, history of treatment with oral medications, and past and current treatment with insulin shots. Prevalent diabetes at baseline was ascertained by asking participants whether a doctor had diagnosed them with diabetes and prescribed pills or shots (‘treated diabetes’). These individuals were excluded from the analyses. Blood assays were only performed on a 1% sub- sample of WHI OS participants at baseline, and thus are not included as variables in these analyses.
Cases of incident diabetes reported as of 5/26/2011 were included in the present study. Follow-up questionnaires asked about treatment for diabetes using the question, ‘Since the date given on the front of this form, has a doctor prescribed any of the following pills or treatments?’ Choices included ‘pills for diabetes’ and ‘insulin shots for diabetes’. Incident treated diabetes was ascertained and defined as the self-report of a new physician diagnosis of diabetes treated with oral pills or insulin. Unfortunately, information about diabetes without treatment for pills or insulin (i.e. with lifestyle intervention) was not ascertained at follow-up evaluations. Self-reported incident treated diabetes was found to be concordant with medication inventory in 72% of women in the WHI OS study, while more than 99% of those women not reporting diabetes had no evidence of antidiabetic medications or insulin in their medication inventory (16).
Other measures
Dietary habits, medical history, household income, education, health behaviors (e.g. smoking, alcohol intake), ethnicity/race, family history of diabetes, quality of life and psychosocial factors were collected by self-report. Ethnicity and race was included into models as an ordinal covariate after rank ordering the categories according to prior knowledge about risk of diabetes in the WHI OS (Lowest risk in Caucasians and greatest risk in African Americans) (17). Family history of diabetes was coded as the number of immediate relatives (parents, full-blooded siblings and progeny) ever having sugar diabetes or high blood sugar that first appeared as an adult. Moderate to vigorous intensity physical activity (MVPA) was determined by using a detailed questionnaire on the frequency and duration of walking and other types of activity as described elsewhere (18). Walking was assessed by a series of questions about the frequency of walks outside the home for more than 10 minutes without stopping. The average duration of each walk and the usual walking pace was recorded. Vigorous exercise was defined as that in which “you work up a sweat and your heart beats fast,” and examples included aerobics, aerobic dancing, jogging, tennis, and swimming laps. Moderate exercise was defined as that which was “not exhausting,” and examples included biking outdoors, using an exercise machine (such as a stationary bicycle or a treadmill), calisthenics, easy swimming, and popular or folk dancing. Total weekly physical activity energy expenditure was calculated as the summed product of frequency, duration, and intensity for reported activities. The duration, in minutes per week performing MVPA, was categorized into three levels according to the US Department of Health and Human Services physical activity guidelines (3): none (0 minutes), some, but not recommended levels (1–149 minutes per week) and greater than or equal to 150 min/week. Trained staff measured height using a wall-mounted stadiometer and weight using a balance beam scale. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters and categorized into normal (<24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2). Dietary intake was assessed using a food frequency questionnaire described previously (20). Total caloric intake and macronutrient caloric amounts were used to calculate the percent caloric intake as carbohydrate, fat and protein.
Data analysis
Comparisons of participant baseline characteristics between sitting time categories were performed by analysis of variance or chi-square tests. The primary endpoint for this study was incident self-reported treatment of diabetes assessed at annual contacts. We first examined age-adjusted probability of diabetes across the eight sitting time categories and then across a priori effect modifiers of body mass index (BMI) and time performing MVPA. Models adjusted for covariates were developed to evaluate the incidence of diabetes across four sitting categories. Time to event analysis was not performed because it was not possible to ascertain true person time status with annual contacts. Thus, we used modified Poisson regression to estimate incidence rates and risk ratios according to sitting categories as described previously (19). Poisson regression is known to overestimate error variances when using binomial outcomes and thus, as suggested by Zou et al., we conducted the analysis using robust error variances produced using a sandwich estimation (20). We first examined potential effect modification by ethnicities that confer a high incidence of diabetes (Hispanic, Native and African Americans). Effect modification of the sitting time-diabetes association by BMI class and MVPA were also evaluated using product interaction terms in regression model 1 and through stratified analyses.
Four models were constructed to understand how MVPA and BMI influenced the association between sitting time and diabetes risk, while adjusting for demographic, comorbidity and lifestyle confounders. Model 1 adjusted for potential confounding due to age (in years), ethnicity and race (1= Caucasian; 2 = other; 3 = Asian; 4 = Hispanic; 5 = Native American; 6 = African American), college education (yes or no), income less than $35,000 per year, which represents individuals in the lowest third of income in the WHI OS sample (yes or no), marital status (yes or no being married or in a marriage-like relationship), reported history of health conditions (feeling depressed, hypertension, hyperlipidemia, osteoarthritis, history of cancer and cardiovascular disease), currently smoking (yes or no), alcohol intake > 7 drinks per week (yes or no), percent of daily caloric intake as carbohydrate and percent of daily caloric intake as fat. A second model (Model 2) adjusted for all confounders in Model 1 plus MVPA. Model 3 adjusted for all confounders in Model 2 plus BMI. Finally, Model 4 adjusted for all confounders in Model 3 and included both MVPA and BMI. All models were adjusted with a propensity score for comorbid conditions that included the prevalence of: feeling depressed, hypertension, hyperlipidemia, osteoarthritis, cancer and cardiovascular disease. A propensity score reduces a large number of confounders to a single summary measure and was calculated according to methods outlined by Fitzmaurice (21). Tests for a linear trend in diabetes risk across sitting time exposure groups were conducted by treating the categories of sitting time as an ordinal variable. Sensitivity analyses excluded data for the first year of follow-up in order to minimize potential bias on the exposure caused by the presence of subclinical disease. All tests were two-sided and an alpha level less than or equal to 0.05 was set to determine statistical significance.
Results
Characteristics of 88,829 non-diabetic women stratified across self-reported sitting time categories are displayed in Table 1. There were fewer Caucasians who reported sitting at the extremes, ≤7 hours and ≥16 hours per day. Hispanic/Latinos were more likely to report sitting ≤ 7 hours when compared to other sitting time categories. The proportion of African Americans and Asians or Pacific Islander increased with each category of reported sitting time. Marriage was associated with lower amount of time sitting. Women with a college education were less likely to report sitting ≤ 7 and ≥ 16 hours per day (an inverted “U” shaped association). The prevalence of women with an income less than $35,0000 per year was higher at the extremes of sitting categories— ≤ 7 hours and ≥16 hours per day. A higher body mass index was strongly associated with longer sitting time. Longer sitting time was associated with the following behaviors: higher caloric intake, higher fat intake, lower carbohydrate intake, slightly lower protein intake, and lower levels of MVPA. Reporting a poor quality of life, hypertension, hyperlipidemia, osteoarthritis, prevalent cardiovascular disease and cancer were higher among those who reported sitting for longer periods of times. Despite having an overall higher rate of diabetes, the effects of sitting time on incident diabetes were similar in Hispanic, Native and African American (p-values for all ethnicity by sitting time interactions > 0.15).
Table 1.
Demographics and health conditions according to sitting time among women without diabetes baseline.
| Characteristics | Total n = 88,829 | ≤ 7 hours n = 51,622 | 8–11 hours n = 26,713 | 12–15 hours n = 9,471 | ≥16 hours 1,023 | p-value |
|---|---|---|---|---|---|---|
| Age, M (SD) | 88,829 | 64.4 (7.2) | 62.8 (7.4) | 61.1 (7.3) | 61.8 (7.5) | <0.001 |
| Caucasian, N (%) | 74,960 | 43,080 (83.7) | 23,060 (86.5) | 8,057 (85.3) | 763 (74.8) | <0.001 |
| African American, N (%) | 6,568 | 3,944 (7.6) | 1,748 (6.6) | 715 (7.6) | 161 (15.8) | <0.001 |
| Hispanic/Latino, N (%) | 3,229 | 2,249 (4.4) | 709 (2.7) | 242 (2.5) | 29 (2.8) | <0.001 |
| Asian or Pacific Islander, N (%) | 2,513 | 1,373 (2.7) | 773 (2.9) | 321 (3.4) | 46 (4.5) | <0.001 |
| American Indian or Alaskan Native, N (%) | 343 | 229 (0.44) | 79 (0.30) | 29 (.31) | 6 (0.59) | 0.005 |
| Other ethnicity, N (%) | 978 | 601 (1.2) | 277 (1.0) | 85 (0.90) | 15 (1.5) | 0.051 |
| Married, N (%) | 55,297 | 33,725 (65.7) | 15,878 (59.7) | 5,216 (55.3) | 478 (46.9) | <0.001 |
| College education, N (%) | 37,580 | 20,175 (39.4) | 12,298 (46.4) | 4,680 (49.1) | 427 (42.3) | <0.001 |
| Income $35,000 or less, N (%) | 34,311 | 20,106 (42.9) | 9,650 (37.6) | 3,118 (34.1) | 437 (44.5) | <0.001 |
| BMI (kg/m2), M (SD) | 87,778 | 26.6 (5.3) | 27.3 (5.9) | 28.2 (6.5) | 29.8 (7.4) | <0.001 |
| Alcohol intake (drinks/wk), M (SD) | 88,634 | 3.7 (1.6) | 3.8 (1.5) | 3.8 (1.5) | 3.4 (1.5) | <0.001 |
| Current smoker | 5,458 | 2,899 (5.7) | 1,726 (6.5) | 731 (7.8) | 102 (10.1) | <0.001 |
| Felt Depressed | 23,213 | 3,404 (6.6) | 1,914 (7.2) | 885 (9.4) | 159 (15.6) | <0.001 |
| Report a poor quality of life* | 6,845 | 3,824 (7.5) | 2,032 (7.6) | 869 (9.2) | 160 (15.8) | <0.001 |
| Fat intake (%) | 88,743 | 29.8 (8.5) | 30.1 (8.5) | 30.7 (8.6) | 32.5 (8.9) | <0.001 |
| Carbohydrate intake (%) | 88,743 | 52.9 (9.7) | 52.4 (9.6) | 51.8 (9.7) | 50.8 (10.3) | <0.001 |
| Protein intake (%) | 88,743 | 16.9 (3.3) | 16.9 (3.3) | 16.8 (3.2) | 16.5 (3.7) | <0.001 |
| Total calories per day (kcal/d) | 88,743 | 1513 (687) | 1580 (659) | 1636 (707) | 1710 (942) | <0.001 |
| Moderate to vigorous physical activity (minutes/week), M (SD) | 87,922 | 127.8 (163) | 106 (140) | 86.8 (123) | 65.0 (118) | <0.001 |
| Attain 150 min/wk of moderate to vigorous physical activity, N (%) | 28,469 | 17,889 (35.0) | 8,107 (30.6) | 2,296 (24.5) | 177 (17.6) | <0.001 |
| Family history of diabetes, N (%)† | 26,427 | 15,386 (29.8) | 7,923 (29.7) | 2,281 (29.8) | 297 (29.0) | 0.773 |
| Hypertension, N (%) | 48,717 | 28,532 (55.7) | 14,521 (54.6) | 5,061 (53.8) | 603 (59.5) | <0.001 |
| Hyperlipidemia, N (%) | 12,467 | 7,431 (14.7) | 3,632 (13.9) | 1,263 (13.6) | 141 (14.1) | 0.002 |
| Osteoarthritis, N (%) | 39,428 | 23,582 (46.0) | 11,445 (43.1) | 3,950 (41.9) | 451 (44.5) | <0.001 |
| History of CVD, N (%) | 15,995 | 9,201 (18.2) | 4,871 (18.6) | 1,716 (18.5) | 207 (20.8) | 0.144 |
| History of any Cancer, N(%) | 11,340 | 6,535 (12.7) | 3,412 (12.8) | 1,245 (13.2) | 148 (14.6) | 0.221 |
Depression score determined by Center for Epidemiological Studies-Depression questionnaire CES-D/DIS from SF-36
Hypertension determined by medication use
Poor quality of life defined as rating a 5 or lower on a 0–10 scale after asking “Overall, how would you rate your quality of life?”
CVD = cardiovascular disease
Family history of diabetes was defined having one’s mother, father, full-blooded sisters, full-blooded brothers, daughters or sons ever having sugar diabetes or high blood sugar that first appeared as an adult.
There were 7416 cases of incident diabetes during an average of 11.1 ± 3.2 years of follow-up. Figure 1 shows the probability of diabetes across all eight categories of sitting time. Each hour of sitting was positively associated with increased risk of diabetes when sitting time was modeled as a continuous variable (risk ratio (RR): 1.05; 95% confidence interval (CI): 1.02–1.08]. However, as can be seen in Figure 1, the probability of diabetes was similar among those women sitting ≤ 7 hours per day, but increased in a linear fashion in women reporting sitting for more than 8–9 hours per day. Based on these results, sitting time was categorized into four categories (≤7 hrs, 8–11 hrs, 12–15 hrs and 16 or more hours) and examined in adjusted models seen in Table 2. Fully adjusted model of sitting time continued to be associated with incident diabetes after adjusting for demographics and health conditions/behaviors (Table 2: Model 1). The inclusion of MVPA in the model reduced the association slightly (Table 2: Model 2). Adjusting for BMI significantly reduced the association between sitting time and odds of diabetes as seen in Models 3 and 4 in Table 2.
Table 2.
Risk ratios for incident diabetes according to sitting time exposure groups.
| Model 1 | Model 2 | Model 3 | Model 4 | ||
|---|---|---|---|---|---|
| No. of diabetes | |||||
| ≤ 7 hrs (N = 51,251) | 4,057 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 8–11 hrs (N = 26,577) | 2,295 | 1.14 (1.1–1.2) | 1.12 (1.0–1.2) | 1.07 (1.0–1.3) | 1.06 (1.0–1.1) |
| 12–15 hrs (N = 9,408) | 945 | 1.29 (1.2–1.4) | 1.25 (1.6–1.3) | 1.12 (1.0–1.2) | 1.10 (1.0–1.2) |
| 16 or more hrs (N = 1,014) | 129 | 1.41 (1.2–1.7) | 1.36 (1.5–1.6) | 1.15 (0.97–1.4) | 1.13 (0.95–1.3) |
| P-value for trend | <0.001 | <0.001 | <0.001 | 0.001 |
Values are risk ratio’s estimated using Poisson regression (95% Confidence Interval).
Model 1: adjusted for age, ethnicity and race, college education, income less than $35,000 per year, marital status, comorbidity propensity score (feeling depressed, hypertension, hyperlipidemia, osteoarthritis, history of cancer and cardiovascular disease), number of immediate family members with history of diabetes, currently smoking, alcohol intake > 7 drinks per week, percent of daily caloric intake as carbohydrate and percent of daily caloric intake as fat
Model 2: adjusted for variables in Model 1 plus minutes performing moderate to vigorous intensity physical activity
Model 3: adjusted for variables in Model 1 plus body mass index (kg/m2)
Model 4: adjusted for variables in Model 1 plus body mass index (kg/m2) and minutes performing moderate to vigorous intensity physical activity
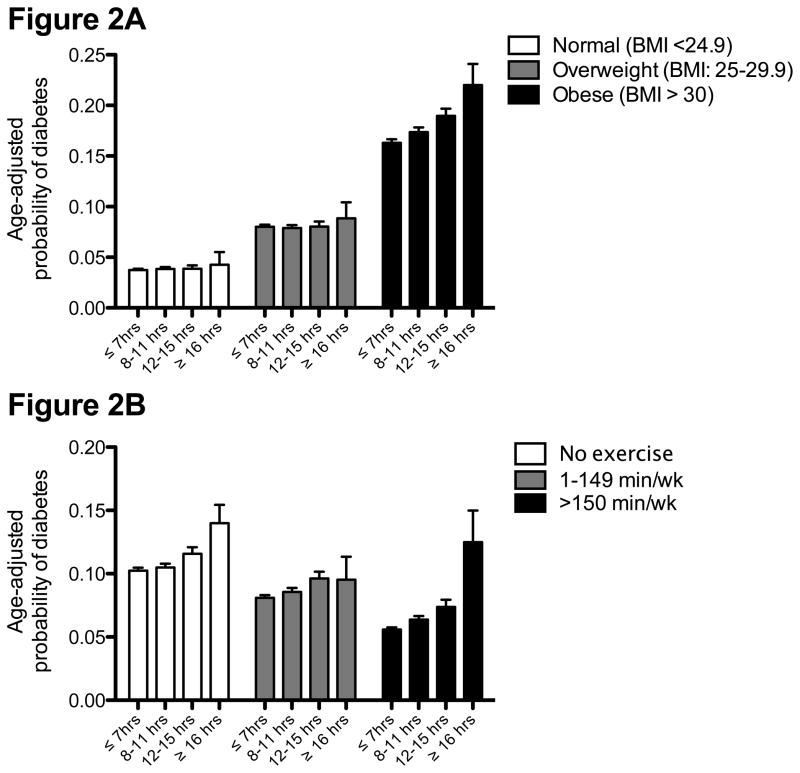
Figures 2A and 2B show the age-adjusted probability of diabetes across categories of BMI and participation in MVPA, respectively. Figure 2A shows no association between sitting time and the probability of diabetes in normal and overweight women. However, obese women reporting longer periods of sitting had a higher incidence of diabetes. This resulted in a significant effect modification by BMI (p = 0.006). Sitting for longer periods of time was associated with a linear increase in incident diabetes among women who did and did not report participating in regular MVPA (Figure 2B; p-value for trend < 0.01).
Figure 2.
Age-adjusted probability of diabetes according to hours of sitting stratified across categories of (A) body mass index (p-value for trend: BMI < 24.9 kg/m2: 0.537; BMI 25–29.9 kg/m2: 0.945; BMI>30 kg/m2: <0.001); (B) minutes performing moderate to vigorous intensity physical activity (all p-values for trend <0.01).
The significant effect modification by BMI was examined more closely in models illustrated in Table 3. The odds of incident diabetes were not different across longer sitting times in normal weight and overweight women after adjusting for demographics, socioeconomic factors and health conditions. These effects were unchanged after adjusting for behaviors (smoking, physical activity, alcohol intake and dietary intake). Obese women who reported sitting 8–11, 12–15 and ≥16 hours had a higher probability of diabetes after adjusting for demographics and health conditions/behaviors. The risk estimates were unchanged after adjusting for MVPA and marginally reduced after adjusting for BMI. For sensitivity analyses, obese women who were treated for diabetes in the first year of follow-up were removed (n = 216). The association between sitting time and incident diabetes was unchanged (OR: 1.08, 1.14, and 1.31 for 8–11 hrs, 12–15 hrs and ≥16 hrs relative to ≤ 7 hrs, respectively; data not shown).
Table 3.
Risk ratios for incident diabetes according to sitting time exposure groups stratified by BMI weight categories.
| Normal weight (<24.9 kg/m2) N = 36,853 | Overweight (25–29.9 kg/m2) N = 30,027 | Obese (>30 kg/m2) N = 20,898 | ||
|---|---|---|---|---|
| Model 1 | No. of diabetes | 1,384 | 2,384 | 3,547 |
| ≤ 7 hrs (N = 51,251) | 4,047 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 8–11 hrs (N = 26,577) | 2295 | 1.08 (0.95–1.2) | 1.04 (0.95–1.1) | 1.10 (1.0–1.2) |
| 12–15 hrs (N = 9,408) | 945 | 1.09 (0.89–1.3) | 1.06 (0.92–1.2) | 1.19 (1.1–1.3) |
| 16 or more hrs (N = 1,014) | 129 | 0.83 (0.40–1.7) | 1.01 (0.70–1.5) | 1.35 (1.1–1.6) |
| P-value for trend | 0.293 | 0.318 | <0.001 | |
| Model 2 | ||||
| ≤ 7 hrs (N = 51,251) | 4,047 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 8–11 hrs (N = 26,577) | 2295 | 1.07 (0.94–1.2) | 1.03 (0.94–1.1) | 1.10 (1.0–1.2) |
| 12–15 hrs (N = 9,408) | 945 | 1.07 (0.87–1.3) | 1.05 (0.93–1.2) | 1.17 (1.1–1.3) |
| 16 or more hrs (N = 1,014) | 129 | 0.80 (0.39–1.7) | 1.00 (0.69–1.4) | 1.33 (1.1–1.6) |
| P-value for trend | 0.427 | 0.447 | <0.001 | |
| Model 3 | ||||
| ≤ 7 hrs (N = 51,251) | 4,047 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 8–11 hrs (N = 26,577) | 2295 | 1.08 (0.95–1.2) | 1.03 (0.94–1.1) | 1.08 (1.0–1.2) |
| 12–15 hrs (N = 9,408) | 945 | 1.08 (0.88–1.3) | 1.06 (0.92–1.2) | 1.14 (1.0–1.2) |
| 16 or more hrs (N = 1,014) | 129 | 0.81 (0.39–1.7) | 1.04 (0.72–1.5) | 1.27 (1.0–1.5) |
| P-value for trend | 0.339 | 0.340 | 0.001 | |
| Model 4 | ||||
| ≤ 7 hrs (N = 51,251) | 4,047 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 8–11 hrs (N = 26,577) | 2295 | 1.07 (0.94–1.2) | 1.03 (0.94–1.1) | 1.08 (1.0–1.1) |
| 12–15 hrs (N = 9,408) | 945 | 1.06 (0.87–1.3) | 1.05 (0.91–1.2) | 1.13 (1.1–1.2) |
| 16 or more hrs (N = 1,014) | 129 | 0.79 (0.38–1.6) | 1.03 (0.72–1.5) | 1.25 (1.0–1.5) |
| P-value for trend | 0.469 | 0.447 | <0.001 |
Values are risk ratio’s estimated using Poisson regression (95% Confidence Interval).
Model 1: adjusted for age, ethnicity and race, college education, income less than $35,000 per year, marital status, comorbidity propensity score (feeling depressed, hypertension, hyperlipidemia, osteoarthritis, history of cancer and cardiovascular disease), number of immediate family members with history of diabetes, currently smoking, alcohol intake > 7 drinks per week, percent of daily caloric intake as carbohydrate and percent of daily caloric intake as fat
Model 2: adjusted for variables in Model 1 plus minutes performing moderate to vigorous intensity physical activity
Model 3: adjusted for variables in Model 1 plus body mass index (kg/m2)
Model 4: adjusted for variables in Model 1 plus body mass index (kg/m2) and minutes performing moderate to vigorous intensity physical activity
Discussion
The results from 88,829 women and 7416 incident cases of diabetes demonstrate that sitting time is moderately associated with the development of diabetes. However, this relationship was clearly most pronounced in obese women. Normal weight and overweight women showed no excess risk of diabetes across sitting time categories. Our hypothesis that participation in MVPA would modify the association between sitting time and diabetes risk was not confirmed— women who performed regular exercise had a similar diabetes risk associated with sitting time as those women not exercising. These results have important implications for targeting obese women for interventions that minimize sitting time.
Time spent in sedentary behaviors such as television and computer work are recognized risk factors for metabolic dysregulation (22). Ford and colleagues showed in the 2003–2006 National Health and Nutrition Examination Survey (NHANES) that insulin concentrations for men and women reporting a total screen time (television + computer time) >4 hours per day were higher than those individuals reporting less than 1 hour per day (5). This association was substantially reduced after adjusting for body mass, although others have not seen such a modifying effect (6, 10, 23). A new report by Yates and colleagues showed that higher sitting time was associated with elevated fasting insulin, leptin, and inflammation (C-reactive protein and interleukin-6) among women (24). Interestingly, this association was not seen in men. Contrary to previous reports, our data suggest that obese women are more prone to diabetes risk when exposed to greater amounts of sitting time as compared to normal weight and overweight women. It’s unclear why the potential harmful effects of prolonged sitting on glucose control are not observed in normal and overweight women. Theoretically, inactivity mediated insulin resistance coupled with a pro-inflammatory state found in obese individuals may play a role (25, 26). Additional observational and experimental studies are needed to confirm these findings.
The etiology of obesity-associated diabetes involves a complex set of biological processes that generally involve physiological impairments to hepatic and muscle tissue that cause insulin resistance (27). Our findings suggest that insulin resistance pathways might be further upregulated when obesity is combined with prolonged sitting. Muscle tissue has long been considered a major site of insulin stimulated glucose uptake in vivo and is responsible for 20% of the body’s blood glucose disposal in a post-absorptive state (i.e. several hours following a meal) (28, 29). Skeletal muscle plays a much larger role in the post-prandial state where it accounts for 80% of the glucose disposal following a meal (30). Additionally, muscle contraction as seen with vigorous exercise is a potent regulator of glucose uptake (31, 32). However, it was recently found that even light physical activity can increase glucose disposal and reduce insulin levels (33). Collectively, results from the current study suggest that prolonged muscle inactivity and obesity likely create an environment that enhances the progression to diabetes.
Physical activity habits have long been noted as important contributors to reduced risk of diabetes (34). The results presented in Figure 2 support this notion. Individuals who regularly performed physical activity experienced approximately half the risk of diabetes than women reporting no physical activity (35). Interestingly, the effect of sitting time on risk of diabetes was consistent even among women who reported being a regular exerciser. Previous research support our findings whereby adjustments for physical activity had only minor effects on the association between sitting time and poor health outcomes (5, 24, 36, 37). These results suggest that a bolus of physical activity might not be sufficient to counteract the enormous amount of time spent in sedentary behaviors such as sitting. For example, some reports suggest that adults spend 70% or more of their day sitting (1); one 30-minute bout of MVPA might not provide a sufficient stimulus to ward off the harmful metabolic effects of sitting. The current data along with prior reports provide evidence that prolonged sitting is an independent risk factor for diabetes in obese women.
There are a number of strengths of this study. The study sample was of a sufficient size to conduct stratified analyses to examine the potential importance of behavioral factors on the association between sitting time and risk of diabetes. Consideration of these behavioral factors is critical because unhealthy behaviors are often linked with sedentary habits (e.g. obesity). Additionally, the WHI OS investigators collected a number of potential confounders that helped to refine the exposure to disease association. These strengths are coupled with limitations. Sitting time estimated questionnaires have a significant but modest correlation with objectively measured sedentary behavior (14, 15). However, it is worth noting that the average hours of sitting time (9–10 hours per day) in our sample of women aged 50–79 years was similar to the average hours of sitting time in 60–69 year old women (8.4 hours per day) estimated by accelerometer data in NHANES (1). Reproducibility and validity of this question has been verified in other studies that demonstrate good test-retest reliability and significant associations compared to monitor-based determination of sedentary time (14, 15). Additionally, these results are restricted to midlife and older women and additional studies are needed to confirm such associations in men. Ascertainment of diabetes did not include measures of fasting glucose and thus we might have missed a substantial number of diabetes cases (38). WHI OS investigators previously reported that about 3% not reporting diabetes had fasting glucose >126 mg/dl, and 88% of those participants subsequently reported being treated for diabetes. Consequently, we expect that self-report of anti-diabetic medications missed approximately 10% of undiagnosed diabetes. Lastly, there is a chance of detection bias because self-reported sedentary behavior is closely linked with health problems that increase the likelihood of having diabetes testing.
In conclusion, data from the WHI OS study suggest that prolonged sitting is preferentially associated with risk for the onset of diabetes in obese women when compared to normal and overweight women. Regular exercise and consuming a low or high fat/carbohydrate diet did not modify the effect of sitting time on risk of diabetes. Coupled with America’s already serious obesity problem, the “sitting pandemic” is expected to contribute independently to excess rates of type 2 diabetes in the future, and thus warrants considerable public health attention.
What is already known about this subject?
Sedentary behaviors such as sitting are extremely common. On average, the US population spends approximately 60% (or 8 hours) of their day in sedentary behaviors. Older adults spend significantly more time being sedentary than young adults.
The public health recommendations focus on promoting moderate to vigorous intensity physical activity. However, there is a growing literature to suggest that the negative health effects of prolonged sedentary behavior are not remedied by performing moderate to vigorous intensity physical activity.
Sedentary behavior is associated with metabolic dysregulation in cross-sectional studies. Such studies are difficult to interpret because there is no temporal connection to the association.
What does this study add?
Sitting time is associated with excess risk of incidence diabetes in post-menopausal women.
Weight status modifies the effect that sedentary behavior has on incidence diabetes in post-menopausal women.
Moderate to vigorous intensity physical activity does not impact the association between sitting time and incident diabetes in obese post-menopausal women.
Acknowledgments
National Heart, Lung, and Blood Institute, Bethesda, Maryland: Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle, Washington: Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan. Medical Research Labs, Highland Heights, Kentucky: Evan Stein. University of California at San Francisco, San Francisco, California: Steven Cummings.
Clinical Centers: Albert Einstein College of Medicine, Bronx, New York: Sylvia Wassertheil-Smoller. Baylor College of Medicine, Houston, Texas: Aleksandar Rajkovic. Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts: JoAnn E. Manson. Brown University, Providence, Rhode Island: Charles B. Eaton. Emory University, Atlanta, Georgia: Lawrence Phillips. Fred Hutchinson Cancer Research Center, Seattle, WA: Shirley Beresford. George Washington University Medical Center, Washington, D.C.: Lisa Martin. Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, California: Rowan Chlebowski. Kaiser Permanente Center for Health Research, Portland, Oregon: Yvonne Michael. Kaiser Permanente Division of Research, Oakland, California: Bette Caan. Medical College of Wisconsin, Milwaukee, WI: Jane Morley Kotchen. MedStar Research Institute/Howard University, Washington, D.C.: Barbara V. Howard. Northwestern University, Chicago/Evanston, Illinois: Linda Van Horn. Rush Medical Center, Chicago, Illinois: Henry Black. Stanford Prevention Research Center, Stanford, California: Marcia L. Stefanick. State University of New York at Stony Brook, Stony Brook, New York: Dorothy Lane. The Ohio State University, Columbus, Ohio: Rebecca Jackson. University of Alabama at Birmingham, Birmingham, Alabama: Cora E. Lewis. University of Arizona, Tucson/Phoenix, Arizona: Cynthia A Thomson. University at Buffalo, Buffalo, New York: Jean Wactawski- Wende. University of California at Davis, Sacramento, California: John Robbins. University of California at Irvine, California: F. Allan Hubbell. University of California at Los Angeles, Los Angeles, California: Lauren Nathan. University of California at San Diego, La Jolla/Chula Vista, California: Robert D. Langer. University of Cincinnati, Cincinnati, Ohio: Margery Gass. University of Florida, Gainesville/Jacksonville, Florida: Marian Limacher. University of Hawaii, Honolulu, Hawaii: J. David Curb. University of Iowa, Iowa City/Davenport, Iowa: Robert Wallace. University of Massachusetts/Fallon Clinic, Worcester, Massachusetts: Judith Ockene. University of Medicine and Dentistry of New Jersey, Newark, New Jersey: Norman Lasser. University of Miami, Miami, Florida: Mary Jo O’Sullivan. University of Minnesota, Minneapolis, Minnesota: Karen Margolis. University of Nevada, Reno, Nevada: Robert Brunner. University of North Carolina, Chapel Hill, North Carolina: Gerardo Heiss. University of Pittsburgh, Pittsburgh, Pennsylvania: Lewis Kuller. University of Tennessee Health Science Center, Memphis, Tennessee: Karen C. Johnson. University of Texas Health Science Center, San Antonio, Texas: Robert Brzyski. University of Wisconsin, Madison, Wisconsin: Gloria E. Sarto. Wake Forest University School of Medicine, Winston-Salem, North Carolina: Mara Vitolins. Wayne State University School of Medicine/Hutzel Hospital, Detroit, Michigan: Michael Simon.
The WHI program is funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, U.S. Department of Health and Human Services through Contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221 This work was also supported by PO1 CA53996, R01AG025441-03, and T32 AG027677.
Dr. Manini worked on this manuscript while employed at the University of Florida. He is supported by the National Institute on Aging (R21AG031974 to TM Manini) and by University of Florida Claude D. Pepper Center awarded by the National Institute on Aging (P30AG028740)
Dr. Manson worked on this manuscript while employed at Brigham and Women’s Hospital, Harvard Medical School.
Dr. Garcia worked on this manuscript while employed at the University of California Davis; she was supported by the Building Interdisciplinary Research Careers in Women’s Health Program (K12 HD051958).
Dr. Hingle worked on this manuscript while employed at the University of Arizona
Dr. Song Dr. Song worked on this manuscript while employed at Brigham and Women’s Hospital, Harvard Medical School; he is supported by the R01-DK088078 (NIDDK).
Dr. Seguin worked on this manuscript while employed at the Fred Hutchinson Cancer Research Center and the Group Health Research Institute; she was supported by the T32 AG027677 fellowship (NIA) and by K01 HL108807 (NHLBI).
Sponsor’s Role: None.
Footnotes
Conflicts of Interest: None (all authors).
Author Contributions: TMM led the writing team; TMM conducted analysis under direction of ML; all authors contributed substantively to the interpretation of the results and development, editing, and/or writing of the manuscript.
Bibliography
- 1.Matthews CE, Chen KY, Freedson PS, et al. Amount of Time Spent in Sedentary Behaviors in the United States, 2003–2004. American Journal of Epidemiology. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanck HM, McCullough ML, Patel AV, et al. Sedentary behavior, recreational physical activity, and 7-year weight gain among postmenopausal U.S. women. Obesity (Silver Spring) 2007;15:1578–1588. doi: 10.1038/oby.2007.187. [DOI] [PubMed] [Google Scholar]
- 3.Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev. 2009;67:114–120. doi: 10.1111/j.1753-4887.2008.00136.x. [DOI] [PubMed] [Google Scholar]
- 4.Owen N, Bauman A, Brown W. Too much sitting: a novel and important predictor of chronic disease risk? British journal of sports medicine. 2009;43:81–83. doi: 10.1136/bjsm.2008.055269. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Li C, Zhao G, Pearson WS, Tsai J, Churilla JR. Sedentary behavior, physical activity, and concentrations of insulin among US adults. Metabolism. 2010;59:1268–1275. doi: 10.1016/j.metabol.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Hu FB. Sedentary lifestyle and risk of obesity and type 2 diabetes. Lipids. 2003;38:103–108. doi: 10.1007/s11745-003-1038-4. [DOI] [PubMed] [Google Scholar]
- 7.Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. European Heart Journal. 2011;32:590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzmarzyk PT, Church TS, Craig CL, BOUCHARD C. Sitting Time and Mortality from All Causes, Cardiovascular Disease, and Cancer. Med Sci Sports Exerc. 2009;41:998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 9.Patel AV, Bernstein L, Deka A, et al. Leisure Time Spent Sitting in Relation to Total Mortality in a Prospective Cohort of US Adults. American Journal of Epidemiology. 2010;172:419–429. doi: 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bankoski A, Harris TB, McClain JJ, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care. 2011;34:497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: a 9-year prospective population-based study. The Melbourne Women’s Midlife Health Project. Climacteric. 2004;7:375–389. doi: 10.1080/13697130400012163. [DOI] [PubMed] [Google Scholar]
- 12.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHI. Design of the Women“s Health Initiative clinical trial and observational study. The Women”s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 14.Clark BK, Sugiyama T, Healy GN, Salmon J, Dunstan DW, Owen N. Validity and reliability of measures of television viewing time and other non-occupational sedentary behaviour of adults: a review. Obes Rev. 2009;10:7–16. doi: 10.1111/j.1467-789X.2008.00508.x. [DOI] [PubMed] [Google Scholar]
- 15.Clark BK, Thorp AA, EAHW, et al. Validity of self-reported measures of workplace sitting time and breaks in sitting time. Med Sci Sports Exerc. 2011;43:1907–1912. doi: 10.1249/MSS.0b013e31821820a2. [DOI] [PubMed] [Google Scholar]
- 16.Margolis KL, Lihong Q, Brzyski R, et al. Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clinical trials (London, England) 2008;5:240–247. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsia J, Wu L, Allen C, et al. Physical activity and diabetes risk in postmenopausal women. AMEPRE. 2005;28:19–25. doi: 10.1016/j.amepre.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Annals of epidemiology. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 19.Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, Groenwold RHH. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. Canadian Medical Association Journal. 2012;184:895–899. doi: 10.1503/cmaj.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Fitzmaurice G. Confounding: propensity score adjustment. Nutrition. 2006;22:1214–1216. doi: 10.1016/j.nut.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Grøntved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality. JAMA. 2011;305:2448. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardiner PA, Healy GN, Eakin EG, et al. Associations between television viewing time and overall sitting time with the metabolic syndrome in older men and women: the Australian Diabetes, Obesity and Lifestyle study. J Am Geriatr Soc. 2011;59:788–796. doi: 10.1111/j.1532-5415.2011.03390.x. [DOI] [PubMed] [Google Scholar]
- 24.Yates T, Khunti K, Wilmot EG, et al. Self-Reported Sitting Time and Markers of Inflammation, Insulin Resistance, and Adiposity. AMEPRE. 2012;42:1–7. doi: 10.1016/j.amepre.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends in immunology. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism. 2010:1–9. doi: 10.1016/j.metabol.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Kahn BB, Flier JS. Obesity and insulin resistance. Journal of Clinical Investigation. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–74. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- 29.Edelman SV, Laakso M, Wallace P, Brechtel G, Olefsky JM, Baron AD. Kinetics of insulin-mediated and non-insulin-mediated glucose uptake in humans. Diabetes. 1990;39:955–964. doi: 10.2337/diab.39.8.955. [DOI] [PubMed] [Google Scholar]
- 30.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Annals of internal medicine. 1990;113:909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 31.Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46:1775–1781. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- 32.Wojtaszewski JF, Lynge J, Jakobsen AB, Goodyear LJ, Richter EA. Differential regulation of MAP kinase by contraction and insulin in skeletal muscle: metabolic implications. Am J Physiol. 1999;277:E724–32. doi: 10.1152/ajpendo.1999.277.4.E724. [DOI] [PubMed] [Google Scholar]
- 33.Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greaves CJ, Sheppard KE, Abraham C, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11:119. doi: 10.1186/1471-2458-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 36.Helmerhorst HJF, Wijndaele K, Brage S, Wareham NJ, Ekelund U. Objectively Measured Sedentary Time May Predict Insulin Resistance Independent of Moderate- and Vigorous-Intensity Physical Activity. Diabetes. 2009;58:1776–1779. doi: 10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorp AA, Healy GN, Owen N, et al. Deleterious Associations of Sitting Time and Television Viewing Time With Cardiometabolic Risk Biomarkers: Australian Diabetes, Obesity and Lifestyle (AusDiab) study 2004–2005. Diabetes Care. 2010;33:327–334. doi: 10.2337/dc09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20–74 yr. Diabetes. 1987;36:523–534. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]