Abstract
Background
Distal revascularization and interval ligation (DRIL) is commonly used to treat ischemic steal syndrome caused by arteriovenous hemodialysis access and has been associated with good outcomes. However, the literature lacks technical details of a successful intervention. We tested the hypothesis that a brachial-level arteriovenous fistula (AVF) generates a zone of low arterial blood pressure in the brachial artery near the AVF origin.
Methods
We identified patients with ischemic steal syndrome caused by an AVF originating from the brachial artery level who were eligible for the DRIL procedure. All patients were studied with invasive pressure monitoring in the brachial artery at the time of digital subtraction angiography. We measured systolic, diastolic and mean arterial blood pressure at 5 cm intervals from a point in the arterial circulation 5 cm distal to the origin of the AVF and continuing proximally into the subclavian artery.
Results
Our series involved 10 patients with a mean age of 66.5 (range 53–81) years. Four patients were women and 8 had diabetes. All patients had grade 3 ischemic steal syndrome with ischemic rest pain and/or ischemic tissue loss. Mean systolic, diastolic and arterial pressures increased from the level of the AVF until central pressures were reached. Systolic blood pressure was significantly lower than central blood pressure until a level 20–25 cm proximal to the AVF.
Conclusion
The benefits of the DRIL procedure in alleviating ischemic steal syndrome associated with hemodialysis access are best achieved with a DRIL bypass for which inflow originates at least 20–25 cm proximal to the origin of the AVF.
Abstract
Contexte
On utilise souvent la revascularisation distale avec ligature intermédiaire (DRIL) pour traiter le syndrome d’hémodétournement ischémique causé par une fistule artérioveineuse pour hémodialyse et elle a été associée à de bons résultats. Or, la littérature donne peu de détails techniques sur les interventions réussies. Nous avons voulu tester l’hypothèse selon laquelle une fistule artérioveineuse (FAV) brachiale génère une zone de tension artérielle réduite dans l’artère brachiale près de la naissance de la FAV.
Méthodes
Nous avons recensé des patients porteurs d’un syndrome de détournement ischémique causé par une FAV de l’artère brachiale qui étaient admissibles à l’intervention DRIL. Nous avons examiné les patients par monitorage endovasculaire de la pression de l’artère brachiale sous angiographie numérique avec soustraction. Nous avons mesuré les tensions artérielles systoliques, diastoliques et moyennes à des intervalles de 5 cm, à partir d’un point distal de la circulation artérielle éloigné de 5 cm de la naissance de la FAV, puis proximalement, jusqu’à la sous-clavière.
Résultats
Notre série a regroupé 10 patients âgés en moyenne de 66,5 ans (de 53 à 81 ans). Quatre patients étaient des femmes et 8 souffraient de diabète. Tous les patients étaient porteurs d’un syndrome d’hémodétournement ischémique de grade 3 accompagné de douleur ischémique au repos et/ou d’ischémie tissulaire. Les tensions artérielles systoliques, diastoliques et moyennes allaient croissant à partir de la FAV, jusqu’à l’atteinte des tensions centrales. La tension systolique s’est révélée significativement plus faible que la tension centrale jusqu’à un point proximal situé à 20–25 cm de la FAV.
Conclusion
Dans le traitement du syndrome d’hémodétournement ischémique associé à une fistule artérioveineuse pour hémodialyse, les avantages de l’intervention DRIL sont plus marqués avec un pontage dont l’influx tire son origine à au moins 20–25 cm de la naissance de la FAV.
In the year 2010, more than 19 000 patients in Canada were receiving hemodialysis for renal replacement therapy, and the prevalence of patients on hemodialysis increased by 51% from 2001 to 2010.1 Canadian and international professional bodies continue to support the use of an autologous arteriovenous fistula (AVF) as the optimal type of vascular access for patients undergoing hemodialysis.2–4 Clinically significant ischemic steal syndrome can be expected in 1%–8% of patients with this type of dialysis access, and risk factors include female sex, diabetes, age older than 60 years, multiple previous AVF access sites in the same extremity and use of the brachial artery for fistula inflow.5–7
Several options are available for the treatment of ischemic steal secondary to AVF dialysis access. These include ligation of the fistula, which sacrifices the access site. Banding of the AVF can be performed to reduce fistula flow, but thrombosis of the access site is common after this treatment.8 Proximalization of arterial inflow (PAI) has been described and involves a graft to maintain patency of the AVF from a proximal inflow source.9 Revision using distal inflow (RUDI) involves a graft to move AVF inflow from the brachial artery to an inflow source 2–3 cm distal to the brachial trifurcation.10
Distal revascularization and interval ligation (DRIL) was first described by Schanzer and colleagues11 and has become one of the more common operations to treat steal syndrome associated with dialysis access. The technique involves ligation of the brachial artery distal to the AVF inflow and revascularization of the distal arm with a bypass taken from a more proximal inflow source.11 Good outcomes following DRIL have been reported by many authors;12–18 however, technical factors associated with failure of the procedure have not been identified. Furthermore, details on operative technique, particularly the level of the bypass inflow, are often lacking. Many authors suggest that a short bypass, originating only a few centimetres proximal to the AVF may be adequate.11,13,16
This study was undertaken to test the hypothesis that a brachial level AVF generates a zone of low arterial blood pressure in the brachial artery near the AVF origin. Such a zone of low pressure might extend over a distance within the brachial artery, suggesting that optimal inflow for the DRIL procedure should be taken at a more proximal level than previously recommended.
Methods
The section of Vascular Surgery in the Regina Qu’Appelle Health Region is responsible for creation and maintenance of hemodialysis access for southern Saskatchewan’s hemodialysis program. Hemodialysis services operate from the Regina General Hospital and several satellite dialysis units in communities across the southern part of the province. In 2008, the program served 273 patients on long-term hemodialysis, of whom 70% had an arteriovenous hemodialysis access, either with a native AVF or with an arteriovenous graft.
Any patient within the hemodialysis population with suspected ischemic steal syndrome secondary to an arteriovenous hemodialysis access is assessed by 1 of 3 vascular surgeons working in the Regina Qu’Appelle Health Region. After appropriate history and physical examination, patients with confirmed ischemic steal syndrome who are considered suitable candidates for the DRIL procedure are referred for catheter angiography of the affected extremity.
Between Apr. 1, 2004, and Oct. 31, 2011, patients with brachial level AVF and ischemic steal syndrome underwent digital subtraction angiography. In brief, each patient underwent transfemoral aortic arch angiography under local anesthesia followed by selective catheterization of the artery to the affected limb with an end-hole angiography catheter. After the acquisition of digital subtraction angiograms, with or without manual compression of the AVF, the catheter was advanced over a guidewire into the dominant artery of the forearm 5 cm distal to the AVF, and the pressure within the artery was electronically transduced with the AVF open. Systolic, diastolic and mean arterial blood pressures were recorded. The catheter tip was then pulled back to the brachial artery at the level of the AVF, and the pressure recording was repeated. Similarly, pressures were recorded at 5 cm intervals from the level of the AVF through the entire length of the brachial artery, into the subclavian artery proximal to the first rib.
Pressure readings were recorded in the patient’s hospital record and retrospectively abstracted, along with the patient’s clinical data (age, sex, presence of diabetes, type of hemodialysis access, time between AVF creation and the onset of ischemic symptoms). The grade of ischemia and the status of the arteries to the affected hand were also recorded, as recommended for current reporting standards.19
We calculated the mean systolic, diastolic and arterial blood pressure for each measurement point in the brachial artery for the entire sample. We calculated 95% confidence intervals (CIs) for each average pressure. The study was approved by the Research Ethics Board of the Regina Qu’Appelle Health Region.
Results
Ten patients with ischemic steal syndrome underwent angiography with pullback pressures in the affected extremity during the study period. Their demographic and clinical characteristics are shown in Table 1. Four patients (40%) were women. The mean age of all the patients was 66.5 (range 53–81) years. Eight patients (80%) had diabetes. Eight patients (80%) had autogenous brachial-cephalic upper arm direct access, and 2 had autogenous brachial-basilic upper arm transposition. The time between fistula creation and presentation with ischemic symptoms was recorded for 9 patients, with a mean interval of 10.2 (range 2–41) months.
Table 1.
Patient demographic and clinical characteristics
| Patient | Sex | Age, yr | Diabetes | Arterial status of the affected limb | AVF type | Time from creation to presentation, mo |
|---|---|---|---|---|---|---|
| 1 | Male | 72 | Yes | Distal disease | Cephalic | 13 |
| 2 | Male | 55 | Yes | Healthy | Cephalic | 2 |
| 3 | Male | 71 | Yes | Healthy | Cephalic | 5 |
| 4 | Female | 75 | No | Healthy | Cephalic | 13 |
| 5 | Female | 81 | Yes | Distal disease | Basilic transposed | 41 |
| 6 | Female | 81 | No | Healthy | Cephalic | 2 |
| 7 | Male | 53 | Yes | Healthy | Cephalic | 10 |
| 8 | Female | 63 | Yes | Healthy | Cephalic | 3 |
| 9 | Male | 60 | Yes | Distal disease | Cephalic | — |
| 10 | Male | 54 | Yes | Distal disease | Basilic transposed | 3 |
AVF = arteriovenous fistula.
All patients had grade 3 ischemic steal syndrome with ischemic rest pain and/or ischemic tissue loss. None had an arterial inflow obstruction proximal to the level of the AVF. Six (60%) had angiographically healthy vasculature distal to the AVF and preferential flow through a large arteriovenous anastomosis. Four (40%) had distal arterial occlusive disease in the forearm and hand in addition to a large arteriovenous anastomosis to account for their symptoms.
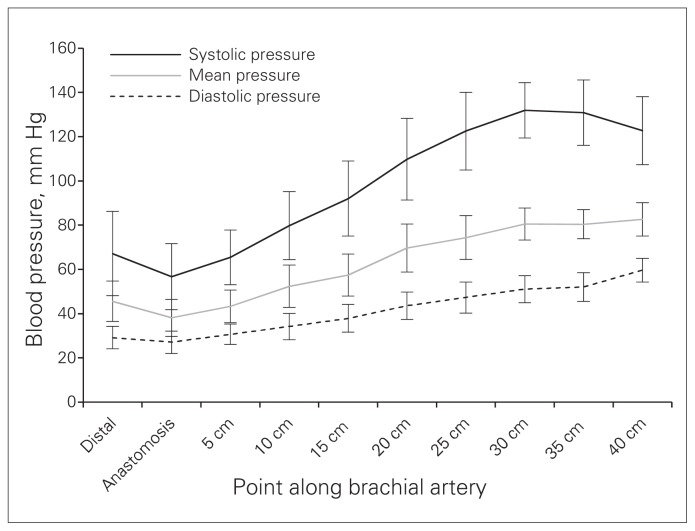
The results of arterial blood pressure measurements along the length of the brachial artery are shown in Figure 1. The graph shows the mean systolic, mean diastolic and average mean blood pressure and 95% CIs measured at 5 cm intervals beginning at a point in the arterial tree distal to the arteriovenous anastomosis.
Fig. 1.
Mean systolic, mean diastolic and average mean blood pressure measured at each point in the brachial arteries of 10 patients with ischemic steal syndrome associated with an arteriovenous fistula for dialysis access, with 95% confidence intervals.
Of the 10 patients in this series, 3 requested ligation of the arteriovenous access and were converted to hemodialysis through tunnelled central venous catheters. Four patients declined further intervention. Of these 4 patients, 3 had ongoing ischemic rest pain or tissue loss at their last follow-up visit, and 1 patient improved spontaneously with no further ischemic symptoms at rest. All 4 of these patients had a functioning arteriovenous hemodialysis access at their last follow-up visit after angiography (5, 24, 36 and 40 mo, respectively).
Three of the patients in this series underwent DRIL with inflow from the axillary artery at the level of the axilla or at the proximal brachial artery at the level of the axillary fold. We used a reversed saphenous vein taken from the patient’s thigh as the conduit in 2 cases. In the patient who did not have an available saphenous vein (due to bilateral in situ vein bypasses of the legs), we used a 6 mm diameter, ring supported, heparin-bonded polytetrafluoroethylene (PTFE) graft (Propaten). All 3 patients had patency of the initial hemodialysis access and the DRIL bypass at the last follow-up visit after the DRIL procedure (7, 12 and 63 mo, respectively). All patients who underwent the DRIL procedure experienced relief of their ischemic hand symptoms.
Discussion
Our results demonstrate a linear rise in arterial pressures over the length of the brachial artery proximal to the AVF. This would be expected from the Hagen–Poiseuille equation, which relates the pressure change in a vessel linearly to the length of the vessel and inversely to the fourth power of its radius. Solved for pressure, the equation is
where ΔP is the change in pressure in the brachial artery between the AVF origin and the pressure measurement point, Q is the blood flow through the artery, η is the viscosity of blood, r is the radius of the brachial artery and l is the length of the arterial segment from the measurement point to the origin of the AVF.
The blood flow in an extremity with a functioning AVF is divided between 2 circuits with a common inflow. Blood enters either into the distal arteries, providing nutrient flow to the tissues, or into the AVF. Nutrient blood flow is in a circuit with high vascular resistance, maintained by vascular tone in the precapillary arterioles. Additional resistance to blood flow may be encountered in the presence of distal atherosclerotic occlusive disease. Nutrient blood flow depends on adequate perfusion pressure to overcome the resistance. Within the AVF, the vascular resistance is low and primarily determined by the radius of the narrowest point in the AVF or the length of the AVF flow circuit, as shown by the Hagen–Poiseuille equation. Proximally, the 2 flow circuits have a common in-flow and, at the point of divergence, a common pressure.
The DRIL procedure moves the divergent point between nutrient flow and AVF flow proximally on the brachial artery to afford the tissues a higher perfusion pressure. Our data show such higher perfusion pressures are not reliably reached until a distance of more than 20–25 cm proximal to the AVF. Only at this distance does the lengthened AVF circuit have enough resistance to maintain adequate pressure in the brachial artery. The concept that lengthening the AVF circuit increases the arterial pressure available for tissue perfusion is consistent with the work of Illig and colleagues,20 who noted that the topologic outcome of the DRIL procedure is to lengthen the AVF circuit. A similar mechanism of circuit lengthening may underlie the efficacy of the PAI procedure.
Our results corroborate those of a recent study with similar methodology, in which the authors also identified a zone of low pressure in association with a stealing AVF.21 As with our results, those of Reifsnyder and Arnaoutakis21 show a gradual rise in arterial pressures transduced in the brachial, axillary and subclavian arteries proximal to a stealing AVF. These authors noted that in only 2 of 9 patients did the brachial arterial pressure rise to adequate levels within the first 5 cm proximal to the AVF. In the remaining 7 patients, the authors observed a gradual rise in blood pressure into the axillary and subclavian arteries. They concluded that optimal inflow requires a DRIL bypass to originate more proximally than previously suggested.
Our study contributes data from an additional 10 patients with ischemic steal syndrome that are reported at fixed distances in relation to the AVF. The pressure data of Reifsnyder and Arnaoutakis are given at relative anatomic locations, such as “mid-brachial” and “proximal brachial.” Like them, we found that the zone of low arterial pressure within the brachial artery extends for a length proximal to the stealing AVF. Our calibrated measurements permit the correlation of the observed pressures with the physical laws governing the applicable hemodynamics.
In our own practice, we use inflow for the DRIL procedure from the third part of the axillary artery within the axilla or from the brachial artery near the anterior axillary fold. In tall patients with long limbs, we use inflow from the brachial artery in the proximal third of the upper arm in order to maintain an inflow source 20–25 cm proximal to the AVF.
Our preferred conduit is a reversed saphenous vein harvested from the thigh. Because of the need for a high-pressure proximal inflow source, this mandates the harvest of an adequate length of conduit. Compared with the practice of performing only a short DRIL bypass, our practice of a long DRIL bypass means a more extensive thigh dissection and potentially increased risk of thigh wound complications. In the situation of an unavailable saphenous vein conduit, we have successfully used heparin-bonded PTFE for the DRIL procedure.
Conclusion
Maximization of the benefits of the DRIL procedure mandate inflow to the DRIL bypass at a proximal level at least 20–25 cm proximal to the AVF.
Footnotes
This work was previously presented in part at the Pacific Northwest Vascular Society 26th Annual Meeting in Seattle, Wash., Nov. 13, 2008, and in whole at the Canadian Society for Vascular Surgery meeting in Québec, Que., Sept. 29, 2012.
References
- 1.Canadian Institute for Health Information. Canadian organ replacement register annual report: treatment of end-stage organ failure in Canada, 2001 to 2010. Ottawa: The Institute; 2012. Jan 31, [Google Scholar]
- 2.Canadian Society of Nephrology. Report of the Canadian Society of Nephrology Vascular Access Working Group. Semin Dial. 2012;25:22–5. doi: 10.1111/j.1525-139X.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis. 2006;48(1 Suppl):S1–S322. [Google Scholar]
- 4.Sidawy AN, Spergel LM, Besarab A, et al. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg. 2008;48(5 Suppl):2S–25S. doi: 10.1016/j.jvs.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Goff CD, Sato DT, Bloch PHS, et al. Steal syndrome complicating hemodialysis access procedures: Can it be predicted? Ann Vasc Surg. 2000;14:138–44. doi: 10.1007/s100169910025. [DOI] [PubMed] [Google Scholar]
- 6.Lazarides MK, Staramos DN, Kopadis G, et al. Onset of arterial “steal” following proximal angioaccess: immediate and delayed types. Nephrol Dial Transplant. 2003;18:2387–90. doi: 10.1093/ndt/gfg346. [DOI] [PubMed] [Google Scholar]
- 7.Sessa C, Pecher M, Maurizi-Balzan J, et al. Critical hand ischemia after angioaccess surgery: diagnosis and treatment. Ann Vasc Surg. 2000;14:583–93. doi: 10.1007/s100169910107. [DOI] [PubMed] [Google Scholar]
- 8.Padberg FT, Calligaro KD, Sidawy AN. Complications of arteriovenous hemodialysis access: recognition and management. J Vasc Surg. 2008;48:55S–80S. doi: 10.1016/j.jvs.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 9.Zanow J, Kruger U, Scholz H. Proximalization of the arterial inflow: a new technique to treat access-related ischemia. J Vasc Surg. 2006;43:1216–21. doi: 10.1016/j.jvs.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Minion DJ, Moore E, Endean E. Revision using distal inflow: a novel approach to dialysis-associated steal syndrome. Ann Vasc Surg. 2005;19:625–8. doi: 10.1007/s10016-005-5827-7. [DOI] [PubMed] [Google Scholar]
- 11.Schanzer H, Schwartz M, Harrington E, et al. Treatment of ischemia due to “steal” by arteriovenous fistula with distal artery ligation and revascularization. J Vasc Surg. 1988;7:770–3. doi: 10.1067/mva.1988.avs0070770. [DOI] [PubMed] [Google Scholar]
- 12.Berman SS, Gentile AT, Glickman MH, et al. Distal revascularization-interval ligation for limb salvage and maintenance of dialysis access is ischemic steal syndrome. J Vasc Surg. 1997;26:393–404. doi: 10.1016/s0741-5214(97)70032-6. [DOI] [PubMed] [Google Scholar]
- 13.Knox RC, Berman SS, Hughes JD, et al. Distal revascularization-interval ligation: A durable and effective treatment for ischemic steal syndrome after hemodialysis access. J Vasc Surg. 2002;36:250–6. doi: 10.1067/mva.2002.125025. [DOI] [PubMed] [Google Scholar]
- 14.Walz P, Ladowski JS, Hines A. Distal revascularization and interval ligation (dril) procedure for the treatment of ischemic steal syndrome after arm arteriovenous fistula. Ann Vasc Surg. 2007;21:468–73. doi: 10.1016/j.avsg.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Mwipatayi BP, Bowles T, Balakrishnan S, et al. Ischemic steal syndrome: a case series and review of current management. Curr Surg. 2006;63:130–5. doi: 10.1016/j.cursur.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Sessa C, Riehl G, Porcu P, et al. Treatment of hand ischemia following angioaccess surgery using the distal revascularization interval-ligation technique with preservation of vascular access: description of an 18-case series. Ann Vasc Surg. 2004;18:685–94. doi: 10.1007/s10016-004-0113-7. [DOI] [PubMed] [Google Scholar]
- 17.Gupta N, You TH, Konig G, et al. Treatment strategies of arterial steal after arteriovenous access. J Vasc Surg. 2011;54:162–7. doi: 10.1016/j.jvs.2010.10.134. [DOI] [PubMed] [Google Scholar]
- 18.Field M, Blackwell J, Jaipersad A, et al. Distal revascularisation with interval ligation (DRIL): an experience. Ann R Coll Surg Engl. 2009;91:394–8. doi: 10.1308/003588409X392153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidawy AN, Gray R, Besarab A, et al. Recommended standards from reports dealing with arteriovenous hemodialysis access. J Vasc Surg. 2002;35:603–10. doi: 10.1067/mva.2002.122025. [DOI] [PubMed] [Google Scholar]
- 20.Illig KA, Surowiec S, Shortell CK, et al. Hemodynamics of distal revascularization-interval ligation. Ann Vasc Surg. 2005;19:199–207. doi: 10.1007/s10016-004-0162-y. [DOI] [PubMed] [Google Scholar]
- 21.Reifsnyder T, Arnaoutakis GJ. Arterial pressure gradient of upper extremity arteriovenous access steal syndrome: treatment implications. Vasc Endovascular Surg. 2010;44:650–3. doi: 10.1177/1538574410376450. [DOI] [PMC free article] [PubMed] [Google Scholar]