Abstract
Purpose
To evaluate the morphological restoration of retinal anatomy after surgery for epiretinal membrane (ERM) peeling using spectral domain optical coherence tomography (SD-OCT). Correlation of retinal structure with visual outcome.
Design
Retrospective consecutive case series
Methods
34 consecutive eyes with epiretinal membrane underwent surgery with one year follow up examination. Spectral domain optical coherence tomography (SD-OCT) scans were analyzed pre-operatively and 1 3, 6, 9 and 12 months post-operative. Best-corrected visual acuity (BCVA) using ETDRS charts was measured at each visit.
Results
All eyes showed a significant improvement of BCVA after ERM-peeling (p=0.002). The timepoint of BCVA and retinal restoration seen on SD-OCT occurred simultaneously and varied between individuals (occurrence of BCVA: mean 4.82 months; retinal restoration: mean 4.24 months). At 3-months, the retinal anatomic restoration rate was 70% and 88% at 6-months.
Conclusion
Restoration of the retinal anatomic structure predominantly occurs within the first three months post ERM peeling. An improvement of BCVA and anatomic retinal restoration after ERM-removal varies in individuals. If retinal layers fully restore in their anatomic structure, BCVA improves at the same time-point.
Introduction
Epiretinal membranes (ERM) commonly develop in the elderly.1 The etiology of ERM is often related to proliferative diseases, inflammation, uveitis or trauma, but also other factors may cause ERM formation. However, the pathology is still not completely known. It is believed that migration of glial cells through defects of the internal limiting membrane (ILM) into the vitreous cavity causes ERM development on the surface of the ILM. This proliferative process is mainly triggered by growth factors and cytokines.2 As primary ERM derive from migrated glial cells, secondary ERM may additionally consist of retinal pigment epithelial cells, hyalocytes, myofibroblasts, fibrous astrocytes or vessels.3,4 ERM membranes can cause tangential traction with retinal changes like thickening of retinal layers, surface wrinkling or nerve fiber layer fibrillation. These changes lead to reduction of visual acuity and metamorphopsia.
Advanced forms of ERM with decrease of visual acuity and progression of clinical symptoms can be treated with pars plana vitrectomy and ERM peeling.5 Surgery often improves vision, but clinical symptoms such as metamorphopsia may persist. Prognostic factors have been studied and shown that the pre-operative visual acuity and the duration of symptoms are the strongest factors.6 Other prognostic factors include macular thickness and pre-operative changes of the neuro-sensory retina.7 Two recent studies have shown that the percentage of photoreceptor disruption is a strong predictive factor for visual acuity after ERM peeling. 8, 9
The use of optical coherence tomography (OCT) with high-speed and high-resolution images has become an important tool to monitor macular diseases.10, 11 Spectral domain-OCT (SD-OCT) has a 3–7-µm axial depth resolution and an 11-µm lateral resolution allowing for very detailed information at inner and outer retinal layers. In patients with ERM, the ability to distinguish retinal layers, may give new insight in the disease concerning the retinal restoration process after ERM peeling.
Our goal was to evaluate the morphological restoration of retinal anatomy after ERM peeling using SD-OCT and to correlate structure to visual outcome including an intense subsequent series of follow-up visits post surgery. No prior studies have focused on anatomic retinal restoration in short term post ERM-removal. Therefore, we combined our study with a short term and a long term follow-up-series. The focus was on retinal anatomy seen in SD-OCT to look for various changes during the first weeks after ERM peeling and its evolution during a 12 months follow-up period.
Methods
Patients
All patients with typical ERM clinically examined at the Shiley Eye Center and Jacobs Retina Center, University of California San Diego, were consented to study enrollment. This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of California, San Diego.
Only patients with ERMs, predominantly of the diffuse type, were included in this study. Pre-operative spectral domain OCT (SD-OCT) images showed intact photoreceptor layer without any inner segment/outer segment (IS/OS) abnormalities, retinal distortion or existence of cotton-wool spots. Patients with recurrent ERMs, any history of uveitis or prior retinal detachment were excluded. Pre-operative fluorescein angiography (FA) was performed to exclude retinal vein occlusion, proliferative diabetic retinopathy and macular diseases such as age related macular degeneration or diabetic macular edema. Also patients with severe media opacities affecting visual acuity were excluded from the study.
All patients underwent pre-operative SD-OCT scanning at the Jacobs Retina Center and ERM removal using 25-gauge pars plana vitrectomy with or without triamcinolone staining (40 mg/ml) or indocyanine green (1.25 mg/ml) as a negative stain12 by a single experienced retinal surgeon (WRF). None of the patients had combined surgery with cataract extraction, neither intraocular lens surgery during the follow-up-time. Additional peeling of internal limiting membrane (ILM) was simultaneously performed in patients that appeared to have a very adherent ERM without plications. No intraoperative complications were noted in our study.
In total, we included 34 eyes with ERM from 34 patients (19 male, 15 female) that had surgery and one year follow up. In these patients we recorded demographic data and best corrected visual acuity (BCVA) of the involved eye. All visual acuity measurements were performed using the ETDRS charts at 4 meters under standard illumination.
Imaging
Pre-operative and post-operative imaging was performed using an eye tracking spectral domain optical coherence tomograph (SD-OCT, Heidelberg Engineering, Heidelberg, Germany). All SD-OCT images were taken at maximal resolution of the ERM structure along the vertical and horizontal meridians over the fovea. We confirmed the location and position of the scans with the simultaneous real-time SLO infrared images of the retina. All patients had follow-up SD-OCT images taken at month 1, 3, 6, 9 and 12. A small sub-group of these patients (n=12) was imaged on a weekly basis after surgery to assess the change in retinal anatomy in the early post-operative period. To perform follow-up SD-OCT scans, the eye tracker of that device rejects any images where a lateral or rotational movement has been detected. Only images that overlap to the baseline image were included in the scan.
Evaluation and quantification of retinal restoration
All SD-OCT images were taken with the follow-up-mode of the Heidelberg tomograph to directly combine the parameters for retinal restoration after ERM-peeling. The parameters to achieve best retinal restoration in SD-OCT consisted of reflectivity changes, fibrillation of the nerve fiber layer (NFL), as well as restoration of the foveal contour. Best retinal restoration was defined as homogenous reflectivity within every retinal layer, even when restoration of the foveal contour was not observed. Therefore pre- and post-operative SD-OCT images were evaluated side to side from two masked observers (KIH; AKS) and the time-point of best anatomic restoration seen in SD-OCT was noted.
Statistical analysis
All statistical analysis was performed using SPSS (Version 19.0, Chicago, IL). Values as mean and standard deviation were calculated and paired and unpaired t-test was used for analysis of time to BCVA and OCT restoration. Pearson’s correlation coefficient was calculated to correlate anatomical retinal restoration with BCVA. A p<0.05 was considered statistically significant.
Results
Of the 34 eyes of 34 patients (19 male, 15 female; mean age 69 ± 7.5 years) follow up was one year with SD-OCT (Spectralis, Heidelberg Engineering) and measurement of BCVA. The primary outcome was defined as best retinal restoration in SD-OCT. All images were pre-operatively checked for changes in reflectivity of the photoreceptor layer. Also the restoration of the foveal contour was observed, but not seen in every patient: 20 eyes (59%) showed restoration of the foveal contour and 13 eyes (38%) showed persisting foveal evagination even when all retinal layers already restored. Therefore, best retinal restoration was achieved when outer and inner retinal layers showed similar reflectivity within every retinal layer compared to pre-operative SD-OCT pictures.
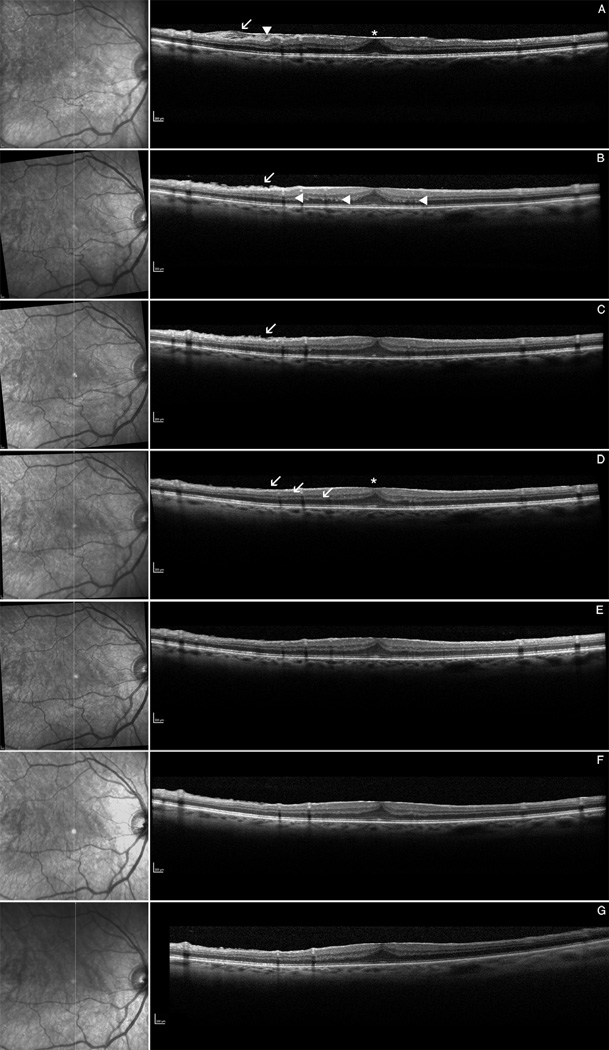
Initially we imaged 12 patients (7 male, 5 female; mean age 68 ± 9.7 years) weekly and observed that the retina slowly changed within the first 4 weeks after surgery (data not quantified). One month post-operatively and thereafter, we observed more anatomic restoration of all retinal layers. Figure 1 gives an example for a patient being imaged 1 day after ERM peeling and intensively followed with SD-OCT.
Figure 1.
Infrared picture (left), spectral domain optical coherence tomography (right): Example of a patient imaged with an intense series of spectral domain optical coherence tomography (SD-OCT). A Pre-operative OCT showing the ERM with fibrillation of the nerve fiber layer = NFL (arrow), surface wrinkling (arrowhead) and retinal thickening with foveal evagination (asterix). B Post-operative OCT at day 2, showing still irregulation of the NFL (arrow) and the outer plexiform layer = OPL (arrowheads) . C OCT at week 2 with persisting of NFL fibrillation. D OCT at 1 month showing for the first time normalization of the NFL (arrow) and the reflectivity y of the OPL. Also note the retinal thinning of the fovea with amelioration of the foveal contour (asterix). The OCT images at 3 months (E), 6 months (F) and 9 months (G) do not show any significant signs for retinal change after surgery anymore. The foveal contour was not completely restored in this patient whereas BCVA was already 60 letters at ETDRS charts at month 1.
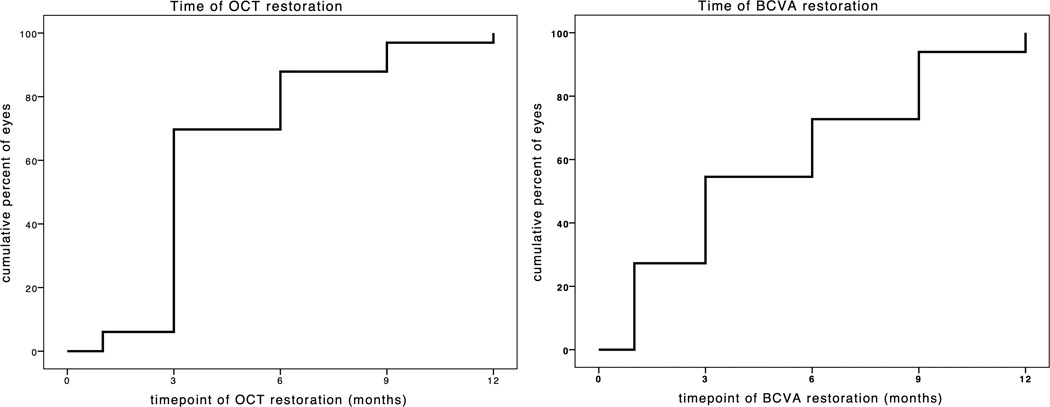
Out of 34 in our cohort, 33 eyes (97%) showed anatomic retinal restoration of both inner and outer retinal layers during the 1 year observation period. One eye did not restore during that time. The mean time for the anatomic retinal restoration was 4.2 ± 2.5 months. Figure 2 shows an example for retinal restoration after ERM-peeling at month 3. Figure 3 is demonstrating pre-operative fibrillation of the nerve fiber layer with restoration post-operatively. Eyes had a 3-month Kaplan-Meier retinal restoration rate of 70%, a 6-month retinal restoration rate of 88% and a 9-month retinal restoration rate of 97% (figure 4A). In the sub-group of 9 eyes with simultaneous ILM-peeling, mean time of retinal restoration was 5.7 ± 3.2 months.
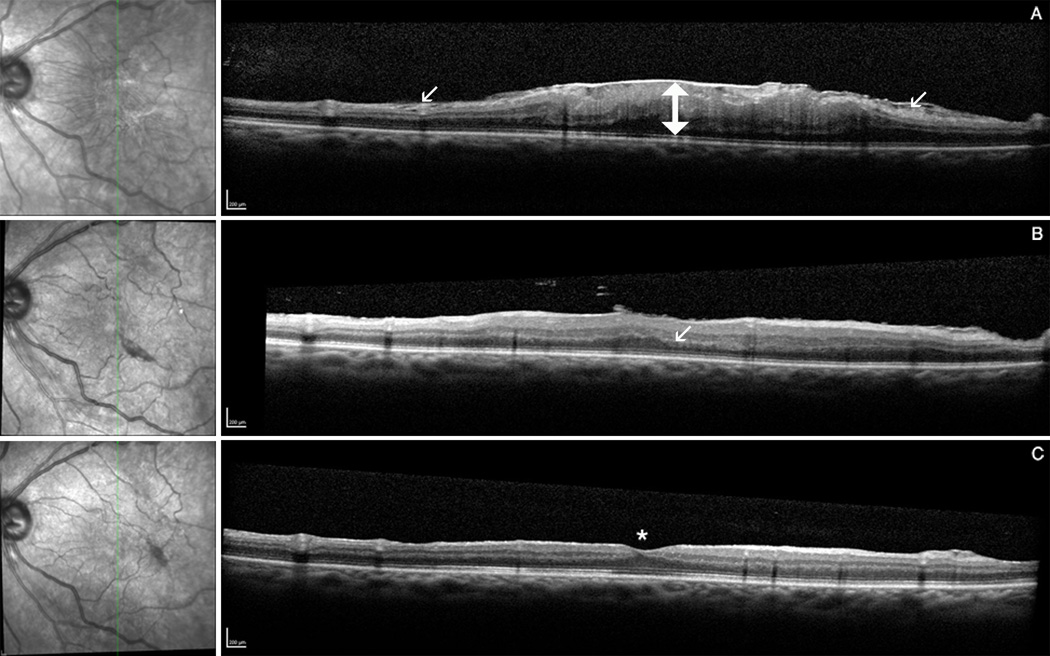
Figure 2.
2A and 2B Example of retinal restoration after ERM-peeling. Infrared picture (left), spectral domain optical coherence tomography (right): Figure 2A SD-OCT pre-operative (A) showing tangential traction of the ERM with thickening of the retinal center. B Post-operative OCT at 1 month with irregularity of the outer plexiform layer (arrow). C OCT at month 3 demonstrating normalization of all retinal layers with recovery of the foveal contour (aterix). Figure 2B SD-OCT pre-operative (A) with wrinkling of inner and outer retinal layers (arrows). B Post-operative OCT at month 3 with restoration of the foveal contour (asterix) and minimal irregularity of the outer plexiform layer (arrow). C OCT at month 6 showing best reflectivity and restoration of retinal layers.
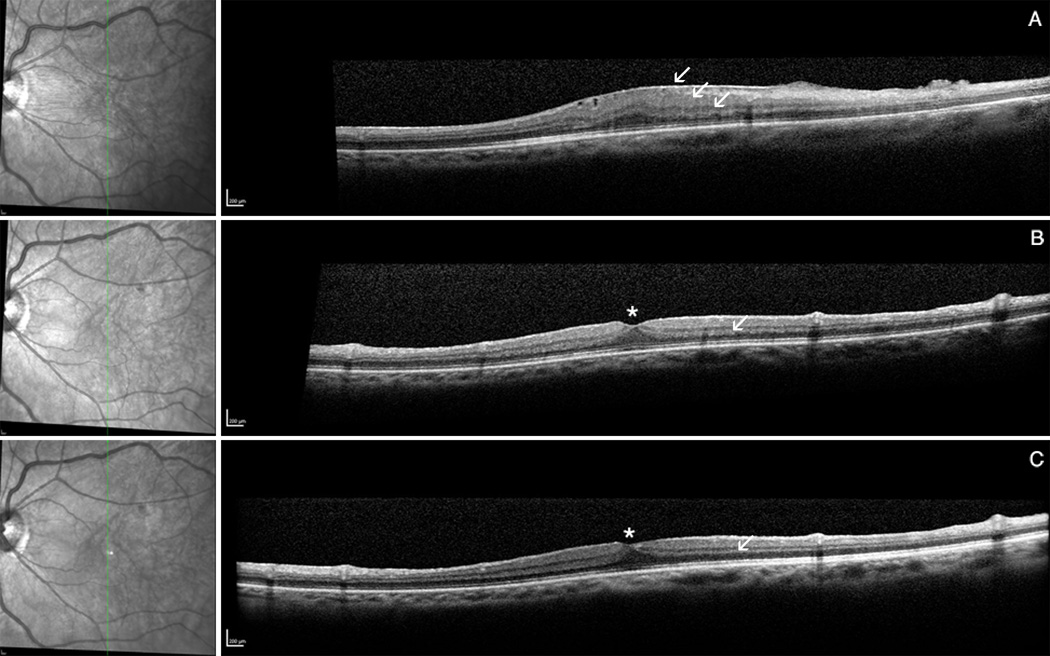
Figure 3.
Example for the pre-operative fibrillation of the nerve fiber layer (arrow) caused by tangential traction of the ERM that restored post-operatively. Infrared picture (left), spectral domain optical coherence tomography (right): SD-OCT pre-operative (A) and post-operative at month 9 (B).
Figure 4.
4A and 4B Kaplan-Meier-Curves for time to retinal restoration (3A) and for time to best corrected visual acuity (3B). Eyes had a 3-month Kaplan-Meier retinal restoration rate of 70%, a 6-month retinal restoration rate of 88% and a 9-month retinal restoration rate of 97%. Eyes had a 3-month Kaplan-Meier BCVA rate of 58%, a 6-month BCVA rate of 76% and a 9-month BCVA rate of 94%.
Mean pre-operative BCVA was 54 ± 13 letters at ETDRS charts, post-operative BCVA was 62 ± 9 letters. Table 1 gives detailed information on BCVA for each patient. All eyes showed a significant improvement of BCVA with a mean of 8 letters after ERM-peeling (p=0.002). The mean time to achieve BCVA was 4.82 ± 3.5 months. Eyes had a 3-month Kaplan-Meier BCVA rate of 58%, a 6-month BCVA rate of 76%, and a 9-month BCVA rate of 94% (figure 4B). The sub-group that had simultaneous ILM-peeling showed a mean time of 7.4 ± 3.8 months to achieve BCVA after surgery.
Table 1.
shows the evolution of BCVA of each patient after ERM-peeling or combined surgery with ILM-peeling. Best post-operative BCVA was seen at different time-points (month of post-operative BCVA). BCVA was taken in letters at ETDRS charts.
| Name | Preoperative BCVA (ETDRS) |
Postoperative BCVA (ETDRS) |
Month of post- operative BCVA |
Procedure |
|---|---|---|---|---|
| Patient 1 | 38 | 43 | 1 | ERM + ILM |
| Patient 2 | 43 | 58 | 1 | ERM |
| Patient 3 | 57 | 69 | 1 | ERM |
| Patient 4 | 61 | 69 | 1 | ERM |
| Patient 5 | 62 | 60 | 1 | ERM |
| Patient 6 | 63 | 64 | 1 | ERM |
| Patient 7 | 65 | 65 | 1 | ERM |
| Patient 8 | 65 | 58 | 1 | ERM |
| Patient 9 | 66 | 65 | 1 | ERM |
| Patient 10 | 46 | 58 | 3 | ERM |
| Patient 11 | 50 | 50 | 3 | ERM |
| Patient 12 | 56 | 60 | 3 | ERM |
| Patient 13 | 57 | 69 | 3 | ERM |
| Patient 14 | 60 | 69 | 3 | ERM |
| Patient 15 | 61 | 59 | 3 | ERM |
| Patient 16 | 63 | 65 | 3 | ERM |
| Patient 17 | 64 | 61 | 3 | ERM + ILM |
| Patient 18 | 70 | 65 | 3 | ERM |
| Patient 19 | 55 | 55 | 6 | ERM |
| Patient 20 | 61 | 73 | 6 | ERM + ILM |
| Patient 21 | 64 | 65 | 6 | ERM |
| Patient 22 | 64 | 73 | 6 | ERM |
| Patient 23 | 64 | 54 | 6 | ERM + ILM |
| Patient 24 | 68 | 69 | 6 | ERM |
| Patient 25 | 20 | 58 | 9 | ERM |
| Patient 26 | 28 | 29 | 9 | ERM + ILM |
| Patient 27 | 44 | 55 | 9 | ERM |
| Patient 28 | 47 | 70 | 9 | ERM + ILM |
| Patient 29 | 52 | 60 | 9 | ERM |
| Patient 30 | 57 | 58 | 9 | ERM |
| Patient 31 | 57 | 70 | 9 | ERM + ILM |
| Patient 32 | 20 | 66 | 12 | ERM + ILM |
| Patient 33 | 43 | 70 | 12 | ERM + ILM |
The time to achieve BCVA (mean: 4.82 months) and anatomical retinal restoration (mean 4.24 months) were not statistically significant different (p=0.29) for all eyes.
BCVA and anatomic retinal restoration occurred simultaneously (mean 4.4 months) in 16 eyes (49%). In 7 eyes (21%) BCVA was seen 3.3 months prior to retinal restoration and in 10 eyes (30%) 3.6 months after retinal restoration (data is mean). In a next step, it was of interest to evaluate if the time points, the time of BCVA and the time of best anatomic retinal restoration, correlated. We found that the time of anatomic restoration correlated significantly with the occurrence of BCVA (Pearson correlation coefficient: r=0.53, p=0.001),
Discussion
Since the evolution of optical coherence tomography (OCT), there have been several publications about the morphology of epiretinal membrane (ERM)13 and functional outcome after surgery.14, 15
Studies using multifocal electroretinogram (ERG) in patients with idiopathic ERM have shown changes in both the inner and outer retinal layers leading to speculation that changes in both inner and outer retinal layers may account for the decrease in visual function associated with ERM.16 Many efforts have been made to correlate retinal thickness to visual acuity in patients with ERM. Although a correlation between macular retinal thickness and BCVA has been observed, there is a stronger correlation between foveal anatomic changes and BCVA.17 In addition, recent studies have shown that good visual outcome after ERM peeling is dependent on intact photoreceptor inner segment/outer segment junction seen in pre-operative spectral domain OCT.8, 9, 18 This is true for tractional ERM involving the IS/OS-junction. In our study, the photoreceptor layer was not affected by the idiopathic ERM’s that we studied (which were mild to moderate in extent) and therefore did not need to be analyzed.
There are patients that do not improve in visual function, but show normal appearance of the photoreceptor layer in SD-OCT.19 It is hypothesized that there might be other factors contributing to distorted vision and metamorphopsia after surgery. A study using adaptive optics scanning laser ophthalmoscopy explains that phenomenon with microfolds detected in the foveal photoreceptor layer.19 Another study, measuring inner retinal changes in patients with ERM showed that metamorphopsia is related to the presence of an edematous inner nerve fiber layer.20
Several attempts have been made to define changes of anatomic retinal structures due to ERM as predictors for visual outcome post surgery. Suh et al. and Oster et al. described distorted outer retinal structures as a predictor for functional outcome after ERM-removal. Watanabe et al. focused on the inner retinal structures being responsible for the functional result post surgery. These studies examined anatomic changes of different retinal structures predominantly outer retinal or inner retinal changes. They did not look at all retinal layers. We in our study hypothesized that the restoration of both inner and outer retinal layer contour is important for retinal function improvement after ERM peeling. Therefore, we focused on the anatomic restoration of all retinal layers and correlated it to visual outcome. We did not measure retinal thickness in this study as this was already performed in many other studies focusing on thickness changes after ERM-peeling. In addition, we did not evaluate the IS/OS/-junction, because all patients had intact photoreceptor layer pre-operatively. Our aim was to describe the changes of all retinal layers, as the rehabilitation and demarcation of the high echogenic layers (nerve fiber layer, inner plexiform layer, outer plexiform layer, external limiting membrane, IS/OS-junction, retinal pigment epithelium=RPE) from the low echogenic layers (ganglion cell layer, inner nuclear layer, outer nuclear layer, interdigitation between outer photoreceptor segments and RPE).
The importance of the ILM-peeling in ERM-surgery and its influence on VA improvement was evaluated in a previous study.21 This study suggests that the interpretation of the ILM may be helpful to predict functional outcome. In our study, the analysis of the sub-group that had simultaneous ILM-peeling showed a slower restoration of the retinal anatomy compared to eyes that had ERM-peeling only. In addition, the recovery of BCVA was observed much later (table 1). This difference was not statistically significant, but the trend of slower restoration was obvious. Given the fact that we performed simultaneous peeling of ILM and ERM in very adherent membranes, it is explainable that the mechanical stress and irritation of the whole retina causes a longer retinal recovery compared to eyes without ILM-peeling. We also observed that the improvement and restoration of the retinal layers is not dependent on ILM-removal. Eyes that had ILM- with ERM-peeling showed as good results in retinal and visual outcome as eyes that had ERM-peeling only. We therefore conclude that the normalization of retinal anatomy and BCVA in eyes with simultaneous membrane removal takes longer time to recover.
We found that the retinal layers restored in anatomy of outer and inner retinal layers in 97% of all eyes after surgery. The focus of our study to analyze all retinal layers makes clear that the process of retinal restoration leading to good vision is dependent on the rehabilitation of all retinal layers. The SD-OCT images clearly show that distortion and fibrillation of the nerve fiber layer or the restoration of the foveal contour alone does not explain retinal restoration after ERM-removal. The rehabilitation of the plexiform layers as well as the nuclear layers is also very important to state that the retinal anatomy fully restored after surgery.
Kwon et al. demonstrated that visual acuity and retinal thickness improved until seven months after surgery.22 In contrast, few studies reported faster recovery of BCVA by month 3.7, 14
Our results make clear that the measurements of BCVA and SD-OCT are necessary to be analyzed pre- and post-operatively. The mean time to achieve BCVA after surgery was 5 months whereas the retinal restoration occurred in a mean time of 4.2 months. There was an association between the time of anatomic restoration and occurrence of BCVA. The Pearson analysis found that the time of anatomic restoration correlated significantly with the occurrence of BCVA. However, this observation can vary and BCVA may also occur prior or after retinal restoration. We therefore conclude that retinal function and retinal morphology are both very important factors to determine after ERM-peeling. Besides the measurement of BCVA as a subjective factor, it is necessary to analyze the SD-OCT to get an objective of success after ERM-peeling.
Morphological changes in OCT after ERM-peeling will help to better understand and predict functional outcome. As technical devices will progress in resolution in the future, even smaller morphological changes will be detected and will give new insight in the pathology and recovery of retinal diseases.
Acknowledgments
Supported in part by NIH grant NEI EY 07366 (WRF), EY 016323 (DUB), RPB Inc. and an unrestricted grant to the Jacobs Retina Center.
References
- 1.McCarty DJ, Mukesh BN, Chikani V, et al. Prevalence and associations of epiretinal membranes in the visual impairment project. Am J Ophthalmol. 2005;140:288–294. doi: 10.1016/j.ajo.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 2.Harada C, Mitamura Y, Harada T. The role of cytokines and trophic factors in epiretinal membranes: involvement of signal transduction in glial cells. Prog Retin Eye Res. 2006;25:149–164. doi: 10.1016/j.preteyeres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Snead DR, James S, Snead MP. Pathological changes in the vitreoretinal junction 1: epiretinal membrane formation. Eye. 2008;22:1310–1317. doi: 10.1038/eye.2008.36. [DOI] [PubMed] [Google Scholar]
- 4.Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol. 2002;86:902–909. doi: 10.1136/bjo.86.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald HR, Verre WP, Aaberg TM. Surgical management of idiopathic epiretinal membranes. Ophthalmology. 1986;93:978–983. doi: 10.1016/s0161-6420(86)33635-2. [DOI] [PubMed] [Google Scholar]
- 6.Pesin SR, Olk RJ, Grand MG, et al. Vitrectomy for premacular fibroplasia. Prognostic factors, long-term follow-up, and time course of visual improvement. Ophthalmology. 1991;98:1109–1114. doi: 10.1016/s0161-6420(91)32169-9. [DOI] [PubMed] [Google Scholar]
- 7.Massin P, Allouch C, Haouchine B, et al. Optical coherence tomography of idiopathic macular epiretinal membranes before and after surgery. Am J Ophthalmol. 2000;130:732–739. doi: 10.1016/s0002-9394(00)00574-2. [DOI] [PubMed] [Google Scholar]
- 8.Suh M, Seo J, Park K, Yu H. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol. 2009;147:473–480. doi: 10.1016/j.ajo.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Oster SF, Mojana F, Brar M, et al. Disruption of the Photoreceptor Inner Segment/Outer Segment Layer on Spectral Domain-Optical Coherence Tomography Is a Predictor of Poor Visual Acuity in Patients with Epiretinal Membranes. Retina. 2010;30:713–718. doi: 10.1097/IAE.0b013e3181c596e3. [DOI] [PubMed] [Google Scholar]
- 10.Wojtkowski M, Srinivasan V, Fujimoto JG, et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2005;112:1734–1746. doi: 10.1016/j.ophtha.2005.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt-Erfurth U, Leitgeb RA, Michels S, et al. Three-dimensional ultrahigh-resolution optical coherence tomography of macular diseases. Invest Ophthalmol Vis Sci. 2005;46:3393–3402. doi: 10.1167/iovs.05-0370. [DOI] [PubMed] [Google Scholar]
- 12.Yuson RM, Nigam N, Mojana F, et al. The use of intraoperative indocyanine green dye to assist in epiretinal membrane removal: a novel application of indocyanine green surgical use. Retina. 2009;29:1367–1370. doi: 10.1097/IOP.0b013e3181b80d7a. [DOI] [PubMed] [Google Scholar]
- 13.Michalewski J, Michalewska Z, Cisiecki S, Nawrocki J. Morphologically functional correlations of macular pathology connected with epiretinal membrane formation in spectral optical coherence tomography (SOCT) Graefes Arch Clin Exp Ophthalmol. 2007;245:1623–1631. doi: 10.1007/s00417-007-0579-4. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Rhee KM, Woo SJ, et al. Long-term temporal changes of macular thickness and visual outcome after vitrectomy for idiopathic epiretinal membrane. Am J Ophthalmol. 2010;150:701–709. doi: 10.1016/j.ajo.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Bovey EH, Uffer S, Achache F. Surgery for epimacular membrane: impact of retinal internal limiting membrane removal on functional outcome. Retina. 2004;24:728–735. doi: 10.1097/00006982-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Parisi V, Coppe AM, Gallinaro G, Stirpe M. Assessment of macular function by focal electroretinogram and pattern electroretinogram before and after epimacular membrane surgery. Retina. 2007;27:312–320. doi: 10.1097/01.iae.0000256039.59142.22. [DOI] [PubMed] [Google Scholar]
- 17.Pilli S, Lim P, Zawadzki RJ, et al. Fourier-domain optical coherence tomography of eyes with idiopathic epiretinal membrane: correlation between macular morphology and visual function. Eye. 2011;25:775–783. doi: 10.1038/eye.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitamura H, Hirano K, Baba T, Yamamoto S. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. Br J Ophthalmol. 2009;93:171–175. doi: 10.1136/bjo.2008.146381. [DOI] [PubMed] [Google Scholar]
- 19.Ooto S, Hangai M, Takayama K, et al. High-resolution imaging of the photoreptor layer in epiretinal membrane using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 2011;116:1788–1793. doi: 10.1016/j.ophtha.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe A, Arimoto S, Nishi O. Correlation between metamorphopsia and epiretinal membrane optical coherence tomography findings. Ophthalmology. 2009;116:1788–1793. doi: 10.1016/j.ophtha.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Falkner-Radler CI, Glittenberg C, Binder S. Spectral domain high-definition optical coherence tomography in patients undergoing epiretinal membrane surgery. Ophthalmic Surg Lasers Imaging. 2009;40:270–276. doi: 10.3928/15428877-20090430-08. [DOI] [PubMed] [Google Scholar]
- 22.Kwon SI, Ko SJ, Park IW. The clinical course of the idiopathic epiretinal membrane after surgery. Korean J Ophthalmol. 2009;23:249–252. doi: 10.3341/kjo.2009.23.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]