Abstract
Purpose
Non-AIDS defining cancers (NADCs) now exceed rates of AIDS-defining cancers in HIV-positive patients. Treatment of NADCs may be complicated by drug-drug interactions between antiretrovirals and chemotherapy. Docetaxel is a widely used anticancer agent that is primarily metabolized by CYP3A4 enzymes and used to treat NADCs. A preclinical in vivo assessment was performed to gain a better understanding of CYP3-mediated drug-drug interactions between antiretrovirals and docetaxel, as well as to assess any alterations in gene expression with these combinations.
Methods
Docetaxel (20mg/kg i.v.) was administered to male FVB mice in the presence and absence of dexamethasone (10mg/kg p.o. ×4d), efavirenz (25mg/kg p.o. ×4d), ketoconazole (50mg/kg p.o.), or ritonavir (12.5mg/kg p.o.). At various time points, plasma and liver tissue were harvested. Docetaxel concentrations were determined by LC/MS/MS. Pharmacokinetic parameters were calculated. Liver tissue RNA was used to evaluate alterations in Cyp3a11 and Abcb1a gene expression.
Results
Docetaxel exposure was altered by CYP3A4 inhibitors but not by inducers. The CYP3A4 inducers efavirenz and dexamethasone did not have a significant effect on docetaxel exposure (AUC). However, the CYP3A4 inhibitors ritonavir and ketoconazole resulted in a 6.9 and 3.1-fold increase in AUC, respectively. Alterations in gene expression did not account for the altered docetaxel exposure.
Conclusions
Docetaxel exposure was significantly altered by CYP3A4 inhibitors. Until a definitive clinical trial is performed, docetaxel should be used with caution in patients on a ritonavir-containing antiretroviral regimen or an alternative antineoplastic therapy or antiretroviral regimen should be considered.
Keywords: docetaxel, HIV, antiretrovirals, pharmacokinetics, drug interaction
INTRODUCTION
Since the advent of combination antiretroviral therapy (cART) in the mid-1990’s, widespread use cART has led to significant improvements in the morbidity, mortality, and life expectancy in patients with HIV disease. At the same time, HIV/AIDS patients are now experiencing higher rates of non-AIDS defining malignancies (NADCs) for reasons that remain poorly understood [1-3]. NADCs include cancers without a strong association with HIV such as anal, esophageal, head and neck, lung, prostate cancers, and Hodgkin’s lymphoma. From 1991 to 2005, the incidence and prevalence of NADCs has increased three-fold, and they account for approximately 50% of all cancers among HIV/AIDS patients today [1].
Current recommendations for the initiation of cART consist of a combination of three to four drugs from different classes including ritonavir-boosted protease inhibitors (PIs), nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), or an integrase strand transfer inhibitor [4]. Actual cART regimens utilized in a given patient will differ based on comorbidities, tolerance of drug regimens, duration of AIDS while maintaining virological control (e.g., a patient diagnosed in 1990 vs. 2013), viral mutations, and drug-drug interaction potential [4]. Antiretrovirals are substrates, inducers, and/or inhibitors of various drug metabolizing isoenzymes, including cytochrome P450 (CYP450) and uridine diphosphate-glucuronyl transferases (UGT), as well as drug efflux and uptake transporters [5-7]. While the protease inhibitor ritonavir is widely utilized as a strong CYP3A4 inhibitor, ritonavir also has been noted as a strong inhibitor of CYP2C8, CYP2D6, and ABCB1 in addition to being a weak inducer of CYP2B6, CYP2C9, CYP3A4, and ABCB1 [8-12]. Ritonavir is most commonly utilized as a potent inhibitor of CYP3A4 in ‘boosted’ cART regimens [5,13,14]. Efavirenz, another commonly used antiretroviral agent, has mixed CYP3A4 inhibition and induction but results in clinically relevant interactions by CYP3A4 induction [15-19]. Efavirenz is also a moderate inhibitor of ABCB1 [12,20]. As patients with HIV/AIDS live longer and progress to develop various cancers, the clinical dilemma will continue to be how to treat them given the high likelihood of a clinically relevant drug-drug interaction between cART and anticancer agents until more evidence-based and definitive dosing recommendations are provided.
Docetaxel is a semi-synthetic microtubule stabilizing agent that is indicated for use in a wide variety of tumors including breast, gastric, head and neck, non-small cell lung, and prostate cancers [21-28]. The main dose-limiting toxicities of docetaxel therapy are myelosuppression and neuropathy. Docetaxel is metabolized primarily by CYP3A4 to at least four inactive metabolites and is susceptible to drug-drug interactions [29,30]. Coadministration of docetaxel with drugs that inhibit CYP3A4 may potentially lead to more pronounced toxicities while drugs that induce CYP3A4 may result in decreased efficacy. Docetaxel is also a substrate for the drug transporter ABCB1 (P-glycoprotein), encoded by the MDR1 gene [31]. cART drugs have been shown to be transported by ABCB1 and therefore drug-drug interactions at the level of this drug transporter is also possible [32]. Despite studies demonstrating the drug interaction potential of chemotherapeutic and cART agents independently [33-35], a better understanding of this complex drug-drug interaction is needed.
In this study, we conducted an in vivo assessment of the pharmacokinetics of intravenous docetaxel when coadministered with CYP3A4 inhibitors and inducers in mice. Specifically, we were interested in studying the extent of drug-drug interaction between docetaxel and the CYP3A4 inducers efavirenz or dexamethasone (positive control) and the CYP3A4 inhibitors ritonavir or ketoconazole (positive control). Ketoconazole was selected as the “index” CYP3A4 inhibitor due to minimal inhibitory effects noted on other CYP450s and ABCB1 and wide utilization in in vitro and in vivo studies. Dexamethasone is a potent inducer of CYP3A4 with weak induction effect on other CYP450s and ABCB1 [36,37]. Additionally, we sought to address whether hepatic gene expression of Cyp3a11 (the mouse equivalent for the human CYP3A4), and the drug transporter Abcb1a was altered by the CYP3A4 inducers and inhibitors and whether these alterations contributed to differences in docetaxel exposure among the various CYP3A4 inducers and inhibitors.
METHODS
Chemicals and Reagents
Docetaxel was generously provided by Sanofi-Aventis Pharmaceuticals (Bridgewater, NJ). Drug-free (blank) mouse plasma was obtained from Innovative Research, Inc. (Novi, MI). cART agents were commercially available and of pharmaceutical grade. All other chemicals and reagents were of the highest grade commercially available.
Docetaxel pharmacokinetics in combination with CYP3A4 inducers and inhibitors
Male FVB mice (6 weeks old, Taconic, Germantown, NY) were maintained in a controlled environment with food and sterilized water available ad libitum. Animal experimentation was conducted under an approved Institutional Animal Care and Use Committee (IACUC) protocol in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facility and complied with local and national guidelines. Docetaxel was supplied at a concentration of 40 mg/mL in polysorbate 80 and further diluted in 0.9% sodium chloride to a final concentration of 2 mg/mL. Docetaxel was administered in the presence and absence of dexamethasone, efavirenz, ketoconazole, or ritonavir by tail vein injection at a dose of 20 mg/kg [38]. Dexamethasone and efavirenz were administered by oral gavage daily for 4 days with the last dose occurring approximately 1 hour prior to docetaxel administration to ensure CYP3A4 induction. Dexamethasone was administered at a dose of 10 mg/kg while efavirenz was administered at a dose of 25 mg/kg [36,19]. Ketoconazole and ritonavir were administered by oral gavage approximately 1 hour prior to docetaxel administration to ensure adequate CYP3A4 inhibition. Ketoconazole and ritonavir were administered at a dose of 50 mg/kg and 12.5 mg/kg, respectively [39,40]. Animals in the control arm were administered corn oil by oral gavage. Mice were humanely killed (3 animals/time point), and blood and l tissues were harvested as a function of time after docetaxel administration. Blood was collected by cardiac puncture under anesthesia into syringes coated with heparin and centrifuged to obtain plasma. Liver lobes were rapidly dissected into 4 pieces and snap frozen on dry ice for subsequent RNA extraction (see Gene Expression method section). Samples were stored frozen at −70°C until analysis. A validated LC/MS/MS method was used to quantitate docetaxel in plasma samples over the range of 0.008-0.808 μg/mL, and dilutions up to 1:100 (v:v) were accurately quantitated [41].
Mean docetaxel concentrations were calculated at each time point. Pharmacokinetic parameters were calculated from mean docetaxel concentration-time data using noncompartmental methods as analyzed in WinNonlin version 5.3 (Pharsight Corporation, Mountain View, CA). Cmax was the observed value, which occurred at the first sample drawn after docetaxel administration at 0.08 hr. The AUClast was calculated using the linear trapezoidal method and extrapolated to infinity (AUC0-∞) by dividing the last quantifiable concentration by the terminal disposition rate constant (λz). λz was determined from at least 3 points on the slope of the terminal phase of the concentration-time profile with a weighting factor of 1/y. The terminal half-life (T1/2) was determined by dividing 0.693 by λz. Clearance was calculated by dividing the dose administered by AUC0-∞. If the percent AUC extrapolated was >25% or the r2 of λz was <0.9, the AUC0-∞, Cl, and T1/2 were not reported.
Gene Expression
RNA extraction was performed by crushing a frozen 1 mm section of liver in a disposable mortar and pestle. Extraction was then continued by using RNeasy® Kit (Qiagen, Germantown, MD). Resultant mRNA was then quantified by Optical Density using a Nanodrop Spectrophotometer (ThermoFisher, Wilmington, DE). The RNA was then diluted or concentrated by dilution or vacuum dehydration to obtain a 0.25 μg/μL. Residual genomic DNA contamination was removed using a combination of a RT2 First Strand Kit (Qiagen, Germantown, MD), followed by a Reverse Transcriptase reaction to produce cDNA. This cDNA was combined with RT2 SYBR Green / ROX qPCR Master Mix (Qiagen, Germantown, MD) and added to 96 well Custom Real Time PCR plates (Qiagen, Germantown, MD) preloaded with proprietary primers for Cyp3a11, Abcb1a, a house keeping control gene, one murine genomic contamination detection primer, as well as positive and negative PCR reaction controls. Real time PCR was then performed using a 7900HT ABIPrism sequence detection system (Applied Biosystems, Foster City, CA) using the SYBR green detection probes. Melting curve analysis of the real time PCR product was subsequently performed to evaluate PCR product purity, and the relative changes in mRNA were measured. Analysis of the data obtained from the RT-PCR was done using QIAGEN’s excel-based data analysis template. Data analysis is based on the ΔΔCt method with normalization of the raw data to either housekeeping genes or an external RNA control. Further confirmation was obtained using TaqMan (Applied Biosystems, Foster City, CA) traditional PCR to validate the previous real time PCR expression data.
Statistical Analysis
For the pharmacokinetic studies, the Method of Bailer was used to estimate the variance of AUClast given the calculated variance of the mean concentration at each time point [42]. This was then followed by a pairwise comparison using a Z-test to determine whether there was a significant difference between docetaxel exposure as expressed by AUClast [43]. Comparisons of the gene expression as well as individual Cmax data were conducted using the nonparametric Wilcoxon signed rank test (across groups within a time point for gene expression) with post-hoc analysis using an All Pairs Tukey-Kramer test. The a priori level of significance was p<0.05.
RESULTS
Docetaxel pharmacokinetics in combination with CYP3A4 inducers and inhibitors
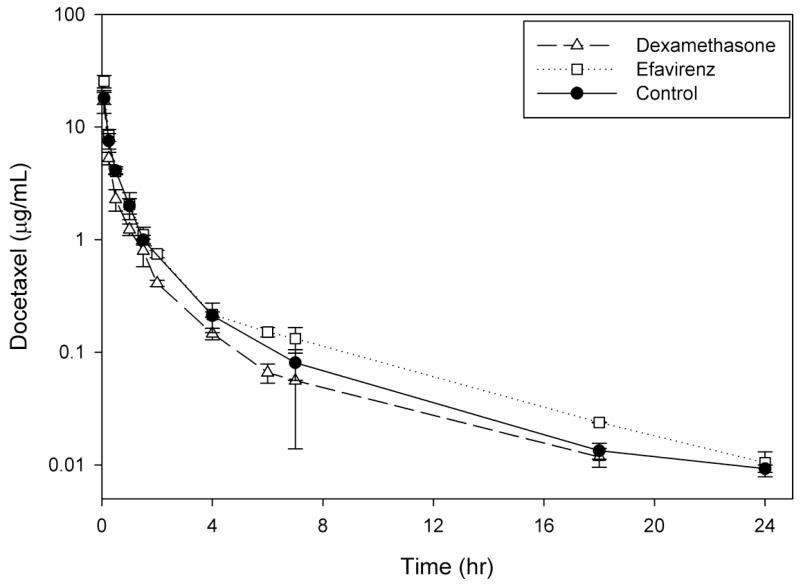
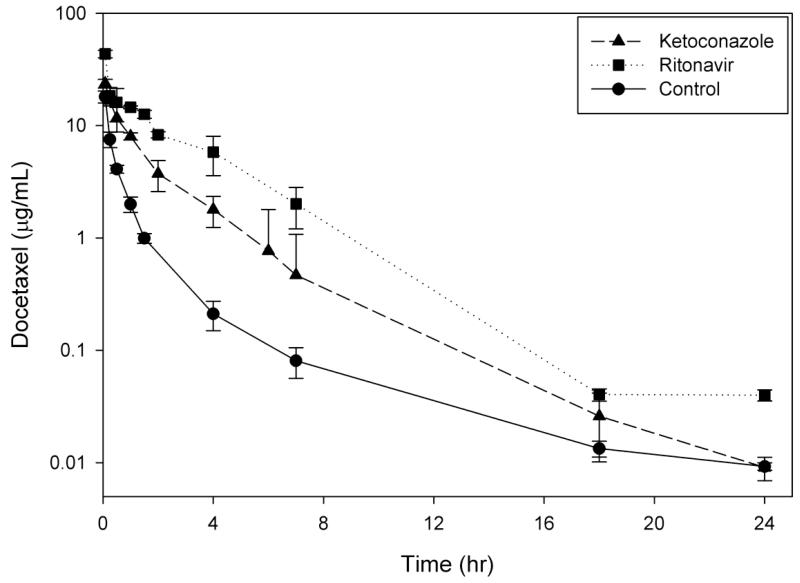
Docetaxel was administered to mice in the presence and absence of CYP3A4 inducers and inhibitors to determine the extent of alterations in the pharmacokinetic profile of docetaxel (Table 1, Fig. 1). When administered alone (control-arm), docetaxel exhibited triphasic elimination with a T1/2,λ of 6.4 hr. Docetaxel concentrations were detectable up to 18 hours for dexamethasone and up to 24 hours for all other conditions thus allowing for accurate comparison of pharmacokinetics.
Table 1.
Plasma Pharmacokinetic Parameters after Intravenous Administration of Docetaxel in Combination with CYP3A4 Inducers & Inhibitors
| Docetaxel | Docetaxel and Dexamethasone |
Docetaxel and Efavirenz |
Docetaxel and Ketoconazole |
Docetaxel and Ritonavir |
|
|---|---|---|---|---|---|
| Cmaxa (μg/mL) | 18.1 ± 2.2 | 17.2 ± 3.9 | 25.5 ± 3.2 | 23.7 ± 2.1 | 43.5 ± 3.4 |
| Fold vs. Control | - | 0.9 | 1.4 | 1.3 | 2.4 |
| C24hra (μg/mL) | 0.009 ± 0.0007 | BLQ | 0.010 ± 0.003 | 0.009 ± 0.002 | 0.040 ± 0.005 |
| Fold vs. Control | - | N.R. | 1.1 | 1.0 | 4.3 |
| AUClast (μg*hr/mL) | 10.3 | 7.6 | 12.4 | 31.4 | 71.0 |
| Fold vs. Control | - | 0.7 | 1.2 | 3.1 | 6.9 |
| AUC0-∞ (μg*hr/mL) | 10.3 | 7.7 | 12.5 | 31.4 | N.R.b |
| Fold vs. Control | - | 0.7 | 1.2 | 3.0 | N.R.b |
| Cl (L/hr/kg) | 1.93 | 2.59 | 1.60 | 0.64 | N.Rb |
| Terminal T1/2 (hr) | 6.4 | 4.9 | 4.7 | 3.5 | N.R.b |
For Cmax and C24hr, individual data is reported (n=3/cohort). For all other pharmacokinetic parameters, the results are from the average concentration-time profile.
Not reported due to >50% extrapolation of the AUC due to poor terminal disposition rate constant (λz).
Abbreviations: AUC0-∞, area under the concentration-time curve extrapolated to infinity; BLQ, below limits of quantitation; Cmax, maximum concentration; %C.V., percent coefficient of variation; Min, minimum; Max, maximum; St. Dev., standard deviation; Terminal T1/2, terminal half-life; Tmax, time to maximum concentration
Fig 1.
Plasma concentration–time curves of docetaxel following administration alone (20 mg/kg, i.v.) or within 1 hr after 4 doses of CYP3A4 inducers (A) or a single dose of CYP3A4 inhibitors (B) to male FVB mice. Dexamethasone (10 mg/kg, p.o.) and efavirenz (25 mg/kg, p.o.) were the CYP3A4 inducers, while ketoconazole (50 mg/kg, i.p.) and ritonavir (12.5 mg/kg, p.o.). Data points and error bars represent the mean and standard deviation of 3 points, respectively.
Administration of docetaxel in the presence of a CYP3A4 inducer did not alter the concentration-time profile with triphasic elimination noted with both dexamethasone and efavirenz (Fig. 1). The CYP3A4 inducers were administered for 4 daily doses prior to the administration of docetaxel. The presence of dexamethasone, our control CYP3A4 inducer, resulted in a 30% decrease (7.6 vs. 10.3 μg*hr/mL; p>0.05) while efavirenz resulted in a 1.2-fold increase (12.4 vs. 10.3 μg*hr/mL; p>0.05) in docetaxel AUClast. While there was no significant alteration in AUClast, the T1/2,λ of docetaxel in the presence of either dexamethasone (4.9 hr) or efavirenz (4.7 hr) did appear to be shorter than with the control (6.4 hr), which is suggestive of induction of CYP3A4. The effect of efavirenz (1.4-fold increase) on the Cmax was more pronounced than dexamethasone (0.9-fold) (p<0.05) but not significantly different than the control arm (p<0.05; post-hoc analysis).
Administration of docetaxel within 1 hour after a CYP3A4 inhibitor resulted in a biphasic elimination in the presence of ketoconazole and mono- to biphasic elimination in the presence of ritonavir (Fig. 1). The presence of ketoconazole, a control CYP3A4 inhibitor, resulted in a 3.1-fold increase (31.4 vs. 10.3 μg*hr/mL; p<0.05) while ritonavir resulted in a 6.9-fold increase (71.0 vs. 10.3 μg*hr/mL; p<0.05) in docetaxel AUClast. This stepwise increase in exposure levels is consistent with our predictions as ritonavir is a more potent inhibitor of CYP3A4. While the T1/2,β of docetaxel in the presence of ritonavir was not calculated due to a poor fit on the terminal slope (a biphasic disposition started to emerge on the last time point), the T1/2,β in the presence of ketoconazole was 3.5 hr. The observed terminal T1/2 was shorter than the control (T1/2,λ = 6.4 hr) which is likely an artifact due to comparing different elimination phases of the concentration-time profile (biphasic vs. triphasic). The effect of ketoconazole (1.3-fold increase) and ritonavir (2.4-fold increase; p<0.05 compared to all other arms) on the Cmax was less pronounced than on AUClast, which is consistent with primary inhibition of the elimination pathway.
Alteration in Cyp3a11 and Abcb1a with CYP3A4 Inducers and Inhibitors
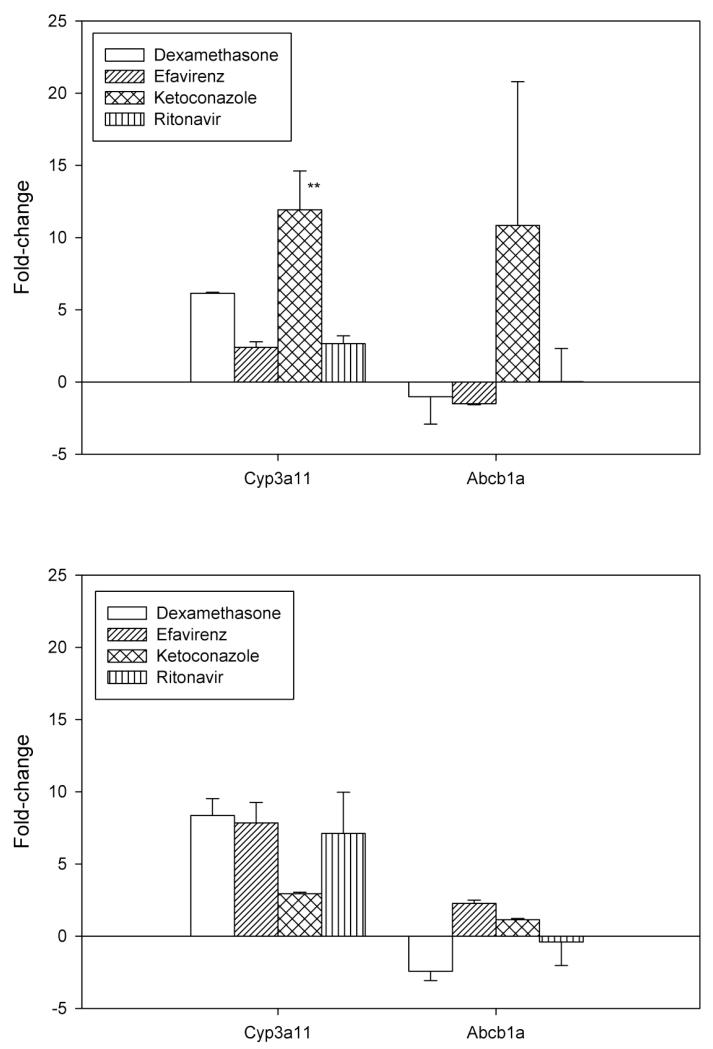
To evaluate possible alterations in Cyp3a11 and Abcb1a gene expression, mouse liver samples were harvested for RNA, and the cDNA was run as described above. At 0.5 hour after docetaxel administration, there was a significant upregulation in Cyp3a11 by ketoconazole compared to all other cohorts (Fig. 2; p=0.02). Otherwise, there were no alterations noted during the time course of the study. There was no association between alterations in gene expression and docetaxel exposure.
Fig 2.
Alteration in Cyp3a11 and Abcb1a gene expression by dexamethasone, efavirenz, ketoconazole, or ritonavir when administered in combination with docetaxel at 0.5 hr (A) and 6 hr (B). The fold-change was normalized to the compared to the control arm. The bars represent the mean, while the standard deviation is noted with the error bars. **, P < 0.05 vs. all groups, Wilcoxon signed rank with post hoc analysis.
DISCUSSION
This study demonstrates that oral administration of the antiretroviral CYP3A4 inhibitor ritonavir significantly altered the systemic exposure of intravenously administered docetaxel but the antiretroviral CYP3A4 inducer efavirenz did not. The magnitude of the interaction with ritonavir (6.9-fold increase in AUClast) was benchmarked against the prototypical CYP3A4 inhibitor ketoconazole (3.1-fold increase in AUClast) demonstrating that ritonavir is a more potent CYP3A4 inhibitor. The alteration of the T1/2 of docetaxel was not determined for ritonavir but the disposition of docetaxel did exhibit a mono- to biphasic pattern in the presence of both CYP3A4 inhibitors. Neither CYP3A4 inducer (efavirenz or dexamethasone) altered the level of exposure but did result in a shorter T1/2, λ of docetaxel, which suggests that CYP3A4 induction was acting on the elimination pathway but not sufficiently to alter total exposure. We found that enzyme inhibition was not due to altered gene expression in vivo, validating what is known to cause such drug-drug interactions.
Our findings differ from reports in the literature in terms of CYP3A4 induction, which is likely due to differences in experimental technique or confounded by other drug metabolism or drug transporter effects. While we chose to use more clinically relevant dosing schedules, we only treated animals for four days with CYP3A4 inducers prior to docetaxel administration. Enzyme induction sufficient to cause altered pharmacokinetics may take longer in the murine model. It may be noted that the slight increase in exposure with coadministration with efavirenz may be due to the mixed inhibition and induction of CYP3A4 or inhibition of ABCB1 noted by efavirenz [12,15,17]. Our results, however, are consistent with CYP3A4 inhibition. While the objective of the studies differed, Bardelmeijer and colleagues have demonstrated that oral coadministration of docetaxel and ritonavir resulted in a 50-fold increase of docetaxel exposure [44]. Therefore, our finding of a 6.9-fold increase in exposure is not surprising. Taken together with our data, ritonavir appears to have a more pronounced effect on docetaxel first-pass metabolism than the elimination.
Based on the results of our study, one would anticipate that standard docetaxel dosing would not be tolerable in patients receiving ritonavir-based cART. As our study suggests a 6.9-fold increase in exposure, one would also anticipate needing a significantly lower dose than what is currently utilized in clinical practice. A case report presented a patient with Kaposi’s sarcoma, an AIDS-defining malignancy, who was receiving ritonavir-based cART and docetaxel 25 mg/m2 experienced febrile neutropenia that did resolve albeit the patient was never re-challenged with docetaxel [45]. In a case report series, three previously discussed patients (all Kaposi’s sarcoma) and three new patients with the diagnoses of Kaposi’s sarcoma (n=4), oropharyngeal carcinoma (n=1), and breast cancer (n=1) were discussed [46]. The three new patients receiving ritonavir-based cART were administered docetaxel at doses ranging from 70 to 100 mg/m2 and experienced severe toxicities including febrile neutropenia (n=3), grade 3 mucositis (n=3), rash (n=2), and hand-foot syndrome (n=2) [46]. The patients were not re-challenged with docetaxel upon resolution of the toxicities. The only other reports of the combination of docetaxel with ritonavir are in an attempt to allow oral administration of docetaxel by circumventing first-pass metabolism. Indeed, ritonavir inhibited CYP3A4 sufficiently to increase the oral bioavailability of docetaxel to ~130-160% of control [47]. No further reports with ritonavir exist but there is further information on ketoconazole which was ~50% less potent of an inhibitor, suggesting that an approximately 3-fold lower dose may be reasonable. Indeed, in a phase I study conducted with the CYP3A4 prototype inhibitor ketoconazole in combination with docetaxel to minimize interpatient pharmacokinetic variability, the recommended dose was substantially lower at a total dose of 70 mg (not normalized to BSA) [48].
Given previous studies demonstrating a correlation between reduced clearance and higher risk for developing febrile and/or grade 4 neutropenia [34] combined with case reports with ritonavir [45,46] and a clinical trial with ketoconazole [48], we would recommend proceeding with caution with docetaxel administration in patients on a ritonavir-based antiretroviral therapy until further clinical studies are performed. If one would opt to use docetaxel, the starting dose should not exceed 70 mg (not normalized to BSA), which is the dose recommended in combination use with ketoconazole [48]. However, given the crucial role that this agent plays in the treatment of NADCs, including non-small cell lung and head and neck cancers, as well as in cancers that patients with HIV will contract at rates similar to the general population, including breast and prostate cancers, further studies of docetaxel in combination with cART are critically needed. Until prospective clinical trials are performed, if a taxane-based chemotherapeutic regimen is needed, choosing an alternative antineoplastic agent or switching the overall antiretroviral regimen may be appropriate. In conclusion, the magnitude of the predicted interaction between docetaxel and ritonavir should provide impetus for and may guide the dose selection in confirmatory phase I dose-finding trials of docetaxel in combination with cART through the Aids Malignancy Consortium (AMC).
Acknowledgments
We would like to thank Dr. Merrill Egorin who aided in the conceptualization and design of this project, Dr. Ming Zhao for his assistance with sample analyses, and Ping He, Rana Rais, and Teresia Wanjiku for their technical support. The project described was supported by the American Cancer Society, NCI contract N02-CM-62212, the Flight Attendants Medical Research Institution (FAMRI) Center for Excellence at the Johns Hopkins University School of Medicine, the Lombardi Comprehensive Cancer Center support grant (NIH grant P30-CA051008) and by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1 RR025005, and the Shared Instrument Grant (1S10RR026824-01)). The project described was supported by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Disclosures
None
Results presented at the 2013 American Society of Clinical Pharmacology and Therapeutics (ASCPT) Annual Meeting.
References
- 1.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, Bhatia K, Uldrick TS, Yarchoan R, Goedert JJ, Engels EA. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753–762. doi: 10.1093/jnci/djr076. doi:10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeken JF, Tjen ALA, Rudek MA, Okuliar C, Young M, Little RF, Dezube BJ. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis. 2012;55(9):1228–1235. doi: 10.1093/cid/cis613. doi:10.1093/cid/cis613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. doi:10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Services DoHaH . Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed March 30, 2013]. Feb 12, 2013. Last updated. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 5.Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol. 2011;12(9):905–912. doi: 10.1016/S1470-2045(11)70056-0. doi:10.1016/S1470-2045(11)70056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minuesa G, Huber-Ruano I, Pastor-Anglada M, Koepsell H, Clotet B, Martinez-Picado J. Drug uptake transporters in antiretroviral therapy. Pharmacol Ther. 2011;132(3):268–279. doi: 10.1016/j.pharmthera.2011.06.007. doi:10.1016/j.pharmthera.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Kis O, Robillard K, Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2010;31(1):22–35. doi: 10.1016/j.tips.2009.10.001. doi:10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277(1):423–431. [PubMed] [Google Scholar]
- 9.Kharasch ED, Mitchell D, Coles R, Blanco R. Rapid clinical induction of hepatic cytochrome P4502B6 activity by ritonavir. Antimicrob Agents Chemother. 2008;52(5):1663–1669. doi: 10.1128/AAC.01600-07. doi:AAC.01600-07 [pii] 10.1128/AAC.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim ML, Min SS, Eron JJ, Bertz RJ, Robinson M, Gaedigk A, Kashuba AD. Coadministration of lopinavir/ritonavir and phenytoin results in two-way drug interaction through cytochrome P-450 induction. J Acquir Immune Defic Syndr. 2004;36(5):1034–1040. doi: 10.1097/00126334-200408150-00006. doi:00126334200408150-00006 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Kageyama M, Namiki H, Fukushima H, Terasaka S, Togawa T, Tanaka A, Ito Y, Shibata N, Takada K. Effect of chronic administration of ritonavir on function of cytochrome P450 3A and P-glycoprotein in rats. Biol Pharm Bull. 2005;28(1):130–137. doi: 10.1248/bpb.28.130. doi:JST.JSTAGE/bpb/28.130 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Storch CH, Theile D, Lindenmaier H, Haefeli WE, Weiss J. Comparison of the inhibitory activity of anti-HIV drugs on P-glycoprotein. Biochem Pharmacol. 2007;73(10):1573–1581. doi: 10.1016/j.bcp.2007.01.027. doi:10.1016/j.bcp.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44(2):190–194. doi: 10.1046/j.1365-2125.1997.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper CL, van Heeswijk RP, Gallicano K, Cameron DW. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin Infect Dis. 2003;36(12):1585–1592. doi: 10.1086/375233. [DOI] [PubMed] [Google Scholar]
- 15.von Moltke LL, Greenblatt DJ, Granda BW, Giancarlo GM, Duan SX, Daily JP, Harmatz JS, Shader RI. Inhibition of human cytochrome P450 isoforms by nonnucleoside reverse transcriptase inhibitors. J Clin Pharmacol. 2001;41(1):85–91. doi: 10.1177/00912700122009728. [DOI] [PubMed] [Google Scholar]
- 16.Gerber JG, Rosenkranz SL, Fichtenbaum CJ, Vega JM, Yang A, Alston BL, Brobst SW, Segal Y, Aberg JA. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of AIDS Clinical Trials Group 5108 Study. J Acquir Immune Defic Syndr. 2005;39(3):307–312. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- 17.Hariparsad N, Nallani SC, Sane RS, Buckley DJ, Buckley AR, Desai PB. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004;44(11):1273–1281. doi: 10.1177/0091270004269142. [DOI] [PubMed] [Google Scholar]
- 18.Mouly S, Lown KS, Kornhauser D, Joseph JL, Fiske WD, Benedek IH, Watkins PB. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72(1):1–9. doi: 10.1067/mcp.2002.124519. doi:10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 19.Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, Wang H. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320(1):72–80. doi: 10.1124/jpet.106.112136. doi:10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burhenne J, Matthee AK, Pasakova I, Roder C, Heinrich T, Haefeli WE, Mikus G, Weiss J. No evidence for induction of ABC transporters in peripheral blood mononuclear cells in humans after 14 days of efavirenz treatment. Antimicrob Agents Chemother. 2010;54(10):4185–4191. doi: 10.1128/AAC.00283-10. doi:10.1128/AAC.00283-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991–4997. doi: 10.1200/JCO.2006.06.8429. doi:10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 22.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ, Murphy BA, Raez LE, Cohen RB, Spaulding M, Tishler RB, Roth B, Viroglio Rdel C, Venkatesan V, Romanov I, Agarwala S, Harter KW, Dugan M, Cmelak A, Markoe AM, Read PW, Steinbrenner L, Colevas AD, Norris CM, Jr., Haddad RI. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. doi:10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 23.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, van den Weyngaert D, Awada A, Cupissol D, Kienzer HR, Rey A, Desaunois I, Bernier J, Lefebvre JL. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–1704. doi: 10.1056/NEJMoa071028. doi:10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 24.Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, Mattson KV, Ramlau R, Szczesna A, Fidias P, Millward M, Belani CP. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21(16):3016–3024. doi: 10.1200/JCO.2003.12.046. doi:10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 25.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L, The TAX 320 Non-Small Cell Lung Cancer Study Group Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18(12):2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 26.Nabholtz JM, Falkson C, Campos D, Szanto J, Martin M, Chan S, Pienkowski T, Zaluski J, Pinter T, Krzakowski M, Vorobiof D, Leonard R, Kennedy I, Azli N, Murawsky M, Riva A, Pouillart P. Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol. 2003;21(6):968–975. doi: 10.1200/JCO.2003.04.040. [DOI] [PubMed] [Google Scholar]
- 27.Sanofi-Aventis . Taxotere Package Insert: Prescribing Information. 2010. [Google Scholar]
- 28.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. doi:10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 29.Marre F, Sanderink GJ, de Sousa G, Gaillard C, Martinet M, Rahmani R. Hepatic biotransformation of docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily in humans. Cancer Res. 1996;56(6):1296–1302. [PubMed] [Google Scholar]
- 30.Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel: recent developments. Clin Pharmacokinet. 2006;45(3):235–252. doi: 10.2165/00003088-200645030-00002. [DOI] [PubMed] [Google Scholar]
- 31.Wils P, Phung-Ba V, Warnery A, Lechardeur D, Raeissi S, Hidalgo IJ, Scherman D. Polarized transport of docetaxel and vinblastine mediated by P-glycoprotein in human intestinal epithelial cell monolayers. Biochem Pharmacol. 1994;48(7):1528–1530. doi: 10.1016/0006-2952(94)90580-0. [DOI] [PubMed] [Google Scholar]
- 32.Lee CG, Gottesman MM, Cardarelli CO, Ramachandra M, Jeang KT, Ambudkar SV, Pastan I, Dey S. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry. 1998;37(11):3594–3601. doi: 10.1021/bi972709x. doi:10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- 33.Engels FK, Ten Tije AJ, Baker SD, Lee CK, Loos WJ, Vulto AG, Verweij J, Sparreboom A. Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther. 2004;75(5):448–454. doi: 10.1016/j.clpt.2004.01.001. doi:10.1016/j.clpt.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16(1):187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 35.Rudek MA, Ambinder RF, Flexner CW, Deeken JF. Systemic therapy for malignancy in patients on anti retroviral medications. UpToDate; Waltham, MA: 2012. UpToDate. [Google Scholar]
- 36.Corcos L. Phenobarbital and dexamethasone induce expression of cytochrome P-450 genes from subfamilies IIB, IIC, and IIIA in mouse liver. Drug metabolism and disposition: the biological fate of chemicals. 1992;20(6):797–801. [PubMed] [Google Scholar]
- 37.Manceau S, Giraud C, Decleves X, Batteux F, Chereau C, Chouzenoux S, Scherrmann JM, Weill B, Perrot JY, Treluyer JM. Expression and induction by dexamethasone of ABC transporters and nuclear receptors in a human T-lymphocyte cell line. J Chemother. 2012;24(1):48–55. doi: 10.1179/1120009X12Z.00000000010. doi:10.1179/1120009X12Z.00000000010. [DOI] [PubMed] [Google Scholar]
- 38.Tampellini M, Filipski E, Liu XH, Lemaigre G, Li XM, Vrignaud P, Francois E, Bissery MC, Levi F. Docetaxel chronopharmacology in mice. Cancer Res. 1998;58(17):3896–3904. [PubMed] [Google Scholar]
- 39.Rudek MA, Zhao M, Smith NF, Robey RW, He P, Hallur G, Khan S, Hidalgo M, Jimeno A, Colevas AD, Messersmith WA, Wolff AC, Baker SD. In vitro and in vivo clinical pharmacology of dimethyl benzoylphenylurea, a novel oral tubulin-interactive agent. Clin Cancer Res. 2005;11(23):8503–8511. doi: 10.1158/1078-0432.CCR-05-1037. doi:10.1158/1078-0432.CCR-05-1037. [DOI] [PubMed] [Google Scholar]
- 40.von Moltke LL, Granda BW, Grassi JM, Perloff MD, Vishnuvardhan D, Greenblatt DJ. Interaction of triazolam and ketoconazole in P-glycoprotein-deficient mice. Drug Metab Dispos. 2004;32(8):800–804. doi: 10.1124/dmd.32.8.800. [DOI] [PubMed] [Google Scholar]
- 41.Baker SD, Zhao M, He P, Carducci MA, Verweij J, Sparreboom A. Simultaneous analysis of docetaxel and the formulation vehicle polysorbate 80 in human plasma by liquid chromatography/tandem mass spectrometry. Anal Biochem. 2004;324(2):276–284. doi: 10.1016/j.ab.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 42.Bailer AJ. Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm. 1988;16(3):303–309. doi: 10.1007/BF01062139. [DOI] [PubMed] [Google Scholar]
- 43.Yuan J. Estimation of variance for AUC in animal studies. J Pharm Sci. 1993;82(7):761–763. doi: 10.1002/jps.2600820718. [DOI] [PubMed] [Google Scholar]
- 44.Bardelmeijer HA, Ouwehand M, Buckle T, Huisman MT, Schellens JH, Beijnen JH, van Tellingen O. Low systemic exposure of oral docetaxel in mice resulting from extensive first-pass metabolism is boosted by ritonavir. Cancer Res. 2002;62(21):6158–6164. [PubMed] [Google Scholar]
- 45.Loulergue P, Mir O, Allali J, Viard JP. Possible pharmacokinetic interaction involving ritonavir and docetaxel in a patient with Kaposi’s sarcoma. Aids. 2008;22(10):1237–1239. doi: 10.1097/QAD.0b013e328300ca98. doi:10.1097/QAD.0b013e328300ca98. [DOI] [PubMed] [Google Scholar]
- 46.Mir O, Dessard-Diana B, Louet AL, Loulergue P, Viard JP, Langlois A, Durdux C, Le Beller C. Severe toxicity related to a pharmacokinetic interaction between docetaxel and ritonavir in HIV-infected patients. Br J Clin Pharmacol. 2010;69(1):99–101. doi: 10.1111/j.1365-2125.2009.03555.x. doi:10.1111/j.13652125.2009.03555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oostendorp RL, Huitema A, Rosing H, Jansen RS, Ter Heine R, Keessen M, Beijnen JH, Schellens JH. Coadministration of ritonavir strongly enhances the apparent oral bioavailability of docetaxel in patients with solid tumors. Clin Cancer Res. 2009;15(12):4228–4233. doi: 10.1158/1078-0432.CCR-08-2944. doi:10.1158/1078-0432.CCR-08-2944. [DOI] [PubMed] [Google Scholar]
- 48.Yong WP, Wang LZ, Tham LS, Wong CI, Lee SC, Soo R, Sukri N, Lee HS, Goh BC. A phase I study of docetaxel with ketoconazole modulation in patients with advanced cancers. Cancer Chemother Pharmacol. 2008;62(2):243–251. doi: 10.1007/s00280-007-0598-1. doi:10.1007/s00280-007-0598-1. [DOI] [PubMed] [Google Scholar]