Abstract
Inorganic arsenic (iAs), a human carcinogen, potentially targets the prostate. iAs malignantly transforms the RWPE-1 human prostate epithelial line to CAsE-PE cells, and a derivative normal stem cell (SC) line, WPE-stem, to As-Cancer SC (As-CSC) line. MicroRNAs (miRNA) are noncoding but exert negative control on expression by degradation or translational repression of target mRNAs. Aberrant miRNA expression is important in carcinogenesis. A miRNA array of CAsE-PE and As-CSC revealed common altered expression in both for pathways concerning oncogenesis, miRNA biogenesis, cell signaling, proliferation, and tumor metastasis and invasion. The KRAS oncogene is overexpressed in CAsE-PE cells but not by mutation or promoter hypomethylation, and is intensely overexpressed in As-CSC cells. In both transformants, decreased miRNAs targeting KRAS and RAS superfamily members occurred. Reduced miR-134, miR-373, miR-155, miR-138, miR-205, miR-181d, miR-181c, and let-7 in CAsE-PE cells correlated with increased target RAS oncogenes, RAN, RAB27A, RAB22A mRNAs, and KRAS protein. Reduced miR-143, miR-34c-5p, and miR-205 in As-CSC correlated with increased target RAN mRNA, and KRAS, NRAS, and RRAS proteins. The RAS/ERK and PI3K/PTEN/AKT pathways control cell survival, differentiation, and proliferation, and when dysregulated promote a cancer phenotype. iAs transformation increased expression of activated ERK kinase in both transformants and altered components of the PI3K/PTEN/AKT pathway including decreased PTEN and increases in BCL2, BCL-XL, and VEGF in the absence of AKT activation. Thus, dysregulated miRNA expression may be linked to RAS activation in both transformants.
Keywords: arsenic, prostate cells, stem/progenitor cells, microRNA, KRAS, cancer
A known human carcinogen, inorganic arsenic (iAs) exposure is associated with skin, lung, bladder and prostate cancers (Benbrahim-Tallaa and Waalkes, 2008; IARC, 2012). There is also evidence of tumor development within the urogenital system of adult mice after exposure to iAs in utero, although not within the prostate (IARC, 2012). In vitro chronic iAs exposure malignantly transforms human prostate epithelial cells into a cancer phenotype that produces aggressive carcinoma in mouse xenograft studies (Achanzar et al., 2002).
Accumulating evidence implicates stem cells (SCs) as key factors in iAs carcinogenesis. Cancer SCs (CSCs) are a small fraction of cells found in solid tumors, and are capable of driving tumor initiation, proliferation, invasion, and metastasis. CSCs share normal SC-like properties such as self-renewal, residence in a niche, and sphere formation in culture. Arsenic induces an overabundance of CSCs in in vivo models of carcinogenesis during tumor formation, including skin carcinomas influenced by in utero arsenic exposure in mice (Waalkes et al., 2008) and in lung and liver malignancies in mice exposed to whole-life iAs (Tokar et al., 2011). Similarly in in vitro models of arsenic-driven acquisition of a malignant phenotype, an overabundance of CSCs occurs in transformed human prostate epithelial cells (Tokar et al., 2010b) and in transformed human skin keratinocytes (Sun et al., 2012). More recently, it was shown that arsenic-induced malignant prostate epithelial cells can convert nearby, but noncontiguous, normal SCs into CSCs, thereby causing CSC accumulation (Xu et al., 2012). Chronic iAs exposure in vitro can also directly induce malignant transformation of human prostate SCs (WPE-stem) producing the As-CSC line (Tokar et al., 2010a). Both in vitro arsenic-transformed prostate epithelial cells (CAsE-PE cells) and arsenic-transformed isogenic SCs (As-CSC cells) produced highly aggressive tumors in mouse xenograft studies (Achanzar et al., 2002; Tokar et al., 2010a), although underlying molecular mechanisms of transformation remain incompletely defined.
MicroRNAs (miRNAs) are small noncoding RNAs that negatively regulate gene expression at a post-transcriptional level. They exert their regulatory function by complementary binding, mostly to the 3′ untranslated region of their target mRNAs, and cause translational repression or degradation of target mRNA depending on the degree they are complementary (Bartel, 2004). A single miRNA is usually capable of negatively regulating the expression of multiple genes. They regulate many cellular processes. Aberrant miRNA expression can influence a variety of pathological events, including cancer (Calin and Croce, 2006). Several miRNAs have been shown to act like oncogenes or tumor suppressor genes (Calin and Croce, 2006; Volinia et al., 2006), and altered expression can lead to cancer development, or enhanced invasion and metastasis. In prostate cancer, there are several reports of aberrant miRNA expression in human tumors, cancer cell lines, or xenograft tumors (Calin and Croce, 2006; Liu et al., 2012; Porkka et al., 2007; Volinia et al., 2006). Accumulating evidence indicates that miRNAs can also regulate CSC properties, including prostate CSCs (Liu and Tang, 2011). Differences in miRNA expression and in their target molecular pathways exist between cancer cells and CSCs. Recently, Liu et al. (2012) showed distinct miRNA expression patterns exist in prostate cancer stem/progenitor cells. Though several miRNAs are aberrantly expressed in different prostate cancer cells and CSCs, the miRNA signatures of prostate cancers induced by different carcinogens might be unique. Therefore, determining the role of miRNA in carcinogen-induced prostatic malignant phenotypes is key to understanding underlying molecular mechanisms of chemical transformation. In this regard, the role of miRNA dysregulation in arsenic-induced prostate oncogenesis is undefined. In prior work, we found no evidence of DNA damage during iAs-induced malignant transformation of human prostate epithelial cells (Kojima et al., 2009). Thus, in this study, we initially profiled the expression of 84 cancer-related miRNAs in arsenic-induced malignantly transformed prostate epithelial and transformed isogenic SCs in hopes to further define potentially nongenotoxic mechanisms of arsenic carcinogenesis. The goal was to investigate the potential role of miRNAs in arsenic-induced acquired malignant phenotype for prostate cancer and to determine if differences in miRNA signature exist between the arsenic-transformed total epithelial cell population and the transformed SC population. Based on these initial data, we focused on the RAS oncogene pathway, exploring the potential role of dysregulated RAS-targeting miRNAs in the activation of KRAS during arsenic-induced malignant transformation.
MATERIALS AND METHODS
Chemicals and reagents
Sodium arsenite (NaAsO2) was purchased from Sigma Chemical Co. (St Louis, MO). Keratinocyte serum-free medium (K-SFM), bovine pituitary extract (BPE), epidermal growth factor (EGF), and 100× antibiotic-antimycotic mixture were purchased from Life Technologies, Inc. (Grand Island, NY). The miRNeasy kit and miScript miRNA PCR array were purchased from Qiagen Inc. (Valencia, CA). Mouse anti-KRAS, rabbit anti-RRAS, rabbit anti-BCL-XL, and rabbit anti-phospho-ERK1/2 (Thr202/Tyr204) were purchased from Santa Cruz Biotech, Inc. (Santa Cruz, CA). Goat anti-NRAS and mouse anti-β-actin were purchased from Sigma Aldrich (St Louis). Rabbit anti-PTEN and mouse anti-VEGF were purchased from Abcam (Cambridge, MA). Mouse anti-BCL2 was purchased from BD Biosciences, Inc. (San Jose, CA). Horseradish peroxidase (HRP) conjugated secondary antibodies were purchased from Cell Signaling Technology (Beverly, MA), and Bradford Protein Assay came from Bio-Rad Laboratories (Hercules, CA).
Cells and cell culture
Four isogenic cell lines, RWPE-1, CAsE-PE, WPE-stem, and As-CSC cells were used. RWPE-1 cells are immortalized nontumorigenic human prostate epithelial cells originally derived from normal adult human prostate (Bello et al., 1997). By single cell dilution cloning, WPE-stem cells were derived from RWPE-1 cells (Tokar et al., 2005). WPE-stem cells are nontumorigenic and serve as a good model for normal SCs due to well-defined SC characteristics (Tokar et al., 2005). Chronic in vitro exposure to 5μM arsenic (as sodium arsenite) for 29 weeks transformed RWPE-1 cells into a malignant phenotype (designated CAsE-PE cells) which showed multiple in vitro signs of malignant transformation and produced tumor xenografts in nude mice (Achanzar et al., 2002). We estimate this cell line contains approximately 2–3% CSCs (Tokar et al., 2010b). Similarly, continuous in vitro exposure of WPE-stem cells to 5μM arsenic for 18 weeks also induced acquisition of a malignant phenotype (designated As-CSC cells) as assessed in vitro and by production of xenograft tumors in nude mice (Tokar et al., 2010a). A schematic diagram showing the genesis of these cell lines is provided (Supplementary fig. 1). All these isogenic cells were grown in K-SFM containing 50 μg/mL BPE and 5 ng/mL EGF, supplemented with antibiotic/antimycotic mixture. The cells were incubated at 37°C in a humidified atmosphere containing 5% carbon dioxide in the presence or absence of arsenic until preconfluence. Due to poor attachment or spreading of SCs (WPE-stem and As-CSC cells) on tissue culture plastic surface, these cells were cultured in flasks coated with a mixture of type IV collagen and fibronectin (2.5 μg each/cm2). To a T-25 flask, 3 ml of the matrix mixture was added to the flask and incubated overnight at 37°C. Cells were grown in triplicates of flasks. RWPE-1 served as passage-matched control for CAsE-PE, whereas WPE-stem served as passage-matched control for As-CSC.
RNA extraction and quantitative Real-Time PCR (qRT-PCR)
Total RNA including miRNAs was isolated using miRNeasy kit (Qiagen Inc.) according to manufacturer's instructions. The quality of total RNA was evaluated using NanoDrop 2000 spectrophotometer (ThermoFisher Scientific, Rochester, NY). For the analysis of miRNA expression, cDNA was generated from RNA by the miScript II RT kit (Qiagen Inc.) according to manufacturer's instructions. The resulting cDNA was used as the template for real-time PCR with the miScript SYBR Green PCR Kit and the Human Cancer Pathway-Focused PCR Array (Qiagen Inc.) following the manufacturer's instructions. The PCR array contains miRNA primers that profile the expression of 84 miRNAs that are differentially expressed in tumor versus normal tissues. Six small RNAs, SNORD 61, 68, 72, 95, 96A, and RNU6B/RNU6–2 served as internal controls, the average of which was used to normalize data. Real-time fluorescence detection was done on an iCycler (Bio-Rad). Data were analyzed using the ΔΔCt method of relative quantification in which cycle times (Ct) of miRNAs were first normalized to that of the average of the internal controls, and then to the passage-matched controls. Fold regulation is compared with miRNA expression in RWPE-1 for CAsE-PE cells, and in WPE-Stem for As-CSC cells.
Target genes of miRNA were predicted using TargetScan 6.2 (http://www.targetscan.org) and microRNA.org (http://www.microrna.org), with main focus on cancer-related genes. For the analysis of mRNA expression, RNA was reverse transcribed with Moloney murine leukemia virus reverse transcriptase and oligo-d (T) primers. The primers for selected genes were designed with ABI Primer Express 3.0 Software (Applied Biosystems, Foster City, CA). Absolute SYBR Green ROX Mix (ThermoFisher Scientific) was used for RT-PCR analysis. Cycle times were normalized with GAPDH from the same sample and then expressed as percentage of passage-matched control. qRT-PCR was performed on an iCycler (Bio-Rad).
Western blot analysis
Total protein was isolated using M-PER reagent (Pierce, Rockford, IL) following manufacturer's protocol. Protein concentration was determined using Bradford assay, and 15–20 μg of each protein sample was separated on 4–12% gradient, or 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis gel, and transferred to PVDF membranes (Invitrogen). Membranes were incubated in antibodies specific for K-RAS, R-RAS, N-RAS, p-ERK (Thr202/Tyr204), BCL2, BCL-XL, VEGF, PTEN, and β-actin followed by incubation in corresponding HRP-conjugated secondary antibodies. β-actin was used to normalize loading. Signals were visualized by incubating membranes in chemiluminescent HRP substrate (SuperSignal West Pico from Pierce, or Immobilon Western from Millipore) followed by exposure to Hyperfilm (Amersham). Densitometric analysis was performed using Quantity One software (Bio-Rad).
Statistical analysis
All data are presented as mean ± SEM from three or more independent experiments. Statistical analyses were performed using an unpaired Student's t-test. A two-tailed p < 0.05 was considered significant in all cases.
RESULTS
Expression Profiling of miRNAs in Arsenic-Transformed Prostate Epithelial and Stem Cells
We used qRT-PCR to determine the expression levels of 84 mature human miRNAs in human prostate epithelial cells (CAsE-PE) and stem cells (As-CSCs) that had been malignantly transformed by chronic iAs exposure in previous work (Achanzar et al., 2002; Tokar et al., 2010a). When compared with their respective controls, the miRNA expression profile showed some interesting instances of differential expression between epithelial cells and SCs for the arsenic-transformed cells. Of the 84 miRNAs profiled, 29 and 13 miRNAs were differentially expressed by > 1.5 fold over control in CAsE-PE and As-CSC cells, respectively (Table 1). There was significantly more downregulated than upregulated miRNAs in both transformants. Among the downregulated miRNAs, miR-134 (14.6-fold), miR-127-5p (13.2-fold), and miR-373 (10.6-fold) were most highly downregulated in CAsE-PE cells, whereas miR-135b (5-fold), miR-143 (4.8-fold), and miR-218 (4.4-fold) were most highly downregulated in As-CSCs. Only a few miRNAs were upregulated in both transformants. In this regard, miR-9 increased the most (7.4-fold) in CAsE-PE cells followed by miR-96 (2.7-fold) and miR-183 (2-fold), whereas in As-CSC, miR-34a was the most increased (2.3-fold) followed by let-29b (1.9-fold), miR-193b (1.8-fold), and miR-7 (1.7-fold). When we compared the miRNA expression patterns in the transformed total population versus SC population, significant differences were found in terms of number of affected miRNAs and levels of change. CAsE-PE total malignant epithelial population showed generally higher level changes (up to 14.6-fold) than transformed As-CSCs (only up to 5-fold). Moreover, seven dysregulated miRNAs were common to both transformants, most of which changed in the same direction. This included miR-34c-5p, miR-135b, miR-138, miR-205, and miR-218 which were downregulated in both transformants, whereas miR-9 and miR-34a were changed in opposite directions in the transformed cell lines. We observed that four let-7 family members (let-7b, let-7c, let-7e, and let-7i) and all miR-181 family members (miR-181a, -181b, -181c, -181d) were downregulated in CAsE-PE cells but did not change in the isogenic As-CSC. These data suggest aberrant miRNA expression might be significant in the malignant transformation process of prostate epithelium by arsenic, and the spectrum of miRNA change could vary in the SC subpopulation when compared with the whole epithelium.
TABLE 1.
Differentially Expressed microRNAs in CAsE-PE and As-CSC Cells Analyzed by microRNA Cancer Array
| Fold regulationa | |||
|---|---|---|---|
| microRNA | CAsE-PE | As-CSC | Predicted targets assayedb |
| miR-134 | 14.6 down | No change | RAN, KRAS, NANOG, RAB27A |
| miR-127-5p | 13.2 down | No change | EREG |
| miR-373 | 10.6 down | No change | RAB22A, VEGFA |
| miR-9 | 7.4 up | 2.6 down | DICER1 |
| miR-34c-5p | 5.9 down | 2.4 down | BCL2, MAP4K4, RRAS |
| miR-146b-5p | 5.7 down | No change | |
| miR-135b | 5.4 down | 5 down | BCL2L2 |
| miR-222 | 5 down | No change | |
| miR-155 | 4.4 down | No change | KRAS |
| miR-138 | 4.2 down | 1.8 down | RAB22A |
| miR-205 | 4.1 down | 2.4 down | VEGFA, RAN, BCL2L2 |
| miR-218 | 2.9 down | 4.4 down | |
| miR-10b | 2.9 down | No change | |
| miR-181d | 2.8 down | No change | KRAS, BCL2 |
| miR-96 | 2.7 up | No change | MTSS1, FOXO4, RRAS |
| miR-125a-5p | 2.6 down | No change | DICER1 |
| let-7b | 2.6 down | No change | RAS, DICER1, BCL2L1 |
| miR-181b | 2.6 down | No change | |
| miR-98 | 2.4 down | No change | |
| let-7i | 2.1 down | No change | RAS, BCL2L1 |
| miR-34a | 2 down | 2.3 up | ITGA10, CTNND1 |
| miR-196a | 2 down | No change | |
| miR-181a | 2 down | No change | BCL2 |
| miR-183 | 2 up | No change | ITGB1 |
| let-7e | 1.9 down | No change | RAS, BCL2L1 |
| miR-181c | 1.9 down | No change | MAPK1, RAN |
| let-7c | 1.8 down | No change | RAS, BCL2L1 |
| miR-125b | 1.8 down | No change | VEGFA, BCL2 |
| miR-126 | 1.6 down | No change | VEGFA |
| miR-143 | No change | 4.8 down | KRAS, MAPK1, BCL2, NRAS |
| let-29b | No change | 1.9 up | PTEN, ITGB1, ITGA6 |
| miR-193b | No change | 1.8 up | ITGB1, PTEN |
| miR-355 | No change | 1.8 down | |
| miR-7 | No change | 1.7 up | CTNND1 |
| miR-148a | No change | 1.7 down | NRAS |
aFold regulation is compared with microRNA expression in RWPE-1 for CAsE-PE and in WPE-stem cells for As-CSC, and are significantly different (p < 0.05).
bPredicted targets by TargetScan 6.0 and miRNA.org assayed; miRNA expression is inversely correlated with expression of target genes.
Relationship Between miRNA Expression and Target Gene Expression in Arsenic Transformants
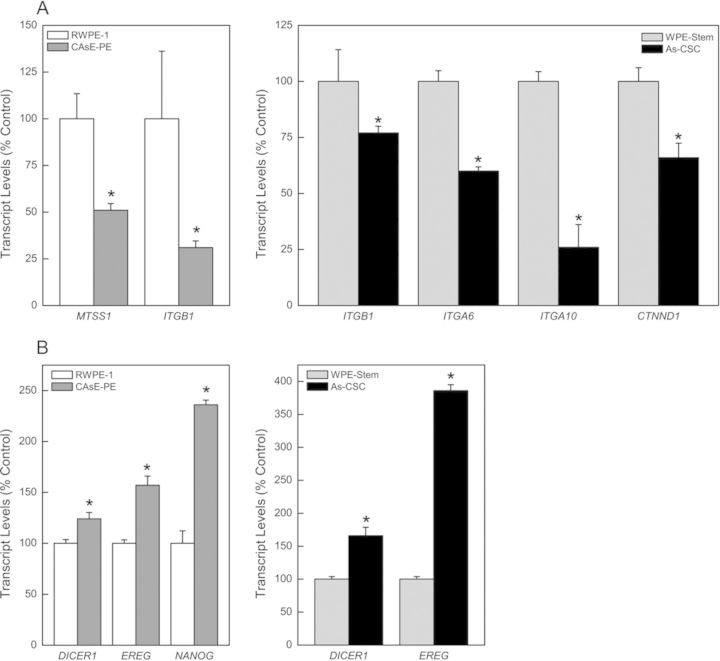
It is thought miRNAs exert their gene regulatory function by mRNA degradation or translational repression of target mRNAs. Using the bioinformatics tools, TargetScan 6.2 (http://www.targetscan.org) and microRNA.org (http://www.microrna.org) which are available online, cancer-related gene targets of selected dysregulated miRNAs in arsenic-transformed prostate epithelial and SCs were identified (Table 1). Predicted or previously confirmed targets were directly analyzed by gene expression (mRNA and/or protein production). As expected, expression of dysregulated miRNAs (Table 1) inversely correlated with target gene expression at the transcript and/or protein level in both transformants (Fig. 1). Although many altered targets were unique to each transformant, some important targets were common to both CAsE-PE and As-CSC cells and shared common pathways including: (1) oncogenesis (RAN and KRAS), (2) miRNA biogenesis (DICER1: 24% increase in CAsE-PE and 66% in As-CSCs), (3) cell signaling (ERK), (4) mitogenesis (EREG: 57% increase in CAsE-PE and 286% in As-CSCs), and (5) tumor metastasis and invasion (ITGB1: 69% decrease in CAsE-PE and 23% in As-CSCs). Distinct targets in CAsE-PE which correlated with miRNA expression include increased RAB22A and RAB27A (oncogenes), increased NANOG by 136% (blocks SC differentiation), increased BCL2L1 and BCL2L2 (apoptosis-related genes), increased VEGF (angiogenesis), and decreased MTSS1 by 49% (metastasis-related gene). Similarly, As-CSC cells showed unique altered targets such as increased NRAS and RRAS (oncogenes); increased MAP4K4 (cell-signaling-related gene); and decreased ITGA6 (40%), ITGA10 (74%), and CTNND1 by 34% (cell-adhesion-related genes). The changes in both CAsE-PE and As-CSC cells would generally favor tumor formation, suggesting a key role in the malignant transformation of prostate epithelial and/or SCs by arsenic.
FIG. 1.
Validation of some miRNA predicted and/or confirmed targets in arsenic transformed CAsE-PE and As-CSCs. mRNA expression of genes associated with: (A) cell adhesion and metastasis; (B) miRNA biogenesis, mitogenesis, and SC pluripotency. Data represent mean ± SEM (n = 3). *p < 0.05 compared with time-matched controls.
Aberrant Expression of RAS-Targeting miRNAs and Target Validation in Arsenic Transformants
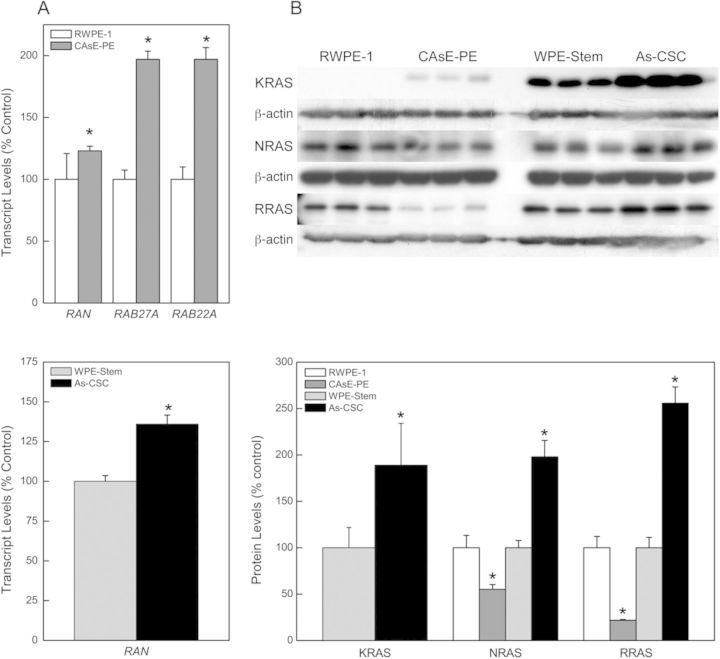
RAS is a very large superfamily of small GTPases that are oncogenes and are often overexpressed in cancer cells. The oncogene RAS superfamily consists of RAS, RAB, RAN, RHO, and ARF families. Multiple reports indicate arsenic induces RAS oncogene expression (Benbrahim-Tallaa et al., 2005; Okoji et al., 2002). In fact, KRAS was previously reported to be induced in arsenic-transformed prostate epithelial cells (CAsE-PE), and this induction precedes malignant transformation and is sustained thereafter (Benbrahim-Tallaa et al., 2005). Although the underlying mechanism of arsenic-induced KRAS expression is incompletely defined, KRAS induction in CAsE-PE cells is not by gene mutation or promoter region hypomethylation (Benbrahim-Tallaa et al., 2005), which are common activation mechanisms. These prior data strongly suggest alternative mechanisms are involved. In the current study, we also found that overexpression of KRAS was very pronounced in the arsenic-transformed prostate SCs (As-CSC), which is a novel finding, clearly implicating relevance to malignant phenotype at the SC level (Fig. 2B). To further assess alternative mechanisms of KRAS induction by arsenic, our list of dysregulated miRNAs was assessed for RAS-targeting miRNAs. In both arsenic-transformed CAsE-PE and As-CSC cells, arsenic exposure downregulated the expression of many miRNAs predicted and/or confirmed to target KRAS and other members of the RAS superfamily (Table 1). Validation by RT-PCR and Western blot showed that miRNA expression inversely correlated with RAS gene expression at the transcript and/or protein level in both transformants. Downregulation of miR-134 (14.6-fold), miR-373 (10.6-fold), miR-155 (4.4-fold), miR-138 (4.2-fold), miR-205 (4.1-fold), miR-181d (2.8-fold), miR-181c (1.9-fold), let-7b (2.6-fold), let-7i (2.1-fold), let-7e (1.9-fold), and let-7c (1.8-fold) in CAsE-PE cells correlated with marked increased expression of target RAS oncogenes, RAN (23%), RAB22A (97%), RAB27A (97%) mRNAs, and KRAS protein (Figs. 2A and B). It was difficult to determine percentage of increased expression for KRAS in CAsE-PE cells because protein expression was below detectable limits in control RWPE-1 cells. Surprisingly, we saw decreased expression of NRAS (45%) and RRAS (22%) proteins in CAsE-PE cells. Decreased RRAS expression might be caused by upregulated (2.7-fold) RRAS-targeting miR-96 (Table 1). None of the upregulated miRNAs in CAsE-PE cells is predicted to target NRAS, suggesting gene expression might be regulated by other factors.
FIG. 2.
Expression of RAS oncogenes in CAsE-PE and As-CSC cells. (A) Transcript levels of RAS mRNAs in CAsE-PE and As-CSC cells. (B) RAS protein levels in CAsE-PE and As-CSC cells. Data represent mean ± SEM (n = 3). *p < 0.05 compared with time-matched controls.
Downregulation of miR-143 (4.8-fold), miR-34c-5p (2.5-fold), and miR-205 (2.4-fold) in As-CSC cells correlated with marked increase in expression of target RAN mRNA (36%), and KRAS (89%), NRAS (98%), and RRAS (156%) proteins (Figs. 2A and B). These data suggest aberrant miRNA expression is impacting activation of RAS oncogenes during arsenic-induced malignant transformation of prostate epithelial and SCs.
Dysregulation of the RAS/ERK Pathway in Arsenic Transformation
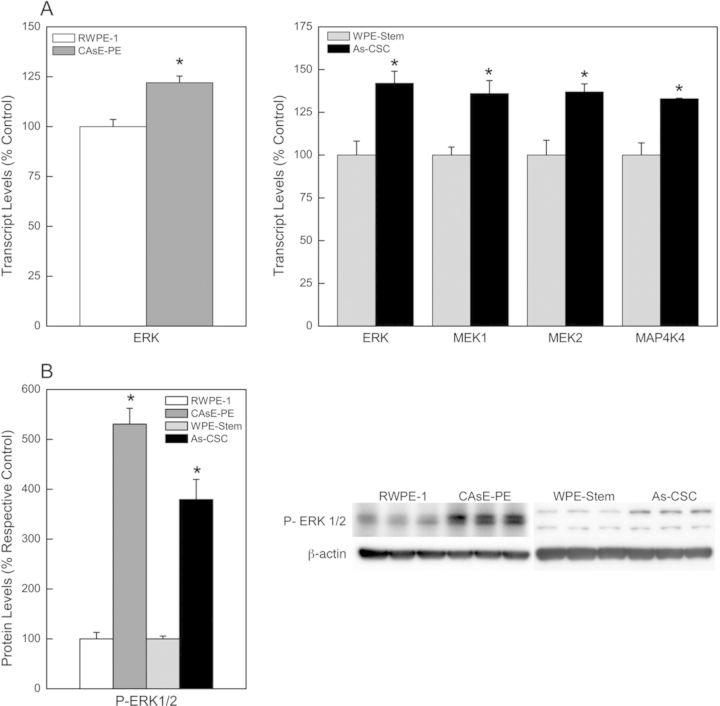
RAS proteins are signaling nodes that are activated in response to several extracellular signals. Upon activation, RAS proteins interact with many downstream effectors, which transduce a signal leading to altered gene expression. The RAS signaling pathway is key in controlling cell survival, differentiation, proliferation, angiogenesis, motility, and metabolism, and if dysregulated promotes oncogenic transformation and progression. One of the best characterized RAS signaling pathways is the RAS/ERK or MAPK pathway. We previously showed that activation of the RAS/ERK pathway occurred in arsenic-transformed total prostate epithelium, CAsE-PE cells (Benbrahim-Tallaa et al., 2007). However, it is unknown whether the RAS/ERK pathway is also activated in the transformed SCs, As-CSCs. Thus, further analysis of events in the RAS/ERK pathway was performed in As-CSC. As shown in Figure 3, there was increased mRNA expression of the ERK kinase (22%) in CAsE-PE; and MEK1/2 (36%), ERK (42%), and MAP4K4 (33%) kinases in As-CSC (Fig. 3A), which are downstream of RAS. Similarly, activated p-ERK (Thr202/Tyr204) was increased in both CAsE-PE and As-CSC cells (Fig. 3B), suggesting pathway activation.
FIG. 3.
Analysis of the RAS/ERK signaling pathway in CAsE-PE and As-CSC cells. (A) Transcript levels of some genes downstream of RAS. (B) Western blot analysis of p-ERK (activated form). Data represent mean ± SEM (n = 3). *p < 0.05.
Dysregulation of Some Components of the PI3K/PTEN/AKT Pathway
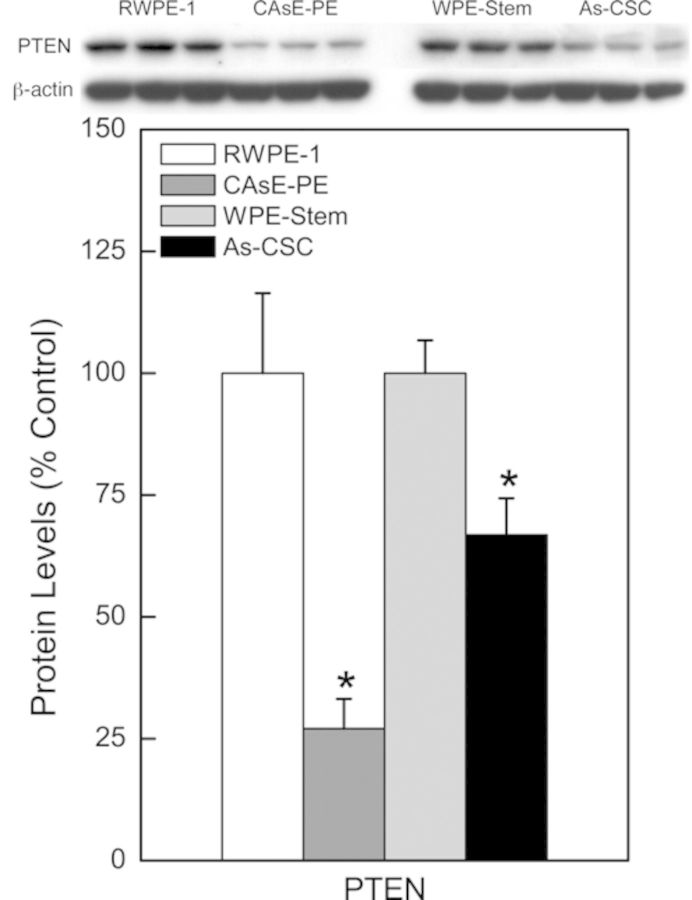
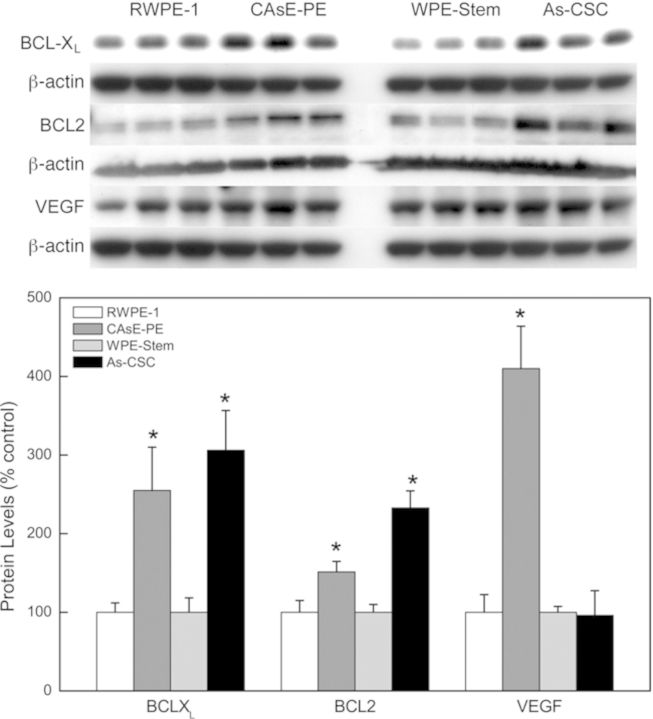
Apart from RAS/ERK pathway, another well-studied signaling pathway triggered by RAS activation is the PI3K/PTEN/AKT pathway. PTEN, a negative regulator of the PI3K/PTEN/AKT pathway, was markedly decreased at the transcript and/or protein level in the arsenic transformants (Fig. 4). PTEN mRNA expression was decreased by 36% in As-CSCs whereas protein expression was decreased 73% in CAsE-PE and 33% in As-CSCs. However, the total or activated AKT, a key substrate in the PI3K/PTEN/AKT pathway, was not induced in either transformants (not shown). Nevertheless, there was induction of genes (anti-apoptotic and angiogenic) further downstream of the PI3K/PTEN/AKT pathway. In CAsE-PE cells, there was increased expression of BCL-XL (155%) and VEGFA (562%) mRNAs; and BCL2 (206%), BCL-XL (155%), and VEGF (310%) proteins (Fig. 5A). Similarly, As-CSC cells showed increased protein expression of BCL-XL (206%) and BCL2 (133%) (Fig. 5). These data suggest that the dysregulation of some components of the PI3K/PTEN/AKT pathway without apparent pathway activation might be a result of direct effects of dysregulated miRNAs targeting these genes (see Table 1).
FIG. 4.
PTEN protein expression in arsenic-transformed CAsE-PE and As-CSC cells. Data represent mean ± SEM (n = 3). *p < 0.05 compared with time-matched controls.
FIG. 5.
Expression of genes associated with and further downstream of the PI3K/PTEN/AKT pathway. Data represent mean ± SEM (n = 3). *p < 0.05 compared with time-matched controls.
DISCUSSION
In the present work, a distinct miRNA expression signature in arsenic-transformed prostate epithelial and transformed isogenic SCs occurred. The dysregulation of miRNAs correlated with expression of critical target genes in the transformed cells, and likely key oncogenic pathways, like RAS. This is consistent with altered miRNA expression playing a pathogenic role in human prostate carcinogenesis (Hassan et al., 2012). Although arsenic-transformed prostate epithelial and SCs are derived from the same parental line, surprisingly, the arsenic transformants showed distinct miRNA signatures, with a few commonly altered miRNAs. This distinct expression pattern is consistent with the notion that subpopulations of prostate cancers exhibit unique miRNA signatures (Liu et al., 2012). Interestingly, regardless of the cell line in question in our study, the number of downregulated miRNAs was higher than upregulated miRNAs, consistent with previous miRNA profiling studies on prostate cancers (Liu et al., 2012; Porkka et al., 2007). The general pattern of downregulation of miRNAs in cancer might reflect poor differentiation of tumor cells. Indeed, the xenograft tumors produced by the As-CSC cells are very poorly differentiated (Tokar et al., 2010a). The miRNA profile of the arsenic transformants showed a combination of miRNAs that are often dysregulated in prostate cancer (e.g., miR-222, miR-205, miR-218, miR-143, miR-96, miR-34a, let-7, miR-125a-5p, miR-125b, and miR-183) and other transformed cell types induced by v-ras-ki oncogene (miR-34a; Mussnich et al., 2013), or cigarette smoke (miR-222 and miR-125; Izzotti et al., 2009), as well as some miRNAs that are not commonly dysregulated or dysregulated in the opposite direction. This suggests that arsenic-induced prostate cancer has a distinct miRNA signature that is specific for the metalloid, and that miRNA expression is potentially cell-type and carcinogen specific.
Evidence indicates that CSCs may be involved in tumor progression and maintenance by aberrant self-renewal and differentiation. However, it is poorly understood how intrinsic CSC properties are regulated at the molecular level. miRNAs control self-renewal and differentiation of embryonic SCs, suggesting they may regulate CSCs (Tay et al., 2008a). Data indicate that miR-34a is downregulated in several CSCs including prostate CSCs (Liu et al., 2011). miR-34a suppresses CSC properties in vitro and in vivo (Liu and Tang, 2011). In prostate CSCs, miR-34a can inhibit CSC properties by directly targeting CD44 inducing inhibition of migration and invasiveness of CD44+ prostate CSCs purified from tumors (Liu et al., 2011). Let-7 also regulates SC properties such as self-renewal and differentiation, and is downregulated in prostate CSCs (Liu et al., 2012). Similarly, miR-134 promotes SC differentiation by translational repression of Nanog, a gene that maintains SC pluripotency (Tay et al., 2008b); thus, decreased miR-134 expression helps maintain SC properties. Consistent with previous reports, arsenic-transformed CAsE-PE cells (the heterogeneous total cell population containing some SCs) showed downregulation of miR-34a, miR-134, and let-7, which may help account for the CSC overabundance observed in these transformants (Tokar et al., 2010b). However, the opposite effects of miR-34a upregulation and no change in miR-134 and let-7 were seen in the transformed SCs, As-CSC. Although it is unclear why in the CSCs miR-34a is overexpressed rather than underexpressed, a plausible explanation is that the regulation is arsenic-specific. These differences in SC regulatory miRNA expression in total population versus SC population indicate that arsenic effects are cell-subpopulation specific.
Studies of miRNAs indicate that they regulate cancer pathways by targeting different oncogenes and/or tumor suppressor genes. RAS is activated in many cancers, and dysregulation leads to abnormal cell proliferation, survival, and motility, favoring tumor progression and metastasis (Fernández-Medarde and Santos, 2011). RAS superfamily members, particularly KRAS, are overexpressed in many cancers including prostate cancer, often due to an activating gene mutation (Silan et al., 2012). However, nonmutational events can also activate KRAS in prostate cancers, and activation appears key in prostate cancer progression (Weber and Gioeli, 2004). Prior work shows KRAS is overexpressed in CAsE-PE cells, and activation precedes malignant transformation (Benbrahim-Tallaa et al., 2005), consistent with other reports of arsenic-induced KRAS activation in vitro and in vivo (Chen et al., 2001; Okoji et al., 2002). However, KRAS activation in CAsE-PE cells was not by common gene mutation or through promoter region DNA hypomethylation (Benbrahim-Tallaa et al., 2005), suggesting alternative mechanisms. In the present study, KRAS is also activated in arsenic-transformed SCs suggesting activation occurs in this key subpopulation of prostate cells. In both transformants, various miRNAs were detected that target KRAS or other members of the RAS superfamily, including miR-134, miR-373, miR-34c-5p, miR-155, miR-138, miR-181d, miR-96, miR-181c, miR-143, miR-148a, and let-7. The dysregulated expression of RAS-targeting miRNAs correlated with RAS overexpression, strongly suggesting a role of dysregulated miRNAs in the activation of KRAS in the epithelium and in the SCs malignantly transformed by arsenic. MiR-143 is a well-established tumor suppressor that is decreased in prostate cancer (Clape et al., 2009; Xu et al., 2011), which suppresses KRAS and the RAS/MAPK signaling pathway, decreasing cell proliferation and migration (Xu et al., 2011). Decreased miR-143 expression would favor prostate cancer progression and invasion. KRAS activation in arsenic-transformed SCs was associated with activation of the RAS/ERK pathway, consistent with the activation of this pathway in the transformed total cell population (Benbrahim-Tallaa et al., 2005).
It is important to note that KRAS activation is not obligatory to malignant transformation of RWPE-1 prostate epithelial cells, and we find that B26 cells, which are RWPE-1 cells transformed by N-methyl-N-nitrosourea, a direct genotoxin, show no evidence of enhanced KRAS signaling (Ngalame et al., unpublished data). Further work directly demonstrating the causative role of KRAS expression in arsenic transformation of human prostate cells is warranted. Such experiments could involve silencing KRAS directly via shRNA or by overexpressing key downregulated RAS-targeting miRNAs and looking for partial or total reversal of malignant phenotype. Arsenic likely works through multiple mechanisms and disrupted RAS signaling, thought important, is probably not the sole factor in acquired malignant phenotype in the cells used in this work. However, the present work now provides a plausible mechanism for RAS activation in iAs carcinogenesis not involving a mutation or methylation change consistent with prior work (Benbrahim-Tallaa et al., 2005).
RAS protein can also activate the PI3K/PTEN/AKT pathway, a key component of which is AKT, which becomes phosphorylated upon activation (De Luca et al., 2012). AKT expression or phosphorylated AKT did not increase, suggesting that the PI3K/PTEN/AKT pathway was not activated in the arsenic transformants, consistent with data that the PI3K/AKT pathway is not activated despite RAS/ERK pathway activation in arsenic-transformed rat lung epithelial cells (Li et al., 2011). Thus, arsenic may preferentially activate the RAS/ERK pathway during malignant cell transformation, at least in these models. The PTEN tumor suppressor gene is often lost in prostate cancer by mutations or deletion events (Verhagen et al., 2006). In this study, arsenic-induced transformation of prostate total cell population and SCs was associated with marked PTEN suppression, consistent with reports of rapid and persistent PTEN suppression during the malignant transformation of normal SCs by arsenic-transformed prostate epithelial in a co-culture system (Xu et al., 2012). PTEN loss leads to increased cell proliferation, increased prostatic SCs, and increased tumor initiation and invasion (Wang et al., 2006). PTEN loss alone appears insufficient to promote prostate cancer, but cooperates with RAS/MAPK activation to enhance epithelial-to-mesenchymal transition and metastasis of prostatic CSCs (Mulholland et al., 2012). PTEN suppression coupled with RAS/MAPK activation appears important for arsenic-induced malignant transformation. PTEN negatively regulates the PI3K/PTEN/AKT pathway, and loss of PTEN results in AKT activation which is key to PTEN-mediated tumorigenesis (Blanco-Aparicio et al., 2007). However, PTEN can mediate tumorigenesis through mechanisms independent of AKT (Blanco-Aparicio et al., 2007), suggesting that the suppression of PTEN in arsenic transformants likely affects targets other than AKT. Further in-depth analysis of the PI3K/PTEN/AKT pathway and alternative PTEN targets in these transformants will be pursued. BCL2, BCL-XL, and VEGF are genes that are regulated by the PI3K/PTEN/AKT pathway, and are often upregulated in prostate cancer, and are associated with prostate carcinogenesis (Chen et al., 2004; Lin et al., 2007; Vilenchik et al., 2002). BCL-2 and BCL-XL were overexpressed in arsenic transformants despite no evidence of pathway activation, suggesting this might be a direct effect of downregulated miRNAs targeting these genes. Upregulation of BCL-2 is not only required for the survival of androgen-independent prostate cancer cells, but also for the progression of prostate cancer cells from androgen-dependent to androgen-independent (Lin et al., 2007). Similarly, knockdown of BCL-XL in prostate cancer cells decreases cell proliferation and resistance to cytotoxic chemotherapeutic agents (Vilenchik et al., 2002). These studies suggest that BCL2 and BCL-XL might trigger survival signals in the arsenic transformants, favoring tumorigenesis. Angiogenesis is critical during tumor initiation and progression. In our study, VEGF expression was markedly increased only in the arsenic-transformed total cell population and not in SCs. VEGF is upregulated in prostate cancer cells and promotes metastasis (Chen et al., 2004). Recently, Beck et al. (2011) showed that overexpression of VEGF increased skin tumor growth by promoting cancer cell “stemness” and symmetric CSC division, leading to CSC expansion. These findings (Beck et al., 2011) suggest that VEGF overexpression in arsenic-transformed total population might be contributing to the overaccumulation of CSCs observed in these transformants relative to their controls.
In conclusion, this work shows that several miRNAs are dysregulated in arsenic-transformed prostate epithelial cells and in transformed SCs. Dysregulated miRNA expression appears to impact RAS activation in the total cell population and SCs transformed by chronic arsenic exposure. KRAS activation appears important to arsenic transformation in these cells, and miRNA dysregulation is a plausible mechanism of this activation.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences of the National Institutes of Health.
Supplementary Material
Acknowledgments
We thank Matt Bell for his assistance in preparation of the graphics and Dr Jonathan Freedman and Dr Yang Sun for their critical review of this manuscript.
REFERENCES
- Achanzar W. E., Brambila E. M., Diwan B. A., Webber M. M., Waalkes M. P. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J. Natl. Cancer Inst. 2002;94:1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- Bartel D. P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Beck B., Driessens G., Goossens S., Youssef K. K., Kuchnio A., Caauwe A., Sotiropoulou P. A., Loges S., Lapouge G., Candi A., et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- Bello D., Webber M. M., Kleinman H. K., Wartinger D. D., Rhim J. S. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18:1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L., Waalkes M. P. Inorganic arsenic and human prostate cancer. Environ. Health Perspect. 2008;116:158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L., Waterland R. A., Styblo M., Achanzar W. E., Webber M. M., Waalkes M. P. Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: Aberrant genomic DNA methylation and K-ras oncogene activation. Toxicol. Appl. Pharmacol. 2005;206:288–298. doi: 10.1016/j.taap.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L., Webber M. M., Waalkes M. P. Mechanisms of acquired androgen independence during arsenic-induced malignant transformation of human prostate epithelial cells. Environ. Health Perspect. 2007;115:243–247. doi: 10.1289/ehp.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Aparicio C., Renner O., Leal J. F., Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- Calin G. A., Croce C. M. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Chen H., Liu J., Merrick B. A., Waalkes M. P. Genetic events associated with arsenic-induced malignant transformation: Applications of cDNA microarray technology. Mol. Carcinog. 2001;30:79–87. doi: 10.1002/1098-2744(200102)30:2<79::aid-mc1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Chen J., De S., Brainard J., Byzova T. V. Metastatic properties of prostate cancer cells are controlled by VEGF. Cell Commun. Adhes. 2004;11:1–11. doi: 10.1080/15419060490471739. [DOI] [PubMed] [Google Scholar]
- Clape C., Fritz V., Henriquet C., Apparailly F., Fernandez P. L., Iborra F., Avances C., Villalba M., Culine S., Fajas L. miR-143 interferes with ERK5 signaling, and abrogates prostate cancer progression in mice. PLoS ONE. 2009;4:e7542. doi: 10.1371/journal.pone.0007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A., Maiello M. R., D'Alessio A., Pergameno M., Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin. Ther. Targets. 2012;16(Suppl. 2):S17–S27. doi: 10.1517/14728222.2011.639361. [DOI] [PubMed] [Google Scholar]
- Fernández-Medarde A., Santos E. Ras in cancer and developmental disease. Genes and Cancer. 2011;2:344–358. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan O., Ahmad A., Sethi S., Sarkar F. H. Recent updates on the role of microRNAs in prostate cancer. J. Hematol. Oncol. 2012;5:1–10. doi: 10.1186/1756-8722-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. (International Agency for Research on Cancer) Arsenic and arsenic compounds. IARC Monogr. Eval. Carcinog. Risk Hum. 2012;100C:41–94. [Google Scholar]
- Izzotti A., Calin G. A., Arrigo P., Steele V. E., Croce C. M., De Flora S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2009;23:806–812. doi: 10.1096/fj.08-121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima C., Ramirez D. C., Tokar E. J., Himeno S., Drobna Z., Styblo M., Mason R. P., Waalkes M. P. Requirement of arsenic biomethylation for oxidative DNA damage. J. Natl. Cancer Inst. 2009;101:1670–1681. doi: 10.1093/jnci/djp414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Lee L. S., Li M., Tsao S. W., Chiu J. F. Molecular changes during arsenic-induced cell transformation. J. Cell. Physiol. 2011;226:3225–3232. doi: 10.1002/jcp.22683. [DOI] [PubMed] [Google Scholar]
- Lin Y., Fukuchi J., Hiipakka R. A., Kokontis J. M., Xiang J. Up-regulation of Bcl-2 is required for the progression of prostate cancer cells from an androgen-dependent to an androgen-independent growth stage. Cell Res. 2007;17:531–536. doi: 10.1038/cr.2007.12. [DOI] [PubMed] [Google Scholar]
- Liu C., Kelnar K., Liu B., Chen X., Calhoun-Davis T., Li H., Patrawala L., Yan H., Jeter C., Honorio S., et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Kelnar K., Vlassov A. V., Brown D., Wang J., Tang D. G. Distinct microRNA expression profiles in prostate cancer stem/progenitor cells and tumor-suppressive functions of let-7. Cancer Res. 2012;72:3393–3404. doi: 10.1158/0008-5472.CAN-11-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Tang D. G. MicroRNA regulation of cancer stem cells. Cancer Res. 2011;71:5950–5954. doi: 10.1158/0008-5472.CAN-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland D. J., Kobayashi N., Ruscetti M., Zhi A., Tran L. M., Huang J., Gleave M., Wu H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussnich P., D'Angelo D., Leone V., Croce C. M., Fusco A. The High Mobility Group A proteins contribute to thyroid cell transformation by regulating miR-603 and miR-10b expression. Mol. Oncol. 2013;7:531–542. doi: 10.1016/j.molonc.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoji R. S., Yu R. C., Maronpot R. R., Froines J. R. Sodium arsenite administration via drinking water increases genome-wide and Ha-ras DNA hypomethylation in methyl-deficient C57BL/6J mice. Carcinogenesis. 2002;23:777–785. doi: 10.1093/carcin/23.5.777. [DOI] [PubMed] [Google Scholar]
- Porkka K. P., Pfeiffer M. J., Waltering K. K., Vessella R. L., Tammela T. L., Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- Silan F., Gultekin Y., Atik S., Kilinc D., Alan C., Yildiz F., Uludag A., Ozdemir O. Combined point mutations in codon 12 and 13 of KRAS oncogene in prostate carcinomas. Mol. Biol. Rep. 2012;39:1595–1599. doi: 10.1007/s11033-011-0898-8. [DOI] [PubMed] [Google Scholar]
- Sun Y., Tokar E. J., Waalkes M. P. Overabundance of putative cancer stem cells in human skin keratinocyte cells malignantly transformed by arsenic. Toxicol. Sci. 2012;125:20–29. doi: 10.1093/toxsci/kfr282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay Y., Zhang J., Thomson A. M., Lim B., Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008a;455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- Tay Y. M., Tam W. L., Ang Y. S., Gaughwin P. M., Yang H., Wang W., Liu R., George J., Ng H. H., Perera R. J., et al. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells. 2008b;26:17–29. doi: 10.1634/stemcells.2007-0295. [DOI] [PubMed] [Google Scholar]
- Tokar E. J., Ancrile B. B., Cunha G. R., Webber M. M. Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differentiation. 2005;73:463–473. doi: 10.1111/j.1432-0436.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- Tokar E. J., Diwan B. A., Waalkes M. P. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ. Health Perspect. 2010a;118:108–115. doi: 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar E. J., Diwan B. A., Ward J. M., Delker D. A., Waalkes M. P. Carcinogenic effects of “whole-life” exposure to inorganic arsenic in CD1 mice. Toxicol. Sci. 2011;119:73–83. doi: 10.1093/toxsci/kfq315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar E. J., Qu W., Liu J., Liu W., Webber M. M., Phang J. M., Waalkes M. P. Arsenic-specific stem cell selection during malignant transformation. J. Natl. Cancer Inst. 2010b;102:638–649. doi: 10.1093/jnci/djq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen P. C., van Duijn P. W., Hermans K. G., Looijenga L. H., van Gurp R. J., Stoop H., van der Kwast T. H., Trapman J. The PTEN gene in locally progressive prostate cancer is preferentially inactivated by bi-allelic gene deletion. J. Pathol. 2006;208:699–707. doi: 10.1002/path.1929. [DOI] [PubMed] [Google Scholar]
- Vilenchik M., Raffo A. J., Benimetskaya L., Shames D., Stein C. A. Antisense RNA down-regulation of bcl-xL Expression in prostate cancer cells leads to diminished rates of cellular proliferation and resistance to cytotoxic chemotherapeutic agents. Cancer Res. 2002;62:2175–2183. [PubMed] [Google Scholar]
- Volinia S., Calin G. A., Liu C. G., Ambs S., Cimmino A., Petrocca F., Visone R., Iorio M., Roldo C., Ferracin M., et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes M. P., Liu J., Germolec D. R., Trempus C. S., Cannon R. E., Tokar E. J., Tennant R. W., Ward J. M., Diwan B. A. Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of tumor stem cell dynamics. Cancer Res. 2008;68:8278–8285. doi: 10.1158/0008-5472.CAN-08-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Garcia A. J., Wu M., Lawson D. A., Witte O. N., Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc. Natl. Acad. Sci. USA. 2006;103:1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J., Gioeli D. Ras signaling in prostate cancer progression. J. Cell. Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- Xu B., Niu X., Zhang X., Tao J., Wu D., Wang Z., Li P., Zhang W., Wu H., Feng N., et al. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol. Cell. Biochem. 2011;350:207–213. doi: 10.1007/s11010-010-0700-6. [DOI] [PubMed] [Google Scholar]
- Xu Y., Tokar E. J., Sun Y., Waalkes M. P. Arsenic-transformed malignant prostate epithelia can convert noncontiguous normal stem cells into an oncogenic phenotype. Environ. Health Perspect. 2012;120:865–871. doi: 10.1289/ehp.1204987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.