Abstract
The previous study has shown that repeated D domain-like (Rdd) proteins, a group of novel secretory proteins consisting of repeated domains of a cysteine-rich sequence, are involved in the process of blood vessel formation in Xenopus embryo. We performed further experiments to examine the localization of Rdd proteins in embryogenesis. Detection of tagged Rdd proteins expressed in blastomeres showed that Rdd proteins formed a high molecular weight complex and existed in the extracellular space. A rabbit antibody against the Rdd synthetic peptide identified a single band of 28 kD in embryonic tissue extract. By whole-mount immunostaining analysis, signal was detected in the regions of inter-somites, vitelline veins, and branchial arches at the tailbud stage. Staining of Rdd was remarkably reduced in the embryos injected with vascular endothelial growth factor Morpholino. We suggest that Rdd proteins interact with a molecule(s) associated with vascular precursor cells.
Keywords: Vascular cells, Frog, Vascular endothelial growth factor, Antibody, Cysteine-rich
Introduction
The process of development in vertebrate embryos consists of cell-autonomous regulation by transcription factors and of non-cell-autonomous regulation by secretory factors. In the Xenopus laevis embryo, primitive blood cells and vascular cells differentiate at first in the ventral blood islands [1, 2], and their differentiation is primarily controlled by bone morphogenetic protein and Wnt signals [3, 4, 5, 6, 7]. Subsequent activation of cell lineage-specific transcription factors determines the hematopoietic and angiogenic lineages. SCL [8, 9, 10], GATA2 [11, 12, 13], GATA1 [9], CEBPα [14], and SpiB [15] are involved in the specification of blood cells, and Fli1 [16], Etv2 [17], Erg [18], and KLF2 [18] are mainly involved in the specification of vascular cells. A major vascular network is then established in the dorsal-lateral plate from which the dorsal aorta and cardinal vein originate. It has been shown recently that the floor region of dorsal aorta has a potency to produce hematopoietic stem cells as revealed by the expression of scl and tel1/etv6 [19].
In addition to intracellular factors, secreted factors in the extracellular environment should play an essential role for the further specification and determination of vascular cells. Vascular endothelial growth factor (VEGF) is a major secretory factor that controls the growth and differentiation of endothelial cells [20, 21, 22, 23]. It was shown in Xenopus that VEGF produced in the hypochord is essential for formation of the vascular structure in the dorsal-lateral plate [24, 25]. Activation and inactivation of the fibroblast growth factor signal control the fate of vascular and blood cell lineages in the ventral blood island mesoderm [26, 27, 28]. A recent study also demonstrated a role of R-spondings and Wnt signal in activation of vascular cell differentiation [29]. Although the above secretory factors are essential for differentiation of vascular cells, roles of secretory factors other than these factors in vasculogenesis and hematopoiesis in embryogenesis have not been elucidated.
We previously identified the expression and function of repeated D domain-like (Rdd) in the Xenopus embryo [30]. rdd1-4 encode the related secretory proteins consisting of the repeated sequence of D domain-like (DL1-DL4) of von Willebrand factor. Transcripts of rdd2-4 are detected in the trunk mesoderm and ectoderm at the neurula and tailbud stages. A knockdown experiment using a Morpholino oligo (MO) indicated that Rdd3 and Rdd4 are necessary for the normal development of blood and vascular cells and especially important for migration of vascular precursor cells at the intermediate mesoderm. These results suggest that Rdd proteins function in the extracellular environment [30]. Although the physiological importance of Rdds in embryogenesis has been demonstrated, no information about the biochemical property of Rdd and the distribution of Rdd proteins in the embryo has been reported. Thus, we generated an anti-serum against the synthetic peptide of an Rdd3 and Rdd4 common sequence and tried to detect the endogenous proteins in the embryo. Whole-mount immunostaining analysis indicated that endogenous Rdd proteins were localized in the regions where primary vasculogenesis occurred. We, therefore, hypothesize that Rdd proteins interact with a molecule(s) associated with vascular precursor cells.
Materials and Methods
cDNA constructs
HA-tagged Rdd2 and Rdd3 in pCS2 (rdd2-ha/pCS2, rdd3-ha/pCS2), Flag-tagged Rdd3 in pCS2 (rdd3-flag/pCS2), and Myc-tagged Mif in pCS2 (myc-mif/pCS2) were described in previous reports [30, 31]. HA-tagged Rdd4 (rdd4-ha/pCS2) was made by polymerase chain reaction amplification using 5'-AAT-TGG-ATC-CTG-GTG-CCT-CAG-AGGAAA-TAC-3' (forward) and 5'-AGC-GTA-ATC-CGGAAC-ATC-GTA-TGG-GTA-AAT-TTT-AGA-AGG-AGGGCA-3' (reverse). The products were further amplified by another reverse primer (5'-AAT-TCT-CGA-GAG-CGTAAT-CCG-GAA-CAT-CGT-3') to add an XhoI site and ligated into pCS2 at the BamHI and XhoI sites. Likewise, Flag-tagged Rdd4 (rdd4-flag/pCS2) and Val (val-flag/pCS2) were made by using primers as follows: 5'-AAT-TGG-ATCCTG-GTG-CCT-CAG-AGG-AAA-TAC-3' (forward) and 5'-CTT-GTC-GTC-ATC-GTC-TTT-GTA-GTC-AAT-TTTAGA-AGG-AGG-GCA-3' (reverse) for rdd-4flag; 5'-AATAGA-ATT-CAT-GTA-CAG-CTC-AGA-CGA-AGA-G-3' (forward) and 5'-CTT-GTC-GTC-ATC-GTC-TTT-GTAGTC-GTAGTC-CTG-TCT-CCT-GTT-GTA-TCT-3' (reverse) for val-flag. Amplified DNAs were added with an XhoI site in the 3' end and inser ted into pCS2 at the BamHI and XhoI sites for rdd4-flag or EcoRI and XhoI sites for val-flag. Capped mRNAs for microinjection were synthesized according to the protocol of the manufacturer (Megascript, Ambion, Grand Island, NY, USA).
Microinjection of synthesized mRNA and Morpholino
Xenopus laevis embryos were obtained as previously described [28]. Developmental stages were determined as described by Niuewkoop and Faber [32]. For expression of Rdd proteins in the embryonic cells, mRNA was injected into the animal pole area of two-cell-stage embryos in 100% Steinberg's solution containing 3% Ficoll by using a micromanipulator (Nanoject, Drummond Scientific Co., Broomall, PA, USA). For immunostaining of tagged proteins in the animal cap explant, myc-mif mRNA was injected with val-flag or rdd3-flag mRNA (1 ng/embryo) into an animal pole blastomere at the 16-cell stage. Macrophage migration inhibitory factor (Mif) is a cytoplasmic protein [33] and ventrally associated leucine-zipper (Val) is a nucleuslocalizing protein [30]. When these embryos reached st. 8, the animal cap was excised and cultured in 50% Marc's Modified Ringer solution (100 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH 7.4) containing an antibiotic (30 g/ml kanamycin sulfate) until st. 20. MOs used in the study are the same as those used in previous studies: Rdd3/4 MO, 5'-TAAAGA-TGG-TGC-TCA-CTC-TCA-GCA-T-3' [30]; VEGF MO, 5'-TGG-CCT-TTT-AGT-ACT-TGA-AAG-ACG-G-3' [28].
Generation of anti-Rdd peptide serum
A common amino acid sequence in Rdd3 and Rdd4, SQCPPSQEQRCPLNQFWE (amino acids 149-166 of Rdd3, amino acids 214-231 of Rdd4), was selected as the antigenic and hydrophilic site to produce anti-Rdd peptide serum. Rdd peptide was synthesized and conjugated with keyhole limpet hemocyanin and then used for immunization. Antigen preparation, immunization to rabbits and affinity purification of the antibody were done by an antibody production service provided by a company (BioSynthesis, Lewisville, TX, USA).
Western blot analysis
For Western blot analysis, protein extracts prepared from the embryos were loaded in 12.5% or 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The transferred membrane was incubated with anti-Rdd antibody or anti-HA antibody (Sigma, St. Louis, MO, USA) as a first antibody and was incubated with AP-conjugated anti-rabbit IgG (Jackson ImmunoResearch, Cambridge, UK) or PO-conjugated antimouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as a second antibody. Positive signals were visualized by NBT/BCIP solution or ECL Western Blotting Detection Reagent (GE Healthcare, Piscataway, NJ, USA) as a substrate.
Immunostaining and histology
For fluorescence-based whole-mount immunostaining, animal caps were fixed in Dent's solution (20% dimethyl sulfoxide, 80% methanol) overnight. Animal caps were incubated with anti-Flag antibody or anti-Myc antibody (9E10, Santa Cruz Biotechnology) in 5% skim milk as a first antibody and were incubated with Alexa488-conjugated anti-mouse or anti-rabbit IgG antibody (Invitrogen, Carlsbad, CA, USA) or Cy3-conjugated anti-mouse or anti-rabbit IgG antibody (Jackson ImmunoResearch) in 95% calf serum/5% DMSO as a second antibody. To detect endogenous Rdd proteins, albino embryos were incubated with anti-rdd antibody as a first antibody and were incubated with AP-conjugated anti-rabbit IgG as a second antibody. The reaction was visualized in NBT/BCIP solution. Rdd pep tides (20 µg/ml) were added with anti-Rdd serum for an antibody absorption experiment. For histological analysis, immunostained albino embryos were re-fixed in MEMFA and embedded in paraffin. Some of serial sections were stained with hematoxylin and eosin.
Whole-mount in situ hybridization
Whole-mount in situ hybridization analysis was performed as described previously [34]. Digoxygenin-labeled antisense ribonucleotide probes were synthesized as follows: tie-2 in pBS (SK+) was linearized by XhoI and RNA was transcribed with T3 polymerase; rdd3 in pCS2+ was linearized by ClaI and RNA was transcribed with T7 polymerase.
Results
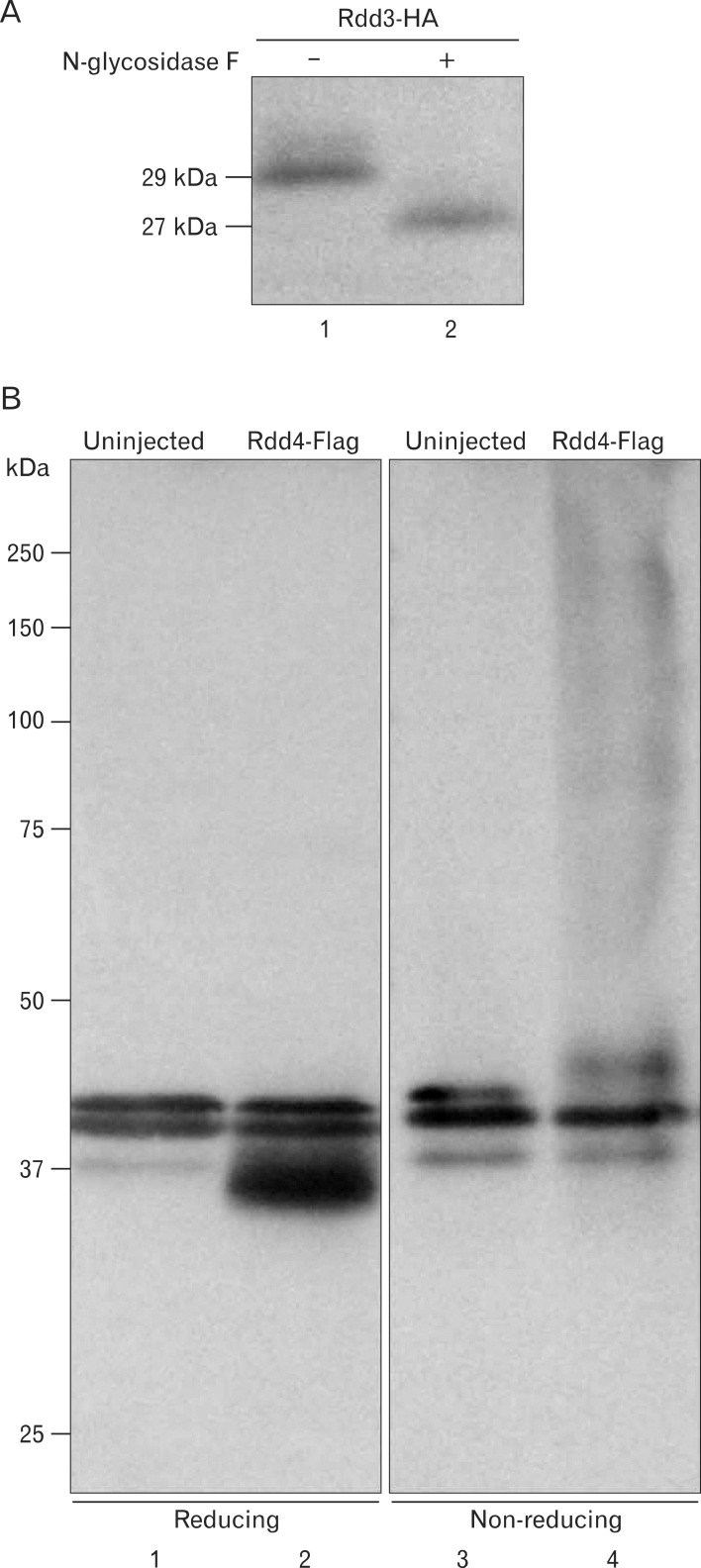
As previously reported, knockdown of Rdd3/4 by Morpholino injection resulted in disruption of blood vessel formation, although vascular precursor cell markers, such as flk-1 and tie-2, were expressed normally [30]. We have speculated that Rdd proteins are involved in the morphogenesis of vascular precursor cells. However, the molecular nature has not been elucidated. In the present study, we examined the biochemical property of Rdd proteins. Rdd proteins were secreted from oocytes [30], and the deduced amino acid sequence of Rdd3 and Rdd4 revealed that these proteins contain potential sites of N-linked glycosylation (amino acids 139 in Rdd3, amino acids 139 and 204 in Rdd4). Therefore, at first, we examined whether the recombinant Rdd3 protein expressed in the embryo is glycosylated or not. Western blot analysis revealed that the signal of Rdd3-HA shifted to a smaller molecular weight after N-glycosidase F treatment, indicating that recombinant Rdd protein was N-glycosylated (Fig. 1A). Since the Rdd proteins may interact with other factor(s) via the D-like (DL) domains that exhibit highly conserved positions of the cysteine residue, we tested whether the Rdd proteins make a complex with covalent bonds in physiological conditions. Embryonic extracts in which Rdd4-Flag had been expressed were electrophoresed under reduced or non-reduced conditions, and Rdd3 protein was detected by Western blot analysis. Rdd4-Flag was detected as a smear at the high molecular weight range in the non-reducing condition, demonstrating that the Rdd protein forms complexes of various molecular sizes in physiological conditions (Fig. 1B).
Fig. 1.
Biochemical properties of Rdd proteins. (A) rdd3-ha mRNA was injected into 2-cell-stage embryos. The embryos were cultured until the late neurula stage. Western blot analysis was performed with extracted proteins after treatment with N-glycosidase F (lane 2) or no treatment (lane 1). The signal of Rdd3-HA was shifted by N-glycosidase F treatment, indicating that recombinant Rdd3 protein was N-glycosylated. (B) Western blot analysis was performed with extracted proteins from uninjected or rdd4-flag mRNA-injected embryos under the reducing (lanes 1 and 2) or non-reducing (lanes 3 and 4) condition. Rdd4-Flag appeared as a single band under the reducing condition (lane 2), but it showed a smear at the high molecular weight range under the non-reducing condition (lane 4).
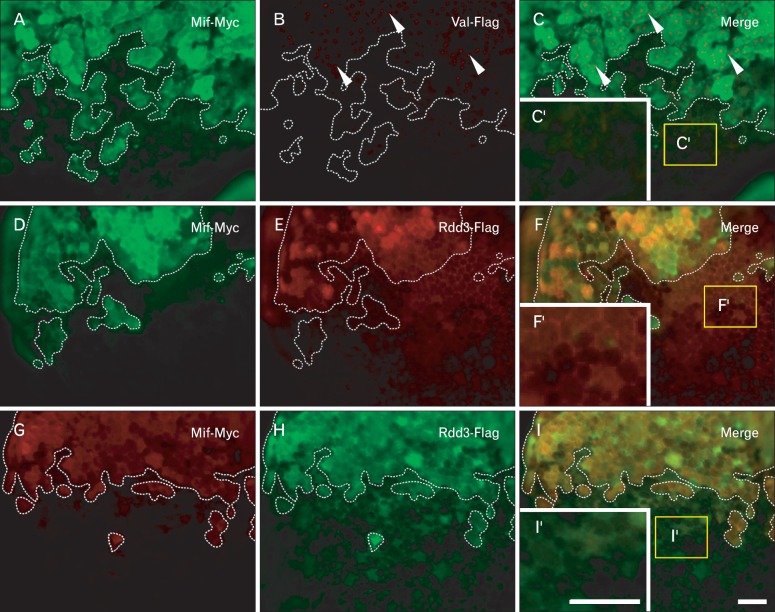
Although Rdd proteins were detected in the culture medium when they were expressed in oocytes, the distribution of Rdd proteins in embryonic tissues was not elucidated. To visualize recombinant Rdd proteins in embryonic tissues, we injected rdd3-flag mRNA together with myc-mif mRNA into an animal blastomere of a 16-cell-stage embryo, and we excised the animal cap explant and cultured it overnight. Macrophage migration inhibiting factor (mif) encodes for a cytoplasmic protein, and we can thus visualize descendant cells of the injected blastomere. Double staining with anti-Myc (mouse IgG) and anti-Flag (rabbit IgG) antibodies, followed by staining with fluorescence-conjugated second antibodies, indicated that Rdd3-Flag protein was positively stained in the cytoplasm of Mif-positive cells and also stained in the intercellular region of Mif-negative cells (Fig. 2D-F). To exclude the possibility that the second antibody binds to embryonic cells non-specifically, Cy3- and Alexa488-conjugated second antibodies were exchanged with each other, and essentially the same results were obtained (Fig. 2G-I). In a control experiment, val-flag mRNA (encoding for a nuclear protein) and myc-mif mRNA were injected together into a blastomere, and Val-Flag protein was stained only in the nucleus of Mif-positive cells (Fig. 2A-C). Thus, it is likely that the secreted Rdd proteins form a high molecular weight complex and exist in the intercellular space in the developing embryo.
Fig. 2.
Localization of recombinant Rdd3 protein in the intercellular region. val-flag mRNA (Flag-tagged Val protein) (A-C) or rdd3-flag mRNA (Flag-tagged Rdd3 protein) (D-I) together with mif-myc mRNA (coding for Myc-tagged Mif protein) were injected into a single blastomere at the 16-cell stage. Animal cap explants were isolated and cultured until the early tailbud stage (st. 20). Explants were stained with anti-Myc and anti-Flag antibodies simultaneously and the reaction was visualized with Alexa488-based fluorescence (A, D, H) and Cy3-based fluorescence (B, E, G). Positive signal for Mif protein shows descendant cells of the RNA-injected single cell at the 16-cell stage (A, D, G). The border between the injected and uninjected cells is indicated by a white dotted line. Merged images of the double staining are also shown (C, F, I). Val-Flag protein was detected in the nuclei of cells that had been injected with mif-myc mRNA (C, C'). On the other hand, Rdd3-Flag protein was stained in the cytoplasm of Mif-positive cells and also stained in the intercellular region of Mif-negative cells (F, F'). Exchange of the second antibodies between Cy3-conjugated IgG and Alexa488-conjugated IgG gave the same results (I, I'). Scale bars in panels I and I'=50 µm (A-I), 50 µm (C', F', I').
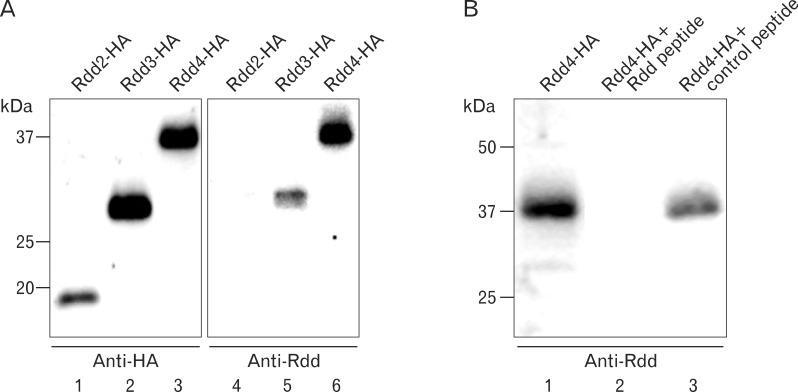
In order to characterize the molecular nature of Rdd proteins and to elucidate localization of endogenous Rdd proteins in developing embryos, we attempted to generate an antibody against the Rdd proteins. Rabbit antisera were raised against a synthetic peptide conjugated with the keyhole limpet hemocyanin (see Materials and Methods), and the immunoglobulin was further purified by a peptide-conjugated affinity column. Western blot analysis showed that the Rdd antibody recognized recombinant Rdd3 and Rdd4 proteins in the embryonic extract but did not react with recombinant Rdd2 (Fig. 3A). Reaction of the antibody with Rdd4 was completely blocked by absorption of the antibody with the synthetic Rdd peptide but was not blocked by adding an unrelated peptide (Fig. 3B). These results confirmed the specific binding of the antibody to Rdd3 and Rdd4 proteins.
Fig. 3.
Production of anti-sera against a synthetic peptide from the Rdd3 amino acid sequence. (A) Western blot analysis was performed with anti-HA antibody or anti-Rdd antibody to detect HA-tagged recombinant Rdd proteins. Anti-HA antibody reacted with Rdd2, Rdd3, and Rdd4 proteins (lanes 1-3), while anti-Rdd antibody reacted with Rdd3 and Rdd4 (lanes 5 and 6). (B) Absorption of anti-serum with Rdd (lane2) or control (lane 3) peptide indicated specific reaction of the antibody with Rdd4 protein in Western blot analysis.
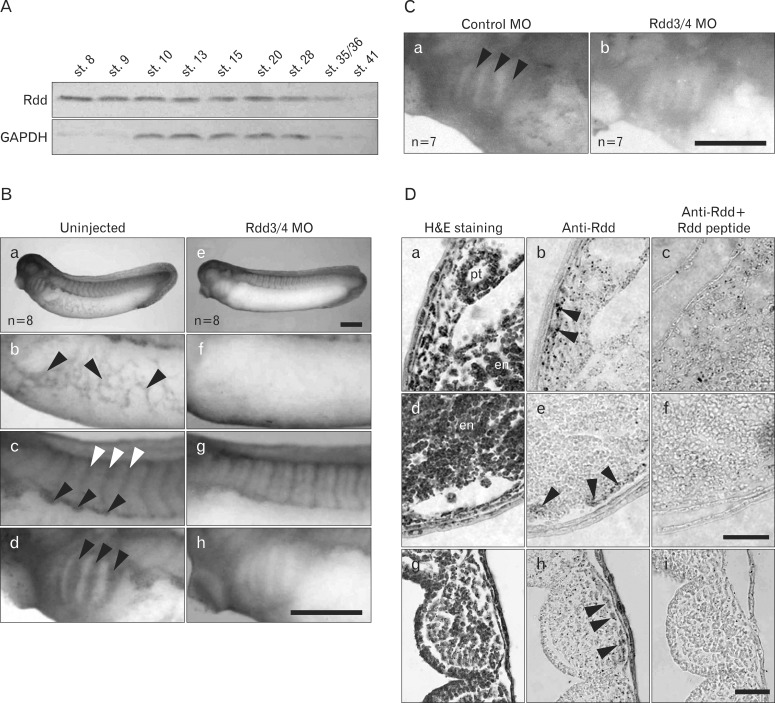
We performed Western blot analysis to detect endogenous Rdd proteins in embryos of different stages. A single positive band at 28 kD was detected in the extracts of embryos from st. 10 to st. 35/36 (Fig. 4A). As a loading control, we detected glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the extract. The signal of GAPDH decreased at st. 35/36 and st. 41, because extraction of proteins was performed without the use of a detergent. Thus, we suggest that the amount of Rdd protein (predicted as Rdd3 because the molecular weight of Rdd3 is estimated to be 24 kD) remains in the extract until st. 28 and decreases gradually as stages advance. Next, we investigated the spatial distribution of Rdd proteins in embryos at the tailbud stage by whole-mount antibody staining. Positive signals were detected in the regions of vitelline veins at the abdomen, inter-somites, ventral border of somites, and branchial arches (Fig. 4B). The antibody reaction was rdd transcript-specific because the signal in the whole-mount staining decreased in the Rdd3/4 MO-injected embryo (Fig. 4B). Signal intensity of Rdd3/4 MO-injected embryo was much less than that of control MO-injected embryo (Fig. 4C). In sections of stained embryos, the signals were found in the lateral plate and ventral mesoderm and also in the pharyngeal pouches where the neural crest-derived cells exist (Fig. 4D). Absorption of the antibody with Rdd peptide resulted in a marked reduction of signals in the mesoderm area, indicating that these signals are specific to Rdd proteins.
Fig. 4.
Detection of endogenous Rdd proteins in the embryo. (A) Western blot analysis was performed using anti-Rdd antibody to detect the Rdd protein in extracts from pooled embryos at various stages. A single band at 28 kDa was detected at the gastrula and tailbud stages. The signal for detecting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is shown as a loading control. (B) Whole-mount immunostaining analysis showed the localization of endogenous Rdd protein in intact (a-d) or Rdd3/4 Morpholino oligo (MO) (9.2 pmol/embryo)-injected (e-h) albino embryos at the tailbud stage (st. 32). Staining was found in the vitelline vein (b, arrowheads), intersomitic region (c, white arrowheads), hypaxial muscle segment (c, arrowheads) and branchial arches (d, arrowheads). The intensity of staining was reduced in the rdd3/4 MO-injected embryo (e-h). (C) Another experiment shows the staining of Rdd protein in control MO (a)- or Rdd3/4 MO (b)-injected wild-type embryos at the tailbud stage (st. 32). (D) Histological views of the lateral (a-c), ventral (d-f ), and pharyngeal (g-i) parts of albino embryos are shown. Sections were stained by hematoxylin and eosin (H&E) (a, d, g) or immunostained using anti-Rdd antibody in the absence (b, e, h) or presence (c, f, i) of Rdd peptide. a, b, c and d, e, f are distinct but neighboring cross sections. g, h, i are horizontal sections. Endogenous Rdd protein was detected in the intermediate mesoderm (b, arrowheads), in the ventral mesoderm (e, arrowheads), and in the branchial arches (h, arrowheads). en, endoderm; pt, pronephric tube. Scale bars=0.5 mm (B, C), 50 µm (D).
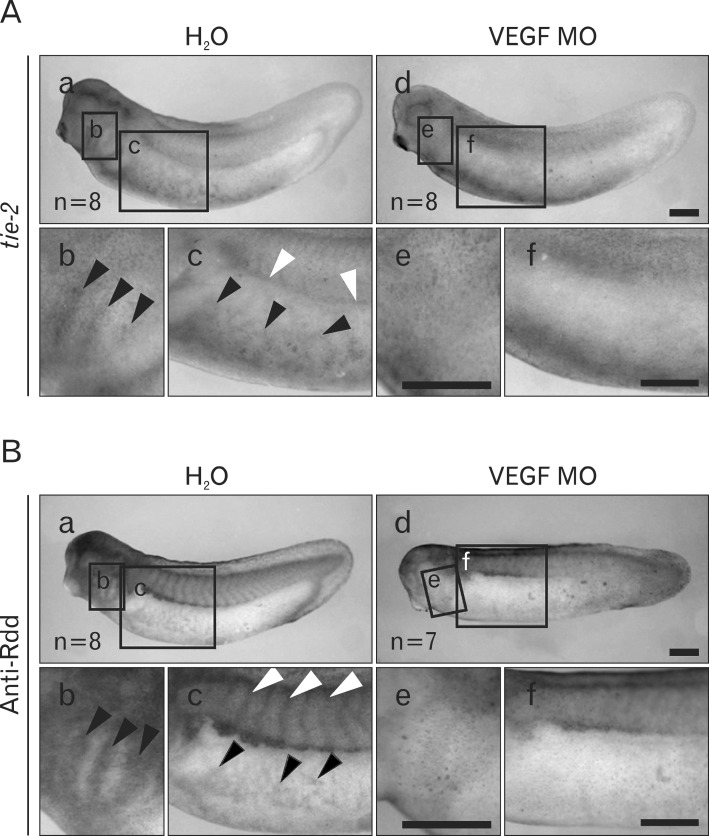
A knockdown experiment in our previous study indicated that Rdd3 and Rdd4 are necessary for morphogenesis of blood vessel formation [30]. The existence of Rdd proteins in the regions for primary vascular formation, therefore, prompted us to further examine the relationship between vascular precursor cells and localization of Rdd protein. For this purpose, we injected VEGF MO into all blastomeres at the 4-cell stage to disturb formation of the vascular structure. This treatment suppressed the expression of tie-2, a vascular precursor cell marker (Fig. 5A), at the tailbud stage, and resultant tadpoles had an impaired morphology of vascularization (data not shown) [28]. The tailbud embryos injected with VEGF MO showed loss of Rdd protein localization at the vascular structures, such as vitelline veins, inter-somitic vasculature, and branchial arches (Fig. 5B). In contrast, staining of Rdd proteins remained at muscle segments found in the ventral border region of somites (Fig. 5B). These observations support the idea that localization of Rdd proteins correlates to morphogenesis of the vascular structure in the Xenopus tailbud embryo.
Fig. 5.
Concomitant localization of Rdd3 protein with blood vessel formation. Whole-mount in situ hybridization (A) and whole-mount immunostaining (B) analyses were performed to show tie-2 expression and Rdd protein localization in tailbud (st. 32) embryos. Embryos were injected with vascular endothelial growth factor Morpholino oligo (VEGF MO) (9.2 pmol/embryo) (d-f ) or H2O (a-c) in the marginal zone at the 4-cell stage. (A) In the control embryo, tie-2 was expressed in the vitelline vein (b, arrowheads), intermediate mesoderm (c, white arrowheads) and branchial arches (c, arrowheads). Localization of tie-2 expression was lost in the VEGF MO-injected embryo (d-f ). (B) Rdd protein was detected in the vitelline vein (b, arrowheads), intersomite region (c, white arrowheads), and branchial arches (c, arrowheads). Localization of Rdd protein was lost in the VEGF MO-injected embryo (d-f ). Scale bars=0.5 mm (A, B).
Discussion
We have reported isolation and functional characterization of repeated D domain-like (rdd) genes in the Xenopus laevis embryo [30]. Although it has been shown that Rdd3 and Rdd4 are involved in the morphogenesis of blood vessels in the frog embryo, the molecular basis of the action of Rdd proteins has not been elucidated. In an attempt to further understand the essential role of Rdd proteins in embryogenesis, we generated an antibody to the Rdd proteins. The Rdd antibody recognizes a single size of molecule in embryonic extracts of embryos of different stages (Fig. 4A). Intensity of the signal gradually decreased after the tailbud stage, partially because the expression of rdd transcripts decreased as the stages advanced. Also, we suggest that extraction of proteins becomes inefficient after the late tailbud stage since extraction of proteins was done without a detergent. Consistently, a decrease in the protein amount of GAPDH was also found in the extract from a swimming tadpole (Fig. 4A). It is possible that the synthetic peptide has a common epitope with the protein from a different gene, and it is therefore difficult to conclude that the signal obtained by the antibody reaction is Rdd-specific. In fact, we performed Western blot analysis for proteins extracted from various adult tissues and found a clear positive signal in the stomach, lung and spleen, although no transcript of Rdd3/4 was expressed in these organs (data not shown). The signals found in the whole-mount immunostaining are derived from Rdd proteins because the embryos injected with Rdd3/4 MO showed a marked reduction of positive signals. In addition, a single size of protein was detected by Western blot analysis in the tailbud embryo. We conclude, therefore, that the antibody specifically recognizes Rdd proteins.
An intriguing finding in the present study is a concomitant pattern of the Rdd protein localization and the primary vasculature structure in embryogenesis. We have shown three observations as evidence that substantiate the above conclusion observation. At first, we compared the distribution of Rdd protein detected by anti-Rdd antibody and the expression pattern of tie-2, a vascular precursor marker. The antibody reacted in particular regions including the intermediate mesoderm, inter-somites, vitellin veins in the abdomen, branchial arches, and optic vesicle. A similar pattern was observed in whole-mount in situ hybridization analysis of tie-2. Second, we suppressed endogenous VEGF expression by injecting a VEGF Morpholino. As also shown in a previous study, the VEGF morphant exhibited reduced expression of tie-2 in the tailbud embryo and hampered formation of the vasculature structure at the following tadpole stage. The localization of Rdd proteins in vascular precursor cells was lost in the morphant animals. Finally, histological analysis of the Rdd-stained embryos showed that positive signals were present in the mesodermal layer at the intermediate and ventral regions.
In addition to the association of Rdd proteins with the vascular structure, Rdd proteins were also stained in the ventral border of somites where hypaxial muscle segments are located. These muscle segments have been shown to migrate toward the ventral region and participate in formation of the ventral muscle wall [35]. Being consistent with that observation, we found that formation of muscle segments was disturbed in Rdd3/4 MO-injected animals (data not shown), suggesting that Rdd proteins are involved not only in vascular morphogenesis but also in muscle cell migration at the ventral region. Furthermore, the Rdd proteins were stained in branchial arches, where endothelial precursor cells and neural crest-derived cells were located (Fig. 4D). Although it is difficult to distinguish which cell type is positive for Rdd staining, we observed that migration of the cranial neural crest was disturbed in the Rdd3/4 MO-injected embryo (data not shown). Taken together, the results indicate that the antiserum to Rdd proteins probably detects endogenous Rdd proteins associated with vascular precursor cells, hypaxial muscle segments, and cranial neural crest cells.
A search for a homologous protein sequence of Rdd using the Metazome program (Joint Genome Research) revealed a homologous amino acid sequence in a beetle (Tribolium castaneum) and a tunicate (Ciona savignyi). A hypothetical protein found in Ciona is a secretory protein consisting of 270 amino acids (NCBI, ENSCSAVT00000012655). This protein showed 41% identity to Rdd4 (286 amino acids) and had highly conserved positions of the cysteine residue, suggesting that these two proteins are functionally related. As previously pointed out, similarity in the cysteine composition was found in the Rdds and the D domain of von Willebrand factor [36]. It has been suggested that the protein-protein interaction through the D domain is essential for the coagulation of the blood plasma [36]. Although a similarity may exist in the sense of biochemical property between Rdds and the D domain of vol Willebrand factor, it is difficult to discuss a common role of the two proteins because the total sizes of the two proteins are so much different. Likewise, there is sequence similarity between Rdd proteins and zonadhesin isolated from the sperm membrane [37, 38], but the functional relationship between these two proteins is unknown. Taken together, results of the analysis of amino acid sequence similarity among the proteins of different species suggest that a group of low molecular weight proteins are conserved in lower vertebrate and invertebrate animals and that such proteins are lost in avian and mammalian animals.
The present study showed localization of endogenous Rdd proteins in particular tissues of developing embryos. Rdd proteins were secreted into the medium when they were expressed in oocytes [30]. Since the rdd transcripts are found in a broad area of the trunk region and are not localized in the vascular structure, we suggest that the Rdd proteins, once secreted from the expressing cells, become localized in particular cells by unknown mechanisms. To trace the proteins after secretion, we overexpressed tag-labeled Rdd proteins in the animal cap system. We detected recombinant Rdd proteins in the intercellular space in the neighboring region of injected cells. However, we found that it is difficult to detect the fluorescent protein in mesodermal and endodermal cells, because these cells show autofluorecence in nature. Further studies will be needed to identify the molecule(s) that binds to the Rdd proteins and that makes the Rdd proteins localize in migrating vascular cells, muscle cells and neural crest cells.
Acknowledgements
This work was partly supported by a grant-in-aid from The Ministry of Education, Science and Culture of Japan (24570234).
References
- 1.Maéno M, Todate A, Katagiri C. The localization of precursor cells for larval and adult hemopoietic cells of Xenopus laevis in two regions of embryos. Dev Growth Differ. 1985;27:137–148. doi: 10.1111/j.1440-169X.1985.00137.x. [DOI] [PubMed] [Google Scholar]
- 2.Maéno M, Tochinai S, Katagiri C. Differential participation of ventral and dorsolateral mesoderms in the hemopoiesis of Xenopus, as revealed in diploid-triploid or interspecific chimeras. Dev Biol. 1985;110:503–508. doi: 10.1016/0012-1606(85)90108-3. [DOI] [PubMed] [Google Scholar]
- 3.Dale L, Howes G, Price BM, Smith JC. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992;115:573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- 4.Jones CM, Lyons KM, Lapan PM, Wright CV, Hogan BL. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992;115:639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- 5.Maeno M, Ong RC, Suzuki A, Ueno N, Kung HF. A truncated bone morphogenetic protein 4 receptor alters the fate of ventral mesoderm to dorsal mesoderm: roles of animal pole tissue in the development of ventral mesoderm. Proc Natl Acad Sci U S A. 1994;91:10260–10264. doi: 10.1073/pnas.91.22.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki A, Thies RS, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran HT, Sekkali B, Van Imschoot G, Janssens S, Vleminckx K. Wnt/beta-catenin signaling is involved in the induction and maintenance of primitive hematopoiesis in the vertebrate embryo. Proc Natl Acad Sci U S A. 2010;107:16160–16165. doi: 10.1073/pnas.1007725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mead PE, Kelley CM, Hahn PS, Piedad O, Zon LI. SCL specifies hematopoietic mesoderm in Xenopus embryos. Development. 1998;125:2611–2620. doi: 10.1242/dev.125.14.2611. [DOI] [PubMed] [Google Scholar]
- 9.Mead PE, Deconinck AE, Huber TL, Orkin SH, Zon LI. Primitive erythropoiesis in the Xenopus embryo: the synergistic role of LMO-2, SCL and GATA-binding proteins. Development. 2001;128:2301–2308. doi: 10.1242/dev.128.12.2301. [DOI] [PubMed] [Google Scholar]
- 10.Walmsley M, Ciau-Uitz A, Patient R. Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development. 2002;129:5683–5695. doi: 10.1242/dev.00169. [DOI] [PubMed] [Google Scholar]
- 11.Kelley C, Yee K, Harland R, Zon LI. Ventral expression of GATA-1 and GATA-2 in the Xenopus embryo defines induction of hematopoietic mesoderm. Dev Biol. 1994;165:193–205. doi: 10.1006/dbio.1994.1246. [DOI] [PubMed] [Google Scholar]
- 12.Maeno M, Mead PE, Kelley C, Xu RH, Kung HF, Suzuki A, Ueno N, Zon LI. The role of BMP-4 and GATA-2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis. Blood. 1996;88:1965–1972. [PubMed] [Google Scholar]
- 13.Dalgin G, Goldman DC, Donley N, Ahmed R, Eide CA, Christian JL. GATA-2 functions downstream of BMPs and CaM KIV in ectodermal cells during primitive hematopoiesis. Dev Biol. 2007;310:454–469. doi: 10.1016/j.ydbio.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Costa RM, Love NR, Soto X, Roth M, Paredes R, Amaya E. C/EBPalpha initiates primitive myelopoiesis in pluripotent embryonic cells. Blood. 2009;114:40–48. doi: 10.1182/blood-2008-11-189159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa RM, Soto X, Chen Y, Zorn AM, Amaya E. spib is required for primitive myeloid development in Xenopus. Blood. 2008;112:2287–2296. doi: 10.1182/blood-2008-04-150268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol. 2008;18:1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 17.Salanga MC, Meadows SM, Myers CT, Krieg PA. ETS family protein ETV2 is required for initiation of the endothelial lineage but not the hematopoietic lineage in the Xenopus embryo. Dev Dyn. 2010;239:1178–1187. doi: 10.1002/dvdy.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meadows SM, Salanga MC, Krieg PA. Kruppel-like factor 2 cooperates with the ETS family protein ERG to activate Flk1 expression during vascular development. Development. 2009;136:1115–1125. doi: 10.1242/dev.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciau-Uitz A, Pinheiro P, Gupta R, Enver T, Patient R. Tel1/ETV6 specifies blood stem cells through the agency of VEGF signaling. Dev Cell. 2010;18:569–578. doi: 10.1016/j.devcel.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 21.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 22.Koibuchi N, Taniyama Y, Nagao K, Ogihara T, Kaneda Y, Morishita R. The effect of VEGF on blood vessels and blood cells during Xenopus development. Biochem Biophys Res Commun. 2006;344:339–345. doi: 10.1016/j.bbrc.2006.03.140. [DOI] [PubMed] [Google Scholar]
- 23.Nagao K, Taniyama Y, Koibuchi N, Morishita R. Constitutive over-expression of VEGF results in reduced expression of Hand-1 during cardiac development in Xenopus. Biochem Biophys Res Commun. 2007;359:431–437. doi: 10.1016/j.bbrc.2007.05.140. [DOI] [PubMed] [Google Scholar]
- 24.Cleaver O, Tonissen KF, Saha MS, Krieg PA. Neovascularization of the Xenopus embryo. Dev Dyn. 1997;210:66–77. doi: 10.1002/(SICI)1097-0177(199709)210:1<66::AID-AJA7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:3905–3914. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- 26.Iraha F, Saito Y, Yoshida K, Kawakami M, Izutsu Y, Daar IO, Maéno M. Common and distinct signals specify the distribution of blood and vascular cell lineages in Xenopus laevis embryos. Dev Growth Differ. 2002;44:395–407. doi: 10.1046/j.1440-169x.2002.00653.x. [DOI] [PubMed] [Google Scholar]
- 27.Walmsley M, Cleaver D, Patient R. Fibroblast growth factor controls the timing of Scl, Lmo2, and Runx1 expression during embryonic blood development. Blood. 2008;111:1157–1166. doi: 10.1182/blood-2007-03-081323. [DOI] [PubMed] [Google Scholar]
- 28.Maéno M, Komiyama K, Matsuzaki Y, Hosoya J, Kurihara S, Sakata H, Izutsu Y. Distinct mechanisms control the timing of differentiation of two myeloid populations in Xenopus ventral blood islands. Dev Growth Differ. 2012;54:187–201. doi: 10.1111/j.1440-169x.2011.01321.x. [DOI] [PubMed] [Google Scholar]
- 29.Kazanskaya O, Ohkawara B, Heroult M, Wu W, Maltry N, Augustin HG, Niehrs C. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development. 2008;135:3655–3664. doi: 10.1242/dev.027284. [DOI] [PubMed] [Google Scholar]
- 30.Shibata T, Takahashi Y, Tasaki J, Saito Y, Izutsu Y, Maéno M. A role of D domain-related proteins in differentiation and migration of embryonic cells in Xenopus laevis. Mech Dev. 2008;125:284–298. doi: 10.1016/j.mod.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Takahashi Y, Izutsu Y, Maéno M. Identification and expression of ventrally associated leucine-zipper (VAL) in Xenopus embryo. Int J Dev Biol. 2010;54:203–208. doi: 10.1387/ijdb.082743ys. [DOI] [PubMed] [Google Scholar]
- 32.Niuewkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: Elsevier; 1994. [Google Scholar]
- 33.Suzuki M, Takamura Y, Maéno M, Tochinai S, Iyaguchi D, Tanaka I, Nishihira J, Ishibashi T. Xenopus laevis macrophage migration inhibitory factor is essential for axis formation and neural development. J Biol Chem. 2004;279:21406–21414. doi: 10.1074/jbc.M311416200. [DOI] [PubMed] [Google Scholar]
- 34.Shain DH, Zuber MX. Sodium dodecyl sulfate (SDS)-based whole-mount in situ hybridization of Xenopus laevis embryos. J Biochem Biophys Methods. 1996;31:185–188. doi: 10.1016/0165-022x(95)00030-u. [DOI] [PubMed] [Google Scholar]
- 35.Martin BL, Harland RM. Hypaxial muscle migration during primary myogenesis in Xenopus laevis. Dev Biol. 2001;239:270–280. doi: 10.1006/dbio.2001.0434. [DOI] [PubMed] [Google Scholar]
- 36.Verweij CL, Diergaarde PJ, Hart M, Pannekoek H. Full-length von Willebrand factor (vWF) cDNA encodes a highly repetitive protein considerably larger than the mature vWF subunit. EMBO J. 1986;5:1839–1847. doi: 10.1002/j.1460-2075.1986.tb04435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardy DM, Garbers DL. A sperm membrane protein that binds in a species-specific manner to the egg extracellular matrix is homologous to von Willebrand factor. J Biol Chem. 1995;270:26025–26028. doi: 10.1074/jbc.270.44.26025. [DOI] [PubMed] [Google Scholar]
- 38.Gao Z, Garbers DL. Species diversity in the structure of zonadhesin, a sperm-specific membrane protein containing multiple cell adhesion molecule-like domains. J Biol Chem. 1998;273:3415–3421. doi: 10.1074/jbc.273.6.3415. [DOI] [PubMed] [Google Scholar]