Abstract
Objective
To assess whether weight loss improves markers of peripheral artery disease and vascular stenosis.
Design and Methods
The Action for Health in Diabetes randomized clinical trial compared intensive lifestyle intervention (ILI) for weight loss to a control condition of diabetes support and education (DSE) in overweight or obese adults with type 2 diabetes. Annual ankle and brachial blood pressures over four years were used compute ankle-brachial indices (ABIs) and to assess inter-artery blood pressure differences in 5018 participants.
Results
ILI, compared to DSE, produced 7.8% (Year 1) to 3.6% (Year 4) greater weight losses. These did not affect prevalence of low (<0.90) ABI (3.60% in DSE versus 3.14% in ILI; p=0.20) or elevated (>1.40) ABI (7.52% in DSE versus 7.59% in ILI: p=0.90), but produced smaller mean (SE) maximum inter-artery systolic blood pressure differences among ankle sites [19.7 (0.2) mmHg for ILI versus 20.6 (0.2) mmHg for DSE (p<0.001)] and between arms [5.8 (0.1) mmHg for ILI versus 6.1 (0.1) mmHg for DSE (p=0.01)].
Conclusions
Four years of intensive behavioral weight loss intervention did not significantly alter prevalence of abnormal ABI, however it did reduce differences in systolic blood pressures among arterial sites.
Keywords: Peripheral artery disease, Weight loss, Diabetes
INTRODUCTION
The ankle-brachial index (ABI) is commonly used for the diagnosis of peripheral arterial disease (1) and is an independent marker of cardiovascular disease (CVD) (2–4). Individuals in the lower tail of the ABI distribution, specifically with values <0.90 or <0.95, are classified as abnormal and considered to be at risk for peripheral artery disease, requiring further work-up for diagnosis. Those in the upper tail of the ABI distribution have been described as having non-compressible arteries or medial calcinosis in lower extremities and have been ignored in many discussions of ABI; however, relatively high values also signal increased CVD risk (5,6) and mortality (1,3,7). Cutpoints of >1.30 (5,6), >1.40 (3,7), and >1.50 (2,4) have been variously used to define this upper tail.
There is growing evidence that, beyond ABI, other differences in systolic pressures among arterial sites (e.g. between left and right arms) may also be markers of atherosclerosis (9–11). ABI evaluations are based on systolic blood pressure measurements taken from six arterial sites (right and left brachial, dorsal pedis, and posterior tibial arteries), which provide us opportunity to assess whether inter-artery differences in systolic blood pressure may be influenced by an intensive lifestyle intervention for weight loss.
While obesity is a strong risk factor for abnormal ABI (8,12), it is not known whether weight loss in overweight and obese individuals with diabetes is effective in reducing its prevalence or in reducing measures of inter-artery systolic blood pressure differences.
METHODS AND PROCEDURES
The Action for Health in Diabetes (Look AHEAD) is a multi-center randomized clinical trial that enrolled 5,145 overweight or obese volunteers with type 2 diabetes between June 2001 and March 2004 (13). Its primary goal was to assess the long-term effects on CVD outcomes of an intensive lifestyle intervention (ILI) program designed to achieve and maintain weight loss by decreased caloric intake and increased physical activity. Participants were randomly assigned by center, with equal probability, to ILI or the control condition of diabetes support and education (DSE).
The ILI included diet modification and physical activity and was designed to induce at least an average 7% weight loss at year 1 and to maintain this weight loss in subsequent years (14). Its participants were assigned a calorie goal (1200–1800 based on initial weight), with <30% of total calories from fat (<10% from saturated fat) and a minimum of 15% of total calories from protein. The physical activity goal was ≥175 minutes per week of activities similar in intensity to brisk walking. Behavioral strategies included self-monitoring, goal setting, and problem solving. Participants in ILI were seen weekly for the first 6 months and 3 times per month for the next 6 months, with a combination of group and individual contacts. During years 2–4, participants were seen individually at least once a month, contacted another time each month by phone or e-mail, and offered a variety of centrally-approved group classes.
DSE participants were invited to three group sessions each year (15). These used standardized protocols with focus on diet, physical activity, or social support. Information on behavioral strategies was not presented and participants were not weighed.
At enrollment, Look AHEAD participants ranged in age from 45–76 years and had a body mass index ≥25 kg/m2, or ≥27 kg/m2 if taking insulin. Other inclusion requirements included a source of medical care, blood pressure <160/100 mmHg (treated or untreated), HbA1c <11%, plasma triglycerides <8.0 mmol/L (600 mg/dl), and willingness to accept random assignment. Potential volunteers judged to be unlikely to be able to carry out the components of the weight loss intervention were excluded.
Data Collection Protocol for Peripheral Artery Disease Risk Factors
Standardized interviewer-administered questionnaires were used to obtain data on demography and medical history. History of cardiovascular disease was defined by self-report of prior myocardial infarction, stroke, coronary or lower extremity revascularization, carotid endarterectomy, or coronary bypass surgery. For calculating body mass index (ratio of weight to height squared), weight was measured in replicate on a digital scale and standing height was determined in replicate with a standard stadiometer. Seated blood pressure was measured twice with an automated device using a common protocol and certified staff. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of anti-hypertensive medications. Hyperlipidemia was defined as LDLcholesterol >3.37 mmol/L (130 mg/dL) or use of lipid-lowering medications. A maximal graded exercise test was administered (16). Fitness was measured as the estimated metabolic equivalents (METS) at 80% maximal heart rate: 4 METS approximates walking on flat ground at just under 4 miles per hour. Additional details have been published previously (13).
Ankle-Brachial Blood Pressures
Ankle and brachial blood pressures were measured at baseline and at each of four annual examinations by trained staff who were masked to intervention assignment. During enrollment, Look AHEAD transitioned through three measurement protocols in an effort to balance the need to streamline ABI data collection with that of obtaining valid data. The most labor-intensive protocol involved three replicate systolic blood pressure measurements at the right arm, left arm, right dorsalis pedis, right posterior tibial, left dorsal pedis, left posterior tibial, and a repeat of the measurements from the original arm (a total of 18 separate measurements). This protocol was used only at baseline for 541 participants. A second protocol involved two replicate measurements from the right arm, left arm, right dorsalis pedis, right posterior tibial, left dorsalis pedis, and left posterior tibial (12 total measurements), and was used only at baseline only for 343 participants. The final protocol involved a single measurement at the right arm, left arm, right dorsalis pedis, right posterior tibial, left dorsalis pedis, and left posterior tibial, and a replicate measure at the arm with the highest initial systolic blood pressure (7 total measurements). This protocol was used for 4259 participants at baseline and for all follow-up measurements on the full cohort. The measurement protocols were used in successive cohorts of enrollees; data from these cohorts were pooled for analysis. Measurements were obtained by trained and certified study personnel using a continuous wave Doppler with a 5–8 mHz probe for ankle pressures and a standard mercury sphygmomanometer for brachial pressures. Our analyses are based on the average of systolic blood pressure measurements at each arterial site.
Leg-specific ABI was calculated according to a standard algorithm reported in guidelines from the American Heart Association (1) in which the ABI for each leg equals the ratio of the higher of the two ankle systolic blood pressure measurements (posterior tibial versus dorsal pedis) divided by either the average of the right and left brachial artery pressures, or if there is a discrepancy ≥10 mmHg in systolic blood pressure values between the two brachial values, the higher of the two brachial values. Cutpoints of 0.90 and 1.40 were used to define low and high ABI (7). The three different measurement protocols used at baseline would be expected to yield slightly different ABI values (17); these differences are minor (i.e. resulting in differences in ABI of <0.03) and will be ignored in our analyses. All follow-up ABIs are calculated from systolic blood pressure measurements using the same protocol.
Statistical Analysis
The prevalence of leg-specific low and high ABI over time between intervention groups was compared using generalized estimating equations with adjustment for baseline status. Alternative analytical approaches (Markov and time-to-event models) produced similar results and are not reported. We also examined two other measures of inter-artery differences in systolic blood pressure: the maximum difference among the four ankle sites and the absolute difference between left and right arms. Generalized linear models were used to compare mean differences in these measures over time between intervention groups, with adjustment for baseline differences. For both the analyses of prevalence and mean differences, a first order autoregressive model was used for longitudinal correlations. The consistency of differences between interventions with respect to measures was examined for subgroups based on gender and baseline history of cardiovascular disease, body mass index, age, and smoking history using tests of interactions.
RESULTS
Table 1 describes the 5,018 (97.5%) participants, of the 5,145 randomized, who contributed follow-up ABI, with respect to baseline risk factors for peripheral arterial disease. Good balance in the distribution of these factors between intervention groups was achieved with the trial’s randomization, with the least balance being a difference in mean systolic blood pressures between groups of 1.2 mmHg (p=0.02). The distribution of ABI and other measures of inter-artery differences in systolic blood pressure were similar between groups.
Table 1.
Baseline risk factors for peripheral artery disease on 5,003 Look AHEAD participants with valid ABI measurements.
| Cardiovascular Disease Risk Factor | DSE N (Percent) or Mean (SD) |
ILI N (Percent) or Mean (SD) |
p-value |
|---|---|---|---|
|
| |||
| Total | 2507 | 2511 | -- |
|
| |||
| Sex | |||
| Female | 1498 (59.8) | 1492 (59.4) | 0.75 |
| Male | 1009 (40.2) | 1019 (40.6) | |
|
| |||
| Age, yrs | |||
| 45–59 | 1413 (56.4) | 1468 (57.7) | 0.35 |
| 60–76 | 1094 (43.6) | 1063 (42.3) | |
|
| |||
| Hypertension | |||
| Yes | 2076 (82.8) | 2113 (84.2) | 0.20 |
| No | 431 (17.2) | 398 (15.8) | |
|
| |||
| Blood pressure, mmHg | |||
| Systolic | 129.4 (17.0) | 128.2 (17.2) | 0.02 |
| Diastolic | 70.3 (9.6) | 69.9 (9.5) | 0.15 |
|
| |||
| Hyperlipidemia | |||
| Yes | 1735 (69.2) | 1724 (68.7) | 0.67 |
| No | 772 (30.8) | 787 (31.3) | |
|
| |||
| Lipid lowering drug use | |||
| Yes | 1205 (49.3) | 1231 (50.2) | 0.69 |
| No | 1241 (50.7) | 1223 (49.8) | |
|
| |||
| Cigarette smoking | |||
| Never | 1280 (51.2) | 1248 (49.8) | |
| Former | 1113 (44.5) | 1145 (45.7) | 0.50 |
| Current | 107 (4.3) | 114 (4.6) | |
|
| |||
| Years with Type 2 diabetes | |||
| <5 | 1120 (44.9) | 1157 (46.5) | |
| 5–9 | 709 (28.4) | 674 (27.1) | 0.47 |
| 10+ | 663 (26.6) | 656 (26.4) | |
|
| |||
| Cardiovascular disease history | |||
| Yes | 336 (13.4) | 354 (14.1) | 0.47 |
| No | 2171 (86.6) | 2157 (85.9) | |
|
| |||
| Body mass index, kg/m2 | |||
| 25–29 | 352 (14.0) | 397 (15.8) | |
| 30–39 | 1593 (63.5) | 1550 (61.7) | 0.19 |
| ≥40 | 562 (22.4) | 564 (22.5) | |
|
| |||
| Fitness (METS at 80% maximal heart rate) | 5.12 (1.51) | 5.19 (1.50) | 0.14 |
|
| |||
| Insulin use | |||
| Yes | 482 (19.2) | 466 (18.6) | 0.55 |
| No | 2025 (80.8) | 2045 (81.4) | |
|
| |||
| HbA1c | |||
|
| |||
| < 7.0% | 1127 (45.0) | 1165 (46.4) | |
| 7.0–8.9% | 1130 (45.1) | 1134 (45.2) | 0.15 |
| 9.0–10.9% | 250 (10.0) | 212 (8.4) | |
|
| |||
| Claudication (Based on Rose scale) | |||
| Yes | 4 (0.2) | 2 (0.1) | 0.45 |
| No | 2502 (99.8) | 2509 (99.9) | |
| ABI | |||
| Right leg | |||
| <0.90 | 55 (2.20) | 62 (2.47) | |
| 0.90–1.40 | 2362 (94.33) | 2364 (94.30) | 0.63 |
| >1.40 | 87 (3.47) | 81 (3.23) | |
| Left leg | |||
| <0.90 | 67 (2.67) | 54 (2.15) | |
| 0.90–1.40 | 2351 (93.85) | 2367 (94.45) | 0.48 |
| >1.40 | 87 (3.47) | 85 (3.39) | |
| Minimum | |||
| <0.90 | 95 (3.79) | 91 (3.62) | |
| 0.90–1.40 | 2369 (94.50) | 2382 (94.86) | 0.81 |
| >1.40 | 43 (1.72) | 38 (151) | |
|
| |||
| Inter-artery SBP differences, mmHg | |||
| Maximum among ankle sites | 19.5 (15.7) | 19.4 (13.8) | 0.10 |
| Absolute difference between left and right arms | 6.2 (6.0) | 6.2 (5.8) | 0.63 |
The ILI participants included in this report lost an average of 8.5% (standard deviation 7.3%) of their body mass index at Year 1, compared to 0.7% (4.8%) for DSE participants. At Years 2–4, mean losses were 6.4% (7.3%), 5.1% (7.8%), and 4.1% (10.0%) for ILI participants compared to 1.0% (6.4%), 1.0 % (7.3%), and 0.5% (10.2%) for DSE participants. Differences between groups were highly significant (p<0.0001) through all four years of follow-up.
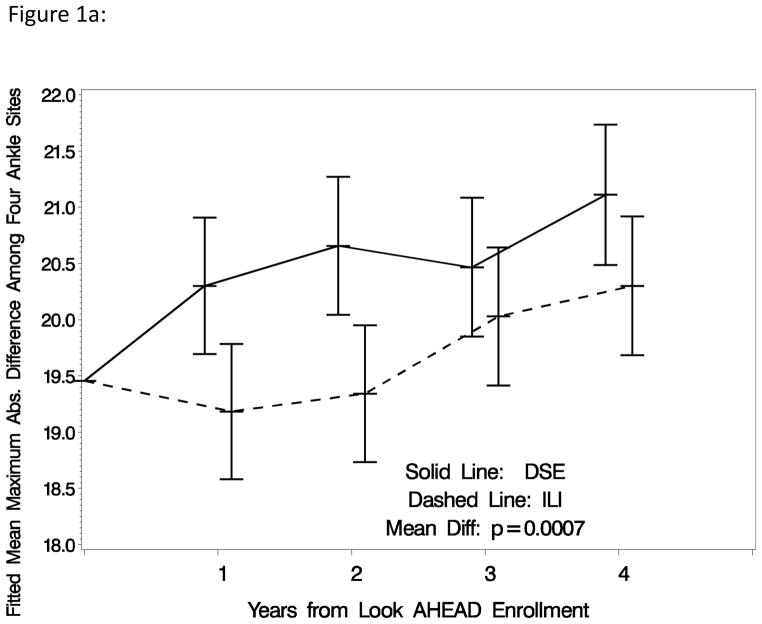
Table 2 presents the prevalence of individuals with a least one low or high leg-specific ABI over follow-up by intervention assignment. At baseline among individuals assigned to DSE, 3.79% had at least one low leg-specific ABI compared to 3.62% in the ILI group. At year 4, these prevalences were 3.86% versus 2.74%. At baseline, the prevalence of individuals with at least on leg-specific ABI>1.40 were 5.23% (DSE) and 5.14% (ILI). The prevalence of high ABI increased over time in both the ILI and DSE groups, rising to 8.16% (DSE) and 7.93% (ILI) at year 4. The mean (SD) maximum differences in systolic blood pressure among ankle sites increased over time in both groups, from 19.5 (15.7) mmHg to 21.1 (16.5) mmHg among DSE participants and from 19.4 (13.8) mmHg to 20.3 (15.7) mmHg among ILI participants. The correlations that these had with baseline maximum differences were r=0.28 at Year 1 and declined to r=0.20 at year 4. Changes in mean (SD) absolute differences systolic blood pressures between arms over time were less pronounced. The correlations with baseline absolute differences between arms ranged from r=0.04 to r=0.08 over time.
Table 2.
Prevalence of leg-specific ABI <0.90 and leg-specific ABI >1.40 over time for participants grouped by intervention assignment.
| Visit | Intervention Assignment (Individuals Providing Measurements) | Prevalence (%) of Individuals with At Least One Leg-specific ABI<0.90 | Prevalence (%) of Individuals with At Least One Leg-specific ABI>1.40 | Mean (SD) Maximum Absolute Systolic Blood Pressure Difference Among Ankle Sites, mmHg | Mean (SD) Absolute Systolic Blood Pressure Difference Between Left and Right Arms, mmHg |
|---|---|---|---|---|---|
|
| |||||
| Baseline | DSE (2507) | 3.79 | 5.23 | 19.5 (15.7) | 6.2 (6.0) |
| ILI (2511) | 3.62 | 5.14 | 19.4 (13.8) | 6.2 (5.8) | |
|
| |||||
| Year 1 | DSE (2410) | 3.36 | 6.14 | 20.3 (13.4) | 5.9 (5.8) |
| ILI (2461) | 2.89 | 6.99 | 19.2 (14.9) | 5.8 (5.6) | |
|
| |||||
| Year 2 | DSE (2345) | 3.20 | 7.93 | 20.6 (16.3) | 6.0 (5.7) |
| ILI (2392) | 3.47 | 8.11 | 19.4 (15.2) | 5.8 (5.8) | |
|
| |||||
| Year 3 | DSE (2324) | 3.96 | 7.75 | 20.4 (16.8) | 6.3 (6.3) |
| ILI (2362) | 3.39 | 7.71 | 20.1 (15.2) | 5.8 (5.4) | |
|
| |||||
| Year 4 | DSE (2280) | 3.86 | 8.16 | 21.1 (16.5) | 6.0 (5.9) |
| ILI (2333) | 2.74 | 7.93 | 20.3 (15.7) | 5.9 (5.2) | |
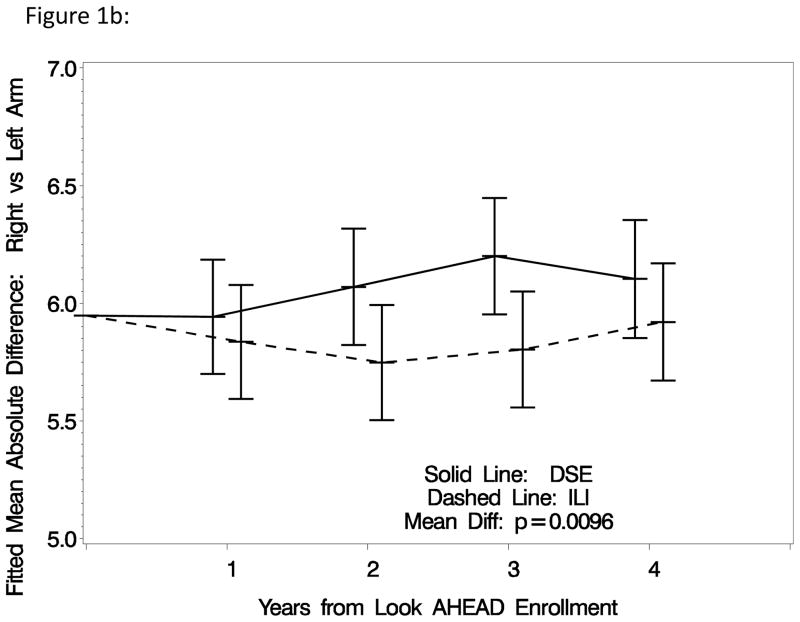
Table 3 summarizes differences in the four measures of inter-artery differences between intervention groups overall, and within subgroups, and reports results of inferences. After covariate adjustment for baseline status, the prevalence (standard error) of individuals with low ABI over time averaged 3.60% (0.26%) among DSE participants compared to 3.14% (0.24%) among ILI participants: non-significant p=0.20. The prevalence (standard error) of individuals with high ABI over time averaged 7.52% (0.36%) among DSE participants compared to 7.59% (0.37%) among ILI participants: nonsignificant p=0.90. The mean maximum inter-artery systolic blood pressure difference among ankle sites was significantly larger among DSE compared to ILI participants: 20.6 (0.2) mmHg versus 19.7 (0.2) mmHg, respectively, p<0.001. Similarly, the mean inter-arm systolic blood pressure difference was significantly larger among DSE compared to ILI participants: 6.1 (0.1) mmHg versus 5.8 (0.1) mmHg, respectively, p=0.01. Including achieved percent weight losses as a time-varying covariate attenuated the statistical significance of these differences: p=0.11 for inter-ankle differences and p=0.29 for inter-arm differences. Figures 1a and 1b portray the pattern of changes in the maximum differences in systolic blood pressure among ankle sites and absolute differences between left and right arms over time. Both of these measures tended to increase over time among individuals assigned to DSE. This progression appeared to be halted during the first two years of ILI, so that overall mean differences across follow-up for both measures reached statistical significance.
Table 3.
Prevalence and mean inter-artery systolic blood pressure differences across follow-up, with adjustment for baseline status: overall and for selected subgroups.
| Subgroup | Intervention Assignment | Average Prevalence (SE) Across Follow-up Adjusted for Baseline ABI | Maximum Difference in Systolic Blood Pressure Among Arterial Sites Controlling for Baseline Difference: Mean (SE) | ||
|---|---|---|---|---|---|
|
| |||||
| ABI < 0.90 | ABI > 1.40 | Among Ankle Sites | Between Left and Right Arms | ||
|
| |||||
| Overall | DSE | 3.60 (0.26) | 7.52 (0.36) | 20.6 (0.2) | 6.1 (0.1) |
| ILI | 3.14 (0.24) | 7.59 (0.37) | 19.7 (0.2) | 5.8 (0.1) | |
| p-value | 0.20 | 0.90 | p<0.001 | 0.01 | |
|
| |||||
| Gender | |||||
| Females | DSE | 3.27 (0.32) | 3.85 (0.30) | 19.4 (0.2) | 6.4 (0.1) |
| ILI | 3.03 (0.28) | 4.12 (0.32) | 18.6 (0.2) | 5.9 (0.1) | |
| p-value | 0.57 | 0.54 | 0.008 | <0.001 | |
| Males | DSE | 4.14 (0.41) | 13.02 (0.74) | 22.5 (0.4) | 5.5 (0.1) |
| ILI | 3.25 (0.42) | 12.66 (0.75) | 21.4 (0.4) | 5.7 (0.1) | |
| p-value | 0.13 | 0.73 | 0.02 | 0.27 | |
| Interaction | p-value | 0.37 | 0.60 | 0.50 | <0.001 |
|
| |||||
| CVD History | |||||
| No | DSE | 2.55 (0.23) | 7.44 (0.37) | 20.1 (0.2) | 6.1 (0.1) |
| ILI | 2.38 (0.22) | 7.14 (0.38) | 19.2 (0.2) | 5.8 (0.1) | |
| 0.58 | 0.93 | <0.001 | 0.006 | ||
| Yes | DSE | 11.07 (1.12) | 9.69 (1.16) | 24.0 (0.7) | 6.1 (0.2) |
| ILI | 8.49 (0.11) | 10.32 (1.10) | 23.0 (0.6) | 6.1 (0.2) | |
| 0.10 | 0.69 | 0.29 | 0.87 | ||
| Interaction | p-value | 0.12 | 0.68 | 0.94 | 0.33 |
|
| |||||
| Baseline BMI | |||||
| 25–29 | DSE | 2.41 (0.05) | 4.75 (0.76) | 20.6 (0.5) | 5.5 (0.2) |
| ILI | 1.94 (0.04) | 4.80 (0.73) | 18.2 (0.5) | 5.3 (0.2) | |
| 0.33 | 0.97 | <0.001 | 0.36 | ||
| 30–39 | DSE | 3.47 (0.32) | 7.34 (0.44) | 20.6 (0.2) | 5.9 (0.1) |
| ILI | 3.38 (0.33) | 7.40 (0.46) | 19.9 (0.2) | 5.8 (0.1) | |
| 0.84 | 0.92 | 0.05 | 0.36 | ||
| ≥40 | DSE | 3.86 (0.57) | 9.86 (0.84) | 20.9 (0.4) | 7.0 (0.2) |
| ILI | 2.55 (0.40) | 10.06 (0.87) | 20.2 (0.4) | 6.3 (0.2) | |
| 0.06 | 0.87 | 0.26 | 0.006 | ||
| Interaction | p-value | 0.37 | 0.99 | 0.07 | 0.07 |
|
| |||||
| Age | |||||
| 45–59 | DSE | 2.28 (0.27) | 7.09 (0.46) | 19.4 (0.2) | 6.0 (0.1) |
| ILI | 1.81 (0.23) | 7.12 (0.46) | 18.4 (0.2) | 5.7 (0.1) | |
| 0.19 | 0.96 | 0.001 | 0.07 | ||
| 60–76 | DSE | 5.33 (0.47) | 8.08 (0.55) | 22.2 (0.3) | 6.2 (0.1) |
| ILI | 4.91 (0.47) | 8.23 (0.60) | 21.5 (0.3) | 6.0 (0.1) | |
| 0.53 | 0.86 | 0.15 | 0.08 | ||
| Interaction | p-value | 0.99 | 0.90 | 0.52 | 0.89 |
|
| |||||
| Smoking | |||||
| Never | DSE | 2.42 (0.30) | 7.36 (0.50) | 20.4 (0.3) | 6.2 (0.1) |
| ILI | 2.10 (0.27) | 7.05 (0.48) | 18.9 (0.3) | 5.7 (0.1) | |
| 0.42 | 0.66 | <0.001 | <0.001 | ||
| Former or Current | DSE | 4.83 (0.43) | 7.71 (0.51) | 20.8 (0.3) | 6.0 (0.1) |
| ILI | 4.18 (0.40) | 8.12 (0.55) | 20.5 (0.3) | 6.0 (0.1) | |
| 0.27 | 0.58 | 0.35 | 0.92 | ||
| Interaction | p-value | 0.61 | 0.48 | 0.04 | 0.007 |
Figure 1.
Figure 1a: Mean maximum absolute pairwise difference among systolic blood pressure measurements taken on four ankle arteries: right and left tibia versus dorsal pedis arteries (with adjustment for baseline absolute difference).
Figure 1b: Mean absolute difference between systolic blood pressures in the left and right arms (with adjustment for baseline absolute difference).
Table 3 also examines the consistency of any intervention effects among subgroups based on gender, cardiovascular disease history, body mass index, age, and smoking history. Individuals assigned to ILI consistently had slightly lower prevalence of low ABI over time compared to DSE, however no differences and no interactions reached nominal statistical significance. There was little evidence of intervention effects on high ABI in any subgroup and no significant interactions. There was some evidence that the impact of ILI on decreasing inter-ankle blood pressure differences was stronger among individuals with no smoking history (interaction p=0.04). Inter-arm differences in systolic blood pressure appeared to be reduced by ILI more among women (interaction p<0.001) and non-smokers (interaction p=0.007).
During the first four years of the trial, 4 DSE and 11 ILI participants were adjudicated as having “peripheral angioplasty or thrombolysis (with or without stent)” or “peripheral vascular surgery:” censoring post-procedure data on these participants did not materially change any of the findings we report.
DISCUSSION
Intervention Effects on Incidence of Low and High ABI
Risk factors for low ABI and peripheral arterial disease are generally similar to those for atherosclerosis and include hypertension, diabetes, smoking, dyslipidemia, and obesity (1,8,12,18–21). Associations with weight change are less clear, perhaps because these may evolve only over extended time frames, may differ by gender, and may be confounded by unintentional weight loss (21–23). High ABI may reflect calcification of arterial walls, in addition to occlusive arterial disease and atherosclerosis (1). Its risk factors have not been as intensively studied as for low ABI, however these include dyslipidemia, smoking, and abnormal BMI (19).
Overall, Look AHEAD achieved 7.9% (Year 1) to 3.6% (Year 4) average difference in weight changes between its intervention groups and marked differences in levels of physical fitness (24,25). This has resulted in relative improvements in several risk factors for vascular disease, including blood pressure, lipids levels, and HbA1c levels (25,26). Despite these associations, prevalence rates of low ABI were only slightly, and not significantly lower among ILI compared to DSE participants. No intervention effect was apparent on the prevalence of high ABI, as well.
It may be that low and high ABI are not sensitive to changes in weight. Changes in ABI may only occur gradually (27): it may also be that four years is too short of a time frame to observe the effects of weight change, even in a large cohort. All participants were provided feedback on measured risk factors during the trial, which may have reduced differences; however, including changes in blood pressure and lipid levels during follow-up in analyses did not materially affect our findings (not reported). ILI did not reduce the risk of major cardiovascular disease events (26), which is consistent for our findings with respect to ABI.
Intervention Effect on Inter-artery Blood Pressure Differences
Larger differences in systolic blood pressure between left and right arms may signal increased risk of cardiovascular events and peripheral arterial disease (10,11). Factors that have been reported as being associated with larger differences include hypertension, diabetes, obesity, and peripheral vascular disease (9–11,28–30), however these associations have not been consistently found (31). Inter-arm systolic blood pressure differences exceeding 10 mmHg or 20 mmHg have been recommended as diagnostic criteria to detect significant localized stenosis (32).
The mean magnitude of absolute inter-arm differences at baseline that we report for the Look AHEAD, about 6 mmHg, is within the range (3 to 11 mmHg) reported for other cohorts (10,28,31). We found that assignment to an intensive weight loss intervention led to a relative reduction in the interarm difference in systolic blood pressure of about 0.2 mmHg (p=0.01), which emerged during the first two years of intervention. Little appears to have been reported about other types of variability in interartery blood pressures. Within Look AHEAD, the maximum differences in systolic blood pressure measurements among ankle sites increased over time in both arms, however ILI was associated with a sustained relative reduction in the maximum differences of about 0.9 mmHg, with overall p<0.001. This difference also emerged within the first two years of intervention. For both of these measures, including weight loss as a time-varying covariate attenuated differences between intervention groups.
What do these intervention effects mean? It is possible that they reflect a reduction in the progression rate of localized stenosis within sites that the cutpoints used to identify low ABI lack the sensitivity to capture. Weight loss has been found to reduce a continuous measure of arterial stiffness (via pulse wave velocity) over one year (33). This effect was mediated by reductions in heart rate and inflammation, both of which were improved by the Look AHEAD intervention (34,35). It may also be that the intervention alters how blood pressures vary in response to existing localized stenosis. For example, weight loss may reduce inter-site differences by lowering systolic blood pressure, which in turn decreases the blood pressure differentials associated with stenosis. In support of this, lower overall variability has been reported for individuals with lower blood pressures (28,29). Look AHEAD found that the interventions produced mean relative decrements in systolic blood pressure of 4.7 mmHg at Year 1, which declined to 1.3 mmHg at 4 years (25); however, while inclusion of changes in systolic blood pressure as a covariate in models attenuated differences between intervention groups, these remained statistically significant (p<0.05). Another possibility follows from decreased blood viscosity arising from weight loss (36): according to Poiseuille’s law, the impact of stenosis on blood pressure is inversely related to blood viscosity (37).
Regardless of the source of the intervention effects that we see on inter-site blood pressure variability, their clinical significance is not clear. Future work will examine if these changes are related to the incidence of other potential adverse consequences of peripheral atherosclerosis, such as neuropathy or frailty (8,31,38).
Subgroup Comparisons
There was little evidence that the ILI differentially affected the prevalence of low or high ABI among the subgroups we examined. The largest difference (nominal interaction p=0.12) was for a more pronounced intervention difference among individuals with a history of CVD, who had the highest overall prevalence of low ABI. For inter-artery differences, three interactions reached nominal significance. Inter-arm differences appeared to be reduced by ILI among women, but not among men, and among never smokers, but not among former or current smokers. It also appeared that the impact of ILI on inter-ankle differences among non-smokers was larger than for those with a smoking history. There is some evidence that markers of obesity are more strongly related to vascular disease among women than men (39). Smoking has been found to invert the expected positive relationship between body mass index and subclinical atherosclerosis in one report (40).
Limitations
As volunteers eligible for a clinical trial, in part based on the ability to complete a maximal graded exercise test, the Look AHEAD cohort may not readily generalize to clinical populations, including those without diabetes. Three different protocols were used to assess ABI at baseline, however the differences in these with respect to bias has been shown to be minor (17).
Summary
Four years of ILI to promote and maintain weight loss did not affect the risk of low or high ABI, however it did reduce differences among inter-arterial systolic blood pressure measurements.
What is already known about this subject?
Obesity is a strong risk factor for abnormal ankle brachial (ABI) and an established risk factor for peripheral vascular disease.
Larger differences in systolic blood pressures between or among arterial sites may signal increased risk of cardiovascular events and peripheral arterial disease.
What this study adds
Look AHEAD is the first randomized controlled clinical trial of long-term intensive lifestyle intervention of sufficient size and duration to examine whether weight loss reduces the prevalence of abnormal ABI. We report its primary findings for this outcome.
We report that the Look AHEAD intensive lifestyle intervention significantly reduced interarterial blood pressure differences compared to diabetes support and education.
Acknowledgments
MAE collaborated on study design, conducted analyses, and led the writing group. CEL, WCK, JGR, and KCJ collaborated on study design, oversaw data collection, and contributed to the interpretation of the data and writing of the manuscript. JB collaborated on study design, oversaw the conduct of the study, and contributed to the interpretation of the data and writing of the manuscript. SAG and DB collaborated on the analysis of data and the writing of the manuscript. MAE serves on Data and Safety Monitoring Boards for KOWA Research Institute and Terumo Medical Corporation and a Steering Committee for Boehringer-Ingelheim Pharmaceuticals. CEL reports grant support from Novo Nordisk.
Funding and Support
This study is supported by the Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Look AHEAD Research Group at Year 4
Clinical Sites
The Johns Hopkins Medical Institutions
Frederick L. Brancati, MD, MHS1; Lee Swartz2; Lawrence Cheskin, MD3; Jeanne M. Clark, MD, MPH3; Kerry Stewart, EdD3; Richard Rubin, PhD3; Jean Arceci, RN; Suzanne Ball; Jeanne Charleston, RN; Danielle Diggins; Mia Johnson; Joyce Lambert; Kathy Michalski, RD; Dawn Jiggetts; Chanchai Sapun
Pennington Biomedical Research Center
George A. Bray, MD1; Kristi Rau2; Allison Strate, RN2; Frank L. Greenway, MD3; Donna H. Ryan, MD3; Donald Williamson, PhD3; Brandi Armand, LPN; Jennifer Arceneaux; Amy Bachand, MA; Michelle Begnaud, LDN, RD, CDE; Betsy Berhard; Elizabeth Caderette; Barbara Cerniauskas, LDN, RD, CDE; David Creel, MA; Diane Crow; Crystal Duncan; Helen Guay, LDN, LPC, RD; Carolyn Johnson, Lisa Jones; Nancy Kora; Kelly LaFleur; Kim Landry; Missy Lingle; Jennifer Perault; Cindy Puckett; Mandy Shipp, RD; Marisa Smith; Elizabeth Tucker
The University of Alabama at Birmingham
Cora E. Lewis, MD, MSPH1; Sheikilya Thomas MPH2; Monika Safford, MD3; Vicki DiLillo, PhD; Charlotte Bragg, MS, RD, LD; Amy Dobelstein; Stacey Gilbert, MPH; Stephen Glasser, MD3; Sara Hannum, MA; Anne Hubbell, MS; Jennifer Jones, MA; DeLavallade Lee; Ruth Luketic, MA, MBA, MPH; L. Christie Oden; Janet Raines, MS; Cathy Roche, RN, BSN; Janet Truman; Nita Webb, MA; Casey Azuero, MPH; Jane King, MLT; Andre Morgan
Harvard Center
Massachusetts General Hospital
David M. Nathan, MD1; Enrico Cagliero, MD3; Kathryn Hayward, MD3; Heather Turgeon, RN, BS, CDE2; Linda Delahanty, MS, RD3; Ellen Anderson, MS, RD3; Laurie Bissett, MS, RD; Valerie Goldman, MS, RD; Virginia Harlan, MSW; Theresa Michel, DPT, DSc, CCS; Mary Larkin, RN; Christine Stevens, RN; Kylee Miller, BA; Jimmy Chen, BA; Karen Blumenthal, BA; Gail Winning, BA; Rita Tsay, RD; Helen Cyr, RD; Maria Pinto
Joslin Diabetes Center
Edward S. Horton, MD1; Sharon D. Jackson, MS, RD, CDE2; Osama Hamdy, MD, PhD3; A. Enrique Caballero, MD3; Sarah Bain, BS; Elizabeth Bovaird, BSN, RN; Barbara Fargnoli, MS, RD; Jeanne Spellman, BS, RD; Ann Goebel-Fabbri, PhD; Lori Lambert, MS, RD; Sarah Ledbury, MEd, RD; Maureen Malloy, BS; Kerry Ovalle, MS, RCEP, CDE
Beth Israel Deaconess Medical Center
George Blackburn, MD, PhD1; Christos Mantzoros, MD, DSc3; Ann McNamara, RN; Kristina Spellman, RD
University of Colorado Health Sciences Center
James O. Hill, PhD1; Marsha Miller, MS, RD2; Brent Van Dorsten, PhD3; Judith Regensteiner, PhD3; Ligia Coelho, BS; Paulette Cohrs, RN, BSN; Susan Green; April Hamilton, BS, CCRC; Jere Hamilton, BA; Eugene Leshchinskiy; Lindsey Munkwitz, BS; Loretta Rome, TRS; Terra Worley, BA; Kirstie Craul, RD, CDE; Sheila Smith, BS
Baylor College of Medicine
John P. Foreyt, PhD1; Rebecca S. Reeves, DrPH, RD2; Henry Pownall, PhD3; Ashok Balasubramanyam, MBBS3; Peter Jones, MD3; Michele Burrington, RD, RN; Chu-Huang Chen, MD, PhD3; Allyson Clark Gardner, MS, RD; Molly Gee, MEd, RD; Sharon Griggs; Michelle Hamilton; Veronica Holley; Jayne Joseph, RD; Julieta Palencia, RN; Jennifer Schmidt; Carolyn White
The University of Tennessee Health Science Center
University of Tennessee East
Karen C. Johnson, MD, MPH1; Carolyn Gresham, RN2; Stephanie Connelly, MD, MPH3; Amy Brewer, RD, MS; Mace Coday, PhD; Lisa Jones, RN; Lynne Lichtermann, RN, BSN; Shirley Vosburg, RD, MPH; and J. Lee Taylor, MEd, MBA
University of Tennessee Downtown
Abbas E. Kitabchi, PhD, MD1; Ebenezer Nyenwe, MD3; Helen Lambeth, RN, BSN2; Amy Brewer, MS, RD, LDN; Debra Clark, LPN; Andrea Crisler, MT; Debra Force, MS, RD, LDN; Donna Green, RN; Robert Kores, PhD
University of Minnesota
Robert W. Jeffery, PhD1; Carolyn Thorson, CCRP2; John P. Bantle, MD3; J. Bruce Redmon, MD3; Richard S. Crow, MD3; Scott Crow, MD3; Susan K Raatz, PhD, RD3; Kerrin Brelje, MPH, RD; Carolyne Campbell; Jeanne Carls, MEd; Tara Carmean-Mihm, BA; Julia Devonish, MS; Emily Finch, MA; Anna Fox, MA; Elizabeth Hoelscher, MPH, RD, CHES; La Donna James; Vicki A. Maddy, BS, RD; Therese Ockenden, RN; Birgitta I. Rice, MS, RPh, CHES; Tricia Skarphol, BS; Ann D. Tucker, BA; Mary Susan Voeller, BA; Cara Walcheck, BS, RD
St. Luke’s Roosevelt Hospital Center
Xavier Pi-Sunyer, MD1; Jennifer Patricio, MS2; Stanley Heshka, PhD3; Carmen Pal, MD3; Lynn Allen, MD; Lolline Chong, BS, RD; Marci Gluck, PhD; Diane Hirsch, RNC, MS, CDE; Mary Anne Holowaty, MS, CN; Michelle Horowitz, MS, RD; Nancy Rau, MS, RD, CDE; Dori Brill Steinberg, BS
University of Pennsylvania
Thomas A. Wadden, PhD 1; Barbara J Maschak-Carey, MSN, CDE 2; Robert I. Berkowitz, MD 3; Seth Braunstein, MD, PhD 3; Gary Foster, PhD 3; Henry Glick, PhD 3; Shiriki Kumanyika, PhD, RD, MPH 3; Stanley S. Schwartz, MD 3; Michael Allen, RN; Yuliis Bell; Johanna Brock; Susan Brozena, MD; Ray Carvajal, MA; Helen Chomentowski; Canice Crerand, PhD; Renee Davenport; Andrea Diamond, MS, RD; Anthony Fabricatore, PhD; Lee Goldberg, MD; Louise Hesson, MSN, CRNP; Thomas Hudak, MS; Nayyar Iqbal, MD; LaShanda Jones-Corneille, PhD; Andrew Kao, MD; Robert Kuehnel, PhD; Patricia Lipschutz, MSN; Monica Mullen, RD, MPH
University of Pittsburgh
John M. Jakicic, PhD1, David E. Kelley, MD1; Jacqueline Wesche-Thobaben, RN, BSN, CDE2; Lewis H. Kuller, MD, DrPH3; Andrea Kriska, PhD3; Amy D. Rickman, PhD, RD, LDN3, Lin Ewing, PhD, RN3, Mary Korytkowski, MD3, Daniel Edmundowicz, MD3; Monica E. Yamamoto, DrPH, RD, FADA 3; Rebecca Danchenko, BS; Barbara Elnyczky; David O. Garcia, MS; George A. Grove, MS; Patricia H. Harper, MS, RD, LDN; Susan Harrier, BS; Nicole L. Helbling, MS, RN; Diane Ives, MPH; Juliet Mancino, MS, RD, CDE, LDN; Anne Mathews, PhD, RD, LDN; Tracey Y. Murray, BS; Joan R. Ritchea; Susan Urda, BS, CTR; Donna L. Wolf, PhD
The Miriam Hospital/Brown Medical School
Rena R. Wing, PhD1; Renee Bright, MS2; Vincent Pera, MD3; John Jakicic, PhD3; Deborah Tate, PhD3; Amy Gorin, PhD3; Kara Gallagher, PhD3; Amy Bach, PhD; Barbara Bancroft, RN, MS; Anna Bertorelli, MBA, RD; Richard Carey, BS; Tatum Charron, BS; Heather Chenot, MS; Kimberley Chula-Maguire, MS; Pamela Coward, MS, RD; Lisa Cronkite, BS; Julie Currin, MD; Maureen Daly, RN; Caitlin Egan, MS; Erica Ferguson, BS, RD; Linda Foss, MPH; Jennifer Gauvin, BS; Don Kieffer, PhD; Lauren Lessard, BS; Deborah Maier, MS; JP Massaro, BS; Tammy Monk, MS; Rob Nicholson, PhD; Erin Patterson, BS; Suzanne Phelan, PhD; Hollie Raynor, PhD, RD; Douglas Raynor, PhD; Natalie Robinson, MS, RD; Deborah Robles; Jane Tavares, BS
The University of Texas Health Science Center at San Antonio
Steven M. Haffner, MD1; Helen P. Hazuda, Ph.D.1; Maria G. Montez, RN, MSHP, CDE2; Carlos Lorenzo, MD3; Charles F. Coleman, MS, RD; Domingo Granado, RN; Kathy Hathaway, MS, RD; Juan Carlos Isaac, RC, BSN; Nora Ramirez, RN, BSN; Ronda Saenz, MS, RD
VA Puget Sound Health Care System/University of Washington
Steven Kahn MB, ChB1; Brenda Montgomery, RN, MS, CDE2; Robert Knopp, MD3; Edward Lipkin, MD3; Dace Trence, MD3; Terry Barrett, BS; Joli Bartell, BA; Diane Greenberg, PhD; Anne Murillo, BS; Betty Ann Richmond, MEd; Jolanta Socha, BS; April Thomas, MPH, RD; Alan Wesley, BA
Southwestern American Indian Center, Phoenix, Arizona and Shiprock, New Mexico
William C. Knowler, MD, DrPH1; Paula Bolin, RN, MC2; Tina Killean, BS2; Cathy Manus, LPN3; Jonathan Krakoff, MD3; Jeffrey M. Curtis, MD, MPH3; Justin Glass, MD3; Sara Michaels, MD3; Peter H. Bennett, MB, FRCP3; Tina Morgan3; Shandiin Begay, MPH; Paul Bloomquist, MD; Teddy Costa, BS; Bernadita Fallis RN, RHIT, CCS; Jeanette Hermes, MS, RD; Diane F. Hollowbreast; Ruby Johnson; Maria Meacham, BSN, RN, CDE; Julie Nelson, RD; Carol Percy, RN; Patricia Poorthunder; Sandra Sangster; Nancy Scurlock, MSN, ANP-C, CDE; Leigh A. Shovestull, RD, CDE; Janelia Smiley; Katie Toledo, MS, LPC; Christina Tomchee, BA; Darryl Tonemah, PhD
University of Southern California
Anne Peters, MD1; Valerie Ruelas, MSW, LCSW2; Siran Ghazarian Sengardi, MD2; Kathryn (Mandy) Graves Hillstrom, EdD, RD, CDE; Kati Konersman, MA, RD, CDE; Sara Serafin-Dokhan
Coordinating Center
Wake Forest University
Mark A. Espeland, PhD1; Lynne E. Wagenknecht, DrPH1; Judy L. Bahnson, BA, CCRP3; David Reboussin, PhD3; W. Jack Rejeski, PhD3; Alain G. Bertoni, MD, MPH3; Wei Lang, PhD3; Michael S. Lawlor, PhD3; David Lefkowitz, MD3; Gary D. Miller, PhD3; Patrick S. Reynolds, MD3; Paul M. Ribisl, PhD3; Mara Vitolins, DrPH3; Haiying Chen, PhD3; Delia S. West, PhD3; Lawrence M. Friedman, MD3; Brenda L. Craven, MS, CCRP2; Kathy M. Dotson, BA2; Amelia Hodges, BS, CCRP2; Carrie C. Williams, MA, CCRP2; Andrea Anderson, MS; Jerry M. Barnes, MA; Mary Barr; Daniel P. Beavers, PhD; Tara Beckner; Cralen Davis, MS; Thania Del Valle-Fagan, MD; Patricia A. Feeney, MS; Candace Goode; Jason Griffin, BS; Lea Harvin, BS; Patricia Hogan, MS; Sarah A. Gaussoin, MS; Mark King, BS; Kathy Lane, BS; Rebecca H. Neiberg, MS; Michael P. Walkup, MS; Karen Wall, AAS; Terri Windham
Central Resources Centers
DXA Reading Center, University of California at San Francisco
Michael Nevitt, PhD1; Ann Schwartz, PhD2; John Shepherd, PhD3; Michaela Rahorst; Lisa Palermo, MS, MA; Susan Ewing, MS; Cynthia Hayashi; Jason Maeda, MPH
Central Laboratory, Northwest Lipid Metabolism and Diabetes Research Laboratories
Santica M. Marcovina, PhD, ScD1; Jessica Chmielewski2; Vinod Gaur, PhD4
ECG Reading Center, EPICARE, Wake Forest University School of Medicine
Elsayed Z. Soliman MD, MSc, MS1; Ronald J. Prineas, MD, PhD1; Charles Campbell2; Zhu-Ming Zhang, MD3; Teresa Alexander; Lisa Keasler; Susan Hensley; Yabing Li, MD
Diet Assessment Center, University of South Carolina, Arnold School of Public Health, Center for Research in Nutrition and Health Disparities
Robert Moran, PhD1
Hall-Foushee Communications, Inc
Richard Foushee, PhD; Nancy J. Hall, MA
Federal Sponsors
National Institute of Diabetes and Digestive and Kidney Diseases
Mary Evans, PhD; Barbara Harrison, MS; Van S. Hubbard, MD, PhD; Susan Z. Yanovski, MD; Robert Kuczmarski, PhD
National Heart, Lung, and Blood Institute
Lawton S. Cooper, MD, MPH; Peter Kaufman, PhD, FABMR
Centers for Disease Control and Prevention
Edward W. Gregg, PhD; David F. Williamson, PhD; Ping Zhang, PhD
Some of the information contained herein was derived from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene for providing vital statistics data.
Footnotes
Principal Investigator
Program Coordinator
Co-Investigator
All other Look AHEAD staff are listed alphabetically by site.
Other authors report no potential conflicts of interest.
Trial Registration clinicaltrials.gov Identifier: NCT00017953
References
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (Lower extremity, renal, mesenteric, and abdominal aortic) Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.Murabito JM, Evans JC, Larson MG, Nieto K, Levy D, Wilson PWF. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death. The Framingham Study. Arch Intern Med. 2003;163:1939–1942. doi: 10.1001/archinte.163.16.1939. [DOI] [PubMed] [Google Scholar]
- 3.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 4.Lee AJ, Price JF, Russell MJ, Smith FB, van Wijk MCW, Fowkes FGR. Improved prediction of fatal myocardial infarction using the ankle brachial index in addition to conventional risk factors. The Edinburgh Artery Study. Circulation. 2004;110:3075–3080. doi: 10.1161/01.CIR.0000143102.38256.DE. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease. The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 6.Kweon SS, Shin MH, Park KS, et al. Distribution of the ankle-brachial index and associated cardiovascular risk factors in a population of middle-aged and elderly Koreans. J Korean Med Sci. 2005;20:373–378. doi: 10.3346/jkms.2005.20.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ankle Brachial Index Collaboration. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott MM, Criqui MH, Ferrucci L, et al. Obesity, weight change, and functional decline in peripheral arterial disease. J Vasc Surg. 2006;43:1198–1204. doi: 10.1016/j.jvs.2006.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CE, Campbell JL, Evans PH, Millward A. Prevalence and clinical implications of the inter-arm blood pressure difference: a systematic review. J Hum Hypertens. 2006;20:923–931. doi: 10.1038/sj.jhh.1002093. [DOI] [PubMed] [Google Scholar]
- 10.Clark CE, Campbell JL, Powell R, Thompson JF. The inter-arm blood pressure difference and peripheral vascular disease: cross-sectional study. Fam Practice. 2007;24:420–6. doi: 10.1093/fampra/cmm035. [DOI] [PubMed] [Google Scholar]
- 11.Clark CE, Campbell JL, Powell RJ. The interarm blood pressure difference as predictor of cardiovascular events in patients with hypertension in primary care: cohort study. J Hum Hypertens. 2007;21:633–638. doi: 10.1038/sj.jhh.1002209. [DOI] [PubMed] [Google Scholar]
- 12.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 13.The Look AHEAD Research Group. Look AHEAD: Action for Health in Diabetes. Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled Clin Trials. 2003;24:610–28. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 14.The Look AHEAD Research Group. The Look AHEAD Study: A description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Look AHEAD Research Group. The development and description of the diabetes support and education (comparison group) intervention for the Action for Health in Diabetes (Look AHEAD) Trial. Clin Trials. 2011;8:320–9. [Google Scholar]
- 16.Jakicic JM, Jaramillo SA, Balasubramanyam A, et al. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes. 2009;33:305–316. doi: 10.1038/ijo.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espeland MA, Regensteiner JG, Jaramillo SA, et al. Measurement characteristics of the ankle-brachial index: results from the Action for Health in Diabetes Study. Vasc Med. 2008;13:225–233. doi: 10.1177/1358863X08091338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasternak RC, Criqui MH, Benjamin EJ, et al. Atherosclerotic vascular disease conference: writing group I: epidemiology. Circulation. 2004;109:2605–2612. doi: 10.1161/01.CIR.0000128518.26834.93. [DOI] [PubMed] [Google Scholar]
- 19.Allison MA, Cushman M, Solomon C, et al. Ethnicity and risk factors for change in the ankle-brachial index: the Multi-Ethnic Study of Atherosclerosis. J Vasc Surg. 2009;50:1049–56. doi: 10.1016/j.jvs.2009.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu B, Zhou J, Waring ME, Parker DR, Eaton CB. Abdominal obesity and peripheral vascular disease in men and women: a comparison of waist-to-thigh ratio and waist circumference as measures of abdominal obesity. Atherosclerosis. 2010;208:253–7. doi: 10.1016/j.atherosclerosis.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Sumner AD, Khali YK, Reed JF. The relationship of peripheral arterial disease and metabolic syndrome prevalence in asymptotic US adults 40 years and older: results from the National Health and Nutrition Examination Survey (1999–2004) J Clin Hypertens. 2012;14:144–148. doi: 10.1111/j.1751-7176.2011.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terzis ID, Papamichail C, Psaltopoulou T, et al. Long-term BMI changes since adolescence and markers of early and advanced subclinical atherosclerosis. Obesity. 2012;20:414–420. doi: 10.1038/oby.2011.137. [DOI] [PubMed] [Google Scholar]
- 23.Ix JH, Biggs ML, Kizr JR, Mukamal KJ, et al. Association of body mass index with peripheral arterial disease in older adults. Am J Epidemiol. 2011;174:1036–1043. doi: 10.1093/aje/kwr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadden T, Neiberg R, Wing R, et al. Four-year weight losses in the Look AHEAD Study: Factors associated with success. Obesity. 2011;19:1987–98. doi: 10.1038/oby.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Look AHEAD Research Group. Effect of intensive lifestyle intervention on cardiovascular morbidity and mortality in type 2 diabetes. Results of the Look AHEAD controlled trial. New Eng J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith FB, Lee J, Price JF, van Wijk MCW, Fowkes FGR. Changes in ankle brachial index in symptomatic and asymptomatic subjects in the general population. J Vasc Surg. 2003;38:1323–30. doi: 10.1016/s0741-5214(03)01021-8. [DOI] [PubMed] [Google Scholar]
- 28.Kimura A, Hashimoto J, Watabe D, et al. Patient characteristics and factors associated with inter-arm difference of blood pressure measurements in a general population in Ohasama, Japan. J Hypertens. 2004;22:2277–2283. doi: 10.1097/00004872-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Poon LCY, Kametas N, Strobl I, Pachoumi C, Nicolaides KH. Inter-arm blood pressure differences in pregnant women. BJOG. 2008;115:1122–1130. doi: 10.1111/j.1471-0528.2008.01756.x. [DOI] [PubMed] [Google Scholar]
- 30.Verberk WJ, Kessels AGH, Thien T. Blood pressure measurement method and inter-arm differences: a meta-analysis. Am J Hypertens. 2011;24:1201–1208. doi: 10.1038/ajh.2011.125. [DOI] [PubMed] [Google Scholar]
- 31.Lane D, Beevers M, Barnes N, et al. Inter-arm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20:1089–1095. doi: 10.1097/00004872-200206000-00019. [DOI] [PubMed] [Google Scholar]
- 32.English JA, Carell ES, Guidera SAS, Tripp HF. Angiographic prevalence and clinical predictors of left subclavian stenosis in patients undergoing diagnostic cardiac catheterization. Catheter Cardiovasc Interv. 2001;54:8–11. doi: 10.1002/ccd.1230. [DOI] [PubMed] [Google Scholar]
- 33.Cooper JN, Buchanich JM, Youk A, et al. Reductions in arterial stiffness with weight loss in overweight and obese young adults: potential mechanisms. Atheroscl. 2012;223:485–490. doi: 10.1016/j.atherosclerosis.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belacazar M, Reboussin S, Haffner S, et al. A 1-year lifestyle intervention for weight loss in persons with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change. Diab Care. 2010;33:2297–2303. doi: 10.2337/dc10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribisl PM, Gaussoin SA, Lang W, et al. Lifestyle intervention improves heart rate recovery from exercise in adults with type 2 diabetes: results from the Look AHEAD study. J Obes. 2012;2012:309196. doi: 10.1155/2012/309196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poggi M, Palareti G, Biagi R, et al. Prolonged very low calorie diet in highly obese subjects reduces plasma viscosity and red cell aggregation but not fibrinogen. Int J Obes Relat Metab Disord. 1994;18:490–496. [PubMed] [Google Scholar]
- 37.Cavendish JJ, Carter LI, Tsimikas S. Recent advanced in hemodynamics: noncoronary applications of a pressure sensor angioplasty guideware. Catheterization Cardiovasc Interv. 2008;71:748–758. doi: 10.1002/ccd.21505. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Bailey KR, Norheria A, Kullo IJ. Frailty across the spectrum of ankle-brachial index. Angiology. 2012;63:229–236. doi: 10.1177/0003319711413457. [DOI] [PubMed] [Google Scholar]
- 39.Budimer D, Jeroncic A, Gunjaca G, Rudan I, Polasek O, Boban M. Sex-specific association of anthropometric measures of body composition with arterial stiffness in a healthy population. Med Sci Monit. 2012;18:CR65–71. doi: 10.12659/MSM.882457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson HM, Piper ME, Jorenby DE, Fiore MC, Baker TB, Stein JB. Risk factors for subclinical carotid atherosclerosis among current smokers. Prev Cardiol. 2010;13:166–71. doi: 10.1111/j.1751-7141.2010.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]