Abstract
Background
Cognitive profiles for pre-clinical Alzheimer's disease (AD) can be used to identify groups of individuals at risk for disease and better characterize pre-clinical disease. Profiles or patterns of performance as pre-clinical phenotypes may be more useful than individual test scores or measures of global decline.
Objective(s)
The aim of this work is to evaluate patterns of cognitive performance in cognitively normal individuals to derive latent profiles associated with later onset of disease using a combination of factor analysis and latent profile analysis.
Methods
The National Alzheimer's Coordinating Centers collect data, including a battery of neuropsychological tests, from participants at 29 NIA funded Alzheimer's Disease Centers across the United States. Prior factor analyses of this battery demonstrated a four-factor structure comprising memory, attention, language, and executive function. Factor scores from these analyses were used in a latent profile approach to characterize cognition among a group of cognitively normal participants (n=3,911). Associations between latent profiles and disease outcomes an average of 3 years later were evaluated with multinomial regression models. Similar analyses were used to determine predictors of profile membership.
Results
Four groups were identified; each with distinct characteristics and significantly associated with later disease outcomes. Two groups were significantly associated with development of cognitive impairment. In post-hoc analyses, both the Trail Making Test Part B, and a contrast score (Delayed Recall - Trails B), significantly predicted group membership and later cognitive impairment.
Conclusions
Latent profile analysis is a useful method to evaluate patterns of cognition in large samples for the identification of preclinical AD phenotypes; however comparable results can be achieved with very sensitive tests and contrast scores.
Keywords: latent profile analysis, factor analysis, neuropsychological test, longitudinal study
1. Objectives
Alzheimer's disease (AD) is neurodegenerative disease that has a gradual onset and progressive course. Without an effective prevention or cure, the prevalence of AD is expected to increase dramatically in the coming years(1). The challenge of identifying individuals who are asymptomatic, yet at increased risk for developing AD has been identified as a critical, rate-limiting factor that is delaying the development and validation of preventive therapies for mild cognitive impairment (MCI) and AD(2). Preventive interventions must take place long before the disease is clinically apparent because the pathology accumulates over many years. For this reason, the focus of research has now shifted to very early detection and characterization of pre-clinical disease (in cognitively normal individuals) in order to facilitate prevention trials. The hope is that new treatments may be effective if applied early, before the level of pathology is too great to overcome.
AD is fundamentally a disease of clinical presentation; therefore, neurocognitive testing is an important tool in the early identification of individuals at risk for MCI and AD. In the absence of an adequate blood test for AD biomarkers, neurocognitive testing with brief, targeted cognitive batteries may be one of the best ways to identify appropriate subjects for research studies and clinical trials because it is less invasive and potentially less expensive to measure than other biomarkers such as amyloid imaging. Furthermore, studies have shown that neuropsychological testing does a better job of predicting conversion to AD than other biomarkers (3, 4). Batteries of neurocognitive tests are designed to measure function in various cognitive domains, nine of which were specifically identified by the original NINCDS-ADRDA criteria as being important in AD(5). To date, there have been few studies of the overall latent patterns of cognitive performance in normal individuals across different cognitive domains (see Mavandadi, et al. for an example in Parkinson's disease)(6). Patterns of performance have been studied by examining multiple cognitive measures concurrently and declines on memory measures and executive function are generally accepted early harbingers of decline to MCI due to AD (7, 8). Contrasts or differences between tests that measure various cognitive domains have also been found to be predictive (9). Contrasts can demonstrate asymmetric cognitive function and have been associated with differences in cortical thickness (10). Given the evidence suggesting that cognitive changes take place years before diagnosis, we sought to identify subtypes of cognitively normal individuals with different patterns of cognition, which may suggest later AD or dementia onset. Using data from the National Alzheimer's Disease Coordinating Center (NACC), we tested the hypotheses that distinct latent profiles can be identified and distinguished from each other, and that group membership will predict later onset of mild cognitive impairment (MCI), AD or dementia.
2. Methods
2.1 Participants & Setting
The NACC is charged with the aggregation of standardized data collected from 29 Alzheimer's Disease Centers (ADCs) across the United States (11). Recruitment methods vary from center to center and participants are generally drawn from the surrounding community. Approximately, 36% of participants were referred by friend or relative, 21% by clinician or clinic sample, 14% by ADC solicitation, 4% by non-ADC media appeal, and the remaining 25% from other sources (12). All protocols are approved by local Institutional Review Boards. Evaluations may take place in a clinical facility or in research participants’ homes.
Basic demographic information, medical history, medication history, and family history of dementia are collected from participants in addition to behavioral and functional assessments. The Uniform Data Set (UDS) Neuropsychological battery (12) is administered at each annual visit by trained psychometricians either in the clinic or in the home. Clinicians review participant's records and assign a global Clinical Dementia Rating (CDR) score. CDR scores were assigned and diagnoses were made according to standard criteria (13-15). See Morris et al., 2006 for a description of clinical and cognitive variables collected for the NACC (15).
2.2 The UDS Neuropsychological Battery
The neuropsychological battery administered as part of the Uniform Dataset (UDS) has 9 neuropsychological tests that are used to characterize normal aging, mild impairments in cognition, and dementia. The battery was designed to be brief and is administered in a fixed fashion (standard administration and order of tests) according to test administration and scoring procedures developed by NACC (12). The major cognitive domains covered by the battery are: attention (Digits Forward(16) and Digits Backward(16)); processing speed (WAIS Digit Symbol(17) and Trail Making Test Part A(18)); executive function (Trail Making Test Part B(18)); memory(Logical Memory Story A, Immediate and Delayed recall); and language (Boston Naming Test (30 item))(19, 20) and semantic fluency (Animals,(21) and Vegetables). Global cognition was measured with the Mini Mental State Examination (MMSE),(22) and the CDR(23, 24) was used as an indicator of dementia severity. The factor structure of the battery was evaluated and four cognitive domains were identified: memory, attention, language, and executive function (25).
2.3 Clinical Dementia Rating and Diagnoses
The CDR (24, 26) was administered to each participant and their collateral informant as part of the diagnostic process. This rating takes decline from a prior functional level into consideration and rates participants on six cognitive domains. The domains measured are memory, orientation, judgment, problem solving, community affairs, home and hobbies, and personal care. Participants were divided into three groups based on global CDR score for these analyses. Individuals with CDR=0.0 were considered cognitively normal; those with CDR=0.5 were defined as having MCI; and those with CDR>0.5 were defined as having dementia. The CDR was chosen to classify participants because the neuropsychological battery results are not specifically used to determine global CDR scores, although clinicians are not blinded to them.
Diagnoses in NACC are based on the neuropsychological and clinical data collected during annual evaluations (see Morris 2006 for a detailed description) (15). The NACC protocol was developed under the guiding principal that dementia and MCI are clinical diagnoses and therefore, the protocol was designed to include all the pertinent elements needed to make clinical decisions. Criteria for MCI are based on Petersen 1999 (27) and modified to allow impairment in other domains (13).
2.4 Statistical methods
There are several steps to these analyses. First, normalizing transformations were applied to the neuropsychological test data (28). Exploratory factor analysis (EFA) and confirmatory factor analyses (CFA)(29) were then performed to identify the factor structure. Factor analysis is a method of data reduction that can be used to evaluate data structure and construct validity. It explains covariation among a set of observed variables and reduces the number of variables to their underlying constructs or factors (30). In this study, EFA was used to empirically define the structure. CFA was used to apply the empirical model, test for invariance across groups, and derive factor scores. Factor scores from the CFA were then used in latent profile analysis (LPA). LPA is a technique for studying latent or unobserved variables that is similar to latent class analysis (31). Latent class analysis is an analytic method used for examining heterogeneity in a sample by grouping together individuals with similar response patterns (32). Both are forms of latent mixture models, however, LPA accounts for the distribution of cases based on observed, continuous variables(33, 34) which are assumed to be independent and normally distributed. LPA allows for variables measured on different scales and different levels of model complexity. Another advantage of this method is that there are a number of rigorous ways to determine the best model fit (35). Factor analyses and LPA were conducted using Mplus statistical software (36).
To validate our methods, we tested longitudinal associations of the latent profiles with diagnostic outcomes for each latent group. Multinomial logistic regression was used to evaluate the association between latent profiles and diagnostic outcomes an average of 3 years later. Multinomial logistic regression was also used to evaluate predictors of group membership. Regression modeling and demographic characteristics were calculated using SAS, version 9.3 (SAS Institute, Cary, NC).
2.5 Evaluation of Model Fit
Standard methods for assessing model fit were used. CFA model fit statistics included: the Comparative Fit Index (CFI)(37); the Tucker-Lewis Index (TLI)(38); the Root Mean Square Error of Approximation (RMSEA)(39); and the Standardized Root Mean Square Residual (SRMR),(40) Satorra-Bentler Scaled χ2 test(41) and the Bayesian Information Criterion (BIC).(42) Latent profile models were evaluated with the following methods: Vuong-Lo-Mendell-Rubin likelihood ratio test, (43, 44) the −2LL χ2(for nested models), sample size adjusted BIC (SABIC), examination of the posterior probability classification table, entropy (45, 46), profile size, and face validity (for review of LPA, see Pastor 2007)(35).
3. Results
3.1 Participants
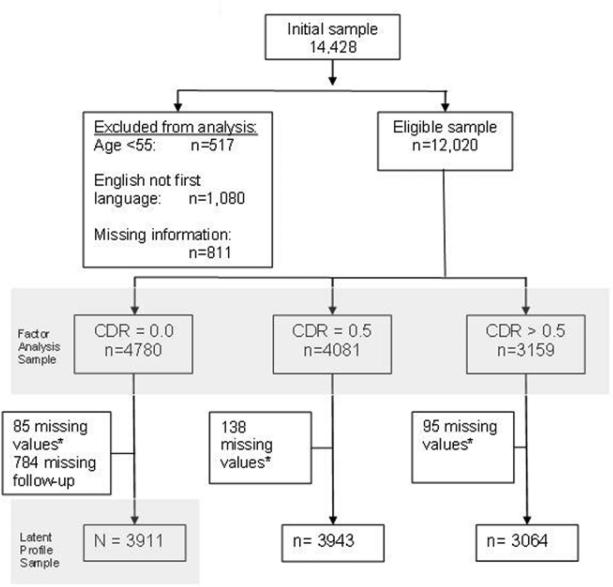
Data from a total of 14,428 participants were available as of May 5, 2008. We set aside data from 517 individuals who were under age 55 at baseline. Participants who reported that English was not their first language (n=1,080) were excluded because of the potential influence of language on test performance. Although complete data are not required for factor analysis using Mplus, we set aside data from 811 individuals who were missing >=10 test scores. The total eligible sample for analysis was n=12,020 (Figure 1). The sample was divided into three groups according to their baseline visit CDR score to roughly approximate normal, MCI, and dementia diagnostic categories. There were n=4,780 participants with CDR scores=0.0; n=4,081 with CDR=0.5; and n=3,159 with CDR>0.5. The group classified as “cognitively normal,” CDR=0.0 was on average younger and more highly educated. The dementia group was the oldest on average and had the lowest mean level of education.
Figure 1.
Sample Selection
3.2 Factor Analysis
Results of the neuropsychological battery factor analyses have been previously reported (25). Briefly, an EFA analysis was performed on the first half of the sample to empirically derive a general model. Confirmatory factor analysis (CFA) was performed with the second half of the sample and the model was tested for invariance. Four factors representing memory, attention, executive function, and language were identified. Logical Memory Immediate and Delayed recall was represented by the memory factor. Digits Forward and Digits Backward were represented by the attention factor. The Trail Making Test parts A & B and the WAIS Digit Symbol were represented by the executive function factor and the Boston Naming Test, animal fluency, and vegetable fluency were represented by the language factor. We then performed a multiple group CFA, accounting for the different CDR groups as described above. Invariance testing was used to evaluate the stability of the factors across CDR groups. This was done by systematically adding constraints to the model to represent levels of invariance (configural, metric, scalar, and strict). Model fit was tested at each level using standard indices. The final model demonstrated a good fit and was relatively invariant (strict invariance results: CFI=0.957, TLI=0.958, RMSEA=0.064, SRMR=0.71, BIC 112.90, and Satorra-Bentler Scaled χ2 test (χ2=112.90, df=202, p<.0001) (compared to a model with fewer constraints). This final model was then applied to the full sample in order to obtain factor scores for the current analysis.
3.3 Latent Profile Analysis
Demographic characteristics of the entire sample are shown in Table 1. To focus the analysis on cognitive performance among cognitively normal individuals, we set aside participants with CDR scores greater than zero (n=3,159 CDR>0.5; n=4,081 CDR=0.5). The sample for this analysis was reduced further (n=85) due to missing data on education, race, or various health indicators of interest including CVD, hypertension, high cholesterol, diabetes, or stroke/TIA. An additional 784 participants from the CDR=0.0 group were dropped from the analysis because no follow-up data were available (Figure 1). There were no statistically significant differences in age, sex, race, education, cardiovascular disease, or hypertension between those who dropped out or were missing information and those who remained in the study (data not shown). However, there was a greater frequency of high cholesterol (χ2=5.64, df=1, p<0.05) and diabetes (χ2=5.22, df=1, p<0.05) among those who did not have follow-up information.
Table 1.
Demographic Characteristics of full sample n=10,918 by CDR group
| Characteristic | Baseline CDR Groups | Total N=10,918 |
||
|---|---|---|---|---|
| CDR=0.0 n=3,911 |
CDR=0.5 n=3,943 |
CDR>0.5 n=3,064 |
||
| Baseline Age (SD) | 74.29 (8.7) | 75.0 (8.7) | 76.5 (9.0) | 75.2 (8.8) F=53.43, df=2, p<.0001 |
| Sex, female | 2564 (65.6) | 1988 (50.4) | 1600 (52.2) | 6152 (56.4) X2=212.49, df=2, p<.0001 |
| Education (SD) | 15.45 (2.8) | 15.0 (3.2) | 14.2 (3.3) | 14.9 (3.2) F=133.80, df=2, p<.0001 |
| Race | X2=24.81, df=4, p<.0001 | |||
| White | 3238 (82.8) | 3349 (84.9) | 2561 (83.6) | 9148 (83.8) |
| African American | 625 (16.0) | 514 (13.0) | 434 (14.2) | 1573 (14.4) |
| Other | 48 (1.2) | 80 (2.0) | 69 (2.3) | 197 (1.8) |
| CVD* | 1012 (25.9) | 1237 (31.4) | 910 (29.7) | 3159 (28.9) X2=30.06, df=2, p<.0001 |
| Hypertension | 2034 (52.0) | 2147 (54.5) | 1628 (53.1) | 5809 (53.2) X2=4.72, df=2, p=0.094 |
| High Cholesterol | 1856 (47.5) | 2092 (53.1) | 1482 (48.4) | 5430 (49.7) X2=212.49, df=2, p<.0001 |
| Diabetes | 397 (10.2) | 501 (12.7) | 359 (11.7) | 1257 (11.5) X2=12.76, df=2, p<.002 |
| Stroke or TIA | 288 (7.4) | 478 (12.1) | 412 (13.5) | 1178 (10.8) X2=77.44, df=2, p<.0001 |
CVD includes history of: heart attack, atrial fibrillation, bypass surgery, congestive heart failure, angioplasty, or pacemaker
Abbreviations: CVD=cardiovascular disease; TIA=transient ischemic attack; MCI= mild cognitive impairment
Values are number(percent) unless indicated as mean(standard deviation)
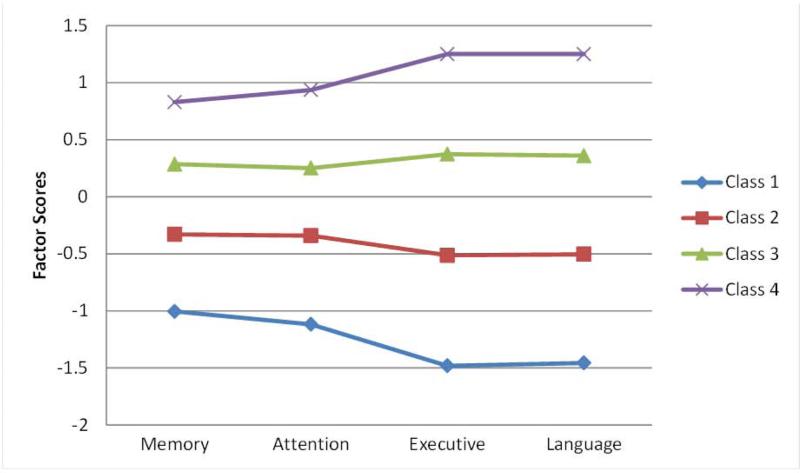
Using the CFA factor scores, baseline age, and sex, we then performed latent profile analyses (LPA). The “cognitively normal” group was best represented by 4 latent profiles as indicated by the Vuong-Lo-Mendell-Rubin likelihood ratio test (43, 47) (Table 2). Figure 2 shows the distribution of factor scores by profile.
Table 2.
Normal Sample Demographic Characteristics by Latent Class
| Characteristic | Latent Class | Total N=3911 |
|||
|---|---|---|---|---|---|
| 1 n=378 |
3 n=1315 |
2 n=1619 |
4 n=599 |
||
| Baseline Age (SD) | 80.7 (8.3) | 77.8 (8.1) | 72.4 (7.6) | 67.6 (6.8) | 74.29 (8.7) F=360.82, df=3, p<.0001 |
| Sex, female | 249 (65.9) | 813 (61.8) | 1088 (67.2) | 414 (69.1) | 2564 (65.6) X2=13.43, df=3, p=.0038 |
| Education (SD) | 13.1 (3.2) | 14.9 (2.7) | 16.0 (2.6) | 16.7 (2.4) | 15.45 (2.8) F=193.27, df=3, p<.0001 |
| Race‡ | X2=465.02, df=6, p<.0001 | ||||
| White | 186 (49.2) | 1034 (78.6) | 1449 (89.5) | 569 (95.0) | 3238 (82.8) |
| African American | 190 (50.3) | 259 (19.7) | 152 (9.4) | 24 (4.0) | 625 (16.0) |
| Other | 2 (.5) | 22 (1.7) | 18 (1.1) | 6 (1.0) | 48 (1.2) |
| CVD | 116 (30.7) | 426 (32.4) | 345 (21.3) | 125 (20.9) | 1012 (25.9) X2=59.14, df=3, p<.0001 |
| Hypertension | 259 (68.5) | 791 (60.2) | 769 (47.5) | 215 (35.9) | 2034 (52.0) X2=151.74, df=3, p<.0001 |
| High Cholesterol | 185 (48.9) | 632 (48.1) | 771 (47.6) | 268 (44.7) | 1856 (47.5) X2=2.32, df=3, p=0.5095 |
| Diabetes | 70 (18.5) | 161 (12.2) | 132 (8.2) | 34 (5.7) | 397 (10.2) X2=55.57, df=3, p<.0001 |
| Stroke or TIA | 44 (11.6) | 140 (10.7) | 85 (5.3) | 19 (3.2) | 288 (7.4) X2=56.94, df=3, p<.0001 |
*CVD includes history of: heart attack, atrial fibrillation, bypass surgery, congestive heart failure, angioplasty, or pacemaker
Abbreviations: CVD=cardiovascular disease; TIA=transient ischemic attack; MCI= mild cognitive impairment
Values are number(percent) unless indicated as mean(standard deviation)
Figure 2. Factor Scores by latent profile in CDR group = 0.0.
Standardized factor scores for each latent profile by cognitive domain.
Analyses to determine the predictive value of the latent profiles an average of three years later were limited by small cell sizes. Therefore, individual models for each profile were derived (Table 3). Members of profile 1 were the least likely to remain cognitively normal an average of three years later. Among members of this profile, the odds of being diagnosed with amnestic MCI were not significant. Odds were greater for amnestic MCI-multiple domain, non-amnestic MCI-single domain, non-amnestic MCI-multiple domain, or dementia. Profile 2 had significantly increased odds of any impairment with the exception of non-amnestic MCI-multiple domain. Profile 3 was a relatively high performing group as reflected by the significantly reduced odds of impairment for all outcomes. Finally, profile 4, the highest performing group had significantly low odds for all outcomes as well. Due to small numbers in profile 4, the diagnostic outcomes of non-amnestic MCI, MCI single domain, and MCI multiple domain had to be combined. We applied the Cochran-Armitage test for trend(48, 49) and found significant trends across profiles for all diagnoses with the exceptions of impaired not MCI and Amnestic MCI.
Table 3.
Multinomial logistic regression results examining the risk of cognitive outcomes by class* among 3,911 study participants rated as CDR=0.0 at the time of assessment
| Diagnosis | Class 1 | Class 2 | Class 3 | Class 4 |
|---|---|---|---|---|
| OR (95% CI) Wald X2, df, p-value |
OR (95% CI) Wald X2, df, p-value |
OR (95% CI) Wald X2, df, p-value |
OR (95% CI) Wald X2, df, p-value |
|
| Normal | Reference | Reference | Reference | Reference |
| Impaired Not MCI | 1.56 (0.85-2.71) 2.25, df=1, p=.1334 |
1.51 (1.08-2.10) 5.91, df=1, p=0.0151 |
0.70 (0.50-0.99) 4.03, df=1, p=0.0447 |
0.34 (0.16-0.62) 10.64, df=1, p=0.0011 |
| Amnestic MCI | 1.45 (0.81-2.46) 1.76, df=1, p=.1844 |
2.43 (1.80-3.29) 33.21, df=1, p<.0001 |
0.41 (0.29-0.58) 25.45, df=1, p<.0001 |
0.49 (0.28-0.80) 7.39, df=1, p=0.0066 |
| Amnestic MCI plus‡ | 3.27 (2.14-4.94) 30.87, df=1, p<.0001 |
2.46 (1.81-3.33) 33.60, df=1, p<.0001 |
0.31 (0.21-0.45) 35.73, df=1, p<.0001 |
0.09 (0.03-0.21)† 26.90, df=1, p<.0001 |
| Non-amnestic MCI, single domain‡ | 2.96 (1.58-5.31) 12.43, df=1, p=.0004 |
1.73 (1.12-2.67) 5.98, df=1, p=0.0144 |
0.52 (0.31-0.84) 6.89, df=1, p=.0086 |
|
| Non-amnestic MCI, multiple domain‡ | 4.26 (2.01-8.81) 14.91, df=1, p=.0001 |
1.71 (0.90-3.21) 2.81, df=1, p=0.0936 |
0.13 (0.03-0.37) 11.15, df=1, p=.0008 |
|
| Dementia‡ | 8.58 (5.64-12.99) 102.12, df=1, p<.0001 |
2.11 (1.52-2.91) 20.45, df=1, p<.0001 |
0.18 (0.11-0.28) 52.37, df=1, p<.0001 |
0.16 (0.06-0.37) 15.36, df=1, p<.0001 |
Abbreviations: OR=Odds ratio; CI=Confidence Interval; MCI=Mild Cognitive Impairment
Separate models were constructed for each class’ probability of multiple diagnostic outcomes. All models are adjusted for education, race, cardiovascular disease, hypertension, high cholesterol, diabetes, stroke or transient ischemic attack, and follow-up time.
Outcomes non-amnestic MCI single and multiple domain were combined due to small numbers.
Cochran-Armitage trend test across classes, 3 df, p<0.05
A review of baseline diagnoses for the overall group revealed that although they all had CDR scores equal to zero, 399 participants had diagnoses other than “normal cognition.” We re-ran the analyses after removing these participants from the sample and our results were essentially unchanged (see Table 5, supplemental materials).
Table 5.
Multinomial logistic regression results examining the risk of cognitive outcome by class, among 3,512 participants rated as cognitively normal at the time of assessment.
| Diagnosis | Class 1 | Class 2 | Class 3 | Class 4 |
|---|---|---|---|---|
| OR (95% CI) Wald X2, df, p-value |
OR (95% CI) Wald X2, df, p-value |
OR (95% CI) Wald X2, df, p-value |
OR (95% CI) Wald X2, df, p-value |
|
| Normal | Reference | Reference | Reference | Reference |
| Impaired Not MCI | 0.92 (0.41-1.85) 0.046, df=1, p=0.8298 |
1.62 (1.12-2.34) 6.65, df=1, p=0.0099 |
0.69 (0.47-1.01) 3.58, df=1, p=0.0584 |
0.38 (0.17-0.74) 6.78, df=1, p=0.0092 |
| Amnestic MCI | 2.71 (1.50-4.70) 11.91, df=1, p=0.0006 |
2.61 (1.82-3.75) 26.85, df=1, p<.0001 |
0.35 (0.22-0.53) 23.47, df=1, p<.0001 |
0.29 (0.12-0.59) 9.59, df=1, p=0.0020 |
| Amnestic MCI plus‡ | 4.68 (2.78-7.70) 35.43, df=1, p<.0001 |
2.16 (1.47-3.16) 15.73, df=1, p<.0001 |
0.30 (0.18-0.48) 23.69, df=1, p<.0001 |
0.12 (0.04-0.29)† 16.89, df=1, p<.0001 |
| Non-amnestic MCI, single domain | 2.07 (0.78-4.81) 2.54, df=1, p=0.1107 |
2.32 (1.33-4.08) 8.72, df=1, p=0.0031 |
0.46 (0.24-0.85) 5.79, df=1, p=0.0161 |
|
| Non-amnestic MCI, multiple domain‡ | 6.60 (2.18-18.46) 12.21, df=1, p=0.0005 |
1.44 (0.56-3.56) 0.62, df=1, p=0.4328 |
0.16 (0.03-0.57) 5.95, df=1, p=.0147 |
|
| Dementia‡ | 7.74 (4.73-12.53) 68.01, df=1, p<.0001 |
1.80 (1.22-2.65) 8.92, df=1, p=0.0028 |
0.21 (0.12-0.35) 32.58, df=1, p<.0001 |
0.21 (0.06-0.52) 9.0, df=1, p=0.0027 |
Abbreviations: OR=Odds ratio; CI=Confidence Interval; MCI=Mild Cognitive Impairment
*Separate models were constructed for each class’ probability of multiple diagnostic outcomes. All models are adjusted for education, race, cardiovascular disease, hypertension, high cholesterol, diabetes, stroke or transient ischemic attack, and follow-up time (baseline age and sex were included in latent profile analysis).
Outcomes amnestic MCI multiple domain, non-amnestic MCI single and multiple domain were combined due to small numbers.
Cochran-Armitage trend test across classes, 3 df, p<0.05
3.4 Predictors of profile membership
Multinomial logistic regression models were used to determine which cognitive test best predicted profile membership. With latent profile groups as the dependent variable and Profile 4 as the reference group, the neuropsychological tests were entered into the equation as independent variables. A stepwise regression method was used to select tests for inclusion in the model; ranks are reported as follows. The Trail Making Test, Part B (χ2 =1473.70, df=3, p<.0001) was the strongest predictor followed by (in order) Logical Memory Delayed Recall (χ2 =485.51, df=3, p<.0001), Verbal fluency (animals)(χ2 332.72, df=3, p<.0001), WAIS Digit Symbol (χ2 133.54, df=3, p<.0001), Verbal fluency (vegetables) (χ2 101.44, df=3, p<.0001), Digits Backward (χ2 98.27, df=3, p<.0001), Trail Making Test Part A (χ2 90.49, df=3, p<.0001), Boston Naming Test (χ2 61.28, df=3, p<.0001), and Logical Memory Immediate recall (χ2 10.09, df=3, p<.002). Digits forward was dropped from the equation.
Based on these findings and after examining plots and standardized values for performance on individual tests, post-hoc analyses were conducted focusing on the Trail Making Test Part B. A survival analysis using Cox Proportional Hazards(50) methods with a stepwise selection procedure and “any impairment” at the 3 year follow-up as the outcome, showed that the Trail Making Test Part B significantly predicted “any impairment” during the follow-up period (using the normalized test score: HR 1.51, 95% CI 1.29-1.78, χ2=25.228, df=1, p<.0001). Other variables retained in the final model were Delayed Recall, Animal and Vegetable Fluency, Digit Symbol, baseline age, race, and self-reported diabetes. The proportionality assumption was tested by examining time interactions for each variable in the model (51). Trails B was ranked second after baseline age in the stepwise procedure. Notably, baseline age and Trails B were also found to be moderately correlated in this sample (Pearson correlation r=0.40, df=3440, p<.0001). A second post-hoc analysis compared the performance of the latent profile approach to a contrast approach. Based on findings presented above, the Delayed Recall test was contrasted with the Trail Making Test Part B. Although diagnostic outcomes were significantly predicted, there was no clear trend in scores indicating severity of outcome (data not shown).
4. Conclusions
Using a single data point from the large NACC database, we defined latent cognitive profiles among cognitively normal individuals (CDR=0.0) that significantly predicted later onset of MCI and dementia. These findings demonstrate the use of latent profile analysis for pre-clinical phenotypes of AD defined by cross-sectional patterns of cognitive performance rather than cognitive decline or performance on a single test of global cognitive function.
In this sample, cognitively normal individuals were best described by 4 profiles of performance which were associated with a range of severity of outcomes. The first profile performed the worst and was consequently more likely to have diagnoses of MCI or dementia three years later. The fourth profile performed the best and was more likely to remain cognitively normal three years later.
The cross-sectional method for case identification that was utilized in this study can be similarly applied by researchers who have the challenging task of identifying the cognitive characteristics of at-risk asymptomatic volunteers based on a single study visit. We chose to evaluate performance at a single point in time because our goal was to evaluate and identify subtle differences in cognitive performance patterns among cognitively normal individuals as opposed to observing early decline over time. Results are consistent with other studies that suggest that variations in the pattern of cognition, i.e. a loss in one area (e.g. executive function) while retaining relative competency in others may be a very early signal of problems (52, 53). In the current study this is evidenced by poor performance on Trail Making Part B, a challenging measure of executive function, motor speed and visual attention. Although these findings are derived from a large sample and cannot be extrapolated to individual test performance, they can serve as a guide for others seeking to identify predictive neuropsychological tests or select samples for prevention studies or subsets of participants for genetic association studies.
Our findings demonstrate an alternative approach to the study of cognitive performance that may predict subsequent onset of cognitive decline and dementia. This serves to fill an important need as researchers are currently looking for better ways to identify asymptomatic research subjects for participation in AD prevention trials. Because individuals who display cognitive problems may have too much pathology to benefit from preventive treatments, it is important to identify the earliest possible cognitive signs and symptoms. These findings also corroborate with and extend the work of others who have found significant associations between performance on the Trail Making Test Part B and conversion from MCI to dementia (3, 7). Thus subtle signs of impending impairment are detectable with the use of challenging neurocognitive testing in the NACC data. It should be noted however, that others have found that the Trail Making Test Part B is significantly correlated with age (54) and this was confirmed in the current sample.
There are a number of limitations in the current study. Participants in this study are self-selected volunteers from ADCs across the United States and do not constitute a representative sample. They are likely to be more highly educated, possibly more affluent, or have better access to healthcare than the general public. Because many of these individuals have a family history of dementia (approximately 38% report that one or both parents had dementia) they are motivated to volunteer in dementia studies. Although the protocol for data collection and diagnosis has been standardized, there is the possibility of differences in practices from center to center. Some participants are interviewed in the home and some in a clinic setting. Although efforts are made to administer the protocol in a standardized fashion, the difference in setting may contribute to performance. The follow-up period is relatively short which may have limited our ability to detect more specific associations between cognitive profiles and dementia outcomes. The memory measures used in the NACC battery may not be the most sensitive to early or mild cognitive changes. The latent cognitive profiles among normals and latent profiles of dementia and MCI cases were all determined from neuropsychological performance at a single point in time. There are a number of factors that may affect an individuals’ performance on cognitive tests on any given day and this variability could dilute the resolution of our results. Finally, the analyses of the MCI and dementia groups involves a clear tautology in that the test performance was used in the determination of diagnosis. Nonetheless, this was an exploratory exercise mainly focused on the performance of individuals who were rated as cognitively normal based on CDR score at baseline. Comparison of CDR scores and baseline diagnoses revealed that a portion of the sample did not have a diagnostic rating of normal at baseline. When we removed these individuals from the CDR=0.0 group and re-ran the latent profile analysis, there were no significant differences in mean factor scores or test scores with the exception of differences in mean language factor scores in profile 2 and profile 3, and a mean difference in Trails B score in profile 2. Profile group membership and results remained relatively unchanged.
These cognitive profiles, while significantly predictive in statistical models, may simply reflect heterogeneity in performance such that they can only delineate increments of severity rather than different patterns of performance as we had hypothesized (e.g., performing well on memory and poorly on executive function or vice versa). Some participants may have already started to decline, yet were still operating within the realm of a “cognitively normal” definition. It seems plausible that the general effects of cognitive decline (i.e., the levels of severity) overwhelmed our ability to detect distinct cognitive patterns. Thus, simpler approaches may be adequate for this purpose.
Contrast measures have been proposed as an approach in cross-sectional data as they allow for the intra-individual heterogeneity that may be indicative of future cognitive difficulties (10). Future research based on the current findings could be used to combine cognitive risk based on contrasts or latent profiles with genetic risk as a means to identify asymptomatic individuals with high risk for developing AD over a short time interval. This may have the effect of reducing the time needed to develop therapies for high-risk populations.
Table 4.
Mean raw test scores by latent profile class
| Test | Group1 | Group2 | Group3 | Group4 |
|---|---|---|---|---|
| Logical Memory | 10.0 (3.6) | 12.8 (3.5) | 15.1 (3.4) | 17.0 (3.3) |
| Delayed | 8.1 (4.0) | 11.3 (3.7) | 14.0 (3.8) | 15.8 (3.7) |
| Digits Forward | 7.5 (1.9) | 8.5 (1.9) | 9.4 (1.9) | 10.4 (1.7) |
| Digits Forward Length | 6.4 (1.1) | 7.0 (1.0) | 7.4 (1.0) | 7.9 (0.8) |
| Digits Backward | 5.0 (1.7) | 6.5 (1.7) | 7.7 (1.9) | 9.2 (2.0) |
| Digits Backward Length | 4.1 (1.0) | 5.0 (1.0) | 5.6 (1.1) | 6.4 (1.1) |
| Animal | 12.9 (3.5) | 17.2 (3.8) | 21.6 (4.2) | 26.9 (5.2) |
| Vegetable | 10.5 (2.9) | 13.1 (3.3) | 15.8 (3.6) | 19.2 (5.1) |
| Boston Naming | 20.1 (5.7) | 26.1 (3.1) | 28.1 (1.9) | 29.0 (1.2) |
| Trails A | 62.7 (25.4) | 41.3 (14.4) | 30.1 (8.4) | 23.8 (6.9) |
| Trails B | 203.4 (72.1) | 113.2 (42.3) | 74.0 (22.9) | 52.6 (14.7) |
| WAIS Digit Symbol | 28.3 (9.3) | 39.5 (8.3) | 49.8 (8.9) | 58.7 (9.2) |
Numbers are mean(standard deviation).
Acknowledgments
Sources of Support:
This work was supported by grants from the National Alzheimer's Coordinating Center (NACC Junior Investigator grant: 2008-JI-02) and from the National Institute on Aging (U01-AG016976, P30-AG028377, and K01-AG029336).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none.
Findings were presented at the Alzheimer's Association International Conference in Vancouver BC, Canada in July 2012.
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, et al. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Khachaturian ZS, Barnes D, Einstein R, et al. Developing a national strategy to prevent dementia: Leon Thal Symposium 2009. Alzheimers Dement. 2010;6:89–97. doi: 10.1016/j.jalz.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomar JJ, Bobes-Bascaran MT, Conejero-Goldberg C, et al. Utility of Combinations of Biomarkers, Cognitive Markers, and Risk Factors to Predict Conversion From Mild Cognitive Impairment to Alzheimer Disease in Patients in the Alzheimer's Disease Neuroimaging Initiative. Arch Gen Psychiatry. 2011;68:961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- 4.Fleisher AS, Sun S, Taylor C, et al. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology. 2008;70:191–199. doi: 10.1212/01.wnl.0000287091.57376.65. [DOI] [PubMed] [Google Scholar]
- 5.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 6.Mavandadi S, Nazem S, Ten Have TR, et al. Use of Latent Variable Modeling to Delineate Psychiatric and Cognitive Profiles in Parkinson Disease. Am. J. Geriatr. Psychiatr. 2009;17:986–995. doi: 10.1097/JGP.0b013e3181b215ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blacker D, Lee H, Muzikansky A, et al. Neuropsychological Measures in Normal Individuals That Predict Subsequent Cognitive Decline. Arch Neurol. 2007;64:862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 8.Chen P, Ratcliff G, Belle SH, et al. Patterns of cognitive decline in presymptomatic alzheimer disease: A prospective community study. Archives of General Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson MW, Delis DC, Bondi MW, et al. Do neuropsychological tests detect preclinical Alzheimer's disease: individual-test versus cognitive-discrepancy score analyses. Neuropsychology. 2002;16:132–139. doi: 10.1037//0894-4105.16.2.132. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson MW, McEvoy LK, Dale A, et al. Cognitive phenotypes, brain morphometry and the detection of cognitive decline in preclinical AD. Behavioural Neurology. 2009;21:29–37. doi: 10.3233/BEN-2009-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer's Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 12.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 14.O'Bryant SE, Lacritz LH, Hall J, et al. Validation of the New Interpretive Guidelines for the Clinical Dementia Rating Scale Sum of Boxes Score in the National Alzheimer's Coordinating Center Database. Arch. Neurol. 2010;67:746–749. doi: 10.1001/archneurol.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler Memory Scale-Revised Manual. Psychological Corp; San Antonio: 1987. [Google Scholar]
- 17.Wechsler D. The Wechsler Adult Intelligence Scale Revised. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 18.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation 2nd. Neuropsychology Press; Tucson, AZ: 1993. [Google Scholar]
- 19.Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination. Third Edition Pro-Ed; Austin, TX: 2001. [Google Scholar]
- 20.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 21.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 24.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 25.Hayden KM, Jones RN, Zimmer C, et al. Factor Structure of the National Alzheimer's Coordinating Centers Uniform Dataset Neuropsychological Battery: An Evaluation of Invariance Between and Within Groups Over Time. Alzheimer Dis Assoc Disord. 2011;25:128–137. doi: 10.1097/WAD.0b013e3181ffa76d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams MM, Roe CM, Morris JC. Stability of the Clinical Dementia Rating, 1979-2007. Arch Neurol. 2009;66:773–777. doi: 10.1001/archneurol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 28.Blom G. Transformations of the binomial, negative binomial, poisson, and chi-square distributions. Biometrika. 1954;41:302–316. [Google Scholar]
- 29.Muthen LK, Muthen BO. Mplus: Statistical Analysis with Latent Variables, 5. Muthen & Muthen; Los Angeles, CA: 2007. [Google Scholar]
- 30.Kim J-o, Mueller CW. Introduction to factor analysis : what it is and how to do it. Sage Publications; Beverly Hills, Calif.: 1978. [Google Scholar]
- 31.Gibson WA. Three Multivariate Models: Factor Analysis, Latent Structure Analysis, and Latent Profile Analysis. Psychometrika. 1959;24:229–252. [Google Scholar]
- 32.McCutcheon AL. Latent class analysis. Sage; Newbury Park, Calif.: 1987. [Google Scholar]
- 33.Lazarsfeld PF, Henry NW. Latent Structure Analysis. Houghton Mifflin; Boston: 1968. [Google Scholar]
- 34.Vermunt JK, Magidson J. Latent GOLD User's Guide. Statistical Innovations, Inc.; Belmont, MA: 2000. [Google Scholar]
- 35.Pastor DA, Barron KE, Miller BJ, et al. A latent profile analysis of college students’ achievement goal orientation. Contemporary Educational Psychology. 2007;32:8–47. [Google Scholar]
- 36.Muthen LK, Muthen BO. Mplus User's Guide, Fifth. Muthen & Muthen; Los Angeles, CA: 1998-2007. [Google Scholar]
- 37.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 38.Tucker LR, Lewis C. A Reliability Coefficient for Maximum Likelihood Factor Analysis. Psychometrika. 1973;38:1–10. [Google Scholar]
- 39.Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research. 1990;25:173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- 40.Joreskog KG, Sorbom D. LISREL 8: User's reference guide. Scientific Software International; Chicago: 1996. [Google Scholar]
- 41.Satorra A, Bentler P. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–514. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafferty AE. Bayesian Model Selection in Social Research. Sociological Methodology. 1995;25:111–163. [Google Scholar]
- 43.Lo YT, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 44.Muthén LK, Muthén BO. Mplus user's guide: Version 3. Muthén & Muthén.; Los Angeles: 2004. [Google Scholar]
- 45.Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification. 1996;13:195–212. [Google Scholar]
- 46.Ramaswamy V, DeSarbo W, Reibstein D, et al. An empirical pooling approach for estimating marketing mix elasticities with PIMS data. Marketing Science. 1993;12:103–124. [Google Scholar]
- 47.Vuong QH. Likelihood Ratio Tests for Model Selection and Non-Nested Hypotheses. Econometrica. 1989;57:307–333. [Google Scholar]
- 48.Cochran WG. Some methods for strengthening the common chi-squared tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 49.Armitage P. Tests for Linear Trends in Proportions and Frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- 50.Cox DR. Regression Models and Life Tables. Journal of the Royal Statistical Society. Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 51.Nagelkerke NJD, Oosting J, Hart AAM. A Simple Test for Goodness of Fit of Cox's Proportional Hazards Model. Biometrics. 1984;40:483–486. [Google Scholar]
- 52.Bondi M, Jak A, Delano-Wood L, et al. Neuropsychological Contributions to the Early Identification of Alzheimer's Disease. Neuropsychology Review. 2008;18:73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobson MW, Delis DC, Lansing A, et al. Asymmetries in global-local processing ability in elderly people with the apolipoprotein e-epsilon4 allele. Neuropsychology. 2005;19:822–829. doi: 10.1037/0894-4105.19.6.822. [DOI] [PubMed] [Google Scholar]
- 54.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]