Abstract
We sought to assess the incidence of aspirin resistance after off-pump coronary artery bypass (OPCAB) surgery, and investigate whether clopidogrel can improve aspirin response and be safely applied early after OPCAB surgery. Sixty patients who underwent standard OPCAB surgery were randomized into two groups. One group (30 patients) received mono-antiplatelet treatment (MAPT) with aspirin 100 mg daily and the other group received dual antiplatelet treatment (DAPT) with aspirin 100 mg daily plus clopidogrel 75 mg daily. Platelet aggregations in response to arachidonic acid (PLAA) and adenosine diphosphate (ADP) (PLADP) were measured preoperatively and on days 1 to 6, 8 and 10 after the antiplatelet agents were administered. A PLAA level above 20% was defined as aspirin resistance. Postoperative bleeding and other perioperative variables were also recorded. There were no significant differences between the two groups in baseline characteristics, average number of distal anastomosis, operation time, postoperative bleeding, ventilation time and postoperative hospital stay. However, the incidence of aspirin resistance was significantly lower in the DAPT group than that in the MAPT group on the first and second day after antiplatelet agents were given (62.1% vs. 32.1%, 34.5% vs. 10.7%, respectively, both P < 0.05). There was no significant difference in postoperative complication between the two groups. DAPT with aspirin and clopidogrel can be safely applied to OPCAB patients early after the procedure. Moreover, clopidogrel reduces the incidence of OPCAB-related aspirin resistance.
Key words: aspirin, clopidogrel, aspirin resistance, off-pump coronary artery bypass (OPCAB)
INTRODUCTION
Previous studies showed that early administration of aspirin after coronary artery bypass grafting (CABG) significantly improved vein graft patency rate, and reduced the risk of death and decreased complications related to ischemia of the heart, brain, kidney and gastrointestinal tract[1]. However, recent studies revealed that after CABG or other major surgery, a large proportion of patients responded poorly to aspirin, the so-called surgery associated aspirin resistance. In such patients, platelet aggregation was not adequately inhibited although patients were receiving a regular dose of aspirin (100 mg/day). This situation occurs in up to 60% of patients within 10 days after surgery and has been reported to be an independent risk factor for early postoperative venous graft thrombosis[2]-[4].
It is well known that dual antiplatelet treatment (DAPT) of aspirin and clopidogrel remarkably reduces cardiovascular risks and improves prognosis in patients with acute coronary syndrome (ACS)[5], especially those with stent implantation[6]. However, the efficacy of the combination of aspirin and clopidogrel after CABG is still controversial[7]-[10]. The most recent guideline from the American College of Cardiology/American Heart Association (ACC/AHA) did not address the use of aspirin with clopidogrel following CABG[11]. This study aimed to assess the incidence of aspirin resistance after off-pump coronary artery bypass (OPCAB) surgery, and investigate whether clopidogrel in addition to aspirin can improve patients' response to aspirin and whether DAPT with aspirin and clopidogrel can be safely applied early after OPCAB surgery.
PATIENTS AND METHODS
Ethics Statement
This study was approved by the Research Ethics Board of the First Affiliated Hospital of Nanjing Medical University, China, and was conducted according to the Declaration of Helsinki. Written consent forms were obtained from all participants.
Subjects
Sixty patients who were going to receive an elective OPCAB surgery and had stopped using antiplatelet agents for more than 1 week were included in this study from April 2011 to April 2012 at the authors' affiliated hospital. The patients were randomly and postoperatively divided into two groups to receive either mono-antiplatelet treatment (MAPT) with 100 mg/day aspirin (Enteric Coated Tablets, Bayer HealthCare AG, Germany) alone or DAPT with 100 mg/day aspirin and 75 mg/day clopidogrel (Clopidogrel Hydrogen Sulphate Tablets, Sanofi-Aventis, France). Antiplatelet treatment was administered to patients at 8:00 am each day early after the surgery according to patient status. All patients received oral atorvastatin 20 mg/day during the perioperative period, as well as subcutaneous injection of low-molecular-weight heparin 7 to 10 days before surgery and 3 days after surgery.
Patients who met the following criteria were excluded: 1) patients who were > 75 years old; 2) patients who were allergic to aspirin or clopidogrel; 3) patients who had myelodysplastic syndrome or an abnormal baseline platelet count of < 80 × 109/ L; 3) patients with hepatic or renal insufficiency (alanine amino transferase [ALT], aspartate amino transferase [AST], serum creatinine [Cr] higher than twice of their upper normal limits); 4) patients who were taking medications that could interfere with the antiplatelet effects of aspirin such as proton pump inhibitors and non-steroidal anti-inflammatory drugs within 10 days before recruitment; 5) patients with a chest drainage of more than 30 mL/hour within 2 hours after surgery; 6) patients who received an perioperative infusion of platelet products; 7) patients with a mechanical ventilation of more than 24 hours; 8) patients who were treated with intra-aortic balloon counter pulsation or hemodialysis.
OPCAB surgery
All patients received OPCAB surgery under combined intravenous inhalational anesthesia. Median sternotomy incision was performed, and the saphenous vein, internal mammary artery, or radial artery were chosen as bypass graft as required. Intraoperative heparin was given at a dose of l mg/kg and was modified to reach the activated clotting time (ACT) level of 300 seconds. Protamine was given to neutralize the anti-coagulant effect of heparin at a ratio of 0.75:1 upon the completion of the procedure, and low molecular weight heparin (Enoxaparin Sodium Injection, Sanofi-aventis France) was used for 3 days from the first day after surgery.
Operation variables including drainage volume, extubation time, postoperative hospitalization stay, severe bleeding, postoperative blood transfusion, second thoracotomy for hemostasis and combined medication, as well as in-hospital cardiac events including death, myocardial infarction and heart failure were recorded.
Sample preparation
Six milliliter venous blood were collected into EDTA and citrate vacutainer tubes at baseline and at days 1 to 6, 8, and 10 after taking antiplatelet agents. Blood samples were subject to platelet function test within 2 hours. Platelet-rich plasma (PRP) was prepared by spinning the sample at 200 relative centrifugal forces (RCF) for 8 minutes. PRP was carefully removed and the remaining blood was centrifuged at 2,465 RCF for 10 minutes to obtain platelet-poor plasma (PPP). Centrifuge temperature was maintained at 22°C. Platelet counts were adjusted by addition of PPP to PRP to achieve a platelet count of 250×109/mm3. Platelet aggregation studies were completed within 3 hours of the preparation of PRP.
Laboratory analysis and platelet aggregation
The whole blood count at each sample point was measured using a Sysme XE-2100D coulter analyzer (Sysme, Japan). Platelet aggregations in response to arachidonic acid (AA) (PLAA) and adenosine diphosphate (ADP) (PLADP) were detected using a Chronolog 560VS/490-2D Aggregometer (Chronolog, Havertown, PA, USA). Immediately after the preparation of PRP, 1 mL PRP was transferred equally into 2 test tubes, with 500 μL PRP set as control. The final concentrations of AA and ADP were 1 mmol/L and 5 µM/L, respectively. PLAA ≥ 20% was defined as aspirin resistance.
Statistical analysis
SPSS statistical software version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for statstistical analysis. Continuous data were presented as mean ± SD and analyzed by either Student's t-test or repeated measures analysis of variance or one-way analysis of variance (ANOVA) as appropriate. Wilcoxon-rank test was performed for analysis of medians. Categorical variables were expressed as frequencies and analyzed by Chi-square test. P-value < 0.05 was considered statistically significant.
RESULTS
Operation variables and clinical outcomes
Three patients dropped out of the study, of which one patient in the MAPT group had postoperative intestinal obstruction, and the other two patients in the DAPT group received postoperative infusion of platelets and developed agranulocytosis, respectively. As a result, 57 patients with an average age of 64.3 ± 8.4 years were available for final analysis. The demographic and clinical characteristics of the patients are reported in Table 1. No significant difference was found between the MAPT and the DAPT groups regarding gender, age, diagnosis, smoking status, presence of hypertension or diabetes, cholesterol levels, left ventricular ejection fraction (LVEF), number of bypass bridges, postoperative hospital stay and clinical outcomes (Table 1 and 2). Three patients in the MAPT group and two patients in the DAPT group developed heart failure.
Table 1. Patient baseline characteristics.
| Variables | DAPT (n = 28) | MAPT (n = 29) | P-value |
| Male (%) | 20 (71.4) | 19 (65.5) | NS |
| Age (years) | 65.7 ± 8.3 | 63.2 ± 8.4 | NS |
| Smoking, n (%) | 11 (39.3) | 8 (27.6) | NS |
| Hypertension, n (%) | 18 (64.3) | 22 (76.9) | NS |
| Hyperlipidemia, n (%) | 8 (28.6) | 9 (31.0) | NS |
| Diagnosis, n (%) | |||
| UAP | 19 (67.9) | 18 (62.1) | NS |
| SAP | 4 (14.3) | 3 (10.3) | NS |
| AMI | 5 (17.9) | 6 (20.7) | NS |
| OMI | 0 (0) | 2 (6.9) | NS |
| Diabetes mellitus, n (%) | 7 (25.0) | 11 (37.9) | NS |
| LVEF (%) | 63.7 ± 3.9 | 63.2 ± 3.5 | NS |
| Serum lipid level (μmmol/L) | |||
| TC | 3.8 ± 0.8 | 3.9 ± 0.8 | NS |
| TG | 1.6 ± 0.9 | 1.5 ± 1.1 | NS |
| LDL-C | 2.5 ± 0.6 | 2.6 ± 0.7 | NS |
DAPT: dual antiplatelet treatment; MAPT: mono-antiplatelet treatment; UAP: unstable angina pectoris; SAP: stable angina pectoris; AMI: acute myocardial infarction; OMI: old myocardial infarction; LVEF: left ventricular ejection fraction; TC: total cholesterol; TG: triglyceride; LDL-C: low density lipoprotein-cholesterol; NS: no statistical significance.
Table 2. Surgical variables and clinical outcomes.
| Variables | DAPT (n = 28) | MAPT (n = 29) | P-value |
| Bypass bridges, (n) | 2.8 ± 0.4 | 3.0 ± 0.5 | NS |
| Operation time (minutes) | 271.1 ± 54.5 | 292.5 ± 59.5 | NS |
| Intubation time (hours) | 17.2 ± 3.6 | 17.0 ± 3.2 | NS |
| Total bleeding volume (mL) | 659.1 ± 215.3 | 525.0 ± 402.7 | NS |
| Red blood cell transfusion (u) | 3.2 ± 1.0 | 3.0 ± 1.7 | NS |
| Plasma infusion (mL) | 395.0 ± 139.5 | 455.0 ± 199.4 | NS |
| Ventilation time (hours) | 17.2 ± 3.6 | 17.0 ± 3.2 | NS |
| Total hospitalization stay (days) | 29.8 + 6.5 | 26.9 ± 3.0 | NS |
| Post-operative hospital stay (days) | 13.2 ± 2.0 | 13.1 ± 0.8 | NS |
| Total drainage volume (mL) | 876.7 ± 247.1 | 823.1 ± 283.8 | NS |
| Cardiac events, n (%) | 2 (7.14) | 3 (10.34) | NS |
DAPT: dual antiplatelet treatment; MAPT: mono-antiplatelet treatment; NS: no statistical significance.
Perioperative alteration of platelet aggregations
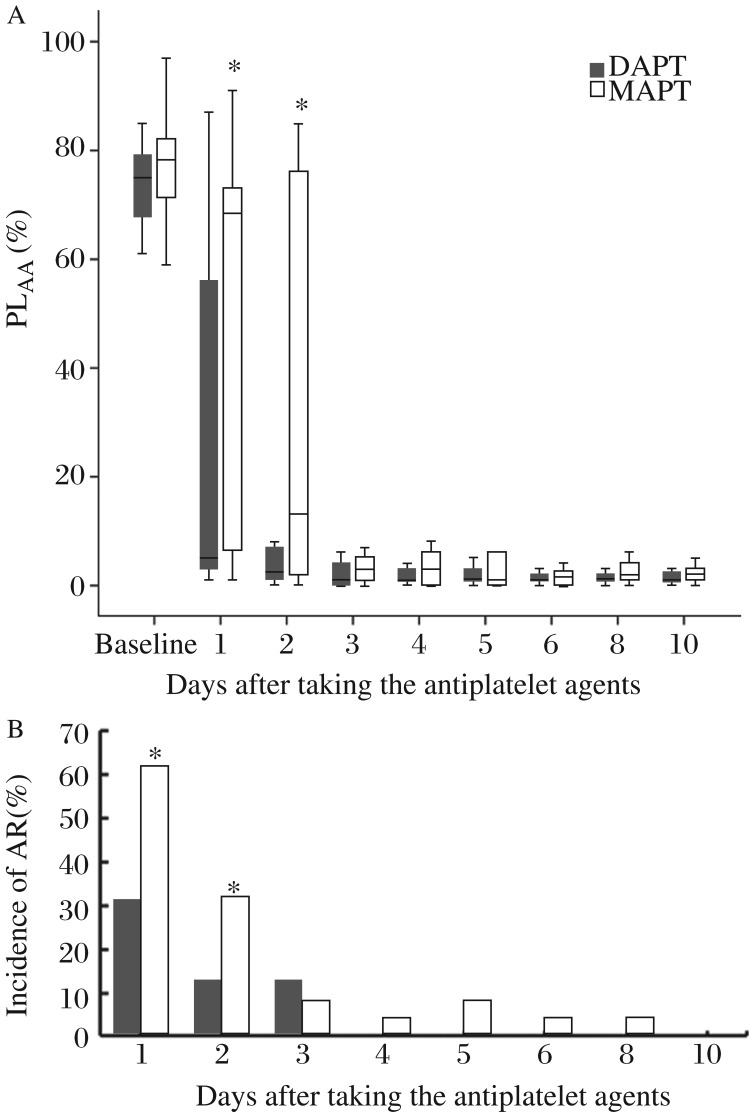
The antiplatelet agents were administered in 3 ± 1.3 days after the surgery. Baseline platelet functions were comparable in the two groups. After taking antiplatelet agents, AA induced platelet aggregations in the DAPT group were significantly lower than those in the MAPT group on day 1 and 2 (Fig. 1A). Aspirin resistance was found in both groups; however, the incidence of aspirin resistance was significantly lower in the DAPT group than that in the MAPT group on day 1 and 2 after taking antiplatelet agents (62.1% vs. 32.1%, 34.5% vs. 10.7%, respectively, both P < 0.05) (Fig. 1B).
Fig. 1. Perioperative alterations of Platelet aggregation and incidence of aspirin resistance.
A: Perioperative alterations of AA-induced platelet aggregations. PLAA, arachidonic acid induced platelet aggregation. Baseline indicates the day before surgery; D1 to D10 indicate days 1 to 10 after taking antiplatelet agents; boxes indicate median values with interquartile ranges, and bars indicate 5% and 95% confidence intervals. *Wilcoxon P < 0.05 DAPT vs. MAPT. B: Perioperative incidence of aspirin resistance. AR, aspirin resistance; D1 to D10 indicate days 1 to 10 after taking antiplatelet agents; *P < 0.05 DAPT vs. MAPT.
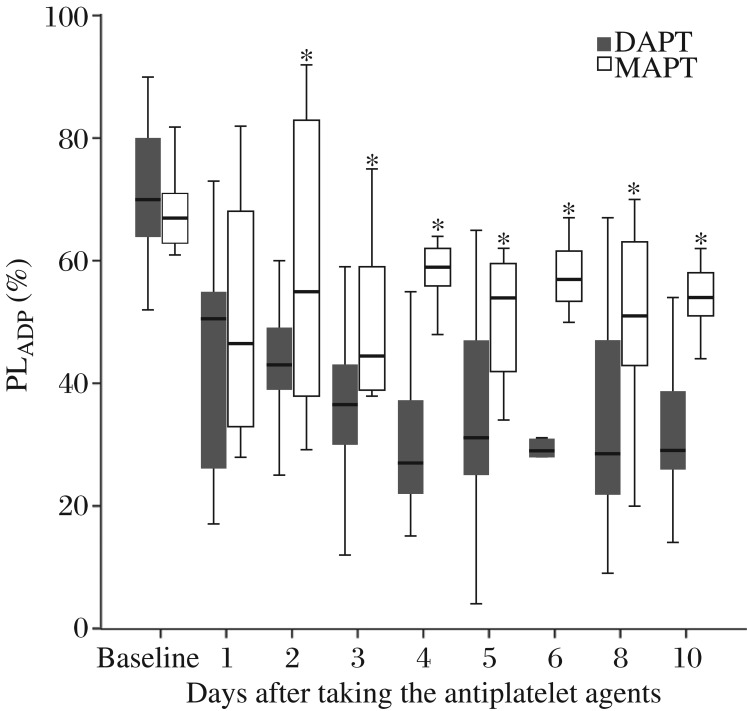
ADP-induced platelet aggregations (median) gradually decreased in the DAPT group after taking clopidogrel and aspirin, which reached an ideal level (PLADP < 40%) from day 3 of antiplatelet treatment (Fig. 2). ADP-induced platelet aggregations (median) in the MAPT group were significantly higher than those of the DAPT group, and none of the postoperative PLADP was lower than 40% during the follow up (Fig. 2).
Fig. 2. Perioperative alterations of ADP-induced platelet aggregation.
ADP, adenosine diphosphate; PLADP, ADP-induced platelet aggregation. Baseline indicates the day before the surgery; D1 to D10 indicate days 1 to 10 after taking the antiplatelet agents; boxes indicate median values with interquartile ranges, and bars indicate 5% and 95% confidence intervals. *Wilcoxon P < 0.05 DAPT vs. MAPT.
Perioperative alterations of blood cell counts
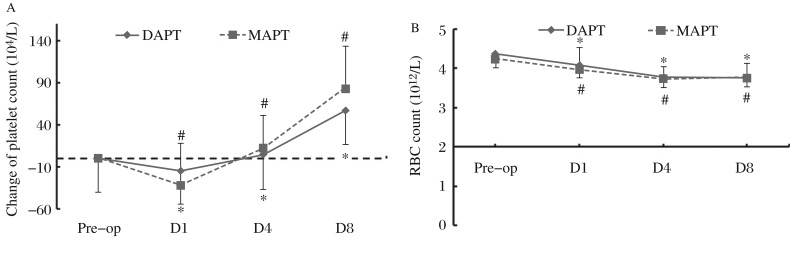
We plotted the time-curves of platelet change calculated by subtracting the platelet each day from the preoperative level, and the results showed that the platelet counts in both groups were significantly decreased after OPCAB, and recovered to the preoperative level on postoperative day 4. The platelet counts significantly increased on postoperative day 8 compared with their baseline levels in both groups. However, there was no significant difference between the MAPT and the DAPT groups in postoperative platelet changes (Fig. 3A). RBC counts decreased on day 1 after OPCAB compared with the baseline levels in both groups (DAPT: 4.1 ± 0.3 vs. 4.4 ± 0.5, P < 0.05; MAPT: 4.0 ± 0.3 vs. 4.2 ± 0.5, P < 0.05). However, there was no significant difference between the two groups concerning postoperative red blood cell changes (P > 0.05) (Fig. 3B).
Fig. 3. Perioperative changes in platelet counts and red blood cell counts.
A: Changes of perioperative platelet counts. Platelet change is calculated by subtracting platelet count on each day from the preoperative level; Pre-op indicates the day before surgery; D1, D4 and D8 indicate days 1, 4 and 8 after the procedure. *P<0.05 vs. the preoperative level in the DAPT group; #P<0.05 vs. the preoperative level in the MAPT group. B: Alterations of perioperative red blood cell counts. RBC, red blood cell; Pre-op indicates the day before the surgery; D1, D4 and D8 indicate days 1, 4 and 8 after the procedure; *P < 0.05 vs. the preoperative level in the DAPT group; #P < 0.05 vs. preoperative level in the MAPT group.
Discussion
This study demonstrated that postoperative aspirin resistance occurs after OPCAB. The postoperative antiplatelet treatment with clopidogrel in addition to aspirin can reduce the incidence of surgery associated aspirin resistance and be safely administered to OPCAB patients early after procedure.
Post-CABG aspirin resistance was first reported by Zimmermann in 2001[12], and acquired aspirin resistance was reported to promote early vein graft failure after bypass surgery[[13]]. The mechanism of postoperative aspirin resistance is still uncertain. Our previous study suggested that greater platelet turnover due to bone marrow hyperplasia caused by hemorrhage may account for impaired postoperative response to aspirin, either due to an increased number of circulating non-aspirinated platelets or an increased number of reticulated platelets that retain the ability to synthesize thromboxane through the COX-2 pathway[14]. From this point of view, higher or more frequent doses of aspirin during the postoperative period may enhance the efficacy of aspirin. However, Frelinger et al. reported that aspirin residual activity was not only related to the COX-1 and COX-2 independent pathway but also the ADP dependent pathway[15]. Thus, an alternative method to treat postoperative aspirin resistance would be administering ADP receptor inhibitor in combination with aspirin. In this study, we found that patients in the DAPT arm had a significantly lower incidence of aspirin resistance compared with those in the MAPT arm, which indicated that clopidogrel in addition to aspirin can reduce the incidence of surgery associated aspirin resistance. This phenomenon could be explained by the ability of clopidogrel to block the ADP dependent pathway associated with aspirin residual activity or its additional inhibitory effect on AA induced platelet aggregation (synergistic effect)[8].
The different incidences of postoperative aspirin resistance between the two groups could also be explained by different platelet turnover. However, we did not find different alterations of either the red blood cell or platelet counts between the DAPT and MAPT arms after operation, which suggested that postoperative hemorrhage induced bone marrow hyperplasia and platelet turnover would be similar between the two groups of patients.
We found that post-OPCAB aspirin resistance occurred mainly on day 1 to 3 after the procedure, suggesting that intensified antiplatelet therapy should be initiated early after OPCAB surgery. In this study, there were no significant differences regarding operation time, number of bridge, intubation time, bleeding volume, infusion of red blood cells and/or plasma, total postoperative drainage volume, postoperative hospital stay and total hospital stay between the MAPT and DAPT groups. Accordingly, dual antiplatelet treatment with clopidogrel in addition to aspirin was relatively safe and can be administered to patients early after OPCAB surgery.
References
- 1.Mangano DT. Aspirin and mortality from coronary bypass surgery. N Engl J Med. 2002;347:1309–17. doi: 10.1056/NEJMoa020798. [DOI] [PubMed] [Google Scholar]
- 2.Gum PA, Kottke-Marchant K, Poggio ED, Gurm H, Welsh PA, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001;88:230–5. doi: 10.1016/s0002-9149(01)01631-9. [DOI] [PubMed] [Google Scholar]
- 3.Sanioglu S, Tetik S, Sokullu O, Deniz H, Aydemir N, Yilmaz M, et al. Aspirin resistance after cabg. Thorac Cardiovasc Surg. 2009;57:281–5. doi: 10.1055/s-0029-1185564. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman TJ, McLean RC, Schulman SP, Kickler TS, Shapiro EP, Conte JV, et al. Effects of aspirin responsiveness and platelet reactivity on early vein graft thrombosis after coronary artery bypass graft surgery. J Am Coll Cardiol. 2011;57:1069–77. doi: 10.1016/j.jacc.2010.08.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without st-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 6.Steinhubl SR, Berger PB, Mann JT, 3rd, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA. 2002;288:2411–20. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 7.de Leon N, Jackevicius CA. Use of aspirin and clopidogrel after coronary artery bypass graft surgery. Ann Pharmacother. 2012;46:678–87. doi: 10.1345/aph.1Q692. [DOI] [PubMed] [Google Scholar]
- 8.Mannacio VA, Di Tommaso L, Antignan A, De Amicis V, Vosa C. Aspirin plus clopidogrel for optimal platelet inhibition following off-pump coronary artery bypass surgery: Results from the cryssa (prevention of coronary artery bypass occlusion after off-pump procedures) randomised study. Heart. 2012;98:1710–5. doi: 10.1136/heartjnl-2012-302449. [DOI] [PubMed] [Google Scholar]
- 9.Elefteriades JA, Meier P. Clopidogrel and cardiac surgery: Enemy or friend? Heart. 2012;98:1685–6. doi: 10.1136/heartjnl-2012-302822. [DOI] [PubMed] [Google Scholar]
- 10.Gurbuz AT, Zia AA, Vuran AC, Cui H, Aytac A. Postoperative clopidogrel improves mid-term outcome after off-pump coronary artery bypass graft surgery: A prospective study. Eur J Cardiothorac Surg. 2006;29:190–5. doi: 10.1016/j.ejcts.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, et al. 2011 accf/aha guideline for coronary artery bypass graft surgery: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;124:2610–42. doi: 10.1161/CIR.0b013e31823b5fee. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann N, Kienzle P, Weber AA, Winter J, Gams E, Schror K, et al. Aspirin resistance after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2001;121:982–4. doi: 10.1067/mtc.2001.111416. [DOI] [PubMed] [Google Scholar]
- 13.Poston RS, Gu J, Brown JM, Gammie JS, White C, Nie L, et al. Endothelial injury and acquired aspirin resistance as promoters of regional thrombin formation and early vein graft failure after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006;131:122–30. doi: 10.1016/j.jtcvs.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Hirsh J, Sloane D, Liang Y, Bai J, Paikin J, et al. Aspirin response variability after major orthopedic surgery. Thromb Res. 2012;130:216–20. doi: 10.1016/j.thromres.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Frelinger AL, 3rd, Furman MI, Linden MD, Li Y, Fox ML, Barnard MR, et al. Residual arachidonic acid-induced platelet activation via an adenosine diphosphate-dependent but cyclooxygenase-1- and cyclooxygenase-2-independent pathway: A 700-patient study of aspirin resistance. Circulation. 2006;113:2888–96. doi: 10.1161/CIRCULATIONAHA.105.596627. [DOI] [PubMed] [Google Scholar]