Abstract
The pelvic ring is stressed by external forces: by partial body weight, by ligament tension, and by muscles forces stabilizing the hip joints. For the symphysis ossis pubis there exist data concerning the type and magnitude of stresses. In one-leg-standing pressure, shear forces are predominant, and in both-leg-standing tensile forces are acting on the pelvic ring. Rupture of the symphysis is problematic due to the variety of its movements. Most literature descriptions of stress in the symphysis reflect only the frontal plane. Our intention was to make morphological as well as experimental investigations on the symphysis ossis pubis to delineate how it will be stressed in the horizontal plane. Twenty pubic bones taken from embalmed adult human cadavers (12 male, 8 female) were used. Horizontal and frontal slices (3 mm thick) of the symphyseal part of the os pubis were made. X-rays and densitometric analysis were performed. The width of the symphysis cartilage in the dorsal and the ventral regions was measured on 15 whole skeleton specimens coming from adult human cadavers. For experimental study an embalmed pelvic ring which had no abnormality was used. The symphysis pubis was cut completely in the midsagittal plane and then the ring was stressed via the cranial sacrum. Our results demonstrate that the symphysis is stressed by bending in the horizontal plane in one-leg-standing. In both-leg-standing the symphysis is stressed by tensile forces.
Keywords: Pubic symphysis, Stress, Biomechanics
Introduction
The bony pelvis is a complex structure with variations in geometrical and mechanical characteristics [1]. The pelvic ring is stressed by external forces: by partial body weight, by ligament tension and muscles stabilizing the hip joints. Little is known about the basic mechanics of the pelvic bones. The pubic symphysis is composed of the paired pubic bones and an intercalated disk. The medial border of the pubic body forms the bony surface of the pubic symphysis. This surface is ovoid, covered by a thin layer of hyaline cartilage, and composed of transversely oriented alternating ridges and grooves that may help protect the joint from shear forces [2].
The disk and four ligaments provide soft tissue support to the osseous structures of the pubic symphysis [2]. The interpubic disk is a fibrocartilaginous structure interposed between the ridges and grooves of the pubic symphyseal surfaces. Its main function during normal motion is to absorb and dissipate axial and shear forces experienced at the joint [2].
The pubic symphysis itself has important functions. First, it stabilizes the anterior pelvis, while allowing a small degree of movement [2]. Stability is usually defined as the pelvis' ability to allow only physiologic displacements under functional loads. Normally the symphysis translates in the transverse and sagittal directions, rotates in the frontal and sagittal planes and moves in vertical direction [3].
The relatively large contact areas allow an even distribution of the superior and inferior shear forces generated during walking and running, thus helping protect the symphysis from injury. The rami also support this function by transmitting compressive forces generated at the symphysis to the remainder of the innominate bone [2]. There are data on the type and magnitude of stresses acting on the symphysis ossis pubis. Strain gauge techniques, finite element analyses and three dimensional finite element analyses are the methods most frequently used for studying pelvic mechanics [4, 5]. In one-leg-standing pressure and shear forces are acting [1, 5,6, 7, 8]; in both-leg-standing tensile forces act on the symphysis pubis [7].
Most literature descriptions of stress in the symphysis reflect only the frontal plane. Therefore the intention was to make morphological and experimental investigations on the symphysis pubis with the aim of defining stresses in the horizontal plane.
Materials and Methods
Morphological and densitometrical analyses
Twenty pubic bones (left, 11; right, 9) taken from embalmed adult human cadavers were used. To harvest the pubic body, the superior and inferior pubic rami and the ischial ramus were separated. The pubic bodies were divided into two groups without regard to right or left. The first group (9 bones) was cut horizontally, the second (11 bones) frontally into three millimeter thick slices with a diamond wire saw. X-rays from frontal and horizontal slices were made. For distribution analysis of the mineral salts inside the cortical bone, densitometry was performed. Finally the width of the symphysis cartilage was measured both in the dorsal and the ventral regions on 15 adult human cadavers.
Experiments
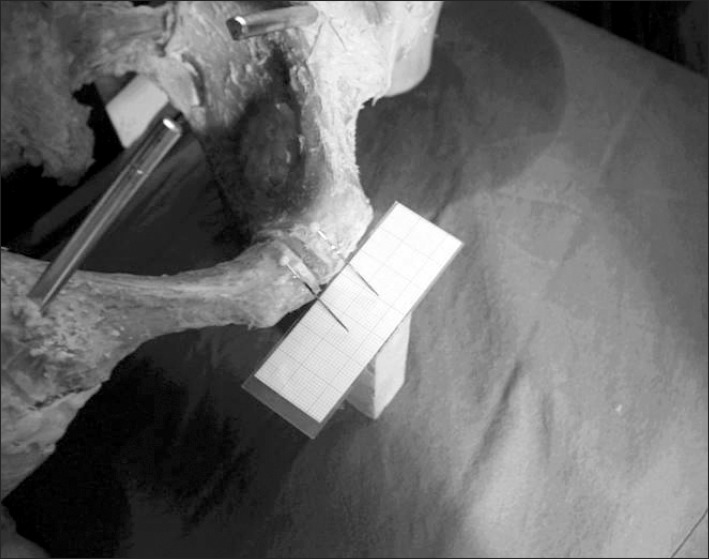
An embalmed cadaveric pelvis (73 years, male) was released from all soft tissue, except the joint capsules and the ligaments. The whole pelvic ring was fixed to a dorsal metal frame by a Steinmann nail inserted into the middle part of the sacrum. The frame allowed movement of the pelvic ring in the vertical plane only. The head and the proximal third of the femurs were fixed to the acetabulum with bone cement (Palakos, Merck, Darmstadt, Germany) in neutral position. This position was held by a Steinmann nail on both sides (Fig. 1). The pubic symphysis was cut in the midsagittal plane and the cartilage was removed. On both sides needles were glued on the pubic tubercle to help to measure the changing distance between the pubic branches.
Fig. 1.
Study experimental design.
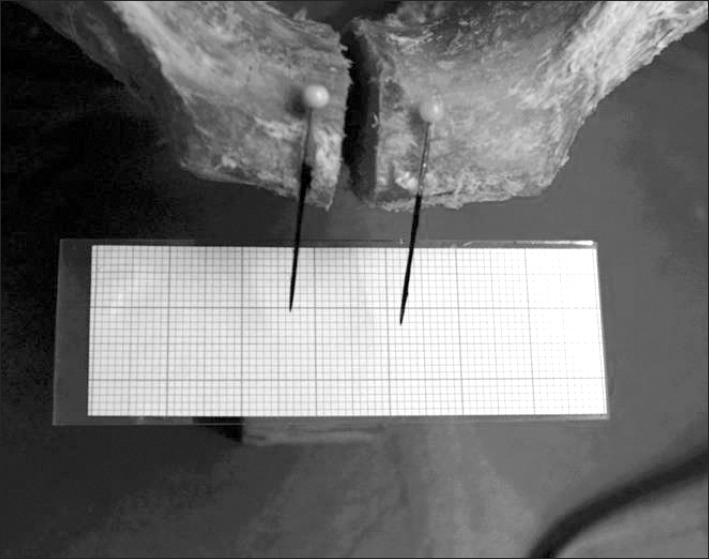
While the pelvis was standing on two legs a 150 kg spinal force was applied on it. Then the pelvis was made to stand on one leg and an adjustable cable was attached to the specimen from the iliac wing to the greater trochanter, to mimic the abductor muscles of the hip (gluteus medius and minimus muscles) and 100 kg spinal force was applied on it. The distance between the pubic branches was measured by a millimeter-scale and documented photographically (Fig. 1).
Results
In the frontal plane the sections show a general arrangement of the bony trabeculae running rectangularly to the compact bone and delineating the symphyseal cleft. The compact bone lamella is thicker and more dense in the caudal part (Fig. 2). In the horizontal plane the dorsal cortical bone was always thicker and more dense than the ventral cortical bone (Fig. 3). In all cases the bone trabeculae form two bundles intersecting each other in a gothic arc like manner. One bundle arises from the inner ventral bone lamella and runs to the dorsal one "orsal bundle" the second bundle runs vice-versa "entral bundle"(Fig. 4). In cases of osteoporosis there is a rarefication of this system with maintaining substantly the dorsal bundle.
Fig. 2.
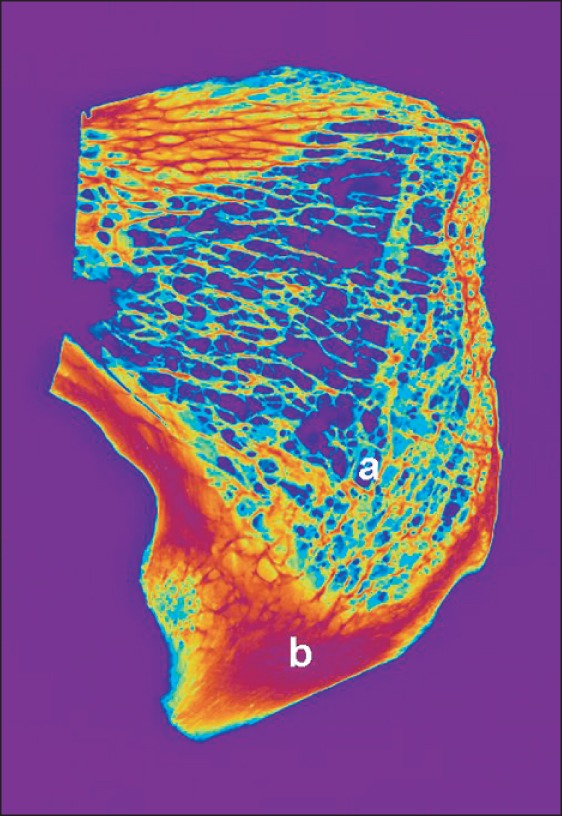
Arrangement of bone trabeculae in the frontal plane. a, run rectangularly to the compact bone delineating the symphyseal cleft; b, compact bone lamella is thicker and denser in the caudal part.
Fig. 3.
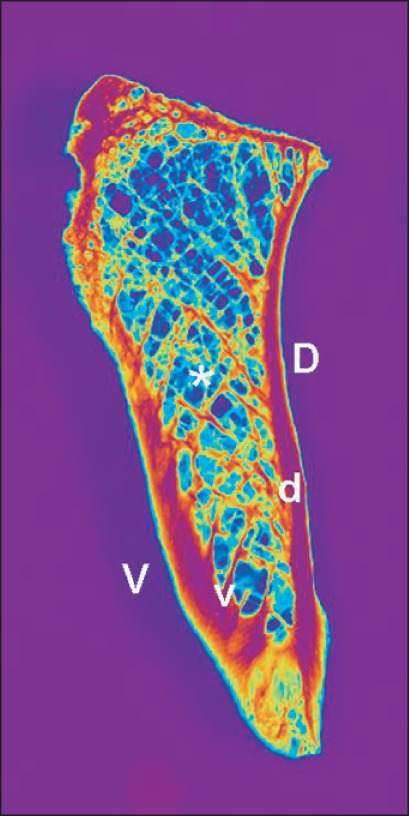
Arrangement of the bone trabeculae in the horizontal plane. d, dorsal bundle; v, ventral bundle; D, dorsal cortical bone; V, ventral cortical bone. The D was thicker and more dense than the V. *Bone trabeculae bundles intersecting each other.
Fig. 4.
In one-leg-standing the symphyseal cleft is narrowest in its caudal part.
The width of the symphysis is different in all 15 whole skeleton specimens. The mean difference between the ventral and dorsal regions is 5.07 mm.
The experiment simulating one-leg-standing leads to a narrowing of the symphysis. Furthermore there is a caudally directed moving of the bone on the side without ground contact and an additional tilting so that the symphyseal cleft is narrowest in its caudal part (Fig. 4). In both-leg-standing the symphyseal cleft widens in all its parts equally (Fig. 5).
Fig. 5.
In both-leg-standing the symphyseal cleft widens in all its parts equally.
Discussion
Many investigations show that the bone is functionally adapted to the magnitude, the distribution and the kind of external stresses acting on it. As described by Wolff [9] and later confirmed by several authors, including Pauwels [8], Kummer [9], and Holm [9], the trabecular structure of the spongious bone is oriented according to the directions of the principal stresses and may be considered to represent the course of the stress trajectories. It should therefore be possible to analyse the trabecular structure in the pelvis and from this to evaluate the stress situation [9]. There are few investigations concerning the architecture of the spongious bone of the pelvis performed by Holm [9] and Sciascia [10]. Only Sciascia [10] gives a short descripition of the internal architecture of the symphyseal bones, but without a functional explanation. Euler [11] analysed the stress of the pubic symphysis using strain gauge or photoelastic methods. But also in his monograph there is no detailed analysis of the symphysis in the horizontal plane [11].
In their study Dalstra and Huiskes [12] stated that the pelvic bone has evolved into a very efficient structure, which is well able to carry these large forces. It resembles a so-called 'sandwich construction' used in engineering to combine high strength and low weight, consisting mainly of low-density trabecular bone, totally covered by a thin layer of cortical bone [12]. In the 3D serial reconstruction of the specimens Dalstra et al. [4] observed a plate-like trabecular structure whereby the plates were always oriented more or less perpendicular to the cortical shells. Since the pelvic trabecular bone will predominantly have to withstand shear-loading modes the plate-like structure gives the best resistance [4].
In the pelvis, however, considerable changes of the force flow will depend upon the position in which the pelvis is loaded. In both-leg-standing it is generally accepted that tensile stresses act on the symphysis, while in one-leg-standing pressure and shearing forces are predominant. [5, 6, 7, 8, 9, 13, 14]. In his histological examinations of the symphysis Pauwels [8] described that bundles of collagen fibers, running oblique from one border to the other, will absorb shear stresses; bundles running horizontally absorb tensile stresses and cartilage tissue absorb pressure forces. Pauwels [8] and Holm [9] stated that the arrangement of the spongious bone is characteristic for bending. However these data are relevant for analysis in the frontal plane only.
The results of this study demonstrated that the symphysis is stressed by bending in the horizontal plane in one-leg-standing. The bending moment is given by pressure mainly in the dorsal part of the symphysis which is narrower than the ventral part. Furthermore the tilting moment will lead to a narrowing of the caudal symphyseal cleft and in consequence to a high local pressure peak in this part. This is reflected by the bundles of fibers, running oblique between the borders and the higher density of cortical and spongy bone in this region as it is demonstrated by the radiological and densitometrical analysis of the frontal sections.
In both-leg-standing we note in accord with the literature a general widening of the cleft, meaning the symphysis is stressed by tensile forces [8, 9, 15]. These will be absorbed by the ligamentous system of the symphysis constituted of the fibers, running horizontally, in horizontal sections.
The problem of determining the distribution of stresses within the skeletal structure has been of interest for surgeons and engineers [13]. Earlier experimental and finite element studies notwithstanding, the load transfer and stress distribution in the pelvic bone in normal conditions are not well understood. This hampers the development of orthopaedic reconstruction methods [12]. The study of the forces and stresses helps in the rational design of the surgical implants such as bone plates, screws and joint replacements [13]. For estimation of the loads of fixation devices, physiological movements and acting forces at the symphysis pubis have to be well understood [16].
References
- 1.Ries M, Pugh J, Au JC, Gurtowski J, Dee R. Normal pelvic strain pattern in vitro. J Biomed Eng. 1989;11:398–402. doi: 10.1016/0141-5425(89)90103-9. [DOI] [PubMed] [Google Scholar]
- 2.Omar IM, Zoga AC, Kavanagh EC, Koulouris G, Bergin D, Gopez AG, Morrison WB, Meyers WC. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28:1415–1438. doi: 10.1148/rg.285075217. [DOI] [PubMed] [Google Scholar]
- 3.Walheim G, Olerud S, Ribbe T. Mobility of the pubic symphysis. Measurements by an electromechanical method. Acta Orthop Scand. 1984;55:203–208. doi: 10.3109/17453678408992338. [DOI] [PubMed] [Google Scholar]
- 4.Dalstra M, Huiskes R, Odgaard A, van Erning L. Mechanical and textural properties of pelvic trabecular bone. J Biomech. 1993;26:523–535. doi: 10.1016/0021-9290(93)90014-6. [DOI] [PubMed] [Google Scholar]
- 5.Fabeck L, Descamps PY, Bourgois R, Dhem A. Contribution to the study of pelvic stress during weight-bearing. Role of the pubic branch and trabecular bone. Rev Chir Orthop Reparatrice Appar Mot. 1994;80:181–187. [PubMed] [Google Scholar]
- 6.MacAvoy MC, McClellan RT, Goodman SB, Chien CR, Allen WA, van der Meulen MC. Stability of open-book pelvic fractures using a new biomechanical model of single-limb stance. J Orthop Trauma. 1997;11:590–593. doi: 10.1097/00005131-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Vrahas M, Hern TC, Diangelo D, Kellam J, Tile M. Ligamentous contributions to pelvic stability. Orthopedics. 1995;18:271–274. doi: 10.3928/0147-7447-19950301-09. [DOI] [PubMed] [Google Scholar]
- 8.Pauwels F. Gesammelte abhandlungen zur funktionellen anatomie des bewegungsapparates. Berlin: Springer-Verlag; 1965. pp. 183–196. [Google Scholar]
- 9.Holm NJ. The internal stress pattern of the os coxae. Acta Orthop Scand. 1980;51:421–428. doi: 10.3109/17453678008990818. [DOI] [PubMed] [Google Scholar]
- 10.Sciascia R. Anatomic and radiologic observations on the general architecture of the spongy substance of the human os coxae. Ann Ital Chir. 1967;43:1087–1109. [PubMed] [Google Scholar]
- 11.Euler E. Das Becken: Anatomie, Biomechanik, Frakturver sorgung und Tumorprothetik. Berlin: Springer-Verlag; 1966. pp. 3–33. [Google Scholar]
- 12.Dalstra M, Huiskes R. Load transfer across the pelvic bone. J Biomech. 1995;28:715–724. doi: 10.1016/0021-9290(94)00125-n. [DOI] [PubMed] [Google Scholar]
- 13.Goel VK, Valliappan S, Svensson NL. Stresses in the normal pelvis. Comput Biol Med. 1978;8:91–104. doi: 10.1016/0010-4825(78)90001-x. [DOI] [PubMed] [Google Scholar]
- 14.Goel VK, Svensson NL. Forces on the pelvis. J Biomech. 1977;10:195–200. doi: 10.1016/0021-9290(77)90058-6. [DOI] [PubMed] [Google Scholar]
- 15.Varga E, Hearn T, Powell J, Tile M. Effects of method of internal fixation of symphyseal disruptions on stability of the pelvic ring. Injury. 1995;26:75–80. doi: 10.1016/0020-1383(95)92180-i. [DOI] [PubMed] [Google Scholar]
- 16.Meissner A, Fell M, Wilk R, Boenick U, Rahmanzadeh R. Biomechanical investigation of the pubic symphysis. Which forces induce mobility of the symphysis under physiological conditions? Unfallchirurg. 1996;99:415–421. [PubMed] [Google Scholar]