Abstract
Objective
To determine the association between preoperative B-type natriuretic peptide (BNP) levels and outcome following TCPC.
Background
Surgical palliation of univentricular cardiac defects requires a series of staged operations, ending in a TCPC. Although outcomes have improved, there remains an unpredictable risk of early TCPC takedown. The prediction of adverse postoperative outcomes is imprecise, despite an extensive preoperative evaluation.
Methods
We prospectively enrolled 50 patients undergoing TCPC. We collected preoperative clinical data, preoperative plasma BNP levels, and postoperative outcomes including the incidence of an adverse outcome within one year of surgery (defined as death, TCPC takedown or the need for cardiac transplantation).
Results
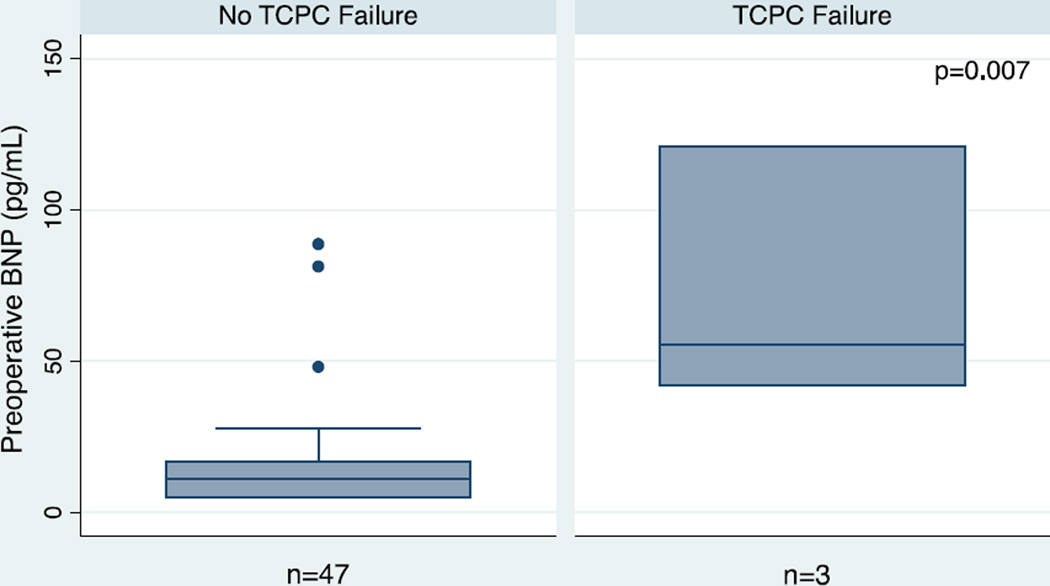
Mean ± SD age was 4.7 ± 2.1 years. Median (IQR) preoperative BNP levels were higher in patients who required TCPC takedown and early postoperative mechanical cardiac support (n=3; 55 (42, 121)) compared with a good outcome (n=47; 11 (5,17)) (p<0.05). In fact, a preoperative BNP ≥ 40 pg/mL was highly associated with the need for TCPC take down (sensitivity = 100%, specificity = 93%; p<0.05), yielding a PPV of 50% and an NPV of 100%. Higher preoperative BNP levels were also associated with longer ICU length of stay, longer hospital length of stay and increased incidence of low cardiac output syndrome (p<0.05).
Conclusions
Preoperative BNP blood levels are uniquely associated with the need for mechanical support early after TCPC and TCPC take down, and thus may provide important information in addition to the standard preoperative assessment.
Introduction
The total cavopulmonary connection (TCPC) is the surgical procedure of choice for suitable patients with a variety of univentricular or non-septable hearts. After the TCPC there is no subpulmonary ventricle between the systemic venous return and the pulmonary circulation. The disadvantages of this circulation include ventricular contractility-afterload mismatch, absence of right ventricular to pulmonary artery coupling and systemic venous return, which is dependent on gravity and respiratory driven forces. This circulation lacks adaptability and often fails in the setting of increased pulmonary vascular resistance (PVR), valvular dysfunction, or decreased ventricular performance [1–5]. Although mortality after TCPC is low with careful patient selection, the accurate prediction of adverse postoperative outcomes is difficult, and there remains an unpredictable risk of early and late TCPC takedown.
B-type natriuretic peptide (BNP) is a 32-amino acid polypeptide hormone secreted by the myocardium in response to various stimuli that has natriuretic, diuretic, and vasoactive properties [6,7]. Recent studies demonstrated that perioperative changes in BNP levels may predict postoperative morbidity and mortality after surgery for the repair or palliation of congenital cardiac defects [8–12]. However, the potential utility of pre-operative BNP levels as predictors of post-operative outcomes in patients undergoing a TCPC has not been investigated previously.
We hypothesized that preoperative BNP levels would be associated with unexpected outcomes after TCPC in patients otherwise deemed to be suitable operative candidates on the basis of standard preoperative clinical, echocardiographic and hemodynamic assessments. Therefore, the objectives of this study were: (1) to determine preoperative BNP levels in children undergoing TCPC, and (2) to investigate the association between preoperative BNP levels and postoperative outcomes.
Methods
Design and subjects
We conducted a prospective cohort study in the pediatric cardiac intensive care unit (PCICU) at the University of California, San Francisco Children’s Hospital between 07/2005 to 07/2006, 04/2008-07/2010, and 06/2011-7/2012 (n= 45) and the PCICU at Lucille Packard Children’s Hospital between 01/2012-06/2012 (n=6). During these time periods, parents of all eligible patients were approached for informed consent and study enrollment. Eligible subjects included all children < 18 years undergoing TCPC. The preoperative anesthesia management, intraoperative surgical strategy, and subsequent PCICU management followed standard institutional practices. In general, a pre-operative decision to utilize a fenestration was based upon the following criteria that would characterize the patient as “higher risk”: depressed ventricular function, modestly increased PVR, pulmonary parenchymal disease and/or lung hypoplasia, and the need to perform additional procedures that would require a prolonged CPB time. A peri-operative decision to perform a fenestration was made for an elevated Fontan pressure and/or poor hemodynamics upon emergence from CPB. The surgical and medical teams involved in the management of the patients were blinded to the BNP values.
Informed consent from the patients’ parents or guardians was obtained before enrollment in the study. The University of California, San Francisco and the Lucille Packard Children’s Hospital review boards approved the study.
Predictor variables
We collected all available preoperative clinical data including: patient demographics and preoperative hemodynamic data obtained at the time of cardiac catheterization, including the ratio of pulmonary blood to systemic blood flow (Qp/Qs), PVR, mean pulmonary artery pressure (mPAP), cardiac index (CI), presence or absence of atrioventricular (A-V) valvular regurgitation, and systemic ventricular end-diastolic pressure (SVEDP), and echocardiographic reports of ventricular function and AV valve regurgitation.
Blood samples were obtained from a venous or arterial catheter < 24 hours preoperatively. The samples were placed immediately on ice in chilled ethylenediamine tetraacetic acid-treated tubes. Whole blood was used immediately to measure BNP levels using a commercially available fluorescence immunoassay (Triage Meter Plus, Biosite Diagnostic, San Diego, CA). The measurable range of BNP on this device is between 5 and 5000 pg/mL. The estimated coefficient of variation for the assay is 9.2% to 11.4%.
Outcome variables
The primary end point was TCPC failure within one year of surgery. TCPC failure was defined as death, or the need for TCPC takedown or cardiac transplantation. Secondary end points were the (1) duration of mechanical ventilation, (2) ICU length of stay (LOS), (3) hospital LOS, and (4) development of low cardiac output syndrome (LCOS) within 48 hours after surgery. The definition of LCOS was derived from criteria published by Hoffman and colleagues which included a combination of changes in clinical signs and biochemical indicators. Specifically, LCOS criteria includes tachycardia, oliguria, poor perfusion, cardiac arrest, or metabolic acidosis, and the need for interventions aimed at augmenting cardiac output, such as increased pharmacologic support relative to the baseline and cardiac pacing [13].
Postoperative clinical and hemodynamic data were collected daily by an observer blinded to the BNP results, included the following: mean systemic arterial pressure, common atrial pressure, and heart rate upon admission to the ICU, postoperative ICU and hospital LOS, time to chest tube removal and chest tube output (CTO), pleural effusions on chest Xray, biochemical evidence of chylothorax, occurrence of LCOS and duration of mechanical ventilation. Postoperative biochemical data collected included hematocrit, arterial and venous blood gases, serum lactate, blood urea nitrogen, and creatinine upon admission to the ICU.
Data collection and management
Study data were collected prospectively and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at UCSF [14]. REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for data downloads to common statistical packages; and 4) procedures for importing data from external sources.
Statistical analysis
We analyzed group differences using the t-test for parametric data and the Wilcoxin rank-sum test for non-parametric data. We used simple and multivariate logistic regression to explore correlations between the continuous predictor variable (BNP) and the binary outcome variables (good/adverse outcome and incidence of LCOS). Next, we fit linear regression models to study correlations between the continuous predictor variable (BNP) and the continuous clinical outcome variables (ICU LOS, hospital LOS and duration of mechanical ventilation). However, considering the general rule of thumb in formal regression methods is to use only one covariate per every ten outcomes, the rarity of the outcome being studied (n = 3) made meaningful interpretation of our exploratory models unreasonable [15].
Statistical analyses were performed using STATA 12, with differences considered significant when two-sided p values were <0.05.
Results
Subjects
We enrolled 50 patients undergoing TCPC. All patients had an extracardiac conduit placed between the inferior vena cava and the pulmonary artery, 26 of which were fenestrated. All patients had a cardiac catheterization performed within 3 months before surgery. The patients’ demographics, cardiac lesions and preoperative hemodynamic data are shown in Table 1.
Table 1.
Preoperative characteristics
| Age (years) | 4.7 ± 2.1 |
| Weight (kilograms) | 16.1 ± 6.0 |
| Male, n (%) | 30 (60) |
| Type of cardiac lesion, n (%) | |
| HLHS | 23 (46) |
| Tricuspid atresia | 7 (14) |
| DORV | 7 (14) |
| Unbalanced AVSD | 6 (12) |
| PA-IVS | 4 (8) |
| DILV | 1 (2) |
| TGA-VSD-PS | 1 (2) |
| Ebstein’s anomaly-HRV | 1 (2) |
| Preoperative hemodynamic data | |
| Heart rate (bpm) | 103 ± 15 |
| Mean arterial pressure (mm Hg) | 77 ± 12 |
| Arterial oxygen saturation (%) | 83 ± 6 |
| Qp:Qs | 0.73 ± 0.26 |
| PVR (Wood’s units) | 2.0 ± 0.9 |
| mPAP (mm Hg) | 11 ± 3 |
| CI (L/min/m2) | 3.5 ± 1.2 |
| SVEDP (mm Hg) | 8 ± 4 |
Qp/Qs: ratio of pulmonary blood flow over systemic blood flow; PVR, pulmonary vascular resistance; mPAP, mean pulmonary artery pressure; CI, cardiac index; SVEDP, systemic ventricular end-diastolic pressure; HLHS, hypoplastic left heart syndrome; DORV, double outlet right ventricle; DILV, double inlet left ventricle; PA-IVS, pulmonary atresia-intact ventricular septum; TGA, transposition of great arteries; VSD, ventricular septal defect; HRV, hypoplastic right ventricle; PS, pulmonary stenosis; AVSD, atrioventricular septal defect. Data are presented as mean ± standard deviation.
Outcomes
Primary end points
Three patients (6%) had an adverse outcome. The preoperative diagnoses, surgical details and specific postoperative events classifying the adverse outcomes are shown in Table 2. All three patients with adverse outcome underwent additional procedures at the time of Fontan (Table 2). Seven (15%) patients with good outcome underwent additional procedures at the time of Fontan. These procedures included: pulmonary artery reconstruction (n=4); repair of right ventricular outflow tract aneurysm (n=1); repair of atrioventricular valve (n=1); and, coronary artery bypass graft with left internal mammary artery to left anterior descending coronary artery (n=1).
Table 2.
Diagnoses and specific postoperative events of patients with an adverse outcome
| Patient | Surgery | Diagnosis | Outcome |
|---|---|---|---|
| 1 | TCPC - extracardiac, fenestrated; and, L pulmonary arteryplasty | HLHS | Fontan circulation taken down after return to the OR on POD 1 because of failed Fontan circulation. Unable to separate from CPB; placed on ECLS. |
| 2 | TCPC - extracardiac, fenestrated; and, R atrial maze, R atrial reduction, oversew of pulmonary valve | Ebstein’s anomaly-HRV | Fontan circulation taken down in the OR because of failed Fontan circulation, unable to separate from CPB; another MBTS was added in the same operation. |
| 3 | TCPC - extracardiac, fenestrated; and, focalization of hepatic veins | Unbalanced AVSD | Fontan circulation taken down 11 mo after operation because of poor hemodynamics requiring prolonged and frequent PCICU hospitalizations and the ultimate development of PLE; also had ECLS during the postoperative course of TCPC because of profound LCOS at PODs 2–5. |
TCPC, total cavopulmonary connection; POD, postoperative day; HLHS, hypoplastic left heart syndrome; HRV, hypoplastic right ventricle; AVSD, atrioventricular septal defect; OR, operating room; CPB, cardiopulmonary bypass; PLE, protein-losing enteropathy; ECLS, extracorporeal life support; LCOS, low cardiac output syndrome.
No enrolled patients died or required cardiac transplantation during the study period. Patients with an adverse postoperative outcome were older and weighed more (Table 3). The presence/absence of AV valve regurgitation and preoperative hemodynamic indices did not differ between patients undergoing TCPC with and without an adverse outcome (Table 3). Use of aortic crossclamping was not significantly associated with preoperative BNP levels. Upon return to the PCICU following surgery, first recorded heart rate, common atrial pressure, serum creatinine and serum lactate were higher in TCPC patients with adverse outcomes while arterial oxygen saturations were lower (Table 3).
Table 3.
Characteristics of patients undergoing total cavopulmonary anastomosis with a good postoperative outcome or an adverse outcome
| Good outcome |
Adverse outcome |
P | |
|---|---|---|---|
| N (%) | 47 (94) | 3 (6) | |
| Age (years) | 4.5 ± 1.9 | 7.2 ± 4.1 | 0.028 |
| Weight (kilograms) | 15.7 ± 5.3 | 23.0 ± 12.2 | 0.039 |
| Male, n (%) | 27 (57) | 3 (100) | 0.151 |
| Preoperative BNP level (pg/mL) | 11 (5,17) | 55 (42, 55, 121) | 0.007 |
| Use of CPB, n (%) | 34 (72) | 3 (100) | 0.299 |
| Duration of CPB (minutes) | 54 (42, 86) | 80 (63, 80, 96) | 0.260 |
| Use of aortic cross-clamp, n (%) | 6 (13) | 2 (67) | 0.013 |
| Duration of aortic cross-clamp (minutes) | 31 (19, 42) | 44 (44, 44) | 0.480 |
| Fenestration, n (%) | 23 (49) | 3 (100) | 0.096 |
| Extracardiac conduit, n (%) | 47 (100) | 3 (100) | |
| Glenn before Fontan, n (%) | 43 (91) | 3 (100) | 0.797 |
| Preoperative hemodynamic and laboratory data | |||
| Mean arterial pressure (mm Hg) | 77 ± 12 | 78 ± 5 | 0.809 |
| Arterial oxygen saturation (%) | 83 ± 6 | 83 ± 4 | 0.839 |
| AV valve regurgitation , n (%) | 19 (40) | 2 (67) | 0.382 |
| Qp:Qs | 0.72 ± 0.26 | 0.80 ± 0.30 | 0.623 |
| PVR (Wood’s units) | 2.0 ± 1.0 | 2.1 ± 0.6 | 0.779 |
| mPAP (mm Hg) | 11 ± 3 | 10 ± 3 | 0.607 |
| CI (L/min/m2) | 3.5 ± 1.2 | 2.8 ± 0.3 | 0.322 |
| SVEDP (mm Hg) | 8 ± 4 | 9 ± 2 | 0.848 |
| Serum creatinine (mg/dL) | 0.42 ± 0.08 | 0.55 ± 0.21 | 0.048 |
| Creatinine clearance | 99 ± 24 | 114 ± 45 | 0.336 |
| Initial postoperative data upon return to PCICU | |||
| Heart rate (bpm) | 119 ± 18 | 152 ± 11 | 0.002 |
| Arterial oxygen saturation (%) | 92 ± 8 | 81 ± 13 | 0.023 |
| Mean arterial pressure (mm Hg) | 75 ± 11 | 70 ± 15 | 0.446 |
| Common atrial pressure (mm Hg) | 7 ± 4 | 15 ± 5 | 0.005 |
| Serum creatinine (mg/dL) | 0.52 ± 0.24 | 1.1 ± 0.32 | 0.001 |
| Creatinine clearance | 83 ± 23 | 51 ± 23 | 0.022 |
| Serum lactate (mmol/L) | 2.8 ± 1.8 | 5.6 ± 2.5 | 0.016 |
| Outcomes | |||
| LCOS, n (%) | 5 (11) | 3 (100) | 0.000 |
| ICU length of stay (days) | 5 (4, 8) | 11 (8, 11, 56) | 0.037 |
| Hospital length of stay (days) | 14 (10, 21) | 36 (14, 36, 64) | 0.079 |
| Duration of mechanical ventilation (hours) | 9 (7, 12) | 7 (7, 7, 24) | 0.850 |
| Duration of chest tubes (days) | 8 (5, 14) | 9 (8, 9, 19) | 0.414 |
BNP, B-type natriuretic peptide; CPB, cardiopulmonary bypass; AV, atrioventricular valve; Qp/Qs, ratio of pulmonary blood flow over systemic blood flow; PVR, pulmonary vascular resistance; mPAP, mean pulmonary artery pressure; CI, cardiac index; SVEDP, systemic ventricular end-diastolic pressure; LCOS, low cardiac output syndrome; ICU, intensive care unit. For the good outcome group (n=47), data are presented as mean ± standard deviation and median (interquartile range). For the adverse outcome group (n=3), data are presented as mean ± standard deviation and median (individual values). Creatinine clearance was estimated using the Shull formula.
Secondary end points
The median (IQR) duration of mechanical ventilation was 8 (7, 12) hours in patients who underwent TCPC. Eight patients (16%) developed LCOS within the first 48 hours after surgery. The median (IQR) ICU LOS and hospital LOS were 5 (4, 9) days and 14 (10, 22) days, respectively. Age, gender and preoperative hemodynamic data obtained from cardiac catheterizations were not associated with any secondary outcomes. Patients with adverse outcomes had a higher incidence of LCOS and longer ICU LOS (Table 3).
Preoperative BNP Levels and Outcomes
Preoperative BNP levels were greater in patients who required TCPC takedown than in patients with a good outcome (Figure 1, Table 3). Preoperative BNP levels were not associated with duration of mechanical ventilation. Preoperative BNP levels correlated with incidence of LCOS and ICU LOS. There was a trend toward longer hospital LOS with higher preoperative BNP levels (p = 0.079). There was no association between preoperative BNP levels and age or gender. Preoperative BNP levels did not correlate with preoperative Qp/Qs, PVR, mPAP, CI, SVEDP, mean arterial pressure, heart rate, arterial oxygen saturation, presence/absence of AV valve regurgitation or postoperative serum lactate.
FIGURE 1. Preoperative BNP levels are higher in patients with adverse outcome in the early postoperative period following TCPC.
Comparisons of preoperative BNP levels between patients with a good outcome (n = 47) and an adverse outcome (n = 3) after TCPC. Horizontal lines represent median values and interquartile ranges are represented with shaded boxes. An adverse outcome is defined as a failed TCPC requiring an intervention within 12 months of the original procedure.
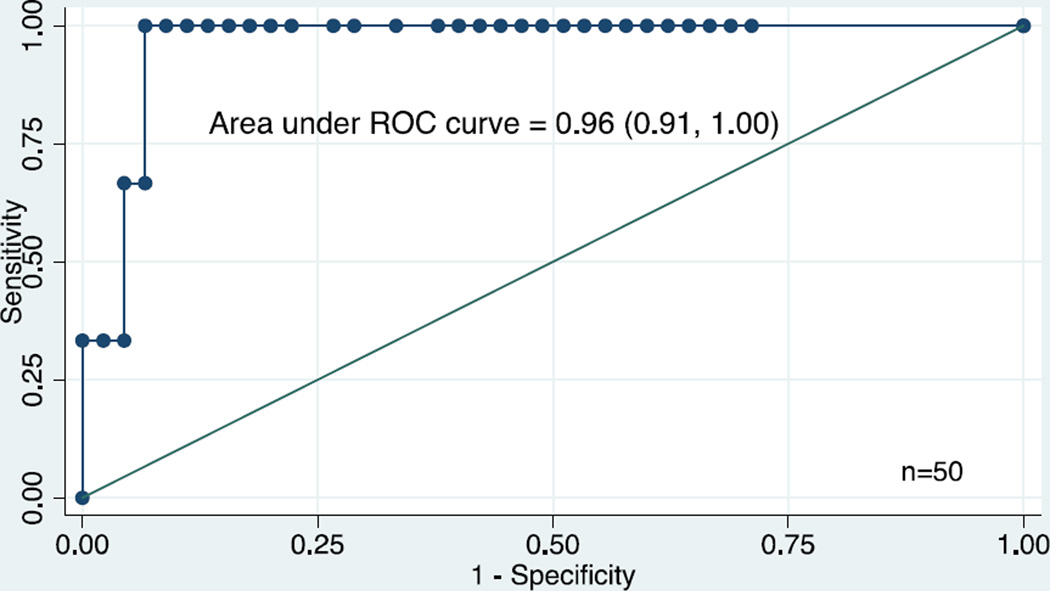
We used receiver operating characteristic curves to evaluate the association between various cutoff values of preoperative BNP and adverse outcome and incidence of LCOS. A preoperative BNP cutoff value of 40 pg/mL was highly associated with adverse outcome, giving a sensitivity of 100% and a specificity of 93% (area under the curve: 0.96, 95% CI 0.91–1.0) (Figure 2). These results yield a positive predictive value (PPV) of 50% and a negative predictive value (NPV) of 100%. Because a preoperative BNP level ≥ 40 pg/mL was strongly associated with adverse outcome, we dichotomized patients by BNP levels of < 40 pg/mL or ≥ 40 pg/mL. The characteristics of patients with a preoperative BNP level < 40 pg/mL and ≥ 40 pg/mL are shown in Table 4. Patients with a preoperative BNP level ≥ 40 pg/mL had a greater incidence of LCOS and a longer ICU and hospital LOS than patients with a preoperative BNP level < 40 pg/mL (Table 4). Among the 47 patients with good outcomes, no differences were identified between the 3 patients with a preoperative BNP ≥ 40 and those 44 with a preoperative BNP < 40 (data not shown).
FIGURE 2. A preoperative BNP level ≥ 40 pg/ml is highly associated with adverse outcome following TCPC (Sensitivity = 100%; Specificity = 93%).
A preoperative BNP cutoff value of 40 pg/mL had a sensitivity of 100% and a specificity of 93% for predicting an adverse outcome (area under the curve: 0.96, 95% CI 0.91–1.0).
Table 4.
Characteristics of patients undergoing total cavopulmonary anastomosis with a preoperative BNP level < or ≥ 40 pg/mL
| Preoperative BNP level < 40 pg/mL |
Preoperative BNP level ≥ 40 pg/mL |
P | |
|---|---|---|---|
| N (%) | 44 (88) | 6 (12) | |
| Age (years) | 4.6 ± 2.0 | 5.0 ± 2.9 | 0.618 |
| Weight (kilograms) | 15.8 ± 5.6 | 18.0 ± 7.9 | 0.333 |
| Male, n (%) | 25 (57) | 5 (83) | 0.086 |
| Use of CPB, n (%) | 31 (70) | 6 (100) | 0.118 |
| Duration of CPB (minutes) | 55 (42, 90) | 63 (52, 96) | 0.423 |
| Use of aortic cross-clamp, n (%) | 6 (14) | 2 (33) | 0.225 |
| Duration of aortic cross-clamp (minutes) | 31 (19, 42) | 44 (44, 44) | 0.480 |
| Fenestration, n (%) | 22 (50) | 4 (67) | 0.486 |
| Extracardiac conduit, n (%) | 44 (100) | 6 (100) | |
| Glenn before Fontan, n (%) | 40 (91) | 6 (100) | 0.707 |
| Preoperative hemodynamic and laboratory data | |||
| Mean arterial pressure (mm Hg) | 77 ± 13 | 76 ± 8 | 0.854 |
| AV valve regurgitation, n (%) | 16 (36) | 5 (83) | 0.982 |
| Arterial oxygen saturation (%)* | 84 ± 6 | 82 ± 6 | 0.502 |
| Qp:Qs | 0.71 ± 0.26 | 0.84 ± 0.27 | 0.191 |
| PVR (Wood’s units) | 2.0 ± 0.9 | 2.1 ± 1.4 | 0.618 |
| mPAP (mm Hg) | 11 ± 3 | 11 ± 2 | 0.812 |
| CI (L/min/m2) | 3.5 ± 1.2 | 3.0 ± 0.4 | 0.281 |
| SVEDP (mm Hg) | 8 ± 4 | 9 ± 1 | 0.556 |
| Serum creatinine (mg/dL) | 0.42 ± 0.08 | 0.47 ± 0.15 | 0.278 |
| Creatinine clearance | 98 ± 24 | 112 ± 31 | 0.231 |
| Initial postoperative data upon return to PCICU | |||
| Heart rate (bpm) | 119 ± 18 | 134 ± 21 | 0.041 |
| Arterial oxygen saturation (%) | 92 ± 8 | 87 ± 11 | 0.133 |
| Mean arterial pressure (mm Hg) | 75 ± 10 | 73 ± 17 | 0.708 |
| Common atrial pressure (mm Hg) | 7 ± 4 | 12 ± 5 | 0.019 |
| Serum creatinine (mg/dL) | 0.50 ± 0.16 | 0.83 ± 0.50 | 0.001 |
| Creatinine clearance | 83 ± 23 | 69 ± 27 | 0.202 |
| Serum lactate (mmol/L) | 2.8 ± 1.9 | 3.9 ± 2.0 | 0.126 |
| Outcomes | |||
| Adverse outcome, n (%) | 0 | 3 (50) | 0.000 |
| LCOS, n (%) | 2 (5) | 6 (100) | 0.000 |
| ICU length of stay (days) | 5 (4, 8) | 8 (6, 18) | 0.020 |
| Hospital length of stay (days) | 12 (10, 19) | 22 (15, 34) | 0.020 |
| Duration of mechanical ventilation (hours) | 9 (7, 12) | 7 (7, 17) | 0.286 |
| Duration of chest tubes (days) | 8 (5, 13) | 10 (7, 17) | 0.392 |
BNP, B-type natriuretic peptide; CPB, cardiopulmonary bypass; AV, atrioventricular valve; Qp/Qs, ratio of pulmonary blood flow over systemic blood flow; PVR, pulmonary vascular resistance; mPAP, mean pulmonary artery pressure; CI, cardiac index; SVEDP, systemic ventricular end-diastolic pressure; LCOS, low cardiac output syndrome; ICU, intensive care unit. Data are presented as mean ± standard deviation and median (interquartile range). Creatinine clearance was estimated using the Shull formula.
Post-operative arterial saturations were significantly higher among the non-fenestrated Fontans compared to those with fenestrations: 94 ± 8 vs. 88 ± 8, p = 0.016.
One patient enrolled was excluded from the analysis after an intraoperative decision was made to not proceed with the TCPC, because of the concern for severe AV valve regurgitation that was underestimated during the pre-operative evaluation. Interestingly, this patient had a pre-operative BNP level of 120 pg/ml.
Discussion
Outcomes following TCPC have improved markedly since 1971, when the Fontan procedure was first described [16]. However, the incidence of early mortality after TCPC remains between 3–8%. Likewise, long-term mortality is estimated to be as high as 10, 14, and 18% at 5, 10 and 15 years, respectively [2,3,17–19]. Contemporary risk factors for early and late adverse outcomes after Fontan type operations include impaired preoperative ventricular function, increased pulmonary artery pressures, and a common AV valve [3,18,19]. In a pilot study, we found that preoperative BNP levels were higher in patients with adverse outcome following TCPC than in patients with good outcomes, and that standard preoperative data were not significantly different between these two groups [9]. Therefore, in the current study we expanded our initial study to test the hypothesis that preoperative BNP blood levels are associated with early adverse outcome following TCPC. We found that a BNP level >40 pg/ml was highly associated with the need for early postoperative ECLS and/or TCPC takedown. Importantly, we have shown that, in low risk patients selected to undergo TCPC based on favorable preoperative hemodynamic assessments, BNP levels better discriminate outcome post TCPC than preoperative cardiac catheterization measurements. This is a meaningful finding since the exact preoperative hemodynamics that predict TCPC takedown remain elusive, and suggest that measurement of BNP blood levels may be important in the preoperative evaluation [20].
BNP is a hormone released by the myocardium that has natriuretic, diuretic and vasoactive properties [6,7]. Produced predominantly in ventricular tissue, BNP has emerged as a powerful biomarker of impaired myocardial performance, and has become an established aid in the diagnosis, prognosis, and treatment of a variety of cardiovascular disease states [8,10–12]. Its use in congenital heart disease is emerging, and recent studies demonstrate that perioperative BNP changes may predict outcomes in an age and lesion-specific fashion, including partial cavopulmonary connections for single ventricular heart disease [9]. However, previous studies in congenital heart disease did not demonstrate associations between outcomes and preoperative levels, only perioperative changes [8–12,21]. In our previous study examining perioperative BNP levels in patients undergoing partial cavopulmonary connections and TCPC, we found that preoperative BNP levels were higher in 2 patients with poor outcome following TCPC compared with 9 patients with good outcome after TCPC. With a five-fold higher sample size, the present study confirms this preliminary finding and to our knowledge is the first to robustly demonstrate the association between a single preoperative BNP value and postoperative outcome in patients undergoing surgery for congenital heart disease.
Although our findings demonstrate the association between an increased preoperative BNP level and poor postoperative outcome in patients undergoing TCPC, the potential mechanisms, particularly at a relatively low cut-off value (40 mg/dl), are unclear. The regulation of the production and release of BNP is not entirely understood, as it depends on multiple, often opposing, factors [10,11,22,23]. Factors known to increase BNP expression and release include increased ventricular wall stress (pressure and volume), hypoxia, increased central blood volume, and neuroendocrine mechanisms [7]. Interestingly, in patients with single ventricular physiology, Law et al demonstrated an elevation in BNP levels in patients with ventricular failure, independent of cavopulmonary failure [24]. We speculate that in the preoperative TCPC setting, BNP values may reflect important clinical parameters in ventricular performance not captured by our current routine evaluations. This requires further investigation.
Potential alterations in BNP expression, release, and clearance in the setting of abnormal cardiac development are unknown. Our results demonstrate that a relatively low preoperative BNP cutoff level (> 40 pg/ml) is associated with adverse outcome after TCPC. This is significantly lower than the cutoff levels first established for the diagnosis of congestive heart failure in adults [25,26], but is concordant with two previously published studies which showed that a threshold value of >30–45 pg/ml showed both sensitivity and specificity for predicting heart failure in children with a single cardiac ventricle. Importantly, this cutoff level is also greater than published values in similarly aged normal children (5.1 to 12.1 pg/ml) [27,28]. Further, our relatively low BNP cutoff level fits with the results of the Pediatric Heart Network Fontan cross-sectional study that demonstrated normal BNP levels in the majority of ambulatory Fontan patients (median time from Fontan 8.2 years), but higher BNP levels in patients with pre-Fontan systolic dysfunction and in patients with late post-Fontan complications[21].
There are several limitations to this investigation that warrant discussion. Most notable is the relatively small sample size, which was associated with a heterogenous group of anatomic lesions and the use of both fenestrated and non-fenestrated surgical techniques. The small sample size makes it difficult to conclude that BNP is a true predictor of adverse outcome following TCPC. However, there is a clear association between increased preoperative BNP levels and adverse postoperative outcome in this unique population. Further, this precludes our ability to demonstrate potential interactions between preoperative BNP levels and outcomes in subjects with specific cardiac lesions after the TCPC. In addition, all three patients experiencing adverse outcome underwent additional procedures at the time of the Fontan compared to 15% of the good outcome group. While there were no significant differences in duration of cardiopulmonary bypass time or aortic crossclamp time as a result, it is challenging to determine if these additional procedures played a role in the observed outcomes. Lastly, our study was undertaken only in children considered eligible for a TCPC. Since we have no data on higher risk patients (i.e. those excluded from TCPC following standard assessment), the associations demonstrated between preoperative BNP and outcomes are only applicable to Fontan patients with favorable preoperative hemodynamics.
We are confident that, due to the robust nature of the data, the results are not due to type I error. However, a small possibility of this always exists in pilot studies with small sample sizes such as this. In addition, the enrolled patients were from two high-volume pediatric cardiothoracic surgery centers, with the obligate subtle differences in surgical approach/technique and postoperative management. Importantly, the strength of the association between postoperative outcome and a BNP cutoff level >40 pg/ml was sustained despite a small sample size from two centers, suggesting it is a robust measurement.
In conclusion, we found that preoperative BNP levels are strongly associated with the need for ECLS and TCPC takedown in the early postoperative period after TCPC. Specifically, we found that a value of >40 pg/ml was a highly sensitive and specific in its’ association with TCPC take down within 12 months of surgery, the need for ECLS and prolonged ICU and hospital LOS. Large multi-centered studies are needed to definitively establish the predictive value of BNP blood levels in the management of patients following TCPC and to overcome the limitations of the present study. However, our current results suggest that preoperative BNP levels have potential to uniquely predict early outcome after TCPC, and thus, after validation in a larger TCPC population, should be considered for inclusion in the standard assessment of candidates for a Fontan type operation.
ACKNOWLEDGEMENTS
The authors would like to thank the pediatric critical care fellows, the cardiac intensive care nurses, Christine Sun, Martina Steurer-Muller, Cynthia Harmon, Ben Ezeokoli and Mathew Bell for their invaluable assistance with the study.
FUNDING SOURCES
This research was supported in part by grants K08 HL086513 (to PEO), U01 HL101798 (to RLK), and HL61284 (to JRF) from the National Institutes of Health, and UL RR024131-01 from the National Center for Research Resources (NCRR). The authors have no relationships to industry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Scheurer MA, Hill EG, Vasuki N, Maurer S, Graham EM, Bandisode V, et al. Survival after bidirectional cavopulmonary anastomosis: analysis of preoperative risk factors. The Journal of Thoracic and Cardiovascular Surgery. 2007 Jul;134:82–9–89.e1–82–9–89.e2. doi: 10.1016/j.jtcvs.2007.02.017. [Internet]. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17599490&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 2.Gentles TL, Mayer JE, Gauvreau K, Newburger JW, Lock JE, Kupferschmid JP, et al. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. The Journal of Thoracic and Cardiovascular Surgery. 1997 Sep 1;114:376–391. doi: 10.1016/s0022-5223(97)70183-1. [DOI] [PubMed] [Google Scholar]
- 3.Hosein RBM, Clarke AJB, McGuirk SP, Griselli M, Stumper O, De Giovanni JV, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The“Two Commandments?”✩. European Journal of Cardio-Thoracic Surgery. 2007 Mar;31:344–353. doi: 10.1016/j.ejcts.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 4.Alejos JC, Williams RG, Jarmakani JM, Galindo AJ, Isabel-Jones JB, Drinkwater D, et al. Factors influencing survival in patients undergoing the bidirectional Glenn anastomosis. AJC. 1995 May 15;75:1048–1050. doi: 10.1016/s0002-9149(99)80722-x. [Internet]. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=7747687&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 5.de Leval MR. The Fontan circulation: a challenge to William Harvey? Nature clinical practice. Cardiovascular medicine. 2005 Apr;2:202–208. doi: 10.1038/ncpcardio0157. [Internet]. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=16265484&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 6.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988 Mar 3;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 7.Epstein FH, Levin ER, Gardner DG, Samson WK. Natriuretic Peptides. N. Engl. J. Med. 1998 Jul 30;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 8.Hsu J-H, Keller RL, Chikovani O, Cheng H, Hollander SA, Karl TR, et al. B-type natriuretic peptide levels predict outcome after neonatal cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery. 2007 Oct;134:939–945. doi: 10.1016/j.jtcvs.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Hsu J-H, Oishi PE, Keller RL, Chikovani O, Karl TR, Azakie A, et al. Perioperative B-type natriuretic peptide levels predict outcome after bidirectional cavopulmonary anastomosis and total cavopulmonary connection. The Journal of Thoracic and Cardiovascular Surgery. 2008 Apr;135:746–753. doi: 10.1016/j.jtcvs.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Cannesson M, Bionda C, Gostoli B, Raisky O, di Filippo S, Bompard D, et al. Time Course and Prognostic Value of Plasma B-type Natriuretic Peptide Concentration in Neonates Undergoing the Arterial Switch Operation. Anesthesia & Analgesia. 2007 May;104:1059–1065. doi: 10.1213/01.ane.0000263644.98314.e2. [DOI] [PubMed] [Google Scholar]
- 11.Shih C-Y, Sapru A, Oishi P, Azakie A, Karl TR, Harmon C, et al. Alterations in plasma B-type natriuretic peptide levels after repair of congenital heart defects: A potential perioperative marker. The Journal of Thoracic and Cardiovascular Surgery. 2006 Mar;131:632–638. doi: 10.1016/j.jtcvs.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 12.Chikovani O, Hsu J-H, Keller R, Karl TR, Azakie A, Adatia I, et al. B-type natriuretic peptide levels predict outcomes for children on extracorporeal life support after cardiac surgery. The Journal of Thoracic and Cardiovascular Surgery. 2007 Nov;134:1179–1187. doi: 10.1016/j.jtcvs.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman TM. Efficacy and Safety of Milrinone in Preventing Low Cardiac Output Syndrome in Infants and Children After Corrective Surgery for Congenital Heart Disease. Circulation. 2003 Feb 10;107:996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009 Apr;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics. 2012 [Google Scholar]
- 16.Fontan F, Baudet E. Surgical repair of tricuspid atresia. 1971;26:240–248. doi: 10.1136/thx.26.3.240. Available from: http://thorax.bmj.com/content/26/3/240.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch JC, Goldberg C, Bove EL, Salehian S, Lee T, Ohye RG, et al. Fontan Operation in the Current Era. Transactions of the … Meeting of the American Surgical Association. 2008;126:52–60. [Google Scholar]
- 18.Gaynor JW, Bridges ND, Cohen MI, Mahle WT, Decampli WM, Steven JM, et al. Predictors of outcome after the Fontan operation: is hypoplastic left heart syndrome still a risk factor? The Journal of Thoracic and Cardiovascular Surgery. 2002 Feb 1;123:237–245. doi: 10.1067/mtc.2002.119337. [DOI] [PubMed] [Google Scholar]
- 19.Rogers LS, Glatz AC, Ravishankar C, Spray TL, Nicolson SC, Rychik J, et al. 18 Years of the Fontan Operation at a Single Institution. Journal of the American College of Cardiology. 2012 Sep;60:1018–1025. doi: 10.1016/j.jacc.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Giglia TM, Humpl T. Preoperative pulmonary hemodynamics and assessment of operability: Is there a pulmonary vascular resistance that precludes cardiac operation? Pediatric Critical Care Medicine. 2010 Mar;11:S57–S69. doi: 10.1097/PCC.0b013e3181d10cce. [DOI] [PubMed] [Google Scholar]
- 21.Atz AM, Zak V, Breitbart RE, Colan SD, Pasquali SK, Hsu DT, et al. Factors associated with serum brain natriuretic peptide levels after the Fontan procedure. Congenit Heart Dis. 2011 Jul;6:313–321. doi: 10.1111/j.1747-0803.2011.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clerico A, Giannoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev. 2011 Apr 27;17:81–96. doi: 10.1007/s10741-011-9249-z. [DOI] [PubMed] [Google Scholar]
- 23.Costello JM, Backer CL, Checchia PA, Mavroudis C, Seipelt RG, Goodman DM. Effect of cardiopulmonary bypass and surgical intervention on the natriuretic hormone system in children. The Journal of Thoracic and Cardiovascular Surgery. 2005 Sep;130:822–829. doi: 10.1016/j.jtcvs.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Law YM, Ettedgui J, Beerman L, Maisel A, Tofovic S. Comparison of Plasma B-Type Natriuretic Peptide Levels in Single Ventricle Patients With Systemic Ventricle Heart Failure Versus Isolated Cavopulmonary Failure. The American Journal of Cardiology. 2006 Aug;98:520–524. doi: 10.1016/j.amjcard.2006.02.058. [DOI] [PubMed] [Google Scholar]
- 25.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002 Jul 18;347:161–167. doi: 10.1056/NEJMoa020233. [Internet]. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=12124404&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 26.Dao Q, Krishnaswamy P, Kazanegra R, Harrison A, Amirnovin R, Lenert L, et al. Utility of B-type natriuretic peptide in the diagnosis of congestive heart failure in an urgent-care setting. Journal of the American College of Cardiology. 2001 Feb;37:379–385. doi: 10.1016/s0735-1097(00)01156-6. [Internet]. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11216950&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 27.Lowenthal A, Camacho BV, Lowenthal S, Natal-Hernandez L, Liszewski W, Hills NK, et al. Usefulness of B-Type Natriuretic Peptide and N-Terminal Pro-B-Type Natriuretic Peptide as Biomarkers for Heart Failure in Young Children With Single Ventricle Congenital Heart Disease. The American Journal of Cardiology. 2012 Mar;109:866–872. doi: 10.1016/j.amjcard.2011.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KOCH A. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003 Aug 1;89:875–878. doi: 10.1136/heart.89.8.875. [Internet]. Available from: http://heart.bmj.com/cgi/doi/10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]