Abstract
Objective
We examined whether midlife body mass index (BMI) and waist circumference (WC) predict successful ageing.
Design and Methods
BMI/WC were assessed in 4869 persons (mean age 51.2, range 42–63 in 1991/93) and survival and successful ageing (alive, no chronic disease at age >60 years, not in the worst age- and sex-standardized quintile of cognitive, physical, respiratory, and cardiovascular, and mental health) ascertained over a 16-year follow-up, analysed using logistic regression adjusted for socio-demographic factors and health behaviours.
Results
507 participants died, 1008 met the criteria for successful ageing. Those with BMI≥30 kg/m2 had lower odds of successful ageing (Odds Ratio (OR)=0.37; 95% Confidence Interval (CI): 0.27, 0.50) and survival (OR=0.55; 95% CI: 0.41, 0.74) compared to BMI between 18.5–25 kg/m2. Those with a large waist circumference (≥102/88 cm in men/women) had lower odds of successful ageing (OR=0.41; 95% CI: 0.31, 0.54) and survival (OR=0.57; 95% CI: 0.44, 0.73) compared to those with a small waist (<94/80 cm in men/women). Analysis with finer categories showed lower odds of successful ageing starting at BMI ≥23.5 kg/m2 and waist circumference 82/68 cm in men/women.
Conclusions
Optimal midlife BMI and waist circumference for successful ageing might be substantially below the current thresholds used to define obesity.
Keywords: obesity, body mass index, waist circumference, ageing
Introduction
500 million adults, corresponding to more than a tenth of the total world adult population, were obese in 2008.(1) There is considerable evidence of the adverse effects of obesity on mortality,(2–4) with concerns growing that the obesity epidemic will reduce gains made in cardiovascular disease prevention(5, 6) and life expectancy.(7) Besides mortality and chronic diseases, obesity is linked to disability,(8–10) poor physical(11–16) and cognitive functioning.(17–20) Given population ageing and the adverse effect of obesity on multiple age-related health conditions,(21) research on the impact of obesity on ageing outcomes has considerable public health significance.
The primary objective of this study is to examine the effect of obesity on health at older ages. Mortality, chronic diseases, and functioning examined as separate outcomes do not allow an evaluation of the extent to which obesity influences overall health at older ages. The concept of ‘successful’(22) or ‘healthy’ ageing allows multiple dimensions of health to be assessed together. In this paper we examine if midlife indicators of adiposity, body mass index (BMI) and waist circumference (WC) predict ‘successful ageing’, defined as being free of major chronic diseases and having good physical, cognitive, respiratory, cardiovascular, and mental health. Besides successful ageing we also estimate associations of adiposity markers with survival in the same cohort, irrespective of the health status of the person, in order to allow better interpretation and comparison with the adiposity-successful ageing associations.
Methods
Data are drawn from the Whitehall II study, established in 1985 on 10,308 employees (response rate 73%), aged 35–55 years (67% men), of 20 London based civil-service departments.(23) All participants in the study were white-collar workers but a wide range of occupations was represented with a salary difference of over 10-fold between the top and bottom of the hierarchy. Study design consisted of a clinical examination approximately every 5 years: 1985/88, 1991/93, 1997/99, 2002/04, and 2008/09. As waist circumference was added to the anthropometry measures in 1991/93, it is the baseline in the present analysis. All participants provided written consent and the University College London ethics committee approved the study.
Baseline measurements (1991/93)
BMI (weight Kg/height metres2)
Weight was measured in underwear to the nearest 0.1 kg on Soehnle electronic scales with digital readout (Leifheit AS, Nassau, Germany). Height was measured in bare feet to the nearest 1 mm using a stadiometer with the participant standing erect with head in the Frankfurt plane. Reproducibility of the weight and height measurements over 1 month (i.e., between-subject variability/total (between + within subject) variability), undertaken on 331 participants, was 0.99. WHO classification was used to categorise BMI(24): 18.5–24.99 kg/m2 (normal weight), 25–29.99 kg/m2 (overweight), and ≥30 kg/m2 (obese).
WC was measured in the standing position with the participant in underwear, using a fiberglass tape measure at 600 g tension. It was taken as the smallest circumference at or below the costal margin. Waist circumference categories used were small (<94/80 cm in men/women), intermediate (94 to <102/80 to <88 cm in men/women), and large (≥102/88 cm in men/women).(21)
Covariates
Baseline covariates were age, sex, marital status (married vs. other) and socioeconomic status (SES), using the measure of occupational position (high, intermediate, low), a comprehensive marker of socioeconomic circumstances related to salary, social status and level of responsibility at work.(23)
Analyses were also adjusted for health behaviours, assessed by questionnaire. Smoking status was categorized as current, ex-, and never smokers. Alcohol consumption was assessed via questions on the number of alcoholic drinks consumed in the last seven days, converted to units of alcohol consumed in a week. Level of physical activity was defined as “active” (≥2.5 hours/week of moderate physical activity or ≥1 hour/week of vigorous physical activity), “inactive” (<1 hour/week of moderate and vigorous activity) and intermediate level of activity for all others. Dietary behaviour was assessed using a question on frequency of fruit and vegetable consumed in a week.
Outcome Ascertainment (follow-up 1991/93 to 2008/09)
Two key outcomes were ascertained at the end of a mean follow-up of 16.3 years (SD=2.4 years): survival (being alive at the end of the follow-up) and successful ageing. Multiple health outcomes, in order to reflect the multidimensionality of the successful ageing concept, were used to define successful ageing. These included (1) being alive at the end of the follow-up and aged ≥ 60, (2) absence of coronary heart disease, stroke, cancer and diabetes over the follow-up, (3) good cardiovascular, metabolic, respiratory, physical and cognitive functioning, and (4) absence of mental health problems at the clinical examination in 2008/09. These components are described below.
Mortality
Mortality data were drawn from the British national mortality register (National Health Services Central Registry). The tracing exercise was carried out using the National Health Service identification number assigned to each British resident.
Chronic diseases
Coronary heart disease (CHD) was identified at baseline (1991/93), to exclude prevalent disease, and over the follow-up using MONICA criteria.(25) Data for this procedure came from the medical examinations carried out every 5 years, hospital records of acute electrocardiograms (ECGs) and cardiac enzymes. Stroke diagnosis was self-reported and included history of stroke or a transient ischemic attack. Cancer status was assessed using the National Health Service cancer registry. At each medical examination, diabetes mellitus was determined by self-report of doctor diagnosis and/or medication or oral glucose tolerance test (a fasting glucose ≥ 7.0 mmol/l, a 2-h postload glucose ≥ 11.1 mmol/l, reported doctor-diagnosed diabetes, or use of diabetes medication).(26)
Functional status
Functional measures were assessed at the 2008/09 clinical examination using standard protocols. Good functional status was defined as not being in the worst quintile of any of the domains assessed, with the worst quintile standardized using age in 5-year bands and sex because of the association of these measures with functioning. Systolic blood pressure (average of 2 measurements in sitting position after 5 minutes of rest with the OMRON HEM 907), a marker of cardiovascular health in our analysis, is a major risk factor for coronary heart disease and the most important risk factor for stroke (27) The highest quintile of systolic blood pressure described poor cardiovascular health. Physical function using walking speed measured over a clearly marked eight foot walking course at usual pace,(28) respiratory function as forced expiratory volume in one second/height2 (FEV1 in L/m2),(29),34 and cognitive functioning using a global cognitive score composed of 5 tests.(30) Mental functioning were indicated by a score of ≤ 42 in the mental health scale of the short form general health survey (SF-36),(31) part of the study questionnaire administered at 2008/09.
Statistical Analysis
Survival and successful ageing were ascertained as described above. As there was no evidence of sex differences in the associations (p for interaction with sex = 0.16 to 0.97 depending on the outcome), men and women were combined in the analysis. Logistic regression was used to examine associations of baseline BMI (18.5–24.99 kg/m2, 25–29.99 kg/m2, and ≥30 kg/m2) and WC categories (men/women: <94/80 cm, 94 to <102/80 to <88 cm, and ≥102/88 cm) with survival and successful ageing.
In analysis where survival was the outcome, cases were participants who survived (comprising both successful and unsuccessful ageing) and non-cases were those who died during the follow-up. When successful ageing was the outcome, non-cases were those who died and those alive but deemed not to have aged successfully. Analyses were adjusted for age, sex, marital status, SES and health behaviours (smoking, alcohol consumption, physical activity and dietary behaviour). We also examined associations of BMI and WC with individual components used to define successful ageing.
We also examined finer categories of BMI and WC for their association with both outcomes. Statistical tests were 2-sided and a P-value of less than 0.05 was considered statistically significant. Sensitivity analysis: One, we first examined the influence of missing data on the results by examining the association of BMI and WC with “missingness” at the end of follow-up using logistic regression. As all participants are linked to the national mortality registers we repeated the mortality analyses in all participants with data on BMI and WC. Two, we examined associations of BMI and WC with successful ageing and survival in non-smokers. Finally, the main analysis was repeated using multinomial logistic regression to analyse both outcomes (successful ageing, mortality) in a single analytic framework. All analyses were performed with STATA statistical software, version 12.
Results
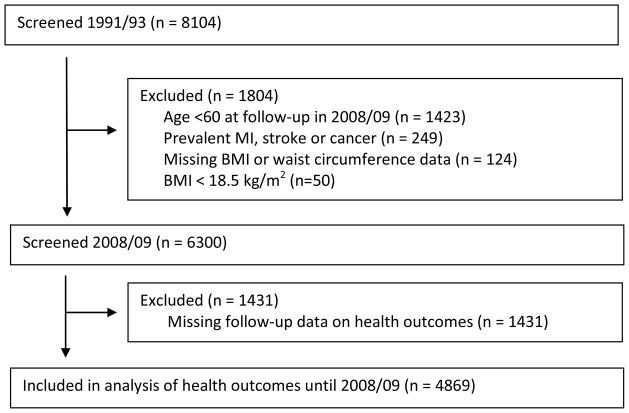
Figure 1 shows a flow chart of the sample selection. Of the 8104 participants at the 1991/93 clinical screening where BMI and WC were assessed, we excluded those who were alive but not yet aged 60 (n=1423) at the end of the follow-up (2008/09) as they were too young to be considered to have aged successfully, those with prevalent cardiovascular disease or cancer at baseline (n=249), those with missing data on BMI or WC (n=124) or BMI below 18.5 (n=50), and those lost to follow-up (N=1431) between the assessment of adiposity (1991/93) and functioning (2008/09). Of the 8104 participants at 1991/93 assessment, mortality along with socio-demographic data were available for 7902 participants. shows that the associations of BMI and waist circumference with mortality were similar in the two samples.
Figure 1.
Flow Chart of Sample Selection
Compared to those with missing data the sample included in the analysis (N=4869) was composed of fewer women (33.2% vs. 29.4% women p<0.001) and more individuals from the higher SES group (32.7% vs. 41.3%, p<0.001); both BMI and WC were only slightly higher in the sample included in the analysis (age and sex adjusted differences of 0.4 kg/m2 and 0.7 cm, respectively). Compared to normal weight participants, missingness was no different in overweight (OR=0.93, 95% CI: 0.83, 1.03) and obese (OR=0.84, 95% CI: 0.71, 1.00) persons in analysis adjusted for age and sex (N=7902). Those with a small waist had similar odds ratio of missingness to those in the intermediate (OR=1.02, 95% CI: 0.90 1.105) and large (OR=0.90, 95% CI: 0.77, 1.05) WC categories. The measures of BMI and WC were strongly correlated (correlation coefficient = 0.74, p<0.001).
Quintiles used to ascertain poor functional status accumulated over the measures so that only 33.8% of participants in the study had good functioning on all measures; 59.4% were free of chronic diseases and combining these two led to 1008 (20.7% of those included in the analysis) successful agers, see Table 1 for sample characteristics. At baseline those ageing successfully had a better socio-demographic and behavioural profile. 507 (10.4%) participants died over the follow-up; 107 were aged less than 60 at the time of death (mean age=55.9, SD=3.2, range=46.2–59.9) and 400 over 60 years (mean age=68.0, SD=4.8, range=60.0–79.0). Participants who died over the follow-up had higher BMI and WC at baseline and were less likely to be married or from the high SES group (p<0.001).
Table 1.
Sample characteristics at baseline (1991/93) a function of ageing outcomes at the end of follow-up (2008/09).
| Characteristic | Successful ageing (n =1008) | Others (n = 3861) | P | |
|---|---|---|---|---|
| Age, y | M (SD) | 49.8 (5.0) | 51.5 (5.3) | <0.0001 |
| Sex, % women | N (%) | 279 (27.7) | 1151 (29.8) | 0.19 |
| BMI, kg/m2 | M (SD) | 24.5 (3.0) | 25.8 (3.9) | <0.0001 |
| Waist circumference, cm | ||||
| Men | M (SD) | 87.2 (8.2) | 90.7 (9.4) | <0.0001 |
| Women | M (SD) | 74.8 (10.5) | 80.5 (12.8) | <0.0001 |
| Married | N (%) | 815 (80.6) | 2979 (77.2) | 0.01 |
| High socioeconomic status* | N (%) | 542 (53.8) | 1441 (37.3) | <0.0001 |
| Smoker | N (%) | 72 (7.2) | 521 (14.1) | <0.0001 |
| Alcohol consumption, units/week | M (SD) | 10.7 (11.2) | 10.1 (12.9) | 0.14 |
| Physical activity, inactive | N (%) | 144 (14.3) | 855 (22.2) | <0.0001 |
| Fruit/vegetable consumed/week | M (SD) | 6.7 (1.6) | 6.4 (1.4) | <0.0001 |
Abbreviations: M: Mean, SD: Standard Deviation, N: Number
Top occupational position, out of three.
The mean (SD) follow-up in those who died was 11.3 (4.7) years and 16.8 (SD=0.6) years in the survivors. Successful ageing and mortality in the BMI groups were as follows: 25.3% and 9% in normal weight persons, 17.8% and 10.7% in the overweight and 9.8% and 16.1% in the obese. In the waist circumference categories these numbers were 24.2% and 9.1% in those in the small, 16.1% and 10.3% in the intermediate and 10.1% and 17.1% in the large category. Table 2 presents the association of BMI and WC with successful ageing and survival in analyses adjusted for socio-demographic measures (model 1) and health behaviours (model 2). Successful ageing had a dose-response relation with both measures of adiposity but the associations with survival were statistically significant only for those with a BMI ≥30kg/m2 and those in the largest WC category. Sensitivity analysis showed similar associations in analysis excluding current smokers at baseline () and that using multinomial logistic regression () yielded results similar to those in table 2. In persons with both BMI ≥30 kg/m2 and a large WC (n=423, 83% of obese and 65% of those with large WC), the associations with successful ageing (OR=0.35, 95% CI: 0.24, 0.49) and survival (OR=0.51, 95% CI: 0.37, 0.69) were similar in fully adjusted analyses.
Table 2.
Association of BMI and waist circumference in 1991/93 with ageing outcomes in 2008/09.
| N | Model 1 OR (95% CI) | Model 2 OR (95% CI) | |
|---|---|---|---|
| BMI | |||
| Successful ageing | successful ageing/N | ||
| Normal weight (18.5–24.99 kg/m2) | 613/2420 | 1 (ref) | 1 (ref) |
| Overweight (25–29.99 kg/m2) | 345/1940 | 0.69 (0.59, 0.80) | 0.71 (0.61, 0.82) |
| Obese (≥30 kg/m2) | 50/509 | 0.35 (0.26, 0.48) | 0.37 (0.27, 0.50) |
| Survival | survival/N | ||
| Normal weight (18.5–24.99 kg/m2) | 2203/2420 | 1 (ref) | 1 (ref) |
| Overweight (25–29.99 kg/m2) | 1732/1940 | 0.87 (0.71, 1.06) | 0.90 (0.73, 1.11) |
| Obese (≥30 kg/m2) | 427/509 | 0.53 (0.40, 0.71) | 0.55 (0.41, 0.74) |
|
| |||
| Waist circumference | |||
| Successful ageing | successful ageing/N | ||
| Small (men: <94 cm, women: <80 cm) | 788/3255 | 1 (ref) | 1 (ref) |
| Intermediate (men: 94–<102 cm, women: 80–<88 cm) | 154/958 | 0.65 (0.53, 0.79) | 0.66 (0.55, 0.81) |
| Large (men: ≥102 cm, women: ≥ 88 cm) | 66/656 | 0.38 (0.29, 0.51) | 0.41 (0.31, 0.54) |
| Survival | survival/N | ||
| Small (men: <94 cm, women: <80 cm) | 2959/3255 | 1 (ref) | 1 (ref) |
| Intermediate (men: 94–<102 cm, women: 80–<88 cm) | 859/958 | 0.92 (0.72, 1.18) | 0.96 (0.75, 1.23) |
| Large (men: ≥102 cm, women: ≥ 88 cm) | 544/656 | 0.52 (0.41, 0.67) | 0.57 (0.44, 0.73) |
Abbreviations: OR: Odds Ratio, CI: Confidence Interval.
Model 1: Analyses adjusted for age, sex, SES, and marital status.
Model 2: Model 1 + smoking, alcohol consumption, physical activity and dietary behaviour.
Table 3 shows results of the association of BMI and WC with the components used to define successful ageing. Individuals classified as obese were less likely to have good functioning on all components except mental health (OR=0.96, 95% CI: 0.68, 1.36), they also had lower odds of being free of chronic diseases (OR=0.38, 95% CI: 0.31, 0.46). Both BMI and WC had the strongest association with chronic diseases. However, functioning components did contribute to the associations as when with successful ageing was defined without taking into consideration chronic diseases, the overweight (OR=0.85, 95% CI: 0.73, 0.98) and the obese (OR=0.56, 95% CI: 0.43, 0.72) had lower odds ratio of successful ageing in fully adjusted analyses. Similarly, odds ratio of successful ageing were lower in those with an intermediate (OR=0.81, 95% CI: 0.68, 0.96) and large (OR=0.58, 95% CI: 0.46, 0.72) compared to small WC.
Table 3.
Association of BMI and waist circumference (1991/93) with components of successful ageing (2008/09).*
| Good lung function | Good cognitive function | Good physical function | Good systolic blood pressure | Good mental health | No Chronic disease | |
|---|---|---|---|---|---|---|
|
| ||||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| BMI | ||||||
| Normal weight (18.5–24.99 kg/m2) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Overweight (25–29.99 kg/m2) | 0.92 (0.76, 1.10) | 0.78 (0.65, 0.93) | 0.81 (0.68, 0.96) | 0.90 (0.77, 1.06) | 1.06 (0.85, 1.33) | 0.66 (0.58, 0.75) |
| Obese (≥30 kg/m2) | 0.64 (0.47, 0.86) | 0.71 (0.53, 0.96) | 0.41 (0.32, 0.53) | 0.61 (0.48,0.79) | 0.96 (0.68, 1.36) | 0.38 (0.31, 0.46) |
| Waist Circumference | ||||||
| Small (men: <94 cm, women: <80 cm) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Intermediate (men: 94–<102 cm, women: 80–<88 cm) | 0.85 (0.68, 1.06) | 0.70 (0.57, 0.86) | 0.76 (0.62, 0.92) | 0.96 (0.79, 1.17) | 0.96 (0.74, 1.26) | 0.71 (0.61, 0.82) |
| Large (men: ≥102 cm, women: ≥ 88 cm) | 0.59 (0.45, 0.76) | 0.93 (0.71, 1.22) | 0.47 (0.37, 0.59) | 0.63 (0.50, 0.80) | 0.80 (0.59, 1.08) | 0.45 (0.37, 0.53) |
Abbreviations: OR: Odds Ratio, CI: Confidence Interval.
Analysis adjusted for age, sex, marital status, SES, smoking, alcohol consumption, physical activity and dietary behaviour.
Good lung, cognitive, and physical function defined as scores not in the lowest age- and sex-standardized quintile of FEV1/height2, global cognitive score, and walking speed, respectively. Good systolic blood pressure defined as not being in the highest age- and sex-standardized quintile of systolic blood pressure. Good mental health defined as the SF36 mental component score < 42. Chronic diseases assessed were cancer, CHD, stroke, and/or diabetes. Ns vary from 3346 to 4922 depending on the outcome.
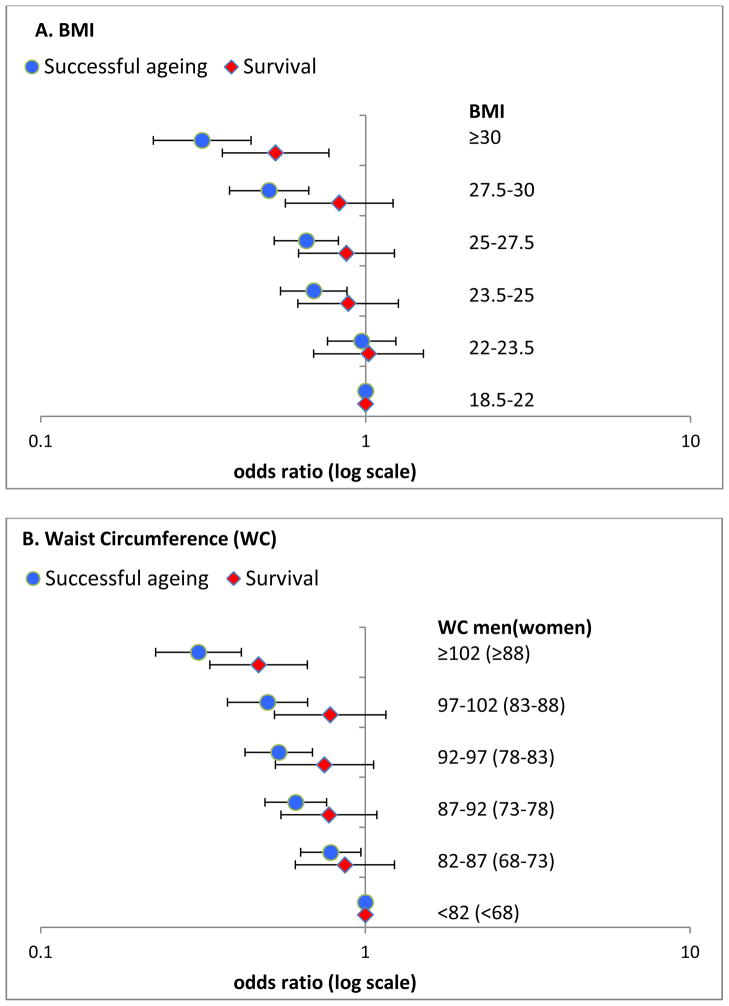
Figure 2 shows results of analysis using finer categories of BMI (Panel A) and WC (Panel B) with successful ageing and survival as the outcomes. In these data, higher BMI was significantly associated with lower survival only when ≥ 30 kg/m2 (OR=0.53; 95% CI: 0.36, 0.77). However, the odds ratio of successful ageing was significantly lower in those with BMI starting at 23.5–25 kg/m2 (OR=0.69, 95% CI: 0.55, 0.88). The results for WC are similar (Figure 2B); here again poorer survival was observed in the highest WC category, ≥102/88 cm in men/women, but the odds ratio of successful ageing were lower (OR=0.78, 95% CI: 0.63, 0.97) starting at 82–87/68–73 cm in men/women.
Figure 2.
Odds ratio of successful ageing and survival as a function of BMI (A) and waist circumference (B) categories.
Analyses adjusted for age, sex, marital status, SES, smoking, alcohol consumption, physical activity and dietary behaviour.
Discussion
In this 16-year follow-up of nearly 5000 British men and women we found both high BMI and WC in midlife to be associated with substantially reduced likelihood of successful ageing. The obese (BMI ≥30 kg/m2) had 63% and those with a large WC (men: ≥102 cm, women: ≥88 cm) 59% lower odds of successful ageing than those of normal weight and small WC, respectively. Associations of adiposity markers with survival were consistently weaker than those with successful ageing, obesity reduced the odds ratio of survival by 45% and large WC by 43%. Exploratory analysis of narrower categories of BMI and WC suggested that successful ageing was less likely in those with BMI>23.5 kg/m2 and waist circumference greater than 82/68 cm in men/women; much below the threshold for low risk of disease and mortality, i.e. 25kg/m2 for BMI and 94/80 cm WC in men/women.
The doubling of the human lifespan this last century(32) has led to a rapid increase in the research on ageing. Rowe & Kahn’s seminal paper in 1987 highlighted the heterogeneity in the health of older persons and the importance of understanding “successful ageing”.(22) A recent review by Depp and colleagues (33) concluded that despite subsequent attempts to define successful ageing there is little consensus on the appropriate terminology, the components of such a concept, and whether it needs to be defined using objective or subjective criteria. Physical functioning, usually measured as the absence of reported disability is central to many definitions of successful ageing, used in 26 out of 29 definitions reviewed by Depp et al. while 13 definitions included measures of cognitive function.(33) Life satisfaction/well-being and social engagement measures were used in a third of the studies. Many of the outcome measures in these studies were self-reported and the analysis did not take into account mortality and chronic diseases. In contrast, our outcome measure was assessed using objective data on physical and cognitive functioning, in addition to morbidity and death.
A third of the individuals (35.8%, SD= 19.8%) included in the 29 studies in the review by Depp et al. qualified as successful agers.(33) Nearly 21% in our study sample qualified as successful agers, this number would have been larger had we used a less restrictive definition. Our definition of successful ageing requires good health in a greater number of components than any previous publication; however, utilizing fewer components would have increased heterogeneity in the group of successful agers, and potentially a less comprehensive assessment of the impact of obesity on ageing outcomes.
Our study shows that midlife obesity shapes a range of ageing outcomes, from morbidity and mortality to chances of successful ageing. We used a prospective design to assess the impact of body weight as age is known to modify the association between obesity and death due to survivor effect and competing causes of death at older ages.(34, 35) There is increasing evidence of the importance of midlife bodyweight for adverse health outcomes at older ages. Associations in cross-sectional studies on older adults could be because body weight is a risk factor or because it is a correlate or consequence of the health outcome under investigation. Our use of a prospective design with a long follow-up ensures that the observed results are not due to reverse causation bias. Two recent reviews highlight the importance of midlife BMI for dementia (36) and limitations in activities of daily living.(37) Our use of “successful ageing” as the outcome suggests that bodyweight influences multiple dimensions of health, the risk of chronic disease but also lung, cognitive, physical functioning and chronic diseases.
A BMI threshold of 25 kg/m2 has been used extensively to define normal weight but there is already some evidence that individuals with lower values have better cardiovascular outcomes.(5) In the Nurses’ Health Study midlife BMI greater than 23 kg/m2 was associated with worse odds of healthy aging.(38) Our data show decrease in the odds ratio of successful ageing to start at BMI≥23.5 kg/m2 in midlife, suggesting that carrying excess weight might accelerate the rate of ageing in those well below the obesity threshold. Similarly, for waist circumference successful ageing was less likely starting much below the threshold used to define risk (≥102/88 cm in men/women).(21)
The main strengths of this study include the conceptualization of successful ageing based on multiple outcomes, including validated measures of chronic disease and objective measures of functioning. The estimates for successful ageing where examined against those for survival showing that both BMI and WC have a stronger effect on survival in good health (successful ageing) rather than survival alone. The large sample size and the long follow-up are further advantages. Finally, we were able to use linkage of all participants to national mortality register to show that associations of obesity measures with mortality were similar in those included and not included in the analysis. Thus, despite the loss to follow-up over the course of the study it is unlikely that our results are influenced by missing data.
There are also several limitations to the results reported here. One, despite the long follow-up, our study does not cover the oldest age-groups. The extent to which the results apply to the 80+ age-groups remains to be explored. Two, our effect estimates might lack precision as there is emerging evidence of the effect of duration of obesity, every 2 obese-years increases mortality risk by 6%.(39) Three, the use of population specific cut-offs to define poor functional status is not ideal. Further research is required to define cut-offs for measures of functioning so that they are consistent across studies. Four, a large number of participants with data at baseline were not included in the analysis either by design (less than 60 years old at the end of follow-up) or due to missing data on the functioning measures. However, this is unlikely to have biased the results as BMI and WC were not associated with missing data. Finally, these findings from an occupational cohort may not be fully generalizable to the general population.
In conclusion, our study highlights the importance of midlife adiposity for health at older ages. Although overweight and obesity may become less disabling due to improvement in the treatment of chronic diseases associated with obesity, our data suggest this might not be the case for overall health and functioning at older ages. These results highlight the importance of low BMI and waist circumference, below the current thresholds used to define general and central obesity associated with risk of mortality and chronic diseases, for good health at older ages.
What is already known?
Obesity is a risk factor for mortality and chronic diseases, with emerging evidence of its effect on multiple ageing outcomes.
It is unclear if obesity in midlife is a risk factor for global measures such as ‘successful ageing’, defined as being free of major chronic diseases and good physical, cognitive, respiratory, cardiovascular functions and good mental health.
It is unknown whether the risk is contained at thresholds used to define obesity (BMI≥30Kg/m2 & and large waist circumference:≥102/88 cm in men/women).
What does this study add?
Both obesity measures were more strongly associated with successful ageing than with mortality.
Lower odds of successful ageing starting at BMI ≥23.5 kg/m2 and waist circumference 82/68 cm in men/women, considerably below the threshold used to define obesity.
Acknowledgments
We thank all of the participating civil service departments and their welfare, personnel, and establishment officers; the British Occupational Health and Safety Agency; the British Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team. The Whitehall II Study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants and data entry staff, who make the study possible.
Funding
This research is supported by the US National Institutes of Health (R01AG013196 to ASM; R01AG034454 to ASM & MK, R01HL036310 to MK), the UK Medical Research Council (K013351 to MK), the Economic and Social Research Council (to Mk) and the British Heart Foundation (to EB).
Footnotes
Author contribution
ASM had full access to the data, and takes responsibility for the integrity of the data and accuracy of the data analyses. All authors contributed to the concept and design of study, drafting and critical revision of the manuscript.
Competing interests: the authors have no competing interests.
Reference List
- 1.World Health Organization. Fact sheet N°311. World Health Organisation; 2011. Obesity and overweight. Ref Type: Online Source. [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 3.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al MA, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138(1):24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.Hardoon SL, Morris RW, Whincup PH, et al. Rising adiposity curbing decline in the incidence of myocardial infarction: 20-year follow-up of British men and women in the Whitehall II cohort. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361(23):2252–60. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferraro KF, Su YP, Gretebeck RJ, Black DR, Badylak SF. Body mass index and disability in adulthood: a 20-year panel study. Am J Public Health. 2002;92(5):834–40. doi: 10.2105/ajph.92.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins KR. Obesity’s effects on the onset of functional impairment among older adults. Gerontologist. 2004;44(2):206–16. doi: 10.1093/geront/44.2.206. [DOI] [PubMed] [Google Scholar]
- 10.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298(17):2020–7. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 11.Fine JT, Colditz GA, Coakley EH, et al. A prospective study of weight change and health-related quality of life in women. JAMA. 1999;282(22):2136–42. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 12.Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med. 2000;15(11):789–96. doi: 10.1046/j.1525-1497.2000.90906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156(2):110–21. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 14.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11(8):568–79. doi: 10.1111/j.1467-789X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 15.Stenholm S, Sainio P, Rantanen T, et al. High body mass index and physical impairments as predictors of walking limitation 22 years later in adult Finns. J Gerontol A Biol Sci Med Sci. 2007;62(8):859–65. doi: 10.1093/gerona/62.8.859. [DOI] [PubMed] [Google Scholar]
- 16.Houston DK, Ding J, Nicklas BJ, et al. Overweight and obesity over the adult life course and incident mobility limitation in older adults: the health, aging and body composition study. Am J Epidemiol. 2009;169(8):927–36. doi: 10.1093/aje/kwp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging. 2005;26 (Suppl 1):11–6. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Gustafson D. Adiposity indices and dementia. Lancet Neurol. 2006;5(8):713–20. doi: 10.1016/S1474-4422(06)70526-9. [DOI] [PubMed] [Google Scholar]
- 19.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabia S, Kivimaki M, Shipley M, Marmot M, Singh-Manoux A. Body mass index over the adult lifecourse and cognition in late midlife: the Whitehall II cohort study. Am J Clin Nutr. 2009;89(2):601–7. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 22.Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237(4811):143–9. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 23.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. Int J Epidemiol. 2005;34(2):251–6. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 25.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90(1):583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 26.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.WHO. The Atlas of Heart Disease and Stroke. 2004. Ref Type: Report. [Google Scholar]
- 28.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 29.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 30.Singh-Manoux A, Marmot MG, Glymour M, Sabia S, Kivimaki M, Dugravot A. Does cognitive reserve shape cognitive decline? Ann Neurol. 2011;70(2):296–304. doi: 10.1002/ana.22391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware JE, Snow KK, Kosinski M. SF-36 health Survey: manual and interpretation guide. Boston MA: The Health Institute, New England Medical Centre; 1993. [Google Scholar]
- 32.Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296(5570):1029–31. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- 33.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14(1):6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- 34.Zamboni M, Mazzali G, Zoico E, et al. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005;29(9):1011–29. doi: 10.1038/sj.ijo.0803005. [DOI] [PubMed] [Google Scholar]
- 35.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 36.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12(5):e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 37.Backholer K, Wong E, Freak-Poli R, Walls HL, Peeters A. Increasing body weight and risk of limitations in activities of daily living: a systematic review and meta-analysis. Obes Rev. 2012;13(5):456–68. doi: 10.1111/j.1467-789X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 38.Sun Q, Townsend MK, Okereke OI, Franco OH, Hu FB, Grodstein F. Adiposity and weight change in mid-life in relation to healthy survival after age 70 in women: prospective cohort study. BMJ. 2009;339:b3796. doi: 10.1136/bmj.b3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdullah A, Wolfe R, Stoelwinder JU, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40(4):985–96. doi: 10.1093/ije/dyr018. [DOI] [PubMed] [Google Scholar]