Abstract
Background
Elderly patients undergoing acute gastrointestinal (GI) surgery experience increased morbidity and mortality compared with younger and elective patients. Prognostic factors can be used to counsel patients of these risks and, if modifiable, to minimize them. We reviewed the literature on prognostic factors for adverse outcomes in elderly patients undergoing acute GI surgery.
Methods
We searched PubMed and Embase using a strategy developed in collaboration with an expert librarian. Studies examining independent associations between prognostic factors and morbidity or mortality in patients aged 65 and older undergoing acute GI surgery were selected. We extracted data using a standardized form and assessed study quality using the QUIPS tool.
Results
Nine cohort studies representing 2958 patients satisfied our selection criteria. All studies focused on postoperative mortality. Thirty-four prognostic factors were examined, with significant variability across studies. There was limited or conflicting evidence for most prognostic factors. Meta-analysis was only possible for the American Society of Anesthesiologists (ASA) score, which was found to be associated with mortality in 4 studies (pooled odds ratio 2.77, 95% confidence interval 0.92–8.41). Conclusion: While acute GI surgery in elderly patients is becoming increasingly common, the literature on prognostic factors for morbidity and mortality in this patient population lags behind. Further research is needed to help guide patient care and potentially improve outcomes.
Abstract
Contexte
On constate une morbidité et une mortalité accrues chez les patients âgés soumis à une chirurgie gastro-intestinale (GI) urgente, comparativement aux patients plus jeunes et ceux qui subissent une intervention non urgente. Certains facteurs pronostiques peuvent servir à conseiller les patients au sujet des risques et, s’ils sont modifiables, au sujet de leur atténuation. Nous avons passé en revue la littérature sur les facteurs pronostiques qui sous-tendent l’issue négative d’une chirurgie GI urgente chez des patients âgés.
Méthodes
Nous avons interrogé les bases de données PubMed et Embase à l’aide d’une stratégie mise au point en collaboration avec un expert bibliothécaire. Nous avons sélectionné les études portant sur les liens indépendants entre facteurs pronostiques et morbidité ou mortalité chez les patients de 65 ans et plus soumis à une chirurgie GI urgente. Nous avons extrait les données à l’aide d’un formulaire standardisé et évalué la qualité des études au moyen de l’outil QUIPS.
Résultats
Neuf études de cohorte regroupant 2958 patients répondaient à nos critères de sélection. Toutes les études faisaient état de la mortalité postopératoire. Trente-quatre facteurs pronostiques ont été analysés et la variabilité entre les études était significative. Les preuves se sont révélées limitées ou divergentes pour la plupart des facteurs pronostiques. Il n’a été possible d’effectuer une méta-analyse que pour le score ASA (American Society of Anesthesiologists), qui s’est révélé associé à la mortalité dans 4 études (rapport des cotes regroupées 2,77, intervalle de confiance de 95 % 0,92–8,41).
Conclusion
La chirurgie GI urgente est de plus en plus courante chez les patients âgés, mais la littérature sur les facteurs pronostiques de morbidité et de mortalité chez cette population de patients a pris du retard. Il faudra approfondir la recherche pour orienter le soins des patients et améliorer les résultats.
Elderly patients (age ≥ 65) are the fastest growing subset of the population in industrialized countries.1,2 This has had an impact on the health care system as the proportion of discharged patients older than 65 has increased from 10% in 1970 to 37% in 2007.3,4 This trend will likely continue, as 25% of North Americans are expected to be older than 65 by 2040.5 This changing demographic will impact the delivery of health care, including surgical care, in many ways.6 Of particular concern to the field of general surgery is that 40% of gastrointestinal (GI) surgeries in elderly patients occurs on an acute (urgent or emergent) basis.7 Nonelective surgery in older adults is associated with a 10- to 15-fold increase in morbidity and a 3- to 5-fold increase in mortality compared to elective surgery in this age group.8–11 Furthermore, nonelective surgery in this cohort is also associated with increased morbidity (28% v. 10%) and mortality (15.2% v. 2.5%) compared with younger cohorts1,2,12 This high potential for poor outcomes has implications for patient care and autonomy as well as cost and resource planning.
Prognostic factors for perioperative morbidity and mortality are useful to clinicians and patients in several ways.3,4,13 At the most basic level, prognostic factors can inform care and convey the probability of expected risks to the patients and their families. Once identified, factors associated with adverse outcomes can potentially be modified. Finally, prognostic factors can be used to inform the development of risk prediction models in order to more accurately assess risk for individual patients. To our knowledge, no previous review articles have explored prognostic factors for morbidity and mortality in this patient population. With these views in mind, the purpose of our study was to systematically review and synthesize the available evidence on prognostic factors associated with morbidity and mortality in elderly patients undergoing acute GI surgery.
Methods
Literature search
We used a strategy developed in collaboration with an expert librarian (see the Appendix, available at canjsurg.ca) to search PubMed and Embase (all years through June 11, 2012). Search terms (medical subject headings, Emtree headings and free text words) related to acute GI surgery, elderly patients, postoperative outcomes, risk prediction and prognosis were used with Boolean logic to identify all potentially relevant articles. No language restrictions were applied.
Search results were combined using Ref Works software version 2.0 (ProQuest), and duplicates were removed. One of us (J.S.) initially screened titles for potential relevance, and citations were excluded if they did not pertain to the study population of interest. Abstracts were independently screened for relevance by 2 of 3 reviewers (P.D., J.S., and J.B.). Full text review was then performed by 2 reviewers (J.S. and P.D.). At this stage articles were limited to those published in English or French. When there was disagreement about study selection, an attempt at consensus was made. In the rare instance that consensus could not be reached, adjudication was done by the third reviewer (J.B.). Reference lists of all included studies were searched for additional studies of potential relevance. If relevant information was unclear or missing, up to 3 attempts were made to contact the primary author and obtain the pertinent information.
Study selection
Study population
Patients aged 65 years and older who were undergoing acute GI surgery constituted the population of interest. In order to be consistent with current North American models of acute care surgery, at least 90% of the included cohorts had to have undergone GI surgery, with at least 75% of these surgeries being acute. The definition of acute surgery was any unscheduled or unplanned surgery.
Outcomes of interest
The primary outcomes of interest were postoperative morbidity and mortality. Postoperative mortality was defined as in-hospital or 30-day mortality. Morbidity was defined as any deviation from the normal postoperative course, using the classification scheme proposed by Dindo and colleagues.5,14 Major complications (Clavien III–IV) were defined as those requiring surgical, endoscopic or radiologic intervention and/or those requiring intensive care. Minor complications (Clavien I–II) were defined as any complication that was not major, including ileus, wound infection, the need for blood transfusion, systemic infection not requiring intensive care unit (ICU) intervention, cardiac arrythmia or the need for parenteral nutrition. Secondary outcomes of interest were length of stay (LOS) in hospital and discharge to an institution (rehabilitation hospital, assisted living situation or nursing home).
Prognostic factors
All prognostic factors evaluated in previous studies were considered in this systematic review. Prognostic factors were classified into 3 groups for synthesis and clear presentation: patient factors, disease factors and perioperative factors. Patient factors were any underlying condition or demographic characteristic present before the acute illness (e.g., age, sex, comorbidities). Disease factors were any prognostic feature related to the acute illness (e.g., laboratory values, presence of sepsis, peritonitis, obstruction, malignancy). Perioperative factors were aspects related to the surgical admission (e.g., postoperative complications, time to surgery, need for blood transfusion, type of surgery).
Study designs
Clinical cohort studies were included if there was a longitudinal component between prognostic factor measurement and outcomes of interest, including cohort studies or randomized controlled trials (if analyzed to identify important prognostic variables). Study data could be collected prospectively or retrospectively. Selection of studies was limited to those that included multivariate analysis (studies that reported only univariate, or crude analysis were excluded).
Critical appraisal of included studies
Prognostic factor studies were categorized into 3 groups based on phase of investigation.6,15,16 Phase one studies were exploratory studies in which associations between prognostic factors and outcomes were sought out. Phase 2 studies were exploratory studies based on prior hypotheses to test the association between prognostic factors and outcomes of interest. Finally, phase 3 studies were those that aimed to explain how relationships between prognostic factors influence the outcome.
Risk of bias (ROB) was assessed by 2 reviewers (J.B. and P.D.) using the Quality in Prognostic Studies (QUIPS) tool.7,17 The QUIPS tool examines ROB in 6 domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and presentation. Cohen’s κ was used to assess inter-observer reliability for agreement on all 6 domains. Where there was disagreement about assessment of individual items and judgment about domain risk of bias, reviewers attempted to reach consensus through discussion. In the rare instance that consensus was not achieved, adjudication was done by a third reviewer (J.S.).
Data extraction
Data extraction was performed with consensus by 2 independent reviewers (P.D. and J.S.), using a standardized data extraction form (see the Appendix, available at canjsurg.caa). Extracted information included study characteristics (type of study, number of patients, type of surgery, outcomes of interest), patient characteristics (age, sex, body mass index, and comorbid conditions), and strength of association (odds ratios [OR], relative risks [RR] and hazard ratios [HR]) between prognostic factors and outcomes of interest.
Data synthesis
When data were available, multivariate associations between prognostic factors and postoperative outcomes were synthesized. For clarity, associations were recalculated to be in the same direction, as necessary, with associations above 1 indicating a worse prognosis. Where 3 or more studies reported an association between a prognostic factor and outcome of interest, we performed random-effects generic inverse variance meta-analysis using Review Manager version 5.1 (Cochrane Collaboration). We calculated standard errors (SEs) from confidence intervals (CIs) and appropriately transformed the individual study association and SE to their natural logarithms to normalize their distributions. Heterogeneity among studies was assessed using a χ2 test and the I2 statistic. Heterogeneity was considered significant when the χ2 test had a p < 0.10 or if I2 was greater than 50%.
When meta-analysis was not possible, qualitative synthesis of studies was used to explore heterogeneity due to population source and setting, definitions of prognostic factor and outcomes. Strength of association was defined based on effect size as weak (OR < 1.5), moderate (OR 1.5–2.9) or strong (OR ≥ 3). Consistency of findings was assessed using the following schema.
Strong evidence: consistent findings (defined as > 75% of studies showing the same direction of effect) in multiple high-quality (defined as low ROB in all domains) studies.
Moderate evidence: consistent findings in multiple low-quality (moderate to high ROB in 4 of 6 domains) studies and/or 1 high-quality study.
Limited evidence: 1 study.
Conflicting evidence: inconsistent findings across studies.
No evidence: lack of association between the prognostic factor and outcome of interest.
Results
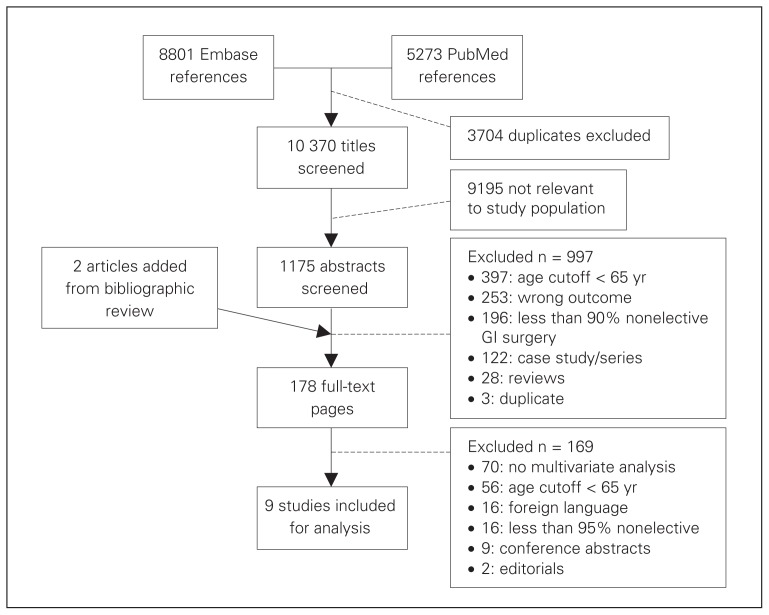
Nine studies met all of our selection criteria (Fig. 1). Sixteen papers (8 Russian, 3 Japanese, 2 German, 1 Chinese, 1 Bulgarian and 1 Norwegian) were excluded as per protocol; most were published in the late 1970s and early 1980s and did not contain a multivariate analysis. Two additional studies were identified through bibliographic review of included studies;8–11,18,19 neither was included in the review because they did not include multivariate analyses.
Fig. 1.
Overview of literature review and study selection. GI = gastrointestinal.
Study characteristics
Table 1 summarizes the characteristics of the 9 included studies9,20–27 representing a total of 2958 patients. Four studies22–25 focused exclusively on acute colorectal surgery, while 5 studies9,20,21,26,27 focused on acute GI surgery. All of these studies included mortality as the primary outcome of interest. Two studies22,24 examined prognostic factors for morbidity using multivariate analysis. No included study examined the association between prognostic factors, LOS or discharge to institution.
Table 1.
Study and patient characteristics of included studies
| Study | Location | No. of patients | Age cut-off, yr | Type of surgery | Outcomes of interest | Average age, yr or median (range) | Sex, % male | Average LOS, d or median (range) | Mortality, % | Morbidity, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Arenal et al.20 | Valladolid, Spain | 710 | ≥ 70 | GI surgery | In-hospital mortality | 79.4 | 46.8 | NR | 21.5 | 58.3 |
| Cook et al.9 | Bristol, UK | 107 | ≥ 65 | GI surgery | In-hospital mortality | 80.2 | 50.4 | NR | 43.9 | NR |
| Fukuda et al.21 | Kawasaki, Japan | 94 | ≥ 80 | GI surgery | 30-day mortality | 85.6 | 38.3 | NR | 16.0 | 43.6 |
| Kwok et al.23 | Boston, USA | 1358 | ≥ 80 | Colorectal | 30-day mortality | 85.3 | 34.3 | NR | 28.9 | 26.9* |
| Leong et al.22 | Singapore | 58 | ≥ 80 | Colorectal | 30-day morbidity/mortality | 83 (80–96) | 41.4 | 17.5 (3–108) | 27.6 | 81.0 |
| McGillicuddy et al.24 | New Haven, USA | 292 | ≥ 65 | Colorectal | In-hospital morbidity/mortality | 78.1 | 41 | 20.9 | 15.0 | 34.6 |
| Modini et al.25 | Rome, Italy | 215 | > 65 | Colorectal | 30-day mortality | 78 | 47 | NR | 16.3 | 17.2 |
| Okubo et al.26 | Niigata, Japan | 36 | ≥ 80 | GI surgery | In-hospital mortality | 84 (80–97) | 44.4 | 38 (2–150) | 27.8 | 83.3 |
| Vaughan-Shaw et al.27 | Southampton, UK | 88 | ≥ 80 | GI surgery | 30-day mortality | 84 (80–95) | 51.1 | 15 (0–72) | 33.0 | NR |
COPD = chronic obstructive pulmonary disease; CRF = chronic renal failure; DM = diabetes mellitus; GI = gastrointestinal; IHD = ischemic heart disease; LOS = length of stay; NR = not reported.
Major morbidity reported.
Study designs
Table 2 summarizes the ROB assessment for all included studies. Inter-rater reliability was good (κ = 0.76, 95% CI 0.59–0.92). Two studies9,23 were prospective cohorts and the rest were retrospective. All studies were exploratory, phase 1, investigations. Most studies were of low to moderate quality. The main issues with study quality were related to prognostic factor measurement, study confounding and statistical analysis. The ROB was reported as moderate in 7 studies9,20–25 owing to incomplete reporting on how prognostic factors were measured and in 5 studies9,20,21,24,25 owing to partial reporting on confounder measurement. One study22 was rated as having a ROB owing to partial reporting of confounder measurement and partial reporting on how adjustment was made. Finally, with respect to statistical analysis, most studies were considered to have a moderate ROB, as step-wise regression was used. One study received a high ROB rating as, in addition to using step-wise regression, model presentation was incomplete.24
Table 2.
Risk of bias assessment for included studies
| Study | Data acquisition | Study participation | Study attrition | Prognostic factor measurement | Outcome measurement | Study confounding | Statistical analysis and presentation |
|---|---|---|---|---|---|---|---|
| Arenal et al.20 | Retrospective | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Cook et al.9 | Prospective | Low | Low | Moderate | Low | Moderate | Moderate |
| Fukuda et al.21 | Retrospective | Moderate | Low | Moderate | Moderate | Moderate | Moderate |
| Kwok et al.23 | Prospective | Moderate | Moderate | Moderate | Low | Low | Moderate |
| Leong et al.22 | Retrospective | Low | Low | Moderate | Moderate | High | Moderate |
| McGillicuddy et al.24 | Retrospective | Low | Low | Moderate | Moderate | Moderate | High |
| Modini et al.25 | Retrospective | Low | Low | Moderate | Low | Moderate | Low |
| Okubo et al.26 | Retrospective | Low | Low | Low | Low | Low | Low |
| Vaughan-Shaw et al.27 | Retrospective | Low | Low | Low | Low | Low | Low |
Prognostic factors associated with perioperative mortality
Patient factors associated with postoperative mortality are summarized in Table 3. Nine patient factors were investigated across studies. There is limited evidence of an association between a history of chronic obstructive pumonary disease,23 a history of congestive heart failure,23 dependent functional status23 and mortality. All studies examined age as a prognostic factor. Evidence for an association between age and mortality was conflicting, as only 4 studies9,23–25 found an association on multivariate analysis (5 studies reported negative/neutral associations with outcome). The American Society of Anesthesiologists (ASA) score was considered in 7 of 9 studies,9,20,22,24–27 and results were also inconsistent. Three studies treated the ASA score as an ordinal variable; a pooled analysis is summarized in Fig. 2 (pooled OR 2.77, 95% CI 0.92–8.41). An additional 3 studies21,24,27 treated the ASA Score as an ordinal variable; however, they used stepwise regression and the ASA score was removed during modelling. The remaining study treated the ASA score as dichotomous, showing an association between an ASA of 3 or greater and mortality.22 Contradictory evidence also existed for sex9 and history of neurologic disease.25 There is no evidence of an association between the Eastern Cooperative Oncology Group physical status and mortality.
Table 3.
Patient factors associated with postoperative mortality, OR (95% CI)
| Patient factor | Arenal et al.20 | Cook et al.9 | Fukuda et al.21 | Kwok et al.23 | Leong et al.22 | McGillicuddy et al.24 | Modini et al.25 | Okubo et al.26 | Vaughan-Shaw et al.27 |
|---|---|---|---|---|---|---|---|---|---|
| Age | OR 1.03 (0.97–1.09) | OR 1.15 (1.04–1.27)* | NS | Age < 90 yr OR 0.62 (0.43–0.88)* |
NS | p = 0.001*† | Age ≥ 80 yr OR 3.77 (1.32–10.7)* |
NS | NS |
| Sex, male | OR 1.05 (0.99–1.11) | OR 0.21 (0.19–0.23)* | NS | NS | NS | NS | NS | NS | NS |
| ASA score | OR 1.15 (1.11–1.19)* | OR 5.88 (2.40–14.43)* | — | — | ASA ≥ 3 OR 10.41 (1.48–73.19)* |
NS | OR 3.87 (2.05–7.93)* | NS | NS |
| Presence of comorbidities | — | NS | NS | — | — | NS | NS | NS | — |
| Hx of COPD | — | — | — | OR 1.79 (1.28–2.50)* | — | — | — | — | NS |
| History of CHF | — | — | — | OR 1.87 (1.21–2.90)* | — | — | — | — | — |
| History of neurologic disease | — | — | — | — | — | — | OR 4.47 (1.73–11.41)* | — | NS |
| ECOG physical status | — | — | NS | — | — | — | — | NS | — |
| Totally dependent functional status | — | — | — | OR 2.54 (1.88–3.43)* | — | — | — | — | — |
ASA = American Society of Anesthesiologists; CHF = congestive heart failure; CI = confidence interval; COPD = chronic obstructive pulmonary disease; ECOG = Eastern Cooperative Oncology Group; NS = not significant; OR = odds ratio.
p < 0.05.
Data treated as continuous and no OR produced.
Fig. 2.
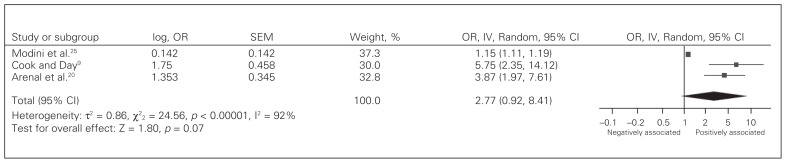
Pooled analysis for American Society of Anesthesiologists (ASA) score as a prognostic factor for postoperative mortality. CI = confidence interval; IV = inverse variance; OR = odds ratio; SEM = standard error of the mean.
Disease factors associated with postoperative mortality are summarized in Table 4. A total of 11 disease factors were analyzed. There was limited evidence of an association between the physiologic component of the Physiologic and Operative Severity Score for the enumeration of Mortality and Morbidity (POSSUM) score,21 the neutrophil to lymphocyte ratio,27 presence of 2 or more failing organs26 and mortality. Conflicting evidence for an association between mortality and serum creatinine,23,24 mesenteric ischemia,20,23,25 the presence of the systemic inflammatory response (SIRS) or sepsis,9,23–25 and metastatic disease20,23,25,26 was shown. There was no evidence of an association between the Acute Physiology and Chronic Health Evaluation-II (APACHE-II) score,21 the operative severity component of the POSSUM score,21 the presence of GI bleeding,20,23,25 intestinal obstruction,9,20,23 or the presence of peritonitis20,25 and mortality.
Table 4.
Disease factors associated with postoperative mortality, OR (95% CI)
| Disease factor | Arenal et al.20 | Cook et al.9 | Fukuda et al.21 | Kwok et al.23 | McGillicuddy et al.24 | Modini et al.25 | Okubo et al.26 | Vaughan-Shaw et al.27 |
|---|---|---|---|---|---|---|---|---|
| APACHE-II score | — | — | OR 1.13 (0.92–1.38) | — | — | — | — | — |
| POSSUM score | — | — | PS: OR 1.20 (1.03–1.42)* | — | — | — | — | — |
| OSS: OR 1.02, (0.85–1.23) | ||||||||
| Presence of intestinal obstruction | OR 1.04 (0.97–1.12) | NS | — | NS | — | — | — | — |
| Creatinine > 1.5 mg/dL | — | — | OR 2.57 (1.97–3.36)* | NS | — | — | — | |
| N/L ratio | — | — | — | — | — | — | — | OR 1.03 (1.01–1.06)* |
| ≥ 2 failing organs | — | — | OR 5.51 (1.97–15.4)* | — | ||||
| Presence of GI bleeding | OR 1.12 (0.96–1.30) | — | — | NS | — | NS | — | — |
| Presence of mesenteric ischemia | OR 1.29 (1.08–1.53)* | — | — | NS | — | OR 4.33 (0.89–21.11) | — | — |
| Presence of SIRS/sepsis | — | NS | — | OR 2.13 (1.60–2.82)* | OR 5.26 (1.21–22.5)* | NS | — | — |
| Presence of peritonitis | OR 1.04 (0.96–1.13) | — | — | — | — | NS | — | — |
| Metastatic disease | OR 1.03 (0.91–1.17) | — | — | OR 2.00 (1.08–3.71)* | — | NS | NS | — |
APACHE-II = Acute Physiology and Chronic Health Evaluation II; CI = confidence interval; GI = gastrointestinal; N/L = neutrophil/lymphocyte; NS: not significant; OR = odds ratio; OSS = operative severity score; POSSUM = Physiologic and Operative Severity Score for the enUmeration of Mortality and Morbidity; PS = physiologic score; SIRS = systemic inflammatory response syndrome.
Prognostic factor not considered in analysis.
p < 0.05.
Perioperative factors associated with patient mortality are summarized in Table 5. A total of 14 perioperative factors were considered across studies. There was moderate evidence of an association between duration of symptoms before admission and mortality.20,21 There was limited evidence for an association between mortality and palliative resection,20 nontherapeutic laparotomy,20 need for invasive monitoring,9 need for ICU admission9 and midline laparotomy.27 Conflicting evidence for an association between time from admission to surgery,20,24–26 postoperative complications,22,24–26 pre-operative steroid use23,24 and estimated blood loss24,25 was shown. There was no evidence of an association with GI resection,9,20,22,23 suture repair of perforation,20,22 time from symptom onset to surgery,20 or adequate resuscitation9 and mortality.
Table 5.
Perioperative factors associated with postoperative mortality, OR (95% CI)
| Perioperative factor | Arenal et al.20 | Cook et al.9 | Fukuda et al.21 | Kwok et al.23 | Leong et al.22 | McGillicuddy et al.24 | Modini et al.25 | Okubo et al.26 | Vaughan-Shaw et al.27 |
|---|---|---|---|---|---|---|---|---|---|
| Duration of Symptoms before admission, h | OR 1.10 (1.03–1.18)* | — | > 24 h of symptoms: OR 9.60 (1.82–50.60)* | — | — | — | — | — | — |
| Time from admission to operating room, h | OR 1.05 (0.98–1.12) | — | — | — | — | p = 0.002*† | NS | NS | — |
| Time from symptom onset to operating room, h | OR 0.97 (0.89–1.05) | — | — | — | — | — | — | — | — |
| Patient adequately resuscitated | — | NS | — | — | — | — | — | — | — |
| EBL | — | — | — | — | — | p = 0.02*† | NS | — | — |
| Preoperative steroid use | — | — | — | OR 0.61 (1.06–2.45)* | — | NS | — | — | — |
| Postoperative complications | — | — | — | — | NS | OR 36.17 (11.48–113.9)* | Anastomotic leak OR 39.51 (5.17–301.63)* |
NS | — |
| GI resection | OR 1.04 (0.97–1.12) | NS | — | NS | NS | — | — | — | — |
| Suture closure of GI perforation | OR 1.08 (0.96–1.21) | — | — | — | NS | — | — | — | — |
| Palliative procedure | OR 1.18 (1.06–1.31)* | — | — | — | — | — | — | — | — |
| Nontherapeutic laparotomy | OR 1.26 (1.11–1.43)* | — | — | — | — | — | — | — | — |
| Need for invasive monitoring | — | OR 6.25 (1.59–24.55)* | — | — | — | — | — | — | — |
| Need for ICU admission | — | OR 11.11 (1.95–64.21)* | — | — | — | — | — | — | — |
| Midline laparotomy | — | — | — | — | — | — | — | — | OR 8.86 (1.20–65.46)* |
CI = confidence interval; EBL = estimated blood loss; GI = gastrointestinal; ICU = intensive care unit; NS: not significant; OR = odds ratio.
p < 0.05.
Data treated as continuous and no OR produced.
Prognostic factor not considered in analysis.
Prognostic factors associated with postoperative complications
Only 2 exploratory studies19,21 examined potential prognostic factors for postoperative morbidity. One study22 evaluated the association between patient age, sex, surgeons’ expertise, ASA grade, hemoglobin on admission, the need for blood transfusion, duration of the operation and type of operation and postoperative morbidity. On multivariate analysis only high ASA score (≥ 3) was associated with postoperative morbidity (OR 37.29; 2.31–602.60). A second study24 examined the association between 17 prognostic factors and the development of any postoperative complication (including pneumonia, respiratory failure, myocardial infarction, deep venous thrombosis, pulmonary embolus and stroke). The authors used a stepwise regression model and found wound contamination (OR 3.22, 95% CI 1.55–6.67, p < 0.001), shock (OR 2.23, 95% CI 1.05–4.88, p = 0.04), chronic renal insufficiency (OR 1.47, 95% CI 1.06–2.04, p = 0.02) and time in the operating room (no OR reported as data continuous, p = 0.01) to be associated with postoperative complications.
Taken together, these studies provide limited evidence of an association between an ASA score of 3 or greater, wound contamination, shock, chronic renal insufficiency, time in the operating room and postoperative morbidity in this patient population.
Discussion
As the population ages, issues related to the surgical care of elderly patients are becoming increasingly common.2,7,10,28 Elderly patients may differ from younger patients in several ways, including the number and severity of comorbid conditions they have, the types of surgical problems that develop and the treatments that are offered to them. Accordingly, there has been considerable interest in risk assessment specifically for elderly patients undergoing abdominal surgery.29–32 However, currently available preoperative risk assessment tools33–42 lack sufficient accuracy and reliability and often are not applicable in the acute clinical setting in this patient population.27,29,30 Thus surgeons are left with very little in their armamentarium to counsel patients regarding postoperative risks and must rely on clinical judgment.43 Better risk prediction models are needed to guide the care of older patients, particularly in areas associated with high morbidity and mortality.44
To our knowledge, this is the first systematic review on prognostic factors associated with mortality among elderly patients undergoing acute GI surgery. A total of 34 potential prognostic factors were analyzed, and there was significant variability with regards to which factors were examined in each study. The majority of evidence suggesting an association between prognostic factors and mortality was limited or conflicting. Only age at the time of surgery, the ASA score, the presence of SIRS/sepsis, duration of symptoms before admission and postoperative complications were shown to be associated with mortality in more than 1 study. Quantitative meta-analysis was only possible for the ASA score,9,20,25 and there was significant heterogeneity in effect size (I2 = 92%).
Although there was an association between increased ASA score and postoperative mortality in elderly patients undergoing acute abdominal surgery, 3 studies,21,24,27 not incorporated in the quantitative meta-analysis, did not show an association between ASA score and mortality. Given the limited number of studies available, sensitivity analysis was not possible, and the reasons for this variation are unclear. As the covariates examined across studies are not dissimilar, a possible explanation for this discrepancy is related to the study design. As all 3 studies21,24,27 used step-wise regression, it is possible that the results differ due to the statistical method used, as step-wise regression techniques are very sensitive to small changes in the data and the results are highly dependent on the cohort make-up. Therefore, the true association between the ASA score and mortality in elderly patients is unclear and further research is required.
The present systematic review highlights the lack of quality research for potential prognostic factors for morbidity and mortality in this high-risk population. Importantly, none of the studies examined variables associated with postoperative LOS, postoperative quality of life, loss of independence or the need for nursing home placement. Given that many elderly patients consider quality of life to be more important than quantity of life,45,46 research that addresses these patient-centred outcomes is needed. This issue was highlighted in a recent quality improvement guideline for optimal preoperative assessment of geriatric surgical patients.32 Future research evaluating prognostic factors for perioperative outcomes in elderly patients should also include frailty, which has been associated with postoperative morbidity, postoperative LOS and loss of independence among elderly patients undergoing elective GI surgery.47–49
Identification of prognostic factors that can be used to create predictive models may help surgeons counsel patients regarding their postoperative risks. In addition it may help to identify strategies to improve outcomes in this patient population.44 While factors such as patient age and comorbidities are not modifiable, other factors, such as postoperative complications, might be. In addition, in trying to improve outcomes associated with emergency procedures, the potential to decrease the need for emergency surgery should be examined. Older patients with chronic conditions, such as incisional hernias or biliary tract disease, who are often managed expectantly with the hope that they might not need therapy, could be the group who benefit the most from elective surgery. With the increasing proportion of elderly patients, a better understanding of the risks and the benefits associated with elective versus emergency surgery is needed for common existing conditions that can lead to acute events requiring urgent surgery.
Conclusion
The literature on prognostic factors for postoperative morbidity and mortality in elderly patients undergoing nonelective GI surgery is very limited. At present, there are no established models that can assist in predicting adverse outcomes in this group. The majority of available studies are exploratory, most evidence is of limited quality and the results are conflicting. Given the aging population and associated future need for emergency surgery in elderly patients, there is a need for high-quality research in this area.
Footnotes
Competing interests: None declared.
Contributors: P. Davis, J. Hayden and P. Johnson designed the study. P. Davis, J. Springer and J. Bailey acquired the data, which P. Davis, J. Hayden and P. Johnson analyzed. P. Davis wrote the article, which all authors reviewed and approved for publication.
References
- 1.Ingraham AM, Cohen ME, Raval MV, et al. Variation in quality of care after emergency general surgery procedures in the elderly. J Am Coll Surg. 2011;212:1039–48. doi: 10.1016/j.jamcollsurg.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Peden CJ. Emergency surgery in the elderly patient: a quality improvement approach. Anaesthesia. 2011;66:440–5. doi: 10.1111/j.1365-2044.2011.06769.x. [DOI] [PubMed] [Google Scholar]
- 3.DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;13(165):1–209. [PubMed] [Google Scholar]
- 4.Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010;(29):1–21. [PubMed] [Google Scholar]
- 5.Health Resources and Skills Development Canada. Canadians in context — aging population. [accessed 2013 May 22]. Available: www4.hrsdc.gc.ca/.3ndic.1t.4r@-eng.jsp?iid=33.
- 6.Menon KV, Young FM, Galland RB. Emergency surgical admissions in patients aged more than 80 years: a study over four decades. Ann R Coll Surg Engl. 2000;82:392–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Massarweh NN, Legner VJ, Symons RG, et al. Impact of advancing age on abdominal surgical outcomes. Arch Surg. 2009;144:1108–14. doi: 10.1001/archsurg.2009.204. [DOI] [PubMed] [Google Scholar]
- 8.Keller SM, Markovitz LJ, Wilder JR, et al. Emergency and elective surgery in patients over age 70. Am Surg. 1987;53:636–40. [PubMed] [Google Scholar]
- 9.Cook TM, Day CJ. Hospital mortality after urgent and emergency laparotomy in patients aged 65 yr and over. Risk and prediction of risk using multiple logistic regression analysis. Br J Anaesth. 1998;80:776–81. doi: 10.1093/bja/80.6.776. [DOI] [PubMed] [Google Scholar]
- 10.Abbas S, Booth M. Major abdominal surgery in octogenarians. N Z Med J. 2003;116:U402. [PubMed] [Google Scholar]
- 11.Neary WD, Foy C, Heather BP, et al. Identifying high-risk patients undergoing urgent and emergency surgery. Ann R Coll Surg Engl. 2006;88:151–6. doi: 10.1308/003588406X94896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bufalari A, Ferri M, Cao P, et al. Surgical care in octogenarians. Br J Surg. 1996;83:1783–7. doi: 10.1002/bjs.1800831239. [DOI] [PubMed] [Google Scholar]
- 13.Riley RD, Hayden JA, Steyerberg EW, et al. Prognosis research strategy (PROGRESS) 2: Prognostic factor research. PLoS Med. 2013;10:e1001380. doi: 10.1371/journal.pmed.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden JA, Côté P, Steenstra IA, et al. Identifying phases of investigation helps planning, appraising, and applying the results of explanatory prognosis studies. J Clin Epidemiol. 2008;61:552–60. doi: 10.1016/j.jclinepi.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Altman DG, Lyman GH. Methodological challenges in the evaluation of prognostic factors in breast cancer. Breast Cancer Res Treat. 1998;52:289–303. doi: 10.1023/a:1006193704132. [DOI] [PubMed] [Google Scholar]
- 17.Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–37. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 18.Karanikas ID, Liakakos TD, Koundourakis SS, et al. Emergency operations in the elderly: management and outcome. Int Surg. 1996;81:158–62. [PubMed] [Google Scholar]
- 19.Schöön IM, Arvidsson S. Surgery in patients aged 80 years and over. A retrospective comparative study from 1981 and 1987. Eur J Surg. 1991;157:251–5. [PubMed] [Google Scholar]
- 20.Arenal JJ, Bengoechea-Beeby M. Mortality associated with emergency abdominal surgery in the elderly. Can J Surg. 2003;46:111–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda N, Wada J, Niki M, et al. Factors predicting mortality in emergency abdominal surgery in the elderly. World J Emerg Surg. 2012;7:12. doi: 10.1186/1749-7922-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leong QM, Aung MO, Ho CK, et al. Emergency colorectal resections in Asian octogenarians: factors impacting surgical outcome. Surg Today. 2009;39:575–9. doi: 10.1007/s00595-008-3925-1. [DOI] [PubMed] [Google Scholar]
- 23.Kwok AC, Lipsitz SR, Bader AM, et al. Are targeted preoperative risk prediction tools more powerful? A test of models for emergency colon surgery in the very elderly. J Am Coll Surg. 2011;213:220–5. doi: 10.1016/j.jamcollsurg.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 24.McGillicuddy EA, Schuster KM, Davis KA, et al. Factors predicting morbidity and mortality in emergency colorectal procedures in elderly patients. Arch Surg. 2009;144:1157–62. doi: 10.1001/archsurg.2009.203. [DOI] [PubMed] [Google Scholar]
- 25.Modini C, Romagnoli F, De Milito R, et al. Octogenarians: an increasing challenge for acute care and colorectal surgeons. An outcomes analysis of emergency colorectal surgery in the elderly. Colorectal Dis. 2012;14:e312–8. doi: 10.1111/j.1463-1318.2012.02934.x. [DOI] [PubMed] [Google Scholar]
- 26.Okubo R, Yajima K, Sakai Y, et al. Short-and long-term outcomes of surgery for diffuse peritonitis in patients 80 years of age and older. Surg Today. 2008;38:413–9. doi: 10.1007/s00595-007-3658-6. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan-Shaw PG, Rees JRE, King AT. Neutrophil lymphocyte ratio in outcome prediction after emergency abdominal surgery in the elderly. Int J Surg. 2012;10:157–62. doi: 10.1016/j.ijsu.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Ingraham AM, Cohen ME, Raval MV, et al. Variation in quality of care after emergency general surgery procedures in the elderly. J Am Coll Surg. 2011;212:1039–48. doi: 10.1016/j.jamcollsurg.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Rix TE, Bates T. Pre-operative risk scores for the prediction of outcome in elderly people who require emergency surgery. World J Emerg Surg. 2007;2:16. doi: 10.1186/1749-7922-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandra A, Mangam S, Marzouk D. A review of risk scoring systems utilised in patients undergoing gastrointestinal surgery. J Gastrointest Surg. 2009;13:1529–38. doi: 10.1007/s11605-009-0857-z. [DOI] [PubMed] [Google Scholar]
- 31.Ergina PL, Gold SL, Meakins JL. Perioperative care of the elderly patient. World J Surg. 1993;17:192–8. doi: 10.1007/BF01658926. [DOI] [PubMed] [Google Scholar]
- 32.Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement program and the American Geriatrics Society. J Am Coll Surg. 2012;215:453–66. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–4. [Google Scholar]
- 34.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 35.Donati A, Ruzzi M, Adrario E, et al. A new and feasible model for predicting operative risk. Br J Anaesth. 2004;93:393–9. doi: 10.1093/bja/aeh210. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy RH, al-Mufti RA, Brewster SF, et al. The acute surgical admission: Is mortality predictable in the elderly? Ann R Coll Surg Engl. 1994;76:342–5. [PMC free article] [PubMed] [Google Scholar]
- 37.Veltkamp SC, Kemmeren JM, van der Graaf Y, et al. Prediction of serious complications in patients admitted to a surgical ward. Br J Surg. 2002;89:94–102. doi: 10.1046/j.0007-1323.2001.01963.x. [DOI] [PubMed] [Google Scholar]
- 38.Arozullah AM, Khuri SF, Henderson WG, et al. Participants in the National Veterans Affairs Surgical Quality Improvement Program. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135:847–57. doi: 10.7326/0003-4819-135-10-200111200-00005. [DOI] [PubMed] [Google Scholar]
- 39.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 40.Mullen JL, Buzby GP, Waldman MT, et al. Prediction of operative morbidity and mortality by preoperative nutritional assessment. Surg Forum. 1979;30:80–2. [PubMed] [Google Scholar]
- 41.Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–7. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 42.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 43.Hartley MN, Sagar PM. The surgeon’s ‘gut feeling’ as a predictor of post-operative outcome. Ann R Coll Surg Engl. 1994;76(Suppl):277–8. [PubMed] [Google Scholar]
- 44.Al-Temimi MH, Griffee M, Enniss TM, et al. When is death inevitable after emergency laparotomy? Analysis of the American College of Surgeons National Surgical Quality Improvement program database. J Am Coll Surg. 2012;215:503–11. doi: 10.1016/j.jamcollsurg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Cohen-Mansfield J, Droge JA, Billig N. Factors influencing hospital patients’ preferences in the utilization of life-sustaining treatments. Gerontologist. 1992;32:89–95. doi: 10.1093/geront/32.1.89. [DOI] [PubMed] [Google Scholar]
- 46.Lee KF. Patient preference and outcomes-based surgical care among octogenarians and nonagenarians. J Am Coll Surg. 2006;202:356–72. doi: 10.1016/j.jamcollsurg.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 48.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric pre-operative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 49.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011;253:1223–9. doi: 10.1097/SLA.0b013e318214bce7. [DOI] [PubMed] [Google Scholar]