Abstract
Inositol requiring enzyme-1 (IRE1) is highly conserved from yeasts to humans. Upon the endoplasmic reticulum (ER) stress, IRE1 activates X-box-binding protein 1 (XBP1) by unconventionally splicing XBP1 mRNA, which activates the unfolded protein response (UPR) to restore ER homeostasis. In mice, IRE1α inactivity leads to embryonic death and IRE1α plays an essential role in extraembryonic tissues and the placenta. However, its precise action in the embryo proper is still unknown. In this study, the loss of function analysis was performed to investigate the function of Xenopus IRE1α (xIRE1α) during pancreas development. Firstly, the complete open reading frame of xIRE1α was amplified and the expression pattern was detected. The effects of Xenopus IRE1α and XBP1 during embryo development were detected with whole-mount in situ hybridization. The results demonstrated that xIRE1α was much closer to human IRE1α when compared with their sequence alignment. xIRE1α was expressed strongly in developing pancreas and the knockdown of xIRE1α inhibited the differentiation and specification of the pancreas. xIRE1α, which was required for cytoplasmic splicing of XBP1 pre-mRNA and XBP1MO, also showed inhibitory effects on pancreas development. These results suggest that xIRE1α is essential for pancreas development during embryogenesis and functions via the XBP1 dependent pathway.
Keywords: IRE1α, Xenopus laevis, pancreas, XBP1
INTRODUCTION
The endoplasmic reticulum (ER) plays an important role in the synthesis and modification of secretory and membrane proteins in all eukaryotic cells. The accumulation of the unfolded/ misfolded proteins in the ER could cause ER stress and affect the overall integrity of the cell. A series of adaptive responses, called the unfolded protein response (UPR), is the transcriptional/translational regulatory pathway that mitigates such impairment of cellular integrity upon the detection of ER stress by the sensor proteins. The UPR is transduced through 3 forms of ER-resident transmembrane sensors, including inositol requiring enzyme-1 (IRE1), protein kinase RNA-like ER kinase (PERK) and activating transcription factor 6 (ATF6). Each sensor protein senses the ER stress in its own fashion and induces the expression of its target genes, which facilitate the protein-folding capacity in the ER[1]–[3].
The IRE1-dependent branch is highly conserved from yeasts to humans[4]. IRE1 is an ER-located type I transmembrane protein with a kinase domain and an RNase domain in the cytosolic region. It plays a central role in the ER stress response. Upon ER stress, IRE1 is activated and the signal is transduced to the cytosol by the sequential dimerization/multimerization, trans-autophosphorylation and activation of its endoribonuclease[5]–[7]. The specific activity of the endoribonuclease is responsible for the unconventional cytosolic splicing of HAC1 in yeasts and the excision of the 26-nucleotide intron of the X-box-binding protein 1 (XBP-1) transcription factor in metazoan organisms. The removal of intron causes a frame shift and the production of a spliced XBP1 (XBP1s) mRNA, and encodes the active transcription factor XBP1s from unspliced XBP1 mRNA (XBP1U)[8],[9]. The active form of XBP1 up-regulates chaperones to enhance protein folding and genes that mediate ER-associated degradation (ERAD) to target degradation of misfolded proteins in ER stress response[4]. Therefore, the splicing of XBP1 mRNA is a major event to mediate the UPR.
Although PERK, ATF6 and IRE1 have common features as UPR inducers, there are several differences among their functions in vivo. PERK is highly expressed in mouse pancreas and is indispensable in pancreas development, while PERK−/−mice postnatally exhibit a phenotype of diabetes mellitus and exocrine pancreatic dysfunction[10]. ATF6α and ATF6β are ubiquitously expressed, and double knockout of ATF6α and ATF6β in mammals causes embryonic lethality in the early developmental stage (by 8.5 days of gestation), although a single knockout of each gene does not cause developmental abnormality[11],[12]. IRE1α is also known to be ubiquitously expressed in fetal and adult mice[13],[14], especially in the pancreas and the placenta. IRE1α inactivation results in widespread developmental defects, leading to embryonic death after 12.5 days of gestation in mice[15]. Embryo proper-restricted IRE1α conditional KO mice, which specifically express IRE1α in the extra-embryonic tissues, can avoid embryonic lethality. It indicates that a defective IRE1α−/− placenta may be one of the reasons for embryonic lethality. However, it has been hitherto unclear in which tissues of the embryo proper IRE1α functions during embryogenesis.
In IRE1α conditional KO mice, embryonic viability disruption of IRE1α caused histological abnormality of the pancreatic acinar and increased blood glucose level that started occurring four weeks after birth. In Xenopus, IRE1α was found to be expressed in the domain that probably represents the dorsal pancreas anlagen[16]. These lines of evidence suggested that IRE1α plays a role during pancreas organogenesis.
Pancreas development is conserved and early pancreas development in Xenopus closely resembles that of mice and humans, and is applicable to mammalian cells[17]. In fact, it is becoming clear that the same genes used in mammalian pancreas development are involved in Xenopus pancreas development[17]. In this study, the complete open reading frame (ORF) of Xenopus IRE1α was cloned, and the knockdown of IRE1α was performed to study the role of IRE1α in pancreas formation.
MATERIALS AND METHODS
Embryo manipulation
Xenopus laevis eggs were obtained from in vitro fertilization, dejellied in 2% cysteine hydrochloride (pH 7.8-8.0) and cultured in 0.1×MBSH (8.8 mmol/L NaCl, 0.24 mmol/L NaHCO3, 0.1 mmol/L KCl, 0.082 mmol/L MgSO4, 0.041 mmol/L CaCl2, 0.033 mmol/L Ca(NO3)2, and 1 mmol/L HEPES, pH 7.4). Embryonic stages were determined according to Nieuwkoop and Faber[18].
Plasmids and constructs
To complete the Xenopus IRE1α ORF, we used rapid amplification of cDNA ends (RACE) technique to extend the known partial cDNA to its 5′ and 3′ ends. For the 5′ RACE of xIRE1α, the following primers were used: 5′-TGCTTCTCACCA GTCACCAG-3′ and 5′-GGTTCTGTGACGTGTGTTGG-3′; for the 3′ RACE of xIRE1′, 5′-GTTTTGCAGACAGGGAGGTG-3′ and 5′-GCTATTTCTGCACCGAGAGG-3′ were used. The ORF of xIRE1α encoding 969 amino acids was established by joining the 1963 bp cDNA sequence, the 5′ RACE and the 3′ RACE sequences. To make the pCS2+-xIRE1α expression plasmid, IRE1α ORF was amplified from a cDNA pool consisting of st.1, st.8, st.10, st.15, st.20 and st.28 cDNAs and was subcloned to pCS2+ vector. For monitoring the splicing effect of Xenopus XBP1 (xXBP1) mRNA in vivo, the coding region of 121-254 aa including the stop codon in the unspliced cDNA was fused to the 5′-end of the coding region of EosFP and the resulting construct was designated as xXBP1(U)-EosFP[16].
In vitro transcription of RNA, antisense morpholino oligonucleotide (MO) and microinjection
Plasmids of pCS2+-xIRE1α were linearized with NotI. Capped mRNA for microinjection was synthesized with SP6 mMessage mMachine™ kit (Ambion, Thebarton, SA, Australia). The sequence of antisense MO (Gene Tools, Philomath, OR, USA) used for xIRE1α's functional knockdown (IRE1α MO) was 5′-AAGAGAACCGCCAGAGGCGCCATGT-3′; the sequence of an antisense MO named XBP(C)MO that was used to inhibit the cytoplasmic splicing of xXBP1 was 5′-GACATCTGGGCCTGCTCCTGCTGCA-3′ [16]; standard control MO (CoMO) was 5′-CCTCTTACCTCAGTTACAATTTATA-3′. Fifty ng IRE1α MO or XBP(C)MO was injected into 4 blastomeres at the 4-cell stage for scoring the phenotype and marker gene analysis.
In vivo assay for morpholino function
To detect the specificity of IRE1α MO, the N-terminus coding region of xIRE1α containing the IRE1α MO binding site was fused to green fluorescent protein (GFP) (xIRE1α/GFP). For control, the N-terminus of xIRE1α was mutated at 6 bases and fused to GFP (xIRE1αmut/GFP). The mRNAs were transcribed and injected either alone or with 50 ng IRE1αMO or CoMO, respectively. At the desired stage, the embryos were analyzed by fluorescence microscopy.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA from embryos was extracted and digested with DNaseI, and purified by RNeasy kit (Qiagen, Hilden, Germany). First strand cDNA was synthesized with RevertAid™ first strand cDNA synthesis kit (Fermentas, Ontario, Canada). Semi-quantitative RT-PCR was performed and primers for xXBP1 splicing were detected as previous described[16]. In parallel, ODC was amplified to confirm equal amounts and integrity of different RNA preparations.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed according to standard procedures[19]. The probes were prepared as follows: pDrive-IRE1α was cut with HindIII and transcribed with T7 RNA polymerase. pdx1, ptf1a, insulin and amylase antisense probes were prepared as previous described[20].
RESULTS
Isolation of Xenopus IRE1α
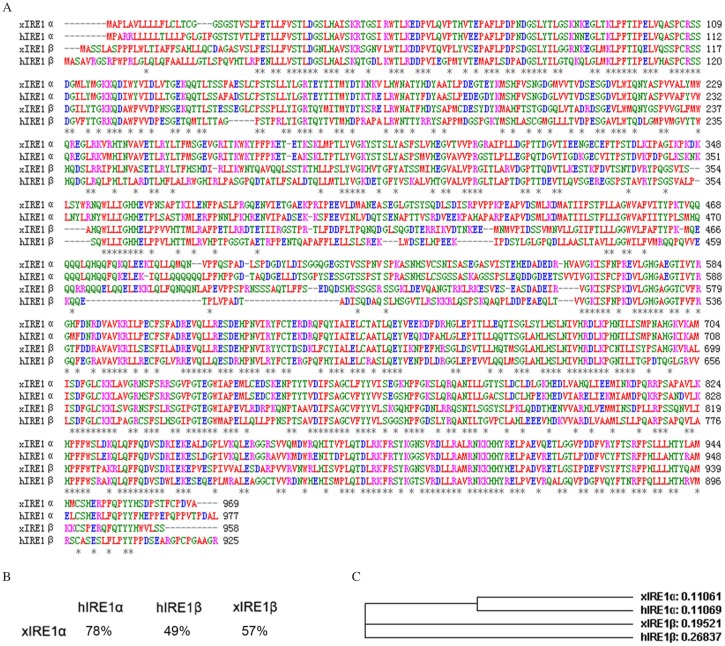
In a previous study, we obtained a piece of 1,963 bp cDNA containing partial xIRE1α ORF[16]. Now, the ORF of xIRE1α coding for 969 amino acids was completed by using RACE. A phylogenetic analysis of xIRE1α with other vertebrate homologues by using ClustalW (MacVector, Cary, NC, USA) showed that this peptide shared 57% identity to the Xenopus IRE1β (xIRE1β) and 49% identity to the human IRE1β (hIRE1β). However, xIRE1α exhibited 78% identity to human IRE1α (hIRE1α) (Fig. 1A and B), which indicated that the isolated xIRE1α sequence and hIRE1α were genetically close (Fig. 1C).
Fig. 1. Xenopus IRE1α sequence analysis.
A: Alignment of Xenopus xIRE1α, xIRE1β, hIRE1α and hIRE1β animo acid sequences. Identical residues are marked by asterisks. Gaps are introduced to achieve optimum alignment. B: Percentage of identity between IRE1 proteins. C: Phylogenetic tree of IRE1 proteins of different species created by ClustalW (h, Homo sapiens; x, Xenopus laevis).
IRE1α expression in the developing pancreas
In tail bud embryos, IRE1α was detected in a domain that is probably representing the dorsal pancreas anlagen[16]. To further explore the spatial expression patterns of IRE1α in Xenopus embryos at later stages, we carried out whole-mount in situ hybridization. During the tadpole stages, high expression of IRE1α was observed in the pancreas (Fig. 2), suggesting a potential role of IRE1α in the Xenopus pancreas development.
Fig. 2. IRE1α is expressed in the developing pancreas.
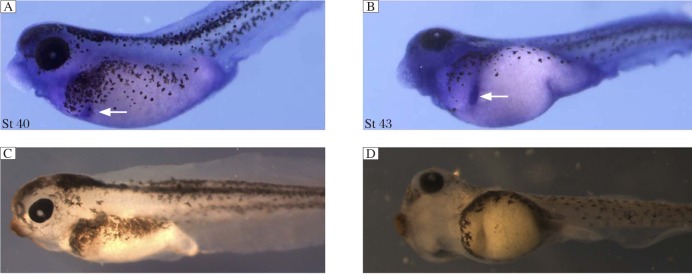
Whole-mount in situ hybridization data for IRE1α expression at stage 40 (St 40) (A) and 43 (B). The arrows indicate pancreas. C and D are negative control.
Xenopus IRE1α knockdown inhibits the expression of pancreatic differentiation marker genes
To perform loss-of-function studies, we designed morpholino antisense oligos for IRE1α, which cover the ATG initiation codon. To test whether IRE1α MO could efficiently block IRE1α translation in vivo, a 300 bp 5′-coding sequence of xIRE1α was fused which contained the putative MO binding site to GFP (xIRE1α/GFP), and a 300 bp with 6 mutation (xIRE1α mut/GFP) was constructed for control. Five hundred pg RNA of these GFP-fusion constructs were injected either alone or together with 50 ng xIRE1α MO or CoMO in each blastomere of 4-cell stage embryos, respectively. Injection of xIRE1α/GFP or xIRE1α mut/GFP and co-injections of these RNAs with CoMO resulted in bright fluorescence (Fig. 3A, B, D and E). In contrast, co-injection of xIRE1α/GFP with 50 ng xIRE1αMO completely abolished fluorescence (Fig. 3C). However, the same amount of xIRE1α MO did not affect the translation of injected xIRE1α mut/GFP RNA (Fig. 3F). These results revealed that IRE1α MO specifically repressed the translation of xIRE1α transcripts within the embryos.
Fig. 3. Inhibition of in vivo translation of a xIRE1α/GFP fusion construct by IRE1α-MO.
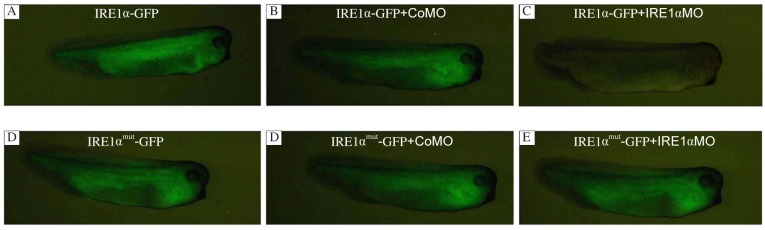
xIRE1α/GFP RNA or xIRE1αmut/GFP RNA was injected into 4 blastomeres at 4-cell stage alone (A, D), or co-injected with 50 ng control MO (B, E) or 50 ng IRE1α MO (C, F). Embryos were collected at stage 31 and GFP was monitored.
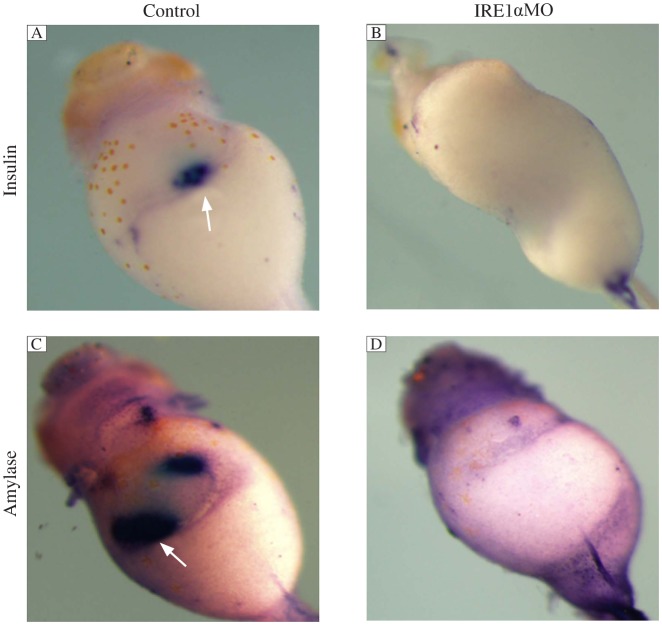
Since the expression pattern showed that xIRE1α was expressed strongly in the pancreas, we further tested whether xIRE1α plays some roles during pancreas development. We injected 50 ng IRE1α MO into 4 blastomeres of 4-cell stage embryos, which were collected at stage 43 and whole-mount in situ hybridization was performed. The results showed that the expressions of insulin and amylase, an endocrine pancreas marker gene and an exocrine pancreas marker gene, were significantly reduced in IRE1α knockdown embryos compared to those in CoMo-injected embryos (Fig. 4).
Fig. 4. IRE1α knockdown specifically inhibits the expression of differentiation marker genes.
Whole mount in situ hybridization analyses revealed that the expression of insulin (B) and amylase (D) was not detected in IRE1α MO injected embryos at stage 43 compared with control MO injected embryos (A, C). The white arrows point to the positive staining of insulin and amylase.
Xenopus IRE1α knockdown suppresses pancreas specification
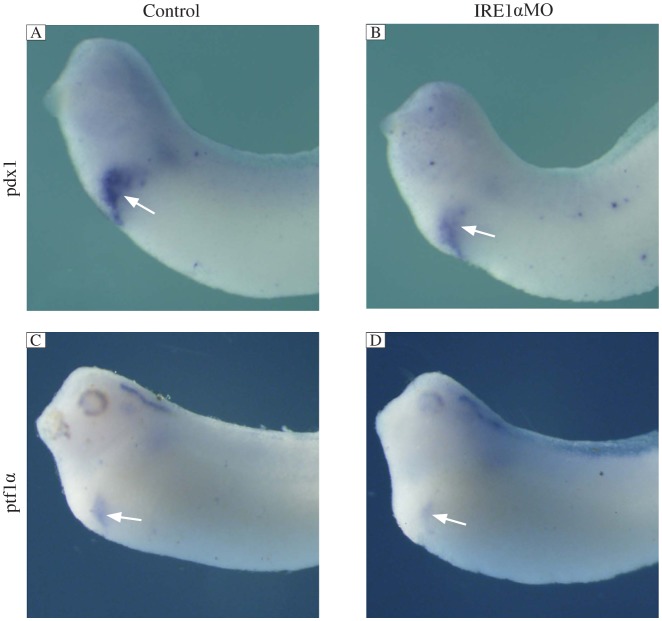
To determine whether the specification of the anterior endoderm was affected by the inhibition of IRE1α, we examined whether there were defects in the early expression of two anterior endoderm markers, pdx1 and ptf1α. At stage 30, when specification of this region occurs, we found that these two marker genes were specifically expressed in pancreatic buds in the control MO injected embryos, while the expression of these two genes was significantly reduced in the IRE1α knockdown embryos (Fig. 5). These results indicated that knockdown of IRE1α affects the specification of the pancreas.
Fig. 5. IRE1α knockdown inhibits the expression of specification marker genes.
Whole mount in situ hybridization analyses revealed that the expression of pdx1 (B) and ptf1α (D) was significantly suppressed in IRE1α MO injected embryos at stage 30 compared with control MO injected embryos (A, C). The white arrows indicate positive staining.
IRE1α is required for the cytoplasmic splicing of XBP1 pre-mRNA in Xenopus laevis
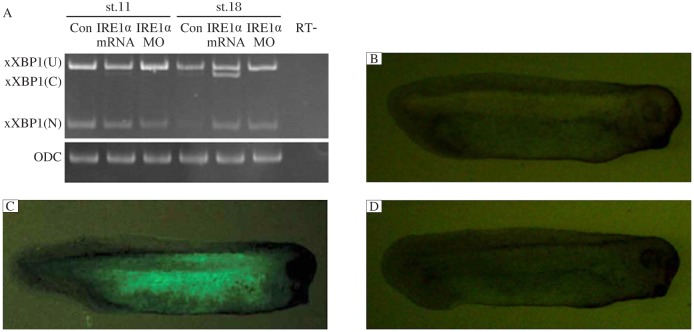
Since cleavage of XBP1 pre-mRNA by IRE1 is a well conserved mechanism throughout all organisms examined[8],[9],[16],[21], we tested whether the IRE1 homologue xIRE1α could also cleave Xenopus XBP1 pre-mRNA and xXBP1(U). Non-injected control and xIRE1α-injected embryos were collected at stages 11 and 18 and subjected to RT-PCR. As previously reported, for non-injected embryos at stage 11, only the unspliced (xXBP1(U)) and the nuclear splice form of xXBP1 (xXBP1(N)) were detected; at later stages, the conventional cytoplasmic splice form of xXBP1 (xXBP1 (C)) was detected[16]. In embryos injected with xIRE1α mRNA, the band representing xXBP1(C) at stage 11 was detected, and xXBP1(C) was significantly increased concomitant with a decrease of xXBP1(U) in comparison to the controls at stages 18. In embryos injected with xIRE1αMO, the band for xXBP1 (C) at stage 18 disappeared (Fig. 6A).
Fig. 6. Effects of xIRE1α on cytoplasmic splicing of xXBP1.
RT-PCR (A) detected an increase of cytoplasmic variant xXBP1 (C) in embryos injected with xIRE1α mRNA and a decrease of the xXBP1(C) in embryos injected with IRE1αMO at stage 11 and 18. ODC (ornithine decarboxylase) served as a loading control. Monitoring the xXBP1 splicing by xIRE1α in vivo (B-D). Embryos injected with 500 pg xXBP1(U)-EosFP RNA individually or in combination with 1 ng xIRE1α RNA and/or 50 ng XBP (C) MO. xXBP1 (U), unspliced xXBP1; xXBP1(N), nuclear spliced xXBP1. RT-: no-reverse transcriptase control.
We further monitored the effects of xIRE1α on xXBP1 splicing in vivo using a fluorescence sensor, by fusing the C terminal coding region of a 121-254 of unspliced xXBP1 with the stop codon to the 5′-end of the coding region of the fluorescent protein, EosFP[22]. In the xXBP1(U)-EosFP mRNA injected embryos, green fluorescence was not detected (Fig. 6B). However, the xXBP1(U)-EosFP mRNA co-injected with xIRE1α mRNA resulted in the appearance of green fluorescence in embryos (Fig. 6C). In the embryos injected with xXBP1(U)-EosFP, xIRE1α mRNA and XBP1(C)MO, no more fluorescence was detected (Fig. 6D). These results suggest that xIRE1α is required for cytoplasmic splicing of xXBP1 pre-mRNA.
Xenopus XBP1 knockdown inhibits pancreas formation
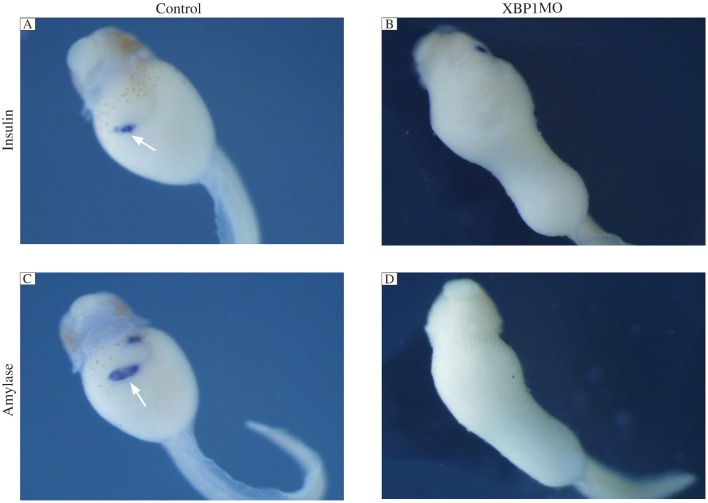
IRE1α is the most evolutionarily conserved branch of the UPR. Upon activation, it initiates the unconventional splicing of mRNA encoding the transcriptional factor XBP1 to attenuate ER stress by mediating UPR. To investigate whether knockdown of XBP1 affects pancreas formation, we injected 50 ng of spliced form of XBP1 (C) MO that bind to the splice site to repress the splice of XBP1 into 4 blastomeres at the 4-cell stage for scoring the phenotype. As shown in Fig. 7, the expression of pancreas specific marker insulin and amylase was significantly reduced compared to the control embryos. Injection of XBP1(C) MO also caused gut-coiling defect.
Fig. 7. IRE1α knockdown inhibits pancreas marker gene expression.
Whole mount in situ hybridization analyses revealed that the expression of insulin (B) and amylase (D) was not detected in 50 ng XBP1MO injected embryos at stage 43 compared with control MO injected embryos (A,C). The white arrows indicate positive staining.
DISCUSSION
Previous studies have established the essential role of IRE1α during embryogenesis[15],[23],[24]; however, it has been hitherto unclear in which tissues in the embryo proper it functions and how IRE1α functions during embryogenesis. Here, we demonstrated that Xenopus IRE1α is essential for pancreas organogenesis. We initially cloned the full length of Xenopus IRE1α and found that it was predominantly expressed in the developing pancreas during Xenopus embryogenesis. Then, we demonstrated that knockdown of IRE1α led to the suppressed expression of pancreas differentiation marker genes and specification marker genes. Finally, we demonstrated that IRE1α functions via the XBP1 dependent pathway.
Only IRE1 is conserved in all eukaryotes of the ER stress sensors, including fungi, plants and animals. Yeasts and nematodes have only one IRE1 gene in their genome, and the inactivation of this gene is not lethal to these organisms under normal conditions[25],[26], while knockout of IRE1α causes embryonic lethality in mice[15]. This evidence suggests that IRE1α has a unique function in the developmental processes. Sequence alignment showed that Xenopus IRE1α is much more similar to human IRE1α. xIRE1α, like mammalian IRE1α[27], cleaves XBP1 pre-mRNA in vivo.
Abundant expression of IRE1α has been reported in the mammalian pancreas[13]. A previous report showed that xIRE1α is expressed in a domain that probably represents the dorsal pancreas anlagen[16]. This study showed that xIRE1α was expressed during the development of the pancreas during Xenopus embryogenesis. The developmental expression of a number of pancreatic markers has been reported in Xenopus including nuclear factors (Pax6, NeuroD, Islet1, Pdx1 and XpabpII), hormones (insulin, glucagon and somatostatin) and digestive enzymes (amylase, elastase, trypsinogen and carboxypeptidase A)[17]. Sox9 and Pdx1 are expressed around stage 25 in the prospective pancreatic rudiments, and most of the other markers are not detected until the pancreatic buds become discernible[17]. Therefore, IRE1α is one of the earliest genes expressed in the developing pancreatic tissue and has the potential role in specification and differentiation of the pancreas.
Pancreas morphogenesis begins with the evagination of the embryonic endoderm for the formation of dorsal or ventral buds whose development is guided by distinct transcription programs[28]. To investigate whether IRE1α plays a role in pancreas development, IRE1α was knocked down and pancreas developmental marker genes were detected with the whole-mount in situ hybridization. Knockdown of IRE1α resulted in dramatic gut defects after stage 40. The expression of the endocrine and exocrine differentiation markers, insulin and amylase at stage 43 was almost completely abolished, which suggested that the pancreas structure was destroyed in IRE1α deficient embryo and the final differentiation of endocrine and exocrine cells was affected in IRE1α knockdown embryos. However, it does not address whether this effect is seen earlier in development when the pancreatic domain is first specified. This is especially important as IRE1α is expressed early in the domain representing the pancreas anlagen.
Pancreatic progenitor cells first express the homeodomain transcription factor Pdx1, then expressing the basic helix-loop-helix (bHLH) factor Ptf1a[29]. The whole-mount in situ hybridization showed that knockdown of IRE1α caused reduced expression of pancreas specification markers, including pdx1 and ptf1α. Both genes are expressed in pancreatic progenitors, and are necessary and sufficient for pancreas development[17]. The defect seen in Pdx1 knockdown Xenopus is similar to that observed in mice; although loss of Pdx1 leads to pancreatic agenesis, there is a small dorsal bud present that produces insulin and glucagons[17]. Knockdown of Ptf1α resulted in a complete loss of acinar cells, and both insulin and glucagons were lost at late stage[30]. Based on the previous report, knockdown of IRE1α has no effect on germ layer formation[31], which suggest that knockdown of IRE1α firstly inhibited the progenitor genes of endocrine and exocrine, and then repressed the differentiation of the pancreas.
In ER stress response, IRE1α and XBP1 function in the same signal transduction pathway[32]. However, some other studies showed that not only a known IRE1α-dependent XBP1 function but also an XBP1-independent IRE1α function exists[23],[24],[33]. We found that the knockdown of IRE1α and XBP1 led to a similar phenotype, which indicated that XBP1 functions downstream of IRE1α. XBP1 is a transcription factor and was reported to physically interact with and negatively regulate the levels of forkhead box O1 (FoxO1)[34]. FoxO1 may play a role on beta cell differentiation in the human fetal pancreas by controlling critical transcription factors, including ngn3 and Nkx6.1[35]. These findings suggest that during pancreas development, IRE1α may function via the XBP1-dependent pathway, and then XBP1 regulates the downstream transcription factors, which needs to be further confirmed.
References
- 1.Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 2.Cao SS, Kaufman RJ. Unfolded protein response. Curr Biol. 2012;22:R622–R626. doi: 10.1016/j.cub.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Moore KA, Hollien J. The unfolded protein response in secretory cell function. Annu Rev Genet. 2012;46:165–83. doi: 10.1146/annurev-genet-110711-155644. [DOI] [PubMed] [Google Scholar]
- 4.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 5.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol Rev. 2011;91:1219–43. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 6.Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–17. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata Y, et al. Translational control by the ER transmembrane kinase/ ribonuclease IRE1 under ER stress. Nat Cell Biol. 2001;3:158–64. doi: 10.1038/35055065. [DOI] [PubMed] [Google Scholar]
- 8.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 10.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–63. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–64. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, et al. Transcriptional induction ofmammalianERquality control proteins is mediated by single or combined action of ATF6 and XBP1. Dev Cell. 2007;13:365–76. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–24. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 15.Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–81. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan L, Cao Y, Oswald F, Knöchel W. IRE1beta is required for mesoderm formation in Xenopus embryos. Mech Dev. 2008;125:207–22. doi: 10.1016/j.mod.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Pearl EJ, Bilogan CK, Mukhi S, Brown DD, Horb ME. Xenopus pancreas development. Dev Dyn. 2009;238(6):1271–86. doi: 10.1002/dvdy.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin), 2nd ed. Elsevier/ North Holland, Amsterdam. 1967:163–88. [Google Scholar]
- 19.Harland RM. In situ hybridization: an improved wholemount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–95. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 20.Wen L, Yang Y, Wang Y, Xu A, Wu D, Chen Y. Appl1 is essential for the survival of Xenopus pancreas, duodenum, and stomach progenitor cells. Dev Dyn. 2010 Aug;239:2198–207. doi: 10.1002/dvdy.22356. [DOI] [PubMed] [Google Scholar]
- 21.Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–9. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Knöchel S, Oswald F, Donow C, Zhao H, Knöchel W. XBP1 forms a regulatory loop with BMP-4 and suppresses mesodermal and neural differentiation in Xenopus embryos. Mech Dev. 2006;123:84–96. doi: 10.1016/j.mod.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Iwawaki T, Akai R, Kohno K. IRE1a Disruption Causes Histological Abnormality of Exocrine Tissues, Increase of Blood Glucose Level, and Decrease of Serum Immunoglobulin Level. PLoS ONE. 2010;5:e13052. doi: 10.1371/journal.pone.0013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwawaki T, Akai R, Yamanaka S, Kohno K. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc Natl Acad Sci U S A. 2009;106:16657–62. doi: 10.1073/pnas.0903775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 26.Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 27.Back SH, Lee K, Vink E, Kaufman RJ. Cytoplasmic IRE1 alpha- mediated XBP1 mRNA splicing in the absence of nuclear processing and endoplasmic reticulum stress. J Biol Chem. 2006;281:18691–706. doi: 10.1074/jbc.M602030200. [DOI] [PubMed] [Google Scholar]
- 28.Jørgensen MC, Ahnfelt-Rønne J, Hald J, Madsen OD, Serup P, Hecksher- Sørensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- 29.Arda HE, Benitez CM, Kim SK. Gene regulatory networks governing pancreas development. Dev Cell. 2013;25:5–13. doi: 10.1016/j.devcel.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afelik S, Chen Y, Pieler T. Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 2006;20:1441–6. doi: 10.1101/gad.378706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo J, Li XX, Feng JJ, Yin CY, Wang XJ, Wang N, et al. Inositol-requiring enzyme 1α is required for gut development in Xenopus lavies embryos. World J Gastroenterol. 2013;19:227–34. doi: 10.3748/wjg.v19.i2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 33.Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–80. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Lee J, Reno CM, Sun C, Park SW, Chung J, et al. Regulation of glucose homeostasis through a XBP 1- FoxO1 interaction. Nature Med. 2011;17:356–65. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee SJ, Cary M, et al. Insulin/IGF 1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A. 2010;107:9730–5. doi: 10.1073/pnas.1002575107. [DOI] [PMC free article] [PubMed] [Google Scholar]