Abstract
Mucoadhesion can be defined as a state in which two components, of which one is of biological origin, are held together for extended periods of time by the help of interfacial forces. Among the various transmucosal routes, buccal mucosa has excellent accessibility and relatively immobile mucosa, hence suitable for administration of retentive dosage form. The objective of this paper is to review the works done so far in the field of mucoadhesive buccal drug delivery systems (MBDDS), with a clinical perspective. Starting with a brief introduction of the mucoadhesive drug delivery systems, oral mucosa, and the theories of mucoadhesion, this article then proceeds to cover the works done so far in the field of MBDDS, categorizing them on the basis of ailments they are meant to cure. Additionally, we focus on the various patents, recent advancements, and challenges as well as the future prospects for mucoadhesive buccal drug delivery systems.
Keywords: buccal mucosa, clinical perspective, mucoadhesion, patents, transmucosal route
INTRODUCTION
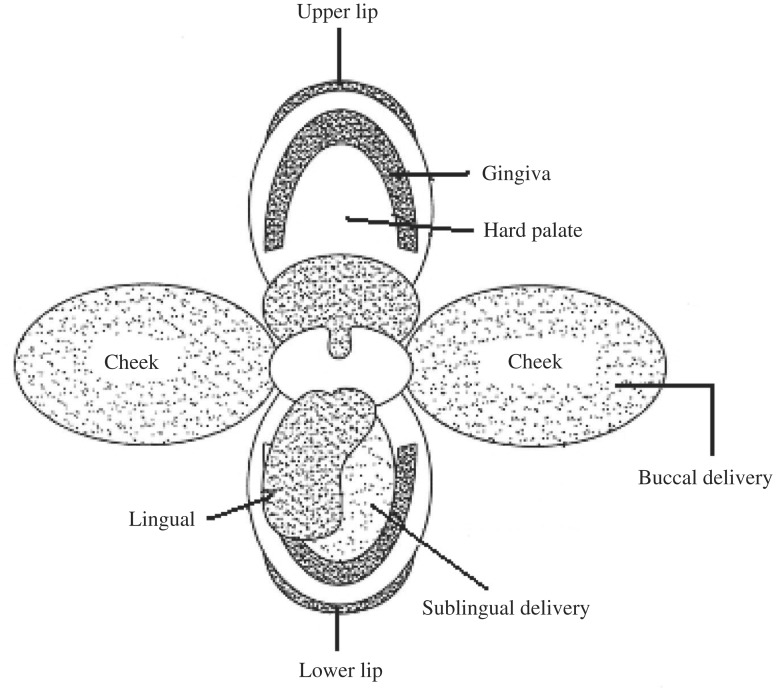
Oral drug administration is the preferred and most common route for drug delivery. Several advantages associated with it include: it is patient-friendly, painless, has the ease of self-medication, and allows for a flexible and controlled dosing schedule in comparison to most other drug delivery systems. Although the oral route is preferred for administration of drugs, it also presents major disadvantages such as first pass effect, gastrointestinal enzymatic degradation and delay between the time of administration and absorption, which is detrimental in the case of drugs with rapid onset requirements. These difficulties have provided the impetus for exploring alternative routes for the delivery of drugs, which include routes such as pulmonary, ocular, nasal, rectal, buccal, sublingual, vaginal, and transdermal. Transmucosal routes of drug delivery which is comprised of the mucosal linings of the nasal, rectal, vaginal, ocular, and oral cavity offer excellent opportunities and potential advantages over peroral administration for systemic drug delivery[1]–[5]. These advantages include possible bypass of first pass effect, avoidance of presystemic elimination within the GI tract and, depending on the particular drug, a better enzymatic flora for drug absorption. Within the oral mucosal cavity, delivery of drugs is broadly classified into two categories: (a) sublingual delivery, which is systemic delivery of drugs through the mucosal membranes lining the floor of the mouth and (b) buccal delivery, which is drug administration through mucosal membranes lining the cheeks (buccal mucosa)[2] (Fig. 1).
Fig. 1. Two main sites for drug delivery in the oral cavity.
The buccal region of oral cavity is an attractive site for the delivery of drugs owing to the ease of administration. Buccal drug delivery involves the administration of the desired drug through the buccal mucosal membrane lining of the oral cavity. This route is useful for mucosal (local effect) and transmucosal (systemic effect) drug administration. In the first case, the aim is to achieve a site-specific release of the drug on the mucosa, whereas the second case involves drug absorption through the mucosal barrier to reach the systemic circulation. Since the drug content within the buccal formulations can be considerably lower than tablets and capsules, toxicity or undesired side effects will potentially be significantly reduced[4],[5].
Theories of bioadhesion and mucoadhesion
Adhesion can be defined as the bond produced by contact between a pressure-sensitive adhesive and a surface. There are many different terminological subsets of adhesion depending upon the environment in which the process occurs. When adhesion occurs in a biological setting it is often termed “bioadhesion”; furthermore, if this adhesion occurs on mucosal membranes, it is termed “mucoadhesion”. Bioadhesion is defined as the state in which two materials, at least one biological in nature, are held together for an extended period of time by interfacial forces[6]–[8].
For drug delivery purposes, the term bioadhesion implies attachment of a drug carrier system to a specified biological location. Bioadhesion and mucoadhesion have been widely promoted as a way of achieving targeted drug delivery to an active site of choice through the incorporation of bioadhesive hydrophilic polymers within pharmaceutical formulations along with the active pharmaceutical ingredient (API). The rationale being that the formulation will be ‘held’ on or at the biological surface and the API will be released close to the absorptive membrane, with a consequent enhancement of bioavailability[6],[9].
Many theories have been proposed to describe mucoadhesion, namely adsorption theory, wetting theory, diffusion theory, electronic theory, and fracture theory[3],[10]. In the “adsorption theory”, primary and secondary chemical bonds of the covalent and non-covalent types are formed upon initial contact between the mucous and the mucoadhesive polymer[3]. The “wetting theory” is mainly applicable to liquid or low viscosity mucoadhesive systems and is essentially a measure of the spreadability of the drug delivery system across the biological substrate[12]. The basis of the “diffusion theory” is chain entanglement between glycoproteins of the mucous and the mucoadhesive polymer to create a semi-permanent adhesive bond. The “electronic theory” describes that adhesion occurs by means of electron transfer between the mucous and the mucoadhesive system arising through differences in their electronic structures. The “fracture theory” is perhaps the most widely used theory in studies on the mechanical measurement of mucoadhesion. It analyzes the force required to separate two surfaces after adhesion is established[11],[12].
Anatomy of the buccal mucosa- considerations for development of mucoadhe-sive buccal drug delivery systems (MBDDS)
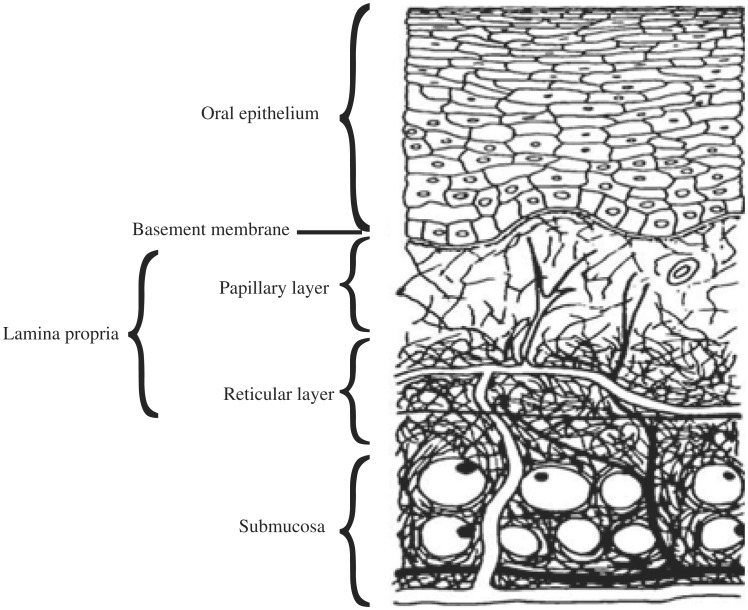
The primary role of the buccal mucosa, like the skin, is to protect underlying structures from foreign agents. The surface of the buccal mucosa consists of a stratified squamous epithelium which is separated from the underlying connective tissue (lamina propria and submucosa) by an undulating basement membrane (Fig. 2). This stratified squamous epithelium consists of differentiating layers of cells (keratinocytes) which change in size, shape, and content as they travel from the basal region to the superficial region, where the cells are shed. Light microscopy reveals several distinct patterns of maturation in the epithelium of the human oral mucosa based on various regions of the oral cavity. The epithelium, as a protective layer for the tissues beneath, is divided into (a) non-keratinized surface in the mucosal lining of the soft palate, the ventral surface of the tongue, the floor of the mouth, alveolar mucosa, vestibule, lips, and cheeks, and (b) keratinized epithelium which is found in the hard palate and non-flexible regions of the oral cavity. The epithelial cells, originating from the basal cells, mature, change their shape, and increase in size while moving towards the surface[3],[13].
Fig. 2. A cross section of the oral mucosa.
The basement membrane forms a distinctive layer between the connective tissues and the epithelium. It provides the required adherence between the epithelium and the underlying connective tissues, and functions as a mechanical support for the epithelium. The underlying connective tissues provide many of the mechanical properties of oral mucosa[3].
Mucus
The tissue layer responsible for formation of the adhesive interface is mucous. Mucus is a translucent and viscid secretion which forms a thin, continuous gel blanket adherent to the mucosal epithelial surface. The mean thickness of this layer varies from about 50 to 450 µm in humans[6]. The thickness of the mucous blanket is determined by the balance between the rate of secretion and the rate of degradation and shedding, and is site dependent[14]. This matrix may actually play a role in cell-cell adhesion, also acting as a lubricant allowing cells to move relative to one another. Similarly, mucus generally plays a critical role in the bioadhesion of mucoadhesive drug delivery systems. Up to 70% of the total mucin found in saliva is contributed by the minor salivary glands. At physiological pH, mucus can form a strongly cohesive gel structure that will bind to the epithelial cell surface as a gelatinous layer. Mucus is composed chiefly of mucins and inorganic salts suspended in water. Mucins contain approximately 95% water, 0.5-5% glycoproteins and lipids, 1% mineral salts and up to 1% free proteins. Mucous glycoproteins are high molecular proteins possessing attached oligosaccharide units. The mucous layer, which covers the epithelial surface, has various roles[6],[15].
The oral mucosa is robust and shows short recovery times after stress or damage. Drug absorption is facilitated by the continuous washing action of saliva (0.5-2 liters per day) over the mucosal surface. This route also allows for accessibility and easy removal of the system in case of an adverse drug reaction. Furthermore, the drug is not subjected to the destructive acidic environment of the stomach; therapeutic serum concentrations of some drugs can be achieved more rapidly. In addition, the drug enters the general circulation without first pass metabolism in the liver. The rich blood supply (20.3 mL/min/100 g tissue) of the oral mucosa offers high permeability to various therapeutic agents (e.g. nitroglycerine). The other functional properties of the buccal mucosa are the relatively high surface area (50.2 cm2) and lower value for membrane thickness (thin membrane) of approximately 500-600 µm, which can, potentially, enhance the rate of drug uptake. A combination of the above factors leads to higher bioavailability. Consequently, these factors support the oromucosal cavity as a highly feasible and rational site for systemic drug delivery [16],[61].
MUCOADHESIVE DOSAGE FORMS: A PROLOGUE
Although the buccal mucosa as a novel drug delivery route is being widely explored recently, its potential as a route for drug delivery was known to mankind centuries ago. Modern day researchers are therefore exploring the various routes available for drug delivery, especially through the oral mucosa, and coming up with novel drug delivery systems.
Tablets
Tablets are small, flat, and oval, with a diameter of approximately 5-8 mm. Unlike conventional tablets, mucoadhesive tablets allow for drinking and speaking without major discomfort. These are placed directly onto the mucosal surface for local or systemic drug delivery. These soften, adhere to the mucosa, and are retained in position until dissolution and or release is complete. Mucoadhesive tablets, in general, have the potential to be used for controlled release drug delivery, but coupling of mucoadhesive properties to tablet has additional advantages. For example, it offers efficient absorption and enhanced bioavailability of the drugs due to a high surface-to-volume ratio and facilitates a much more intimate contact with the mucous layer. Mucoadhesive tablets can be tailored to adhere to any mucosal tissue, including those found in the stomach, thus offering the possibilities of localized as well as systemic controlled release of drugs[17],[18].
In the case of tablets, like other non-wetting solid MDDS, mucoadhesion arises as a result of dehydration of an area of the mucosa. Commercially available tablets are characterized by slow dissolution and maintenance of a therapeutic concentration of the active ingredient in patient's blood for prolonged periods: from 1-2 (Buccastem®) to 8 or more hours (Striant®). Despite the demonstrated efficacy of the local application of mucoadhesive buccal tablets, for example, in the treatment of candidiasis of the oral cavity, the main restriction to their wide use arises from their size and shape, as there is the need for the drug delivery system to make close contact with the mucosal surface[19].
Films/Patches
Mucoadhesive films may be preferred over adhesive tablets in terms of flexibility and comfort. In addition, they can circumvent the relatively short residence time of oral gels on the mucosa, which are easily washed away and removed by saliva. Moreover, in the case of local delivery for oral diseases, the films also help protect the wound surface, thus helping to reduce pain, and treat the disease more effectively. An ideal film should be flexible, elastic, and soft, yet adequately strong to withstand breakage due to stress from mouth movements. It must also possess good mucoadhesive strength in order to be retained in the mouth for the desired duration of action[11],[15].
Buccal patches are described as laminates comprised of an impermeable backing layer, a drug-containing reservoir layer which releases the drug in a controlled manner, and a mucoadhesive surface for mucosal attachment. Patches may be used to deliver drugs directly to a mucosal membrane. These are similar to those used in transdermal drug delivery. They present a greater patient compliance compared with tablets owing to their physical flexibility that causes only minor discomfort to the patient. They also offer advantages over creams and ointments in that they provide a measured dose of drug to the site[11],[20].
Gels and ointments
Semisolid dosage forms, such as gels and ointments, have the advantage of easy dispersion throughout the oral mucosa. However, drug dosing from semisolid dosage forms may not be as accurate as from tablets, patches, or films. Poor retention of the gels at the site of application has been overcome by using mucoadhesive formulations. Certain mucoadhesive polymers, for example, sodium carboxymethylcellulose, carbopol, hyaluronic acid, and xanthan gum, undergo a phase change from liquid to semisolid. This change enhances the viscosity, which results in sustained and controlled release of drugs. Hydrogels are also a promising dosage form for buccal drug delivery[21].
RATIONALIST APPROACH OF MBDDS TOWARDS DIFFERENT DISEASES
Cardio vascular disease
Hypertension, one of the major cardiovascular diseases, needs a lifelong therapy to remain under control. Most of the antihypertensive drugs like carvedilol, metoprolol, propranolol, isosorbide mononitrate etc. have low oral bioavailability and smaller half-life. Two main reasons for low bioavailability are poor aqueous solubility and high first pass metabolism. The buccal mucoadhesive route of drug delivery provides direct access to the systemic circulation through the internal jugular vein by bypassing the first pass metabolism, leading to high bioavailability.
The dose of carvedilol, a model antihypertensive drug, is 25 mg twice a day; however, a lower effective dose is reported to be approximately 3.125 mg. Thus, by increasing the contact time and avoiding the first pass metabolism, a lower amount of drug can effectively produce the normal dose effect. Again, by sustaining the drug release, the frequent administration of drug can be avoided, thereby increasing the patient compliance[22],[23].
Fungal/microbial infections
Oral candidiasis is an opportunistic fungal infection caused by Candida albicans. These yeast infections are usually treated locally by application of gels or suspensions. Release of drugs from these preparations involves an initial burst of activity whose level rapidly declines to subtherapeutic concentrations. Thus, systemic antifungals such as fluconazole are usually preferred for treating oral candidiasis. The oral dose of fluconazole for the treatment of oral candidiasis (100 mg/day for 1 or 2 weeks) results in notable side effects varying from headache, nausea to liver dysfunction, and hepatic failure. Furthermore, oral fluconazole is reported to interact with a number of medications, including oral hypoglycemics, coumarin-type anticoagulants, cyclosporins, terfenadine, theophylline, phenytoin, rifampin, and astemizole. The pathogenic yeasts in oral candidiasis are usually detected in the superficial layers of the oral mucosa. Thus, the effectiveness of the systemic fluconazole may be partially topical through its concentration in oral fluids. The reported topical efficacy of fluconazole together with the adverse effects and drug interaction of systemic fluconazole justifies the design of MBDDS containing a small dose of fluconazole to increase the contact between the drug and the pathogenic yeast for a long time[24],[25].
Migraine
Migraines are thought to occur when certain blood vessels in the brain become swollen (dilated). Drugs used for the treatment include the “triptan” group, comprising of sumatriptan, zolmitriptan, and rizatriptan. These drugs work by helping blood vessels in the brain to return to normal size. It may also block pain signals in the brain. The model drug, sumatriptan is administered orally, in doses of 25, 50 or 100 mg as a single dose, nasally in doses of 10 mg or 20 mg and also subcutaneously as two 6-mg doses over 24 hours. However, a substantial proportion of patients suffer from severe nausea or vomiting during their migraine attack, and also low oral bioavailability (15%) due to high first-pass metabolism may make oral treatment unsatisfactory. Nasal route and subcutaneous route have their own limitations, like lower retention time for nasal solution and inability of self- administration for injectables, respectively[26],[27].
This justifies a need to develop an effective formulation, which allows the drug to directly enter the systemic circulation, bypassing the first-pass metabolism, thereby increasing bioavailability of sumatriptan succinate. Buccal mucosal route is one such alternative.
Nausea and vomiting
Ondansetron HCl, chosen as a model drug for treating postoperative nausea and vomiting associated with emetogenic cancer chemotherapy, possesses certain characteristics that a drug should have to get absorbed through buccal mucosa viz., biphasic solubility and low molecular weight. Moreover, the primary route of ondansetron clearance is by hepatic phase I metabolism, so its bioavailability may be improved when delivered through the buccal mucosal route. Patients may have frequent vomiting following chemotherapy and they may be unable to swallow a tablet to prevent vomiting. It justifies the need to develop a buccal patch/film of ondansetron hydrochloride, which increases patient compliance. Its bioavailability when administered by oral route is only 50% to 60% and its dose is low i.e., 4-8 mg; hence, it can be conveniently loaded onto a patch[28]–[30].
A CLINICAL/THERAPEUTIC APPROACH
Cardio vascular diseases
Carvedilol is a non-selective beta-adrenergic antagonist used in the treatment of hypertension and stable angina pectoris. Yamsani et al. proposed the utilization of carvedilol mucoadhesive tablets for the treatment of hypertension. In this hydrophilic polymer formulation, hydroxypropyl methylcellulose (HPMC K4M and K15M) and Carbopol 934 (CP 934) were used to obtain controlled and zero order release. Studies revealed that increasing the concentration of the polymer in the formulations showed a sustained effect on carvedilol release. The rapidly hydrating polymer dominated in controlling the release of carvedilol from the buccal tablets[31]. Propranolol hydrochloride (PRO-HCl), a nonselective beta-adrenergic blocking agent, has been widely used in the treatment of hypertension, angina pectoris, and many other cardiovascular disorders. Exploring PRO-HCl as a model drug, Patel et al. compared mucoadhesive bilayer buccal tablets with multilayered buccal tablets. An ex-vivo evaluation of the bilayered tablets in natural human saliva revealed sufficient stability criteria by showing no appreciable change in color and shape, and maintaining integrity of the device. Similar results observed in multilayered buccal tablets suggested for a good way to bypass the extensive hepatic first-pass metabolism and to improve the bioavailability of PRO-HCl through buccal mucosa[32]. Buccoadhesive patch of PRO-HCl was developed by the same workers using the hydrophobic polymer Eudragit L-100 as the base matrix. A stability study of optimized Eudragit patches was done in natural human saliva; it was found that both drug and buccal patches were stable in human saliva[33]. Using nifedipine and PRO-HCl, as slightly and highly water-soluble drugs, respectively, Carmen et al. prepared bilaminated films as well as bilayered tablets and demonstrated that these new devices show promising potential for use in controlled delivery of drugs to the oral cavity. The double-layered structure design was expected to provide drug delivery in a unidirectional fashion to the mucosa and avoid loss of drug due to wash-out with saliva[34].
Atenolol, a β-blocker, is prescribed widely in diverse cardiovascular diseases, e.g., hypertension, angina pectoris, arrhythmias, and myocardial infarction. Administration of conventional tablets of atenolol has been reported to exhibit fluctuations in the plasma drug levels, resulting either in manifestation of side effects or reduction in drug concentration at the receptor site[35]. Atenolol was used as a model drug to design oral controlled release mucoadhesive tablet by Singh et al. whereas buccal patches were developed by Mohanty et al. in order to provide sustained buccal delivery for prolonged periods in the management of hypertension[36],[37]. Pravastatin sodium lowers plasma cholesterol levels in hypercholesterolemia subjects. It was used by Shidhaye et al. to develop mucoadhesive bilayered buccal tablets[38]. Lercanidipine hydrochloride (LER) is used in treatment of hypertension because of its selectivity and specificity on the smooth vascular cells[39],[40]. The drug is administered orally in a dose of 10-20 mg daily as its hydrochloride salt, reducing significantly the diastolic blood pressure40]. After oral administration, LER is completely but erratically absorbed from the gastrointestinal tract[41]. However, absolute bioavailability is reduced to approximately 10% because of extensive first pass metabolism to inactive metabolites[40]. Literature suggests mean half-lives of 2.8 and 4.4 h in humans after single dose of 10 and 20 mg of LER, respectively[41]. These pharmacokinetic parameters advocate the suitability of LER as a potential candidate for buccal delivery. Charde et al. developed and evaluated buccal mucoadhesive controlled release tablets of LER using polyethylene oxide and different viscosity grades of HPMC individually and in combination. In vivo studies of selected formulations in rabbits demonstrated significant enhancement in bioavailability of LER relative to orally administered drug. Moreover, in human acceptability studies of placebo formulations, the designed tablets adhered well to the buccal mucosa for more than 4 h without causing any discomfort[42]. Boateng et al. investigated the dissolution and release of hydrochlorothiazide as a model insoluble drug from freeze-dried wafers and solvent-cast films formulated from sodium carboxymethylcellulose (CMC). It was observed that the release of hydrochlorothiazide was generally faster from the wafers than from the corresponding films. This was particularly true during the initial 60% of release. These differences in release rate could be attributed to the differences between the physical properties of the wafers and films, which affect their initial rate of hydration and swelling[43].
Antimicrobial therapy
The clinical treatment of oral candidosis (a common pathological condition of the oral cavity) using conventional pharmaceutical dosage forms-such as solutions, gels, suspensions, and mouthwashes-is usually not very effective, mainly because drugs are quickly removed from the oral cavity. In an attempt to solve this problem, Juan et al. designed a bilayered mucoadhesive tablet using nystatin as the model drug. These tablets released nystatin quickly from the lactose layer and then in a sustained way, during approximately 6 hours, from the polymeric layer[44].
Yehia et al. formulated buccal discs[24] and films of fluconazole for topical treatment of oral candidosis to ensure satisfactory drug level in the mouth for prolonged duration of time and to reduce side effects and possibility of drug interaction encountered during systemic therapy of fluconazole[45]. Buccoadhesive erodible discs of cetylpyridinium chloride were prepared by Ahuja et al. in an attempt to treat various oro-dental infections[46]. Chlorhexidine diacetate was used by Giunchedi et al. to formulate buccal tablets based on chitosan microspheres[47]. Domb et al. prepared iodine complexes with EC and HPC and incorporated them into a mucoadhesive tablet for potential use as antimicrobial agent for treating oral infections[48]. In an attempt to get rid of oral malodor, Sterer et al. formulated a palatal mucoadhesive tablet containing a herbal formulation (i.e. sage, Echinacea, Lavender and Mastic gum) and tested it on human volunteers[49]–[51]. A novel mucoadhesive gel of chlorhexidine was formulated by Fini et al. using HPMC, CMC and HPC as the gel forming materials[52].
One of the major concerns about antibiotic usage, particularly in long-term, low dosage regimes, is that bacteria may develop resistance to the antibiotic. Recently, a combination of two or more antibiotics has been recognized as an important method for, at least, delaying the emergence of bacterial resistance. Besides, antibiotic combinations may also produce desirable synergistic effects in the treatment of infections[53],[54]. This approach was taken by Obaidat et al. to prepare mucoadhesive patches containing tetracycline hydrochloride and carvacrol in an attempt to develop a novel oral drug delivery system for the treatment of mouth infections. The formulation exhibited excellent activity against Pseudomonas aeruginosa, indicating a synergistic action between tetracycline and carvacrol since both of them were separately ineffective against Pseudomonas aeruginosa. Also, their inhibition efficiency increased against Bacillus cereus when they were used in combination, which also indicates a synergistic effect[55].
Anti-inflammatory therapy
Inflammatory processes are one of the major reasons for oral cavity diseases[56]. This problem is managed with topical administration of various nonsteroidal, anti-inflammatory drugs, like flurbiprofen, flufenamic acid, ibuprofen etc, in the treatment of a number of oral cavity pathologies, such as gingivitis, periodontitis, stomatitis, oral ulcers, etc. Their advantage is the reduction of drug dose, the virtue of drug localization in the target tissue and consequent minimization of degree of systemic side effects[57]–[59]. Perioli et al. designed sustained-release mucoadhesive bilayered tablets, using mixtures of mucoadhesive polymers and an inorganic matrix (hydrotalcite), for topical administration of flurbiprofen in the oral cavity. The optimized formulation, loaded with 20 mg of the drug, showed the best results, producing good anti-inflammatory sustained release in the buccal cavity for 12 hours and thus a reduction in daily drug dosage (40 mg vs 70 mg)[56]. Ibuprofen was used as a model compound by Perioli et al. to develop mucoadhesive patches using several film-forming and mucoadhesive polymers. The statistical investigation of in vitro release data revealed that diffusion was the mechanism of drug release[59]. Mura et al. developed mucoadhesive films of flufenamic acid using complexation with hydroxypropyl-β-cyclodextrin (HPβCD) to improve drug dissolution and release rate. KollicoatIR®, a new polyvinyl alcohol- polyethylene glycol graft copolymer, was evaluated as film-forming polymer owing to its ability to form very flexible films with much elongation at break than cellulose derivatives (due to the polyvinyl alcohol moiety), combined to its plasticizing and surfactant properties (due to the polyethylene glycol moiety). The work successfully demonstrated that cyclodextrin complexation could be a suitable strategy to optimize the drug release feature from the system. In fact, introduction of drug as complex with HPβCD enabled a clear improvement of drug release with respect to the film containing the plain drug, allowing achievement of complete release within 4-5 h, which is considered the usual maximum duration for buccal drug delivery[60]. Kianfar et al. formulated and characterized buccal films using Carrageenan (CAR), poloxamer (POL) 407, various grades of PEG (plasticizer), and loaded with paracetamol and indomethacin as model soluble and insoluble drugs, respectively. The results also showed the conversion of crystalline drugs to the amorphous form during film formation and the film matrix demonstrated the ability to maintain the two model drugs in a stable amorphous form during storage over a 12 month period. The films showed ideal release patterns within suitable time periods, following swelling and diffusion of the polymer matrix, under conditions simulating those of saliva. These show the potential of CAR 911 and POL 407 based films for buccal delivery of drugs with varying physicochemical characteristics[61].
Boateng et al. formulated freeze-dried wafers and solvent-cast films prepared from sodium alginate (ALG) and sodium carboxymethylcellulose (CMC) using paracetamol as a model soluble drug. A key finding of the current study was the partial conversion of monoclinic polymorph of paracetamol to the metastable orthorhombic form and the preservation of this metastable polymorph. This observation could be attributed to the polymer (CMC) used to prepare the formulations rather than the freeze-drying or air drying process for wafers and films respectively. The transitions observed seem to counter the well-publicized monotropic property of paracetamol polymorphism and suggests that other factors may be involved that allow the conversion of form I to the metastable form II. It was found that the rate of drug release from the wafers (porous) and films (non-porous) was dependent on their physical structure and the amount of polymer present. These differences present the possibility of using these formulations in different mucosal applications. The wafers which can absorb moisture at a faster rate can be useful for applying onto, and delivering active agents, to suppurating wounds. The faster release rate of drug from wafers and films containing low polymer levels also make them suitable as drug delivery systems such as fast dissolving tablets and films for buccal administration of drugs[62],[63].
Kianfar et al. developed and characterized lyophilized wafers prepared by freeze-drying gels comprising the natural polysaccharide polymer- carrageenan, pluronic acid and polyethylene glycol 600 (PEG 600), loaded with model soluble (paracetamol) and insoluble (ibuprofen) drugs for buccal delivery purposes. The results showed the conversion of crystalline drugs to the amorphous form during gel formation and freeze-drying and the wafer's matrix demonstrated the ability to maintain the two model drugs in a stable amorphous form during storage over a 6 month period. The wafers showed ideal release patterns in conditions simulating those of saliva and, coupled with the desirable mucoadhesive characteristics, have potential for buccal drug delivery[64].
Antiemetics
Ondansetron hydrochloride is a 5HT3 serotonin antagonist used in the prevention of nausea and vomiting associated with emetogenic cancer chemotherapy[65],[66]. As administering drug by buccal route avoids hepatic first-pass metabolism, delivery of ondansetron to the systemic circulation via the buccal route would improve its bioavailability. Ali et al. developed and evaluated a buccal adhesive tablet containing ondansetron using CP 934, sodium alginate, SCMC low viscosity, and HPMC 15cps as mucoadhesive polymers to impart mucoadhesion and ethyl cellulose to act as an impermeable backing layer. The stability of drug in the optimized adhesive tablet was tested for 6 h in natural human saliva; both the drug and device were found to be stable in natural human saliva[67]. Ondansetron was also explored as a suitable candidate by Koland et al. to formulate fast dissolving films for sublingual administration. Rapidly water-soluble polymers such as PVA and PVP were chosen for the formulation with carbopol for conferring mucoadhesive properties. Maximum swelling was observed for the formulation containing carbopol as compared to those formulations containing PVP as mucoadhesive polymer[68]. Bhalekar et al. prepared buccal bioadhesive hydrophilic matrix tablets of domperidone using HPMC and Carbopol. An increasing trend in the bioadhesive strength was seen with an increase in the amount of polymer(s). Maximum bioadhesive strength was observed with the highest level of the two polymers. Application of two-way ANOVA-based factorial analysis indicated that the polymers had a significant influence on the bioadhesive properties of the compressed matrices. The overall rate of drug release tended to decrease with an increase in the polymer amount. This may be attributed to the fact that with an increase in hydrogel concentration, the viscosity of the gel layer around the tablet tends to limit further the release of the active ingredient[69].
Muscle relaxants
Tizanidine hydrochloride is an imidazoline derivative which acts as agonist on centrally located α2 receptors and this leads to myotonolytic effects on skeletal muscle. Shanker et al. formulated and evaluated bioadhesive buccal tablets of tizanidine using bioadhesive polymers such as HPMC K4M, SCMC alone, and a combination of these two polymers, in an attempt to avoid first-pass effect and provide for prolonged release of the drug. The degree of swelling was increased with the increase in the concentration of SCMC, leading to increased bioadhesion strength. Rapid rate of hydration of SCMC led to higher degree of swelling in a short period of time, which improved entanglement of polymer chains with the mucus. The degree of swelling indicated that the rate of swelling is directly proportional to SCMC content and inversely proportional to HPMC K4M content[70].
Hypoglycaemic agents
Certain hypoglycemic agents like glipizide and glibenclamide have been recently exploited for buccal delivery. The short biological half-life (3.4 h) of glipizide necessitates its administration in 2 or 3 doses of 2.5 to 10 mg per day. Semalty et al. Prepared mucoadhesive buccal films of glipizide by a solvent casting technique using HPMC, SCMC, CP-934P and Eudragit RL-100. The effect of glipizide on the swelling behavior and the residence time of various mucoadhesive polymers were observed. The medicated films showed high swelling index in comparison to plain films. The addition of the water-insoluble drug increased the water uptake of the film. The results suggested that therapeutic levels of glipizide could be delivered through the buccal route efficiently[71]. Mucoadhesive buccal films of glibenclamide were prepared by Muzib et al. using different grades of HPMC with different ratios. The amount and properties of the incorporated drug determine matrix integrity. The films containing HPMCK15 showed a higher percent swelling due to the presence of more hydroxyl groups in the HPMC molecules. The incorporation of the drug induced significant reduction in the residence time of various formulations. During dissolution, the loosely bound polymer molecules with HPMC in these films were readily eroded, allowing the easy release of glibenclamide. It was found that the drug release from the films varied with respect to the proportion of polymers. It was concluded that HPMC3000 at low concentrations could be useful for buccal delivery of glibenclamide in a controlled manner[72].
Protein and hormone delivery
The delivery of peptide drugs across the buccal mucosa is more convenient and safer than other delivery approaches. It is shown that the buccal administration of drugs has some advantages, such as low enzymatic activity compared with the gastro intestinal track, and tolerance to potential sensitizers[73]–[75]. Insulin was used by Cui et al. as a model protein and its release behavior from bilaminated films was evaluated. The insulin loaded bilaminated film showed a pronounced hypoglycemic effect following buccal administration to healthy rats, achieving a 17% pharmacological availability compared with subcutaneous insulin injection[76]. Myoglobin (MHb) was explored by Colonna et al. to formulate cross-linked chitosan mucoadhesive films. 5-Methyl-pyrrolidinone chitosan (MPC) is a chitosan derivative having excellent properties for use in buccal drug delivery systems. It proved to be a promising polymer for the manufacture of bioadhesive films. After ionic cross-linking, these films enabled the modulation of the release of the model protein MHb[77]. Jain et al. developed and evaluated mucoadhesive films for buccal administration of progesterone using film-forming and mucoadhesive polymers[78]. Leutinizing hormone-releasing hormone (LHRH) was formulated into a buccoadhesive tablet by Nakane et al. to study its enhanced/controlled delivery. In vivo evaluations were performed in beagle dogs and pharmacokinetic profiles were monitored to characterize the transmucosal permeation kinetics of LHRH. The plasma LHRH concentrations were observed to reach the plateau level within 30 min and were maintained for 2 hr following application of the dosage form, in contrast to a rapid elimination profile observed after IV administration[79]. Ayensu et al. formulated and characterised the physico-mechanical properties of lyophilized CS wafers as potential drug delivery systems to the buccal mucosa membrane using bovine serum albumin (BSA) as a model protein. Wafers were prepared by lyophilizing aqueous gels of the polymer incorporating varying concentrations of glycerol as plasticizer and d-mannitol as cryoprotectant. Texture analysis was employed to investigate the in vitro mucoadhesive properties in tensile mode, residual moisture content by thermo-gravimetric analysis, while hydration capacity and drug release studies were performed in 0.1 M phosphate buffered saline. The results showed the potential of employing lyophilized chitosan wafers for buccal mucosa delivery of protein-based drugs[80]–[82].
Giovino et al. developed and characterized mucoadhesive chitosan based films, incorporated with insulin loaded nanoparticles (NPs) made of poly(ethylene glycol)methyl ether-block-polylactide (PEG-b-PLA). The optimized formulation was loaded with insulin (model protein) at initial loadings of 2, 5 and 10% with respect to copolymer weight. The in vitro release behavior of both formulations showed a classic biphasic sustained release of protein over 5 weeks which was influenced by pH of the release medium. Optimized chitosan films embedded with 3 mg of insulin loaded NPs were produced by solvent casting with homogeneous distribution of NPs in the mucoadhesive matrix, which displayed excellent physico-mechanical properties[83].
Smoking deterrent
The habitual nature of smoking is partly due to nicotine (NCT) in tobacco, which is categorized as a psychoactive substance[84]. The NCT delivery routes are the skin and mucosal membranes, such as buccal and nasal mucosa, because both the neutral and protonated NCT could readily permeate across the mucosal membranes[85],[86]. Pongjanyakul et al. prepared sodium alginate-magnesium aluminum silicate (SA-MAS) buccal films loaded with NCT as a potential drug delivery system. The study revealed that the NCT-loaded SA-MAS films provided higher NCT content, and lower NCT release rate and permeation rate through the mucosal membrane than the NCT-loaded SA films. The NCT-loaded SA-MAS films prepared at different pH demonstrated a change in crystallinity and thermal properties. In addition, the NCT-loaded SA-MAS films also displayed a bioadhesive property for adhesion to the mucosal membrane. This finding suggested that the NCT-loaded SA-MAS films have a strong potential for use as a buccal delivery system[87]. Rao et al. used NCT to formulate a tri-layered buccal mucoadhesive patch, comprising a medicated dry tablet adhered to a mucoadhesive film[88]. Bilayer NCT mucoadhesive patches were prepared and evaluated by Obaidat et al. to determine the feasibility of the formulation as a nicotine replacement product to aid in smoking cessation. The results of the study demonstrated that xanthan mucoadhesive buccal patches are potential candidates for controlled biphasic nicotine delivery. The promising fast initial drug release followed by a controlled release over a 10-h period and the content uniformity, thickness, swelling and mucoadhesive strength, advocated the use of such a system as a potential candidate for future in vivo studies of the permeation and retention of nicotine in the oral mucosa. Interestingly, the release profile, mucoadhesion and swelling studies indicated that the acid-base reaction of nicotine with carbopol is relatively stronger than its reaction with xanthan gum, which restricts the suitability of medicated carbopol patches for controlled drug delivery[89].
Antimigraine
Sumatriptan succinate; a 5-HT1receptor agonist used in the treatment of migraine was used as a model drug by Shidhaye et al. to develop mucoadhesive bilayered buccal patches as an alternative mode of drug delivery. A corresponding increase in the mucoadhesive strength of patches was observed with increase in concentration of chitosan. It was also revealed that the effect of concentration of chitosan was more significant than the effect of concentration of PVP K30. Increase in chitosan and PVP K-30 led to increase in the extent of swelling of the patches. The drug release was found to increase with increasing concentrations of PVP K30 and decreasing concentrations of chitosan. As sumatriptan succinate exhibits low permeability across buccal mucosa, different penetration enhancers (transcutol, polysorbate 80, and DMSO) were tried to improve its buccal penetration. The study suggested that buccal mucosal delivery of sumatriptan succinate can be efficiently carried out by penetration enhancers and the buccal route can be a promising route for its delivery avoiding the first pass effect[90].
Antihistamine
Chlorpheniramine maleate (CPM) is a histamine H1 receptor antagonist widely used for the treatment of various allergic conditions[91]. Sekhar et al. developed mucoadhesive buccal patches of CPM using HEC as water-soluble polymer with a view to bypass the first-pass metabolism, thereby increasing the bioavailability of CPM. The study demonstrated that the dosage form was nonirritating and did not cause mucosal damage or irritation upon buccal administration. Bioavailability from optimized buccal patch was 1.46 times higher than the oral dosage form and the results showed statistically significant difference[92].
Recent years have seen similar studies carried out by many other workers[93]–[96]. Table 1 showcases some of the most worked upon APIs in the field of MBDDS along with the polymers associated, and Table 2 enlists some commercially available dosage forms meant to be applied in the buccal cavity along with their therapeutic application. Various patents related to MBDDS are enlisted in Table 3 and Table 4.
Table 1. List of buccal mucoadhesive drug delivery systems.
| Dosage forms | Active ingredients | Polymers | Investigators |
| Buccoadhesive Discs | Fluconazole | CP 974P, SCMC, sodium alginate, HPMC | Yehia et al.[24] |
| Buccoadhesive Tablets | Propranolol HCl | SCMC, CP-934P | Patel et al.[32] |
| Buccoadhesive Tablets | Atenolol | CP 934P and SCMC | Singh et al.[36] |
| Buccoadhesive Tablets | Pravastatin Sodium | Carrageenan gum, PVP K30 | Shidhaye et al.[38] |
| Buccoadhesive Tablets | Lercanidipine HCl | HPMC | Charde et al.[42] |
| Buccoadhesive Tablets | Nystatin | Carbomer (CB), and HPMC | Juan et al.[44] |
| Buccoadhesive Tablets | Ondansetron HCl | CP 934P, sodium alginate, SCMC, HPMC | Ali et al.[51] |
| Buccoadhesive Tablets | Domperidone | HPMC, CP | Bhalekar et al.[69] |
| Buccoadhesive Tablets | Tizanidine HCl | HPMC K4M, SCMC | Shanker et al.[70] |
| Buccoadhesive Films | Propranolol HCl | Polycarbophil (PC), sodium alginate, gellan gum | Carmen et al.[34] |
| Buccoadhesive Films | Fluconazole | HPMC, HEC, chitosan, Eudragit and sodium alginate | Yehia et al.[45] |
| Buccoadhesive Films | Ondansetron HCl | PVA, PVP, CP 934P | Koland et al.[68] |
| Buccoadhesive Films | Glipizide | HPMC, SCMC, CP-934P and Eudragit RL-100 | Semalty et al.[71] |
| Buccoadhesive Films | Insulin | Ethylcellulose, chitosan | Cui et al.[76] |
| Buccoadhesive Films | Myoglobin | Chitosan | Colonna et al.[77] |
| Buccoadhesive Films | Progesterone | Chitosan | Jain et al.[78] |
| Buccoadhesive Films | Nicotine | Sodium alginate-magnesium aluminium silicate | Pongjanyakul et al.[87] |
| Buccoadhesive Films | Lidocaine | HPC | Okamoto et al.[97],[98] |
| Buccoadhesive Films | Thiocolchicoside | Gelatin and CMC | Artusi et al.[99] |
| Buccoadhesive Patches | Propranolol HCl | CP 934 and PVP-K30 | Patel et al.[33] |
| Buccoadhesive Patches | Atenolol | CP 934 P, SCMC, HPMC | Mohanty et al.[37] |
| Buccoadhesive Patches | Sumatriptan succinate | Gelatin and PVP-K30 | Shidhaye et al.[90] |
| Buccoadhesive Patches | Lignocaine | Proprietary mucoadhesive support system | Brook et al.[100] |
| Buccoadhesive Patches | Miconazole nitrate | SCMC, chitosan, PVA, HEC, HPMC | Nafee et al.[101] |
| Buccoadhesive Patches | Oxytocin | CP 974P | Li et al.[102],[103] |
| Buccoadhesive Patches | Thyrotropin-releasing hormone | Organic polymers | Li et al.[104] |
| Buccoadhesive Gels | Arecoline | CP 934P | Strickland et al.[105] |
| Buccoadhesive Gels | Chlorhexidine | HEC, PVP, and PC | Jones et al.[106],[107] |
| Buccoadhesive Gels | Diclofenac sodium | Hydroxyethyl Methacrylate (HEMA) | Cassidy et al.[108] |
| Buccoadhesive Gels | Flurbiprofen | HEC, PVP, and PC | Jones et al.[109] |
| Buccoadhesive Gels | Lidocaine | PEG, CP 934P, and PVP | Tan et al.[110] |
| Buccoadhesive Gels | Propolis | HPC | Ceschel et al.[111] |
| Buccoadhesive Gels | Tetracycline | HEC, PVP, and PC | Jones et al.[112] |
| Buccoadhesive Gels | Triamcinolone acetonide | Poloxamer 407 and CP 934 | Shin et al.[113], [114] |
Table 2. Commercially available buccal mucosal drug delivery systems [115]–[117].
| Therapeutic application | Brand name | Active ingredients | Dosage form | Manufacturers |
| Breakthrough cancer pain in opioid tolerant patients on maintenance opioids | Actiq® | Fentanyl citrate | Lozenge | Teva Pharmaceuticals, Sellersville, PA, USA |
| Fentora™ | Fentanyl citrate | Tablet | Cephalon, Inc., Frazer, PA, USA | |
| Onsolis® | Fentanyl citrate | Film | Meda Pharmaceuticals Inc.Somerset, NJ, USA | |
| Pain relief; narcotic for opioid dependent patients | Subutex® | Buprenorphinehydrochloride | Tablet | Reckitt Benckiser |
| Suboxane® | Buprenorphine hydrochloride-naloxoneHCl | Tablet | Reckitt Benckiser | |
| Prevention and treatment of angina pectoris | Nitrostat® | Nitroglycerine | Tablet, Spray | W Lambert-P Davis-Pfizer Pharmaceuticals |
| Suscard buccal | Glyceryl trinitrate | Tablet | Forest laboratories | |
| Nitrogard® | Nitroglycerine | Tablet | Forest Pharmaceuticals,St. Louis, MO, USA | |
| Smoking deterrent | Nicotinelle® | Nicotine | Lozenge | Novartis Consumer Health |
| Nicorette® | Nicotine | Chewing gum | GSK Consumer Health | |
| Topical treatment of oro-pharyngeal candidiasis | Loramyc® | Miconazole | Tablet | BioAlliance Pharma SA |
| Lauriad™ | Miconazole | Tablet | BioAlliance Pharma SA | |
| Hormone therapy | Striant™ SR buccal | Testosterone | Tablet | Columbia Pharmaceuticals |
| Nausea/emesis | Buccastem | Proclorperazine | Tablet | Reckitt Benckiser |
| Relief of migraines and associated symptoms, such as nausea | Maxalt Wafers® | Rizatriptan | Wafer | Merck & Co. Inc., WhitehouseStation, NJ, USA |
Table 3. Patents related to buccal film/patch.
| US Patent No. | Inventors | Work | Reference |
| 20110033541 | Myers et al. | The invention relates to self-supporting film dosage forms which provide a therapeutically effective dosage. Such compositions are particularly useful for treating narcotic dependence while providing sufficient buccal adhesion of the dosage form. | [118] |
| 4900552 | Sanvordeker et al. | A trilaminate film suitable for prolonged and sustained delivery of an active ingredient in a buccal cavity is disclosed. A hydratable mucoadhesive base layer, a non-adhesive reservoir layer and a water-impermeable carrier film sandwiched between and bonded to the base layer and the reservoir layer are the elements which form the trilaminate film. | [119] |
| 20100266669 | Meyer et al. | This invention has led to the development of single layer oral disintegrating films that have at least two different zones, which consist of nicotine that allows sufficient buccal absorption. | [120] |
| 20110033542 | Myers et al. | The present invention relates to compositions, methods of manufacture, products and methods of use relating to films containing therapeutic actives. The invention more particularly relates to self-supporting dosage forms which provide an agonist acting alone or in combination with a buffer system. | [121] |
| 20100063110 | Meyer et al. | This invention relates to the development of muco-adhesive oral disintegrating film that completely disintegrates in mouth with in one to ten minutes. The film is composed of alkaline substance and API which may be present optionally. | [122] |
| 5827525 | Liao et al. | A unidirectional buccal delivery system for the delivery of therapeutic agents over an extended period of time. The delivery system includes a matrix for releasing the drug into the oral cavity at a sustained rate and a means for securing the matrix to the palate or other adequate regions in the oral cavity. | [123] |
| 5750134 | Scholz et al. | A bioadhesive composition that adheres suitably to a mucosal surface and is capable of delivering drugs in sustained fashion, and a patch comprising the bioadhesive composition. A bioadhesive composition that adheres suitably to a mucosal surface and is capable of delivering drugs in sustained fashion, and a patch comprising the bioadhesive composition. | [124] |
Table 4. Patents related to buccal tablets.
| US Patent No. | Inventors | Work | Reference |
| 7985430 | Levine et al. | The present invention relates to an adhesive solid dosage form containing a mixture of herbal extracts as the active ingredient, suitable particularly for the treatment of mucosal lesions. | [125] |
| 7153845 | Levine et al. | The present invention relates to progressive hydration tablets for adhesion to the wall of a body cavity for the sustained release of active ingredients. | [126] |
| 6063404 | Timpe et al. | This invention relates to a bioadhesive tablet containing at least one bioadhesive adjuvant and at least one lubricant, with at least one surface of said tablet comprising concentric or parallel, straight and/or curved depressions, and to a method for producing said bioadhesive tablets. | [127] |
| 6916485 | Aiache et al. | The present invention relates to novel prolonged release bioadhesive therapeutic systems for treating local mucosal infections or the mucitis and candidiasis type. | [128] |
| 20020044964 | Bologna et al. | The present invention relates to progressive hydration tablets for adhesion to the wall of a body cavity [oral, vaginal] for the sustained release of active ingredients without premature degradation of the active ingredients. | [129] |
| 6210699 | Acharya et al. | This invention relates to an improved dosage form which can easily adhere to the inner buccal cavity and sustain transmucosal release of drugs, odorants or any other ingredients, wherein said dosage form is a tablet. | [130] |
| 5330761 | Baichwal et al. | The present invention relates to a controlled release bioadhesive tabletwhich includes a locally active agent, a heterodisperse gum matrix, and pharmaceutically acceptable diluents. | [131] |
| 6242004 | Rault et al. | The invention relates to bioadhesive compounds in the form of multilayers, and having at least one bioadhesive layer with the total charge of bioadhesive material; the bioadhesive layer being directly compressible during the production of the tablet. | [132] |
| 5091184 | Khanna et al. | Objective of the present invention: to provide an improved dosage form for the administration of baclofen, in the form of an adhesive tablet, to the mucosa in the oral cavity that permits uniform administration at constant intervals. | [133] |
FUTURE PROSPECTS AND CHALLENGES
Research in buccal drug delivery has revealed remarkable growth and advances in the past few decades. The buccal mucosa holds a great promise for systemic delivery of orally inefficient drugs as well as a feasible and attractive alternative for non-invasive delivery of potent peptide and protein drug molecules. Mucoadhesive drug delivery systems offer unique carrier system for many pharmaceuticals and can be modified to adhere to any mucosal tissue, including those found in oral cavity, gastrointestinal tract, vagina, eye etc. One of the areas of interest is the novel buccal adhesive delivery system, where the drug delivery is directed towards buccal mucosa by protecting the local environment.
In spite of significant advances in the field of mucoadhesion, there is no consensus between scientists in relation to the mechanisms of the interaction between materials and components of mucosal tissue. Many scientists have addressed the development of MBDDS and studied the efficacy of their use, though here too there remain significant gaps, as there is no generally accepted method for assessing mucoadhesive properties. The lack of standardized techniques often leads to discordant and unclear results. Efforts have to be made to develop standardized in vitro and ex vivo biological models that allow one to characterize and compare different materials and formulations in terms of their capability to promote drug absorption via the buccal route.
Looking into the future, researchers find the fate of buccal adhesive drug delivery turning towards vaccine formulations and delivery of small proteins/peptides. Microparticulate bioadhesive systems are particularly interesting because they offer protection to therapeutic entities as well as the enhanced absorption that result from increased contact time provided by the bioadhesive component. Mucoadhesion can clearly play a fundamental role as non-parenteral drug delivery systems for protein formulations, as well as vaccines able to attach to mucous membranes to stimulate local immunity.
CONCLUSION
At the current global scenario, scientists are finding ways to develop buccal adhesive systems through various approaches to improve the bioavailability of orally less/inefficient drugs by manipulating the formulation strategies. Polymeric science needs to be explored to find newer mucoadhesive polymers with the added attributes of being biodegradable, biocompatible, non-toxic, mucoadhesive for specific cells or mucosa, and which could also function as enzyme inhibitors for the successful delivery of proteins and peptides. However, the invention of new biomaterials, tailor-made copolymers, has excellent potential for mucoadhesive drug delivery, but the formulations based on them still have to go a long way to find their path in actual clinical practice.
References
- 1.Jiménez-castellanos MR, Zia H, Rhodes CT. Mucoadhesive Drug Delivery Systems. Drug Develop. and Indus. Pharm. 1993;19:143–94. http://informahealthcare.com/doi/abs/10.3109/03639049309038765. [Google Scholar]
- 2.Harris D, Robinson JR. Drug delivery via the mucous membranes of the oral cavity. J Pharm Sci. 1992;81:1–10. doi: 10.1002/jps.2600810102. [DOI] [PubMed] [Google Scholar]
- 3.Miller NS, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–91. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Puratchikody A, Prasanth VV, Mathew ST, Ashok KB. Buccal Drug Delivery: Past, Present and Future -A Review. Int. J. Drug Deliv. 2011;3:171–84. http://www.arjournals.org/index.php/ijdd/article/view/212. [Google Scholar]
- 5.Kianfar F, Antonijevic MD, Chowdhry BZ, Boateng JS. Formulation development of a carrageenan based delivery system for buccal drug delivery using ibuprofen as a model drug. J Biomater Nanobiotechnol. 2011;2:582–95. [Google Scholar]
- 6.Ahuja A, Khar RK, Ali J. Mucoadhesive Drug Delivery Systems. Drug Develop Indus Pharm. 1997;23:489–515. [Google Scholar]
- 7.Andrews GP, Laverty TP, Jones DS. Mucoadhesive Polymeric Platforms for Controlled Drug Delivery. Eur J Pharma Biopharm. 2009;71:505–18. doi: 10.1016/j.ejpb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Henriksen I, Green K, Smart J, Smistad G, Karlsen J. Bioadhesion of hydrated chitosans: an in vitro and in vivo study. Int J Pharm. 1996;145:231–40. [Google Scholar]
- 9.Woodley J. Bioadhesion: new possibilities for drug administration? Clin Pharmacokinet. 2001;40:77–84. doi: 10.2165/00003088-200140020-00001. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Leobandung W, Foss A, Peppas NA. Molecular aspects of muco- and bioadhesion: tethered structures and site-specific surfaces. J Control Release. 2000;65:63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 11.Boddupalli BM, Mohammed ZNK, Ravindra NA, Banji D. Mucoadhesive drug delivery system. J Adv Pharm Tech Res. 2010;1:381–7. doi: 10.4103/0110-5558.76436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release. 2011;153:106–16. doi: 10.1016/j.jconrel.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Nicolazzo JA, Reed BL, Finnin BC. Buccal penetration enhancers-How do they really work? J Control Release. 2005;105:1–15. doi: 10.1016/j.jconrel.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Shakya P, Madhav NVS, Shakya AK, Singh K. Palatal mucosa as a route for systemic drug delivery: A review. J Control Release. 2011;151:2–9. doi: 10.1016/j.jconrel.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Bruschi ML, de Freitas O. Oral bioadhesive drug delivery systems. Drug Dev Ind Pharm. 2005;31:293–310. doi: 10.1081/ddc-52073. [DOI] [PubMed] [Google Scholar]
- 16.Rupal Patel, Viness Pillay, Yahya E Choonara, Thirumala Govender, et al. A novel cellulose-based hydrophilic wafer matrix for rapid bioactive delivery. J Bioact Compat Polym. 2007;22:119–42. [Google Scholar]
- 17.Schnurch AB. Mucoadhesive systems in oral drug delivery. Drug Discov Today technol. 2005;2:83–7. doi: 10.1016/j.ddtec.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Rathbone MJ, Drummond BK, Tucker G. The oral cavity as a site for systemic drug delivery. Adv Drug Deliv Rev. 1994;13:1–22. [Google Scholar]
- 19.Kharenko EA, Larionova NI, Demina NB. Mucoadhesive drug delivery systems. Pharm Chem Jour. 2009;43:200–8. [Google Scholar]
- 20.Wong CF, Yuen KH, Peh KK. Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm. 1999;178:11–22. doi: 10.1016/s0378-5173(98)00342-1. [DOI] [PubMed] [Google Scholar]
- 21.Boddupalli BM, Mohammed ZNK, Nath RA, Banji D. Mucoadhesive drug delivery system: An overview. J Adv Pharm Technol Res. 2010;1:381–7. doi: 10.4103/0110-5558.76436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thimmasetty J, Pandey Gs, Sathesh Babu Pr. Design and In Vivo evaluation of carvedilol buccal mucoadhesive patches. Pak. J. Pharm. Sci. 2008;21:241–48. [PubMed] [Google Scholar]
- 23.Naveen C, Kumar YK, Rao PV, Rao TR. Chemical enhancers in buccal and sublingual delivery. Int J Pharm Sci Nanotech. 2011;4:1307–19. [Google Scholar]
- 24.Yehia SA, El-Gazayerly ON, Basalious EB. Design and in vitro/in vivo evaluation of novel mucoadhesive buccal discs of an antifungal drug: relationship between swelling, erosion, and drug release. AAPS PharmSciTech. 2008;9:1207–17. doi: 10.1208/s12249-008-9166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koks C, Meenhorst P, Hillebrand M, Bult A. Pharmacokinetics of fluconazole in saliva and plasma after administration of an oral suspension and capsules. Antimicrob Agents Chemother. 1996;40:1935–37. doi: 10.1128/aac.40.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medline Plus A. service of the U.S National Library of Medicine and the National Institutes of Health. Available at: http://medlineplus.gov. 2008.
- 27.Dechant KL, Clissold SP. Sumatriptan, A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the acute treatment of migraine and cluster headache. Drugs. 1992;43:776–98. doi: 10.2165/00003495-199243050-00010. [DOI] [PubMed] [Google Scholar]
- 28.Shayeda Dharani S. Formulation and in vitro evaluation of mucoadhesive buccal patches of ondansetron hydrochloride. Int J Pharm Sci Nanotech. 2010;3:860–6. http://ijpsnonline.com/Issues/860_full.pdf. [Google Scholar]
- 29.Blake JC, Palmer JL, Minton NA, Burroughs AK. The pharmacokinetics of intravenous ondansetron in patients with hepatic impairment. Br J clin Pharmac. 1993;35:441–3. doi: 10.1111/j.1365-2125.1993.tb04164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lachaine J, Laurier C, Langleben A, Vaillant L. Cost-effectiveness and quality of life evaluation of ondansetron and metoclopramide for moderately emetogenic chemotherapy regimens in breast cancer. J Oncol Hema. 1999;32:105–12. doi: 10.1016/s1040-8428(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 31.Yamsani VV, Gannu R, Kolli C, Rao ME, Yamsani MR. Development and in vitro evaluation of buccoadhesive carvedilol tablets. Acta Pharm. 2007;57:185–97. doi: 10.2478/v10007-007-0015-7. [DOI] [PubMed] [Google Scholar]
- 32.Patel VM, Prajapati BG, Patel MM. Formulation, Evaluation, and Comparison of Bilayered and Multilayered Mucoadhesive Buccal Devices of Propranolol Hydrochloride. AAPS PharmSciTech. 2007;8:E147–E154. doi: 10.1208/pt0801022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel VM, Prajapati BG, Patel MM. Effect of Hydrophilic Polymers on Buccoadhesive Eudragit Patches of Propranolol Hydrochloride Using Factorial Design. AAPS PharmSciTech. 2007;8:E119–E126. doi: 10.1208/pt0802045. [DOI] [PubMed] [Google Scholar]
- 34.Remuñán-López C, Portero A, Vila-Jato JL, Alonso MJ. Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices for buccal drug delivery. J Control Release. 1998;55:143–52. doi: 10.1016/s0168-3659(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 35.Sastry SV, Reddy IK, Khan MA. Atenolol gastrointestinal therapeutic system: optimization of formulation variables using response surface methodology. J Control Release. 1997;45:121–30. [Google Scholar]
- 36.Singh B, Chakkal SK, Ahuja N. Formulation and optimization of controlled release mucoadhesive tablets of atenolol using response surface methodology. AAPS PharmSciTech. 2006;7:E19–E28. doi: 10.1208/pt070103. [DOI] [PubMed] [Google Scholar]
- 37.Adhikari SNR, Nayak BS, Nayak AK, Mohanty B. Formulation and Evaluation of Buccal Patches for Delivery of Atenolol. AAPS PharmSciTech. 2010;11:1038–44. doi: 10.1208/s12249-010-9459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shidhaye SS, Thakkar PV, Dand NM, Kadam VJ. Buccal drug delivery of pravastatin sodium. AAPS PharmSciTech. 2010;11:416–24. doi: 10.1208/s12249-010-9381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luscher TF, Cosentino F. The classification of calcium antagonists and their selection in the treatment of hypertension: a reappraisal. Drug. 1998;55:509–17. doi: 10.2165/00003495-199855040-00003. [DOI] [PubMed] [Google Scholar]
- 40.Bang LM, Chapman TM, Goa KL. Lercanidipine a review of its efficacy in the management of hypertension. Drugs. 2003;63:2449–72. doi: 10.2165/00003495-200363220-00013. [DOI] [PubMed] [Google Scholar]
- 41.Barchielli M, Dolfini E, Farina P, Leoni B, Targa G, Vinaccia V, Tajana A. Clinical pharmacokinetics of lercanidipine. J. Cardiovasc. Pharmacol. 1997;29:S1–S15. [Google Scholar]
- 42.Charde S, Mudgal M, Kumar L, Saha R. Development and evaluation of buccoadhesive controlled release tablets of lercanidipine. AAPS PharmSciTech. 2008;9:182–90. doi: 10.1208/s12249-007-9031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boateng JS, Matthews KH, Auffret AD, Humphrey MJ, Eccleston GM, Stevens HN. Comparison of the in vitro release characteristics of mucosal freeze-dried wafers and solvent cast films containing an insoluble drug. Drug Dev Ind Pharm. 2012;38:47–54. doi: 10.3109/03639045.2011.590496. [DOI] [PubMed] [Google Scholar]
- 44.Llabot JM, Manzo RH, Allemandi DA. Double-layered mucoadhesive tablets containing nystatin. AAPS PharmSciTech. 2002;3:47–52. doi: 10.1007/BF02830620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yehia SA, El-Gazayerly ON, Basalious EB. Fluconazole mucoadhesive buccal films: in vitro/in vivo performance. Curr Drug Deliv. 2009;6:17–27. doi: 10.2174/156720109787048195. [DOI] [PubMed] [Google Scholar]
- 46.Ali J, Khar R, Ahuja A, Kalra R. Buccoadhesive erodible disk for treatment of oro-dental infections: design and characterization. Int J Pharm. 2002;238:93–103. doi: 10.1016/s0378-5173(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 47.Giunchedi P, Juliano C, Gavini E, Cossu M, Sorrenti M. Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug loaded chitosan microspheres. Eur J Pharm Biopharm. 2002;53:233–9. doi: 10.1016/s0939-6411(01)00237-5. [DOI] [PubMed] [Google Scholar]
- 48.Mizrahi B, Domb AJ. Mucoadhesive tablet releasing iodine for treating oral infections. J Pharm Sci. 2007;96:3144–50. doi: 10.1002/jps.20876. [DOI] [PubMed] [Google Scholar]
- 49.Sterer N, Nuas S, Mizrahi B, Goldenberg C, Weiss EI, Domb A, Davidi MP. Oral malodor reduction by a palatal mucoadhesive tablet containing herbal formulation. J Dentistry. 2008;36:535–9. doi: 10.1016/j.jdent.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Applied Micro bio. 1999;86:985–90. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 51.Cavanagh HM, Wilkinson JM. Biological activities of lavender essential oil. Phytotherapy Res. 2002;16:301–8. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- 52.Fini A, Bergamante V, Ceschel GC. Mucoadhesive Gels Designed for the Controlled Release of Chlorhexidine in the Oral Cavity. Pharmaceutics. 2011;3:665–79. doi: 10.3390/pharmaceutics3040665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maheshwari MG, Mali A, Paradkar A, Yamamura S, Kadam S. Development of Tetracycline-Serratiopeptidase-Containing Periodontal Gel: Formulation and Preliminary Clinical Study. AAPS Pharm. SciTech. 2006;7:E162–E171. doi: 10.1208/pt070376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodson JM, Tanner A. Antibiotic resistance of the subgingival microbiota following local tetracycline therapy. Oral Microbiol Immunol. 1992;7:113–17. doi: 10.1111/j.1399-302x.1992.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 55.Obaidat RM, Bader A, Al-Rajab W, Sheikha GA, Obaidat AA. Preparation of Mucoadhesive Oral Patches Containing Tetracycline Hydrochloride and Carvacrol for Treatment of Local Mouth Bacterial Infections and Candidiasis. Sci Pharm. 2011;79:197–212. doi: 10.3797/scipharm.1004-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perioli L, Ambrogi V, Giovagnoli S, Ricci M, Blasi P, Rossi C. Mucoadhesive Bilayered Tablets for Buccal Sustained Release of Flurbiprofen. AAPS PharmSciTech. 2007;28:E20–E27. doi: 10.1208/pt0802034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339–45. doi: 10.1016/S0304-3959(97)00120-6. [DOI] [PubMed] [Google Scholar]
- 58.Heasman PA, Offenbacher S, Collins J, Edwards GG, Seymour RA. Flurbiprofen in the prevention and treatment of experimental gingivitis. J Clin Periodontol. 1993;20:732–38. doi: 10.1111/j.1600-051x.1993.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 59.Mura P, Corti G, Cirri M, Maestrelli F, Mennini N, Bragagni M. Development of mucoadhesive films for buccal administration of flufenamic acid: Effect of cyclodextrin complexation. J Pharma Sci. 2010;99:3019–29. doi: 10.1002/jps.22068. [DOI] [PubMed] [Google Scholar]
- 60.Perioli L, Ambrogi V, Angelici F, Ricci M, Giovagnoli S, Capuccella M, Rossi C. Development of mucoadhesive patches for buccal administration of ibuprofen. J Cont Release. 2004;99:73–82. doi: 10.1016/j.jconrel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Kianfar F, Chowdhry BZ, Antonijevic MD, Boateng JS. Novel films for drug delivery via the buccal mucosa using model soluble and insoluble drugs. Drug Dev Ind Pharm. 2012;38:1207–20. doi: 10.3109/03639045.2011.644294. [DOI] [PubMed] [Google Scholar]
- 62.Boateng JS, Matthews KH, Auffret AD, Humphrey MJ, Stevens HN, Eccleston GM. In vitro drug release studies of polymeric freeze-dried wafers and solvent-cast films using paracetamol as a model soluble drug. Int J Pharm. 2009;378:66–72. doi: 10.1016/j.ijpharm.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 63.Boatenga JS, Auffret AD, Matthews KH, Humphrey MJ, Stevens HN, Eccleston GM. Characterisation of freeze-dried wafers and solvent evaporated films as potential drug delivery systems to mucosal surfaces. Int J Pharm. 2010;389:24–31. doi: 10.1016/j.ijpharm.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Kianfar F, Antonijevic M, Chowdhry B, Boateng JS. Lyophilized wafers comprising κ-carrageenan & pluronic acid for buccal drug delivery using model soluble and insoluble drugs. Colloids Surf B Biointerfaces. 2012;103:99–106. doi: 10.1016/j.colsurfb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Mohan KC, Ravikumar K. Ondansetron hydrochloride: a competitive serotonin 5-HT3 receptor blocker. Acta Cryst Sect C Cryst Struct Comm. 1995;51:2627–29. [PubMed] [Google Scholar]
- 66.Milap NC, Leanna HN, John DK. Efficacy and safety of ondansetron in pediatric patients undergoing bone marrow transplantation. Clinical Therap. 1996;18:466–76. doi: 10.1016/s0149-2918(96)80027-0. [DOI] [PubMed] [Google Scholar]
- 67.Hassan N, Khar RK, Ali M, Ali J. Development and evaluation of buccal bioadhesive tablet of an anti-emetic agent ondansetron. AAPS PharmSciTech. 2009;10:1085–92. doi: 10.1208/s12249-009-9304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koland M, Sandeep V, Charyulu N. Fast Dissolving Sublingual Films of Ondansetron Hydrochloride: Effect of Additives on in vitro Drug Release and Mucosal Permeation. J Young Pharm. 2010;2:216–22. doi: 10.4103/0975-1483.66790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Madgulkar AR, Bhalekar MR, Ner AS, Wable ND. Formulation development of domperidone buccal bioadhesive hydrophilic matrix tablets. Asian J Pharm. 2011;5:21–27. [Google Scholar]
- 70.Shanker G, Kumar CK, Gonugunta CS, Kumar BV, Veerareddy PR. Formulation and evaluation of bioadhesive buccal drug delivery of tizanidine hydrochloride tablets. AAPS PharmSciTech. 2009;10:530–39. doi: 10.1208/s12249-009-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Semalty M, Semalty A, Kumar G. Formulation and characterization of mucoadhesive buccal films of glipizide. Indian J Pharm Sci. 2008;70:43–48. doi: 10.4103/0250-474X.40330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muzib YI, Kumari KS. Mucoadhesive buccal films of glibenclamide: Development and evaluation. Int J Pharma Investig. 2011;1:42–47. doi: 10.4103/2230-973X.76728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery-A promising option for orally less efficient drugs. J ControlRelease. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 74.Martin L, Wilson GG, Koosha F, Uchegbu IF. Sustained buccal delivery of the hydrophobic drug denbufylline using physically cross-linked palmitoyl glycol chitosan hydrogels. Eur J Pharm Biopharm. 2003;55:35–45. doi: 10.1016/s0939-6411(02)00118-2. [DOI] [PubMed] [Google Scholar]
- 75.Enel SS, Hıncal AA. Drug permeation enhancement via buccal route: Possibilities and limitations. J Control Release. 2001;72:133–44. doi: 10.1016/s0168-3659(01)00269-3. [DOI] [PubMed] [Google Scholar]
- 76.Cui F, He C, He M, Tang C, Yin L, Qian F, Yin C. Preparation and evaluation of chitosan-ethylenediaminetetraacetic acid hydrogel films for the mucoadhesive transbuccal delivery of insulin. J Biomed Mater Res A. 2009;89:1063–71. doi: 10.1002/jbm.a.32071. [DOI] [PubMed] [Google Scholar]
- 77.Colonna C, Genta I, Perugini P, Pavanetto F, Modena T, Valli M, Muzzarelli C, Conti B. 5-methyl-pyrrolidinone chitosan films as carriers for buccal administration of proteins. AAPS PharmSciTech. 2006;7:E107–E13. doi: 10.1208/pt070370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jain SK, Jain A, Gupta Y, Kharya A. Design and development of a mucoadhesive buccal film bearing progesterone. Pharmazie. 2008;63:129–35. [PubMed] [Google Scholar]
- 79.Nakane S, Kakumoto M, Yukimatsu K, Chien YW. Oramucosal delivery of LHRH: pharmacokinetic studies of controlled and enhanced transmucosal permeation. Pharm Dev Technol. 1996;1:251–59. doi: 10.3109/10837459609022593. [DOI] [PubMed] [Google Scholar]
- 80.Ayensu I, Mitchella JC, Boatenga JS. Development and physico-mechanical characterisation of lyophilised chitosan wafers as potential protein drug delivery systems via the buccal mucosa. Colloids Surf B Biointerfaces. 2012;91:258–65. doi: 10.1016/j.colsurfb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Ayensu I, Mitchella JC, Boatenga JS. Effect of membrane dialysis on characteristics of lyophilised chitosan wafers for potential buccal delivery of proteins. Int J Biol Macromol. 2012;50:905–09. doi: 10.1016/j.ijbiomac.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 82.Ayensu I, Boateng J. Development and evaluation of lyophilized thiolated-chitosan wafers for buccal delivery of protein. J Science Technol. 2012;32:46–55. [Google Scholar]
- 83.Giovino C, Ayensu I, Tetteh J, Boateng JS. Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): a potential approach for buccal delivery of macromolecules. Int J Pharm. 2012;428:142–51. doi: 10.1016/j.ijpharm.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 84.Palmer KJ, Buckley MM, Faulds D. Transdermal Nicotine: A Review of its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy as an Aid to Smoking Cessation. Drugs. 1992;44:498–529. doi: 10.2165/00003495-199244030-00011. [DOI] [PubMed] [Google Scholar]
- 85.Nair MA, Chetty DJ, Ho H, Chien YW. Biomembrane permeation of nicotine: Mechanistic studies with porcine mucosae and skin. J Pharm Sci. 1997;86:257–62. doi: 10.1021/js960095w. [DOI] [PubMed] [Google Scholar]
- 86.Chen LH, Chetty DJ, Chien YW. A mechanistic analysis to characterize oramucosal permeation properties. Int J Phar. 1999;184:63–72. doi: 10.1016/s0378-5173(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 87.Pongjanyakul T, Suksri H. Alginate-magnesium aluminum silicate films for buccal delivery of nicotine. Colloids Surf B Biointerfaces. 2009;74:103–13. doi: 10.1016/j.colsurfb.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 88.Rao S, Song Y, Peddie F, Evans AM. A novel tri-layered buccal mucoadhesive patch for drug delivery: assessment of nicotine delivery. J Pharm Pharmacol. 2011;63:794–99. doi: 10.1111/j.2042-7158.2011.01283.x. [DOI] [PubMed] [Google Scholar]
- 89.Huwaij RA, Obaidat RM, Sweidan K, Hiari YA. Formulation and In Vitro Evaluation of Xanthan Gum or Carbopol 934-Based Mucoadhesive Patches, Loaded with Nicotine. AAPS PharmSciTech. 2011;12:21–27. doi: 10.1208/s12249-010-9534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shidhaye SS, Saindane NS, Sutar S, Kadam V. Mucoadhesive bilayered patches for administration of sumatriptan succinate. AAPS PharmSciTech. 2008;9:909–16. doi: 10.1208/s12249-008-9125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koch KM, Semmes OC, Davis RL, Yin Y. Stereoselective pharmacokinetics of chlorpheniramine and the effect of ranitidine. J Pharm Sci. 1998;87:1097–00. doi: 10.1021/js980045m. [DOI] [PubMed] [Google Scholar]
- 92.Sekhar KC, Naidu KV, Vishnu YV, Gannu R, Kishan V, Rao YM. Transbuccal delivery of chlorpheniramine maleate from mucoadhesive buccal patches. Drug Deliv. 2008;15:185–91. doi: 10.1080/10717540801952639. [DOI] [PubMed] [Google Scholar]
- 93.Rai D, Maniruzzaman M, Boateng JS. Development and characterisation of sodium alginate and HPMC films for mucosal drug delivery. Int J Biotechnology. 2010;11:169–81. [Google Scholar]
- 94.Desai H, Anghan B, Boateng JS. Development, formulation and optimization of polymer based films as potential buccal delivery systems using paracetamol and ibuprofen as model drugs. AAPS J. 2010;12:W4169. Available from: http://www.aapsj.org/. [Google Scholar]
- 95.Kianfar F, Ayensu I, Boateng JS. Development and physico-mechanical characterization of carrageenan and poloxamer based lyophilized matrix as a potential buccal drug delivery system. Drug Develop Indus Pharm. doi: 10.3109/03639045.2012.762655. (In Press) [DOI] [PubMed] [Google Scholar]
- 96.Ayensu I, Mitchell JC, Boateng JS. Preparation, evaluation and in-vitro characterisation of thiolated chitosan based lyophilized formulations for buccal mucosa delivery of proteins. Carbohydr Polym. 2012;89:935–41. doi: 10.1016/j.carbpol.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 97.Okamoto H, Taguchi H, Iida K, Danjo K. Development of polymer film dosage forms of lidocaine for buccal administration: I. Penetration rate and release rate. J Control Release. 2001;77:253–60. doi: 10.1016/s0168-3659(01)00509-0. [DOI] [PubMed] [Google Scholar]
- 98.Okamoto H, Nakamori T, Arakawa Y, Iida K, Danjo K. Development of polymer film dosage forms of lidocaine for buccal administration: II. Comparison of preparation methods. J Pharm Sci. 2002;91:2424–32. doi: 10.1002/jps.10228. [DOI] [PubMed] [Google Scholar]
- 99.Artusi M, Santi P, Colombo P, Junginger HE. Buccal delivery of thiocolchicoside: in vitro and in vivo permeation studies. Int J Pharm. 2003;250:203–13. doi: 10.1016/s0378-5173(02)00545-8. [DOI] [PubMed] [Google Scholar]
- 100.Brook IM, Tucker GT, Tuckley EC, Boyes RN. A lignocaine patch for dental analgesia safety and early pharmacology. J Control Release. 1989;10:183–88. [Google Scholar]
- 101.Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int J Pharm. 2003;264:1–14. doi: 10.1016/s0378-5173(03)00371-5. [DOI] [PubMed] [Google Scholar]
- 102.Li C, Bhatt PP, Johnston TP. In vitro release and permeation of oxytocin from a mucoadhesive buccal patch. Pharm Dev Technol. 1996;1:357–64. doi: 10.3109/10837459609031430. [DOI] [PubMed] [Google Scholar]
- 103.Li C, Bhatt PP, Johnston TP. Transmucosal delivery of oxytocin to rabbits using a mucoadhesive buccal patch. Pharm Dev Technol. 1997;2:265–74. doi: 10.3109/10837459709031446. [DOI] [PubMed] [Google Scholar]
- 104.Li C, Koch RL, Raul VA, Bhatt PP, Johnston TP. Absorption of thyrotropin-releasing hormone in rats using a mucoadhesive buccal patch. Drug Dev Ind Pharm. 1997;23:239–46. [Google Scholar]
- 105.Strickland SS, Veena GV, Houghton PJ, Stanford SC, Kurpad AV. Areca nut, energy metabolism and hunger in Asian men. Ann Hum Biol. 2003;30:26–52. doi: 10.1080/03014460210157448. [DOI] [PubMed] [Google Scholar]
- 106.Jones DS, Woolfson AD, Brown AF. Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm Res. 1997;14:450–57. doi: 10.1023/a:1012091231023. [DOI] [PubMed] [Google Scholar]
- 107.Jones DS, Brown AF, Woolfson AD, Dennis AC, Matchett LJ, Bell SEJ. Examination of the physical state of chlorhexidine within viscoelastic, bioadhesive semisolids using raman spectroscopy. J Pharm Sci. 2000;89:563–71. doi: 10.1002/(SICI)1520-6017(200005)89:5<563::AID-JPS1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 108.Cassidy J, Berner B, Chan K, John V, Toon S, Holt B, Rowland M. Human transbuccal absorption of diclofenac sodium from a prototype hydrogel delivery device. Pharm Res. 1993;10:126–29. doi: 10.1023/a:1018941517355. [DOI] [PubMed] [Google Scholar]
- 109.Jones DS, Irwin CR, Woolfson AD, Djokic J, Adams V. Physicochemical characterization and preliminary in vivo efficacy of bioadhesive, semisolid formulations containing flurbiprofen for the treatment of gingivitis. J Pharm Sci. 1999;88:592–98. doi: 10.1021/js9803095. [DOI] [PubMed] [Google Scholar]
- 110.Tan YTF, Peh KK, Al-Hanbali O. Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological, and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech. 2000;1:69–78. doi: 10.1208/pt010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ceschel GC, Maffei P, Sforzini A, Lombardi BS, Yasin A, Ronchi C. In vitro permeation through porcine buccal mucosa of caffeic acid phenetyl ester (CAPE) from a topical mucoadhesive gel containing propolis. Fitoterapia. 2002;73:S44–S52. doi: 10.1016/s0367-326x(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 112.Jones DS, Woolfson AD, Djokic J, Coulter WA. Development and mechanical characterization of bioadhesive semi-solid, polymeric systems containing tetracycline for the treatment of periodontal diseases. Pharm Res. 1996;13:1734–38. doi: 10.1023/a:1016413428473. [DOI] [PubMed] [Google Scholar]
- 113.Shin SC, Bum JP, Choi JS. Enhanced bioavailability by buccal administration of triamcinolone acetonide from the bioadhesive gels in rabbits. Int J Pharm. 2000;209:37–43. doi: 10.1016/s0378-5173(00)00542-1. [DOI] [PubMed] [Google Scholar]
- 114.Shin SC, Kim JY. Enhanced permeation of triamcinolone acetonide through the buccal mucosa. Eur J Pharm. Biopharm. 2000;50:217–20. doi: 10.1016/s0939-6411(00)00101-6. [DOI] [PubMed] [Google Scholar]
- 115.Pather SI, Rathbone MJ, Senel S. Current status and the future of buccal drug delivery systems. Expert Opin Drug Deliv. 2008;5:531–42. doi: 10.1517/17425247.5.5.531. [DOI] [PubMed] [Google Scholar]
- 116.Hearnden V, Sankar V, Hull K, Juras DV, Greenberg M, Kerr R, Lockhart PB, Patton LL, Porter S, Thornhill MH. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv Drug Deli Reviews. 2012;64:16–28. doi: 10.1016/j.addr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 117.Rossi S, Sandri G, Caramella CM. Buccal drug delivery: A challenge already won? Drug Discovery Today: Technologies. 2005;2:59–65. doi: 10.1016/j.ddtec.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 118.Myers GL, Hilbert SD, Boone BJ, Bogue BA, Sanghvi P, Hariharan M. Sublingual and buccal film compositions. 2011 U. S. Pat. Applic. Pub US 2011/0033541 A1. [Google Scholar]
- 119.Sanvordeker DR, Leung SS. Mucoadhesive buccal dsage forms. 1990 U. S. Pat. 4,900, 552. [Google Scholar]
- 120.Meyer S, Slominski G, Fankhauser CE, Ouis N. Multi-zone films. 2010 U. S. Pat. Applic. Pub. US 2010/0266669 A1. [Google Scholar]
- 121.Myers GL, Hilbert SD, Boone BJ, Bogue BA, Sanghvi P, Hariharan M. Sublingual and buccal film compositions. 2011 U. S. Pat. Applic. Pub. US 2011/0033542 A1. [Google Scholar]
- 122.Meyer S, Slominski G, Fankhause CE. Orally Administrable films. 2010 U. S. Pat. Applic. Pub. US 2010/0063110 A1. [Google Scholar]
- 123.Liao WC, Kydonieus A, Shah KR. Buccal delivery system for therapeutic agents. 1998 U. S. Pat. 5,827, 525. [Google Scholar]
- 124.Scholz MT, Scherrer RA, Marecki NM, Chen YL, Barkhaus JK. Bioadhesive composition and patch. 1998 U. S. Pat. 5,750,134. [Google Scholar]
- 125.Levine W, Satter A. Solid mucoadhesive composition. 2011 U. S. Pat. US 7,985, 430 B2. [Google Scholar]
- 126.Levine HL, Bologna WJ, Cartier PJ, Ziegler DD. Bioadhesive progressive hydration tablets. 2006 U. S. Pat. US 7,153,845 B2. [Google Scholar]
- 127.Timpe C, Dittgen M, Grawe D, Schumacher J, Zimmermann H, Hoffmann H. Bioadhesive tablet. 2000 U. S. Pat. 6,063, 404. [Google Scholar]
- 128.Aiache JM, Costantini D, Chaumont C. Prolonged release bioadhesive therapeutic systems. 2005 U. S. Pat. 6,916,485 B2. [Google Scholar]
- 129.Bologna WJ, Levine HL, Dezeigler D. Bioadhesive progressive hydration tablets. 2002 U. S. Pat. Applic. Pub. US 2002/0044964 A1. [Google Scholar]
- 130.Acharya RN, Baker JL. Oral transmucosal delivery of drugs or any other ingredients via the inner buccal cavity. 2001 U. S. Pat. US 6,210,699 B1. [Google Scholar]
- 131.Baichwal AR. Bioadhesive tablet for non-systemic use products. 1994 U. S. Pat. 5,330,761. [Google Scholar]
- 132.Rault I. Bioadhesive tablets. 2001 U. S. Pat. US 6,242,004 B1. [Google Scholar]
- 133.Khanna SC. Coated adhesive tablets. 1992 U. S. Pat. 5,091,184. [Google Scholar]